Abstract
Background
Therapeutic efficacy of various mesenchymal stromal cell (MSC) types for orthopaedic applications is currently being investigated. While the concept of MSC therapy is well grounded in the basic science of healing and regeneration, little is known about individual MSC populations in terms of their propensity to promote the repair and/or regeneration of specific musculoskeletal tissues. Two promising MSC sources, adipose and amnion, have each demonstrated differentiation and extracellular matrix (ECM) production in the setting of musculoskeletal tissue regeneration. However, no study to date has directly compared the differentiation potential of these 2 MSC populations.
Purpose
To compare the ability of human adipose- and amnion-derived MSCs to undergo osteogenic and chondrogenic differentiation.
Study Design
Controlled laboratory study.
Methods
MSC populations from the human term amnion were quantified and characterized via cell counting, histologic assessment, and flow cytometry. Differentiation of these cells in comparison to commercially purchased human adipose-derived mesenchymal stromal cells (hADSCs) in the presence and absence of differentiation media was evaluated via reverse transcription polymerase chain reaction (PCR) for bone and cartilage gene transcript markers and histology/immunohistochemistry to examine ECM production. Analysis of variance and paired t tests were performed to compare results across all cell groups investigated.
Results
The authors confirmed that the human term amnion contains 2 primary cell types demonstrating MSC characteristics—(1) human amniotic epithelial cells (hAECs) and (2) human amniotic mesenchymal stromal cells (hAMSCs)—and each exhibited more than 90% staining for MSC surface markers (CD90, CD105, CD73). Average viable hAEC and hAMSC yields at harvest were 2.3 × 106 ± 3.7 × 105 and 1.6 × 106 ± 4.7 × 105 per milliliter of amnion, respectively. As well, hAECs and hAMSCs demonstrated significantly greater osteocalcin (P = .025), aggrecan (P < .0001), and collagen type 2 (P = .044) gene expression compared with hADSCs, respectively, after culture in differentiation medium. Moreover, both hAECs and hAMSCs produced significantly greater quantities of mineralized (P < .0001) and cartilaginous (P = .0004) matrix at earlier time points compared with hADSCs when cultured under identical osteogenic and chondrogenic differentiation conditions, respectively.
Conclusion
Amnion-derived MSCs demonstrate a greater differentiation potential toward bone and cartilage compared with hADSCs.
Clinical Relevance
Amniotic MSCs may be the source of choice in the regenerative treatment of bone or osteochondral musculoskeletal disease. They show significantly higher yields and better differentiation toward these tissues than MSCs derived from adipose.
Keywords: stromal cell, perinatal, adipose, differentiation, regenerative medicine, cartilage, bone, orthopaedics
Focal defects in articular cartilage and the underlying bone caused by wear and tear, traumatic injury, or metabolic disorders can result in persistent pain, disability, and osteoarthritis.10,16 It has been estimated that approximately 4 million knee arthroscopies are performed annually worldwide and that 20% to 60% of these procedures reveal the presence of focal chondral or osteochondral defects.7 Despite the availability of various treatment options, significant limitations remain.8 Alternatively, researchers continue to develop and evaluate tissue engineering strategies that use scaffolds seeded with alternative cell sources in an attempt to regenerate cartilage and bone for the management of these lesions.20,28
Mesenchymal stromal cells (MSCs) represent an alternative cell source for use in cartilage and bone reconstruction due to their capacity for osteogenic and chondrogenic differentiation. 9,23,30 While the majority of adult tissues have been shown to contain resident MSCs, studies have illustrated that MSC numbers decline with increasing age.24 Human MSCs, including those isolated from adipose (hADSCs) and bone marrow–derived (hBMSCs) tissue, have demonstrated varying degrees of therapeutic efficacy in preclinical models and human clinical trials. Reduced pain5 and/or improved chondroprotection has been observed after MSC treatment. 15,27 Moreover, enhanced cartilage healing was observed in MSC-treated defects compared with those receiving autologous chondrocytes. Despite these promising outcomes, drawbacks to using hBMSCs and hADSCs include associated donor site morbidity and low cell yields during harvest requiring significant ex vivo expansion before implantation. Additionally, early clinical investigations using MSCs for orthopaedic applications suggest that administration of large quantities of adult MSCs (ie, ≥10 × 107 per dose) are required to realize improved outcomes4,13,19,29; such quantities are not easily achieved with hBMSCs or hADSCs. Identifying alternative MSC sources that can yield a significant number of cells at the time of harvest and that demonstrate the ability to form bone and cartilage tissue would be clinically advantageous.
Recently, researchers have begun investigating the potential therapeutic efficacy of 2 cell populations isolated from the term human amnion (amniotic epithelial and amniotic mesenchymal stromal cells; hAECs and hAMSCs, respectively) for orthopaedic applications due to their MSC-like characteristics. 14 Reported advantages of these cells include significantly greater cell yields at harvest, a lack of donor site morbidity, the presence of a more “youthful” phenotype, compatibility for use in allogenic transplants, and enhanced immunomodulatory properties compared with hBMSCs and hADSCs.1,21,26 However, to date no studies have been conducted to compare the osteogenic and chondrogenic differentiation and the subsequent generation of mineralized and cartilaginous extracellular matrix (ECM) by human amnion-derived MSCs and hADSCs.
Thus, the purpose of this study was to evaluate and compare the temporal expression of genes and ECM production for bone and cartilage in hAECs and hAMSCs with that of hADSCs cultured under identical in vitro differentiation conditions.
METHODS
Amnion Harvest
Human placentas were obtained from consenting patients immediately after delivery via elective cesarean sections of full-term babies under an Institutional Review Board–approved protocol (Greenville Health System: Pro00031185).
Isolation and Enrichment of Amnion-Derived MSC Populations (hAECs and hAMSCs)
Human AECs and AMSCs were isolated from placentas (n = 3) according to methods adapted from Barbati et al2 (Appendix Figure A1, available in the online version of this article). Briefly, amnion were rinsed in sterile saline before 2 sequential digestions in 0.25% trypsin for 30 minutes at 37°C with agitation (150 RPM) to completely liberate only the hAECs followed by digestion in 2 sequential changes of collagenase (2 mg/mL collagenase [249 U/mg]) for 30 minutes at 37°C with agitation to subsequently liberate only the hAMSCs. All digests were filtered through a 100-μm cell strainer before cell counting. Amnion-derived MSCs were subsequently frozen in liquid nitrogen before thawing, pooling of each respective amnion MSC populations from all donors, and expansion to passage 2 before running experiments. Hereafter, replicates of these pooled amnion MSCs were used (ie, n denotes the number of replicates of the pooled amnion MSCs) for each experimental group.
Histological Confirmation Demonstrating Selective Isolation of hAECs and hAMSCs
Amnion sections were fixed with 10% neutral buffered formalin before standard tissue processing, paraffin embedding, and sectioning to 5 μm thicknesses. Cross-sections were stained with hematoxylin and eosin (H&E) for visualization of ECM and cell nuclei in both the epithelial and underlying stromal layers to demonstrate removal of cells from each layer.
Generation of a Mixed Population of Amnion MSCs
A study group consisting of co-cultured hAECs and hAMSCs (termed mixed) was generated by incomplete dissociation of the hAECs from the amnion before complete digestion of the membrane by use of collagenase (Appendix Figure A1). Briefly, amnion (n = 1) was digested twice in a reduced concentration of trypsin (0.125% wt/vol) for 30 minutes at 37°C to incompletely liberate hAECs followed by complete digestion in collagenase, as described above, to liberate the remaining hAECs and hAMSCs. The mixed population of amnion cells was collected from the collagenase digest. This mixed population of amnion-derived MSCs was subsequently frozen in liquid nitrogen before thawing and expansion to passage 2 before running experiments.
Flow Cytometric Analysis of Amnion-Derived MSC Populations
Passage 2 expanded amnion MSCs were resuspended at 1 × 105 cells/mL in phosphate-buffered saline (PBS) and incubated for 30 minutes in the following antibodies for MSC and hemato-lymphoid markers, respectively (final antibody concentrations, 5 μg/mL): mouse anti-CD105 (BD Biosciences: 555690), mouse anti-CD73 (BD Biosciences: 550256), mouse anti-CD90 (BD Biosciences: 555593), and mouse anti-CD45 (BD Biosciences: 555480). A phycoerythrin conjugated mouse anti-EpCAM (BD Biosciences: 347198) was used at a concentration of 0.3 μg/mL to detect the epithelial phenotype. After incubation in primary antibodies, cell suspensions were rinsed and resuspended in PBS containing a goat anti-mouse secondary antibody conjugated with fluorescein isothiocyanate (abD Serotec: STAR117F; 5 μg/mL) for 30 minutes. Samples were subsequently analyzed on a Guava easyCytesingle sample flow cytometer. An isotype control (mouse IgG1 negative control: AbD Serotec MCA928) was included in the analysis to account for nonspecific binding of primary antibodies.
MSC Osteogenic Differentiation Potential
Culture Conditions
Human ADSCs (purchased from Invitrogen) and isolated amnion cells were expanded in standard culture conditions (37°C with 5% CO2) with media changes occurring every 3 days until passage 2. Culture medium for hAECs expansion consisted of Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 ng/mL epidermal growth factor (EMD Millipore), 10% fetal bovine serum (FBS), and 1% antibiotic/antimitotic (Ab/Am). Culture media for hAMSCs and mixed cell expansion consisted of DMEM supplemented with 10% FBS and 1% Ab/AM. Culture medium consisting of MesenPro RS complete media (ThermoFisher) was used for expanding hADSCs per manufacturer’s instructions. To induce osteogenic differentiation, cells were seeded at a density of 2.1 × 104/cm2 into tissue culture–treated 12-well plates and cultured in monolayer for up to 28 days in osteogenic differentiation media (DMEM, 10% FBS, 1% Ab/AM, 0.1 μM dexamethasone, 50 μM ascorbate-2-phosphate, 10 mM β-glycerophosphate). Cells were analyzed for gene expression in “basal” culture media (DMEM + 10% FBS + 1% Ab/AM) for the study duration. Differentiation capacity was assessed via histological staining (n = 2 per condition) and gene transcript expression (n = 4 per condition) as described below.
Gene Transcript Analysis
Total RNA from all samples (n = 4 per condition) was isolated by use of Trizol reagent per the manufacturer’s instructions. RNA purity (260/280 ratio ≥ 1.8) and quantity were assessed by use of a BioTek Epoch Gen 5 spectrophotometer. A total of 600 μg of RNA was reverse transcribed with an Ambion RETROscript kit. Resulting cDNA was amplified by use of a Rotogene 3000 thermocycler. Reaction products were detected with validated human QuantiTect primer assays (Qiagen) (Table 1) in conjunction with a QuantiTect SYBRgreen (Qiagen) polymerase chain reaction kit. Relative differences in gene expression comparing between amnion MSCs and hADSCs in basal medium and differentiation medium were calculated and reported as 2^− (CtGene of interest/CtGapdh).
TABLE 1.
Osteogenic and Chondrogenic Gene Transcript Summary Information
| Gene Name | Species | Gene Symbol | Amplicon Size | Polymerase Chain Reaction Protocol |
|---|---|---|---|---|
| Aggrecan | Human | ACAN | 62 bp | Activation: 95°C for 15 minutes |
| Sex Determining Region y-box 9 | Human | SOX9 | 111 bp | Cycling (45 cycles): |
| Type II Collagen | Human | COL2A1 | 94 bp | Denaturation: 94°C for 10 seconds |
| Runt Related Transcription Factor 2 | Human | RUNX-2 | 101 bp | Anneal: 52.4°C for 20 seconds |
| Osteocalcin | Human | OCN/BGLAP | 90 bp | Extension: 72°C for 20 seconds |
| Glyceraldehyde 3-Phosphate Dehydrogenase | Human | GAPDH | 95 bp | Melt Analysis: 72°C–99°C at 1°C per step |
| Detected Transcript (NCBI Reference Sequence) | ||||
ACAN: NM_001135, NM_013227, XM_001131727, XM_001131734, XM_006720419
SOX-9: NM_000346
COL2A1: NM_001844, NM_033150, XM_006719242
RUNX-2: NM_001015051, NM_001024630, NM_004348, NM_001278478, XM_006715231, XM_006715233, XM_006715234
OCN/BGLAP: NM_199173
GAPDH: NM_001256799, NM_002046, NM_001289745, NM_001289746
Alizarin Red Histological Staining for Mineralization
Well plates were fixed in 4% formaldehyde for 30 minutes at room temperature before incubation in Alizarin Red (2% aqueous Alizarin Red, pH 4.2) for visualization of calcium deposition. The average percentage of the total well-plate area (n = 3 per condition) stained positive was quantified via color threshold analysis by use of National Institutes of Health (NIH) ImageJ software by 2 blinded observers and expressed as a mean.
MSC Chondrogenic Differentiation Potential
Culture Conditions for MSC Differentiation
To induce chondrogenic differentiation, MSCs were seeded in pellets (1 × 105 cells per pellet) and cultured in differentiation medium (DMEM, 1% FBS, 1% Ab/AM, 6.25 μg/mL insulin transferrin selenium, 50 nM ascorbate-2-phosphate, 10 ng/mL human tumor growth factor β [TGF-β]). Cells analyzed for gene expression in basal medium were plated at a density of 2.1 × 104/cm2 into tissue-treated 12-well plates (ie, 1 × 105 cells per well) and cultured in monolayer by use of basal culture media (DMEM, 10% FBS, 1% Ab/AM). Differentiation capacity was assessed via histological staining (n = 3) and gene transcript expression (n = 4 per condition) by use of target specific primer assays (Table 1) as described above.
Alcian Blue Histological Staining for ECM Glycosaminoglycan Content
Chondrogenic cell pellets (n = 3 pellets per condition) were fixed in 10% phosphate buffered formalin for 30 minutes before undergoing serial washes in graded ethanol, followed by xylene and paraffin before embedding and sectioning to 5-μm thickness. Slides were stained with Alcian Blue (1% Alcian Blue in 3% aqueous acetic acid, pH 2.5) and counterstained with 0.1% aqueous Nuclear Fast Red for visualization of glycosaminoglycan (GAG) deposition and cell nuclei, respectively. The average percentage of the total cell pellet area positive for GAG was quantified via color threshold analysis by use of NIH ImageJ software by 2 blinded observers.
Immunohistochemistry for Collagen Type 2
Immunohistochemistry (IHC) on rehydrated paraffin sections was performed to detect collagen type 2 in chondrogenic cell pellets (n = 3 pellets per condition). Briefly, after antigen retrieval via incubation in 10 mM citric acid at 90°C, sections were permeabilized (0.025% Triton X-100 for 10 minutes), and nonspecific binding was blocked with normal serum. Polyclonal rabbit anti-collagen type 2 antibody (Abcam: ab85266, 5 μg/mL) was applied for 1 hour. Negative controls did not receive primary antibody. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide before incubation in an anti-rabbit secondary antibody, which was visualized by diaminobenzidine tetrahydrochloride peroxidases substrate kit (Vector Laboratories). Sections were subsequently counterstained with a 50:50 mixture of hematoxylin in distilled water.
Microscopic Imaging
All images were captured on a Zeiss Axio Vert.A1 microscope with AxioVision software (Release 4.9.1 SP08-2013).
Statistical Analysis
Results are represented as a mean ± standard error of the mean (SEM). All statistical analyses were performed by use of an analysis of variance (ANOVA) with a Tukey’s post hoc analysis performed within each time point to compare across all study groups by use of GraphPad Prism 7 software. Significance was defined in all cases as P < .05.
RESULTS
MSC Yields From Human Amnion
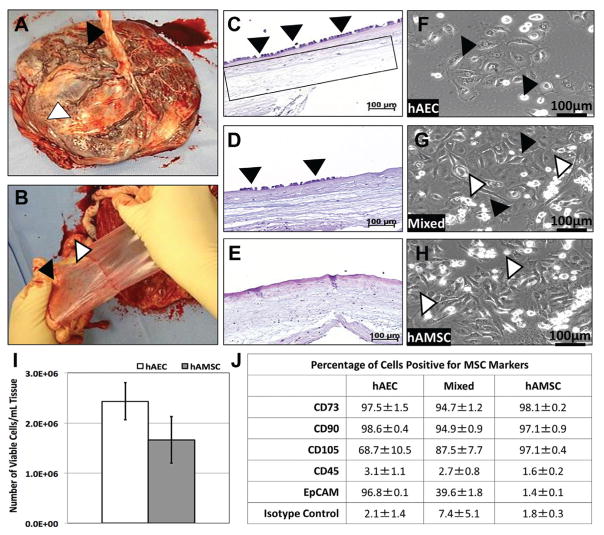
Human AECs and AMSCs from the term amnion were successfully isolated and expanded (Figure 1, A–H). These cells demonstrated positive expression for MSC markers (Figure 1J). Average viable hAEC and hAMSC yields at harvest were 2.3 × 106 ± 3.7 × 105 and 1.6 × 106 ± 4.7 × 105 per milliliter of amnion (Figure 1I), respectively, resulting in total cell yields of 4.2 × 107 ± 8.2 × 106 and 2.8 × 107 ± 7.2 × 106 per membrane, respectively.
Figure 1.
Amniotic membrane harvest, mesenchymal stromal cell (MSC) isolation, and characterization. (A) Representative image of a human placenta with umbilical cord (black arrowhead) and epithelial layer of the amniotic membrane (white arrowhead) facing upward. (B) Representative images depicting the separation of the amniotic membrane (white arrowhead) from the chorion (black arrowhead). Representative hematoxylin and eosin (H&E) histological sections: (C) fresh amniotic membrane exhibiting a continuous layer of human amniotic epithelial cells (hAECs) (arrowheads) and intact stroma containing human amniotic mesenchymal stromal cells (hAMSCs) (box), (D) amniotic membrane after incomplete removal of hAECs (arrowheads) by use of 0.125% trypsin yielding a “mixed” population of amniotic MSCs, and (E) amniotic membrane after complete removal of hAECs by use of 0.25% trypsin. Polarized light microscopic images of monolayer culture expanded (passage 2) amnion cells: (F) enriched hAECs (cuboidal morphology—black arrowheads), (G) a mixed population of hAECs (black arrowheads) and hAMSCs (white arrowheads) after incomplete dissociation of hAECs, and (H) enriched hAMSCs (spindle morphology—white arrowheads). (I) Graph of average viable cell yields from term human amniotic membranes. (J) Flow cytometric analysis of enriched hAECs, mixed, and enriched hAMSCs illustrated positive expression for MSC markers (CD73, CD90, and CD105) and the absence of the hematolymphocyte common antigen, CD45. Additionally, enriched hAECs were positive for the epithelial marker, EpCAM, while enriched hAMSCs further confirmed their identity (data represented as percentage of cells with positive staining ± SEM).
Comparison of MSC Osteogenic Differentiation Potential
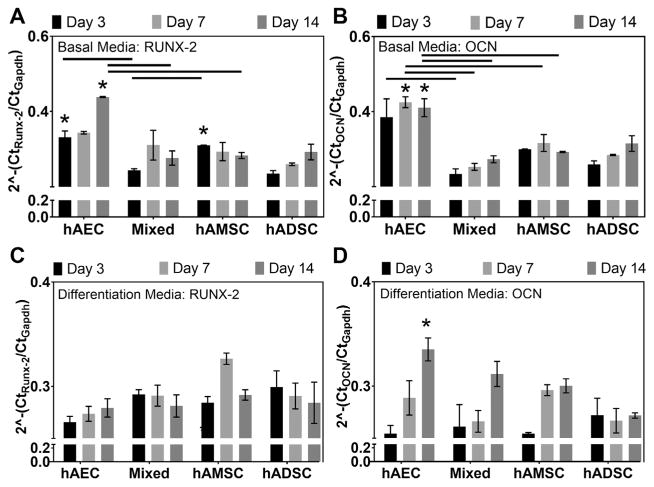
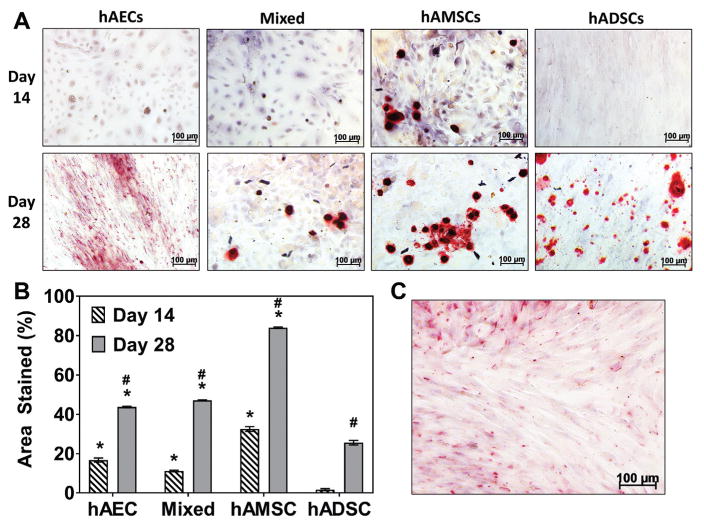
Gene transcript expression of MSCs in basal medium indicated that runx-2 expression was greater in hAECs and hAMSCs compared with hADSCs; these values were significant at day 3 (P = .008) and day 14 (P = .005) for hAECs and at day 3 (P = .019) for hAMSCs (Figure 2A). Furthermore, hAECs demonstrated the highest expression of osteocalcin of all groups investigated when cultured in basal medium (Figure 2B). After culture in osteogenic differentiation media, no statistical differences were found in comparisons of runx-2 expression across all groups investigated; however, expression tended to increase with time in hAEC and hAMSC groups whereas it decreased in hADSCs (Figure 2C). The average relative osteocalcin expression observed in hAEC and hAMSC groups tended to be greater at day 7 and 14 compared with hADSCs at the same time points; this was found to be significant (P = .025) at day 14 for the hAEC group (Figure 2D). Semiquantitative analysis of Alizarin Red staining of MSCs cultured in osteogenic differentiation medium indicated that all amnion MSC groups had significantly (P < .0001) greater percentages of areal staining for mineralized ECM (hAEC, 16.74% ± 1.82%; mixed, 11.17% ± 0.67%; hAMSC, 32.54% ± 2.97%) at day 14 compared with hADSC (1.68% ± 0.88%) (Figure 3A). Additionally, all amnion groups demonstrated significantly (P < .0001) increased mineralized ECM (hAEC, 43.72% ± 0.43%; mixed, 47.11% ± 0.21%; hAMSC, 84.05% ± 0.37%) compared with hADSCs (25.60% ± 0.1.16%) at day 28 (Figure 3B). Of note, hAECs cultured in basal medium demonstrated ECM mineralization after 28 days (37.25% ± 0.75%) as indicated by small red nodules and diffuse staining (Figure 3C), whereas hAMSC, mixed and hADSCs did not (data not shown).
Figure 2.
Osteogenic gene transcript expression of human amnion-derived mesenchymal stromal cells and epithelial cells (hAMSCs and hAECs) and human adipose-derived stromal cells (hADSCs). Gene transcript expression levels of (A) runx-2 and (B) osteocalcin (OCN) in hAEC, mixed (hAEC + hAMSC co-culture), and hAMSC groups relative to hADSCs (dotted line) cultured in basal medium. (C) Runx-2 and (D) osteocalcin expression of amnion-derived mesenchymal stromal cell groups cultured in osteogenic differentiation medium compared with respective expression levels in hADSCs cultured at the same time points (n = 4 per group per time point). Lines indicate statistical difference (P < .05) between connected groups. *Statistical differences compared with the hADSC group at the same time point (P < .05).
Figure 3.
Production of mineralized matrix in human amnion-derived mesenchymal stromal cells and epithelial cells (hAMSCs and hAECs) and human adipose-derived stromal cells (hADSCs). (A) Alizarin Red staining of monolayer cultures of hAECs, mixed (hAEC + hAMSC co-culture), hAMSCs, and hADSCs cultured in osteogenic differentiation medium (red = positive staining for mineralization) at day 14 and 28 (n = 3 per group per time point). (B) Graph of percentage area of the well that stained positive for mineralization. Alizarin Red staining of (C) hAECs cultured in basal medium for 28 days. *Statistical differences compared with the hADSC group at the same time point (P < .05). #Statistical difference (P < .05) within each cell group compared with its respective day 14 value.
Comparison of MSC Chondrogenic Differentiation Potential
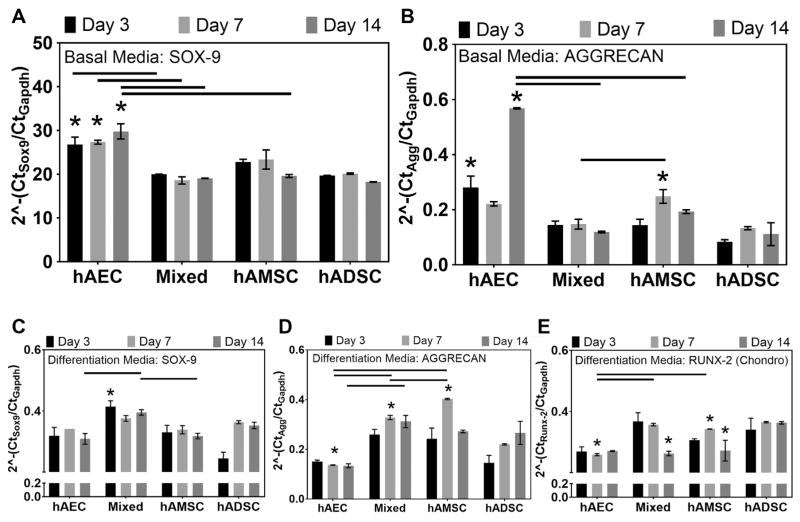
Gene transcript analysis of MSCs in basal medium illustrated that sox-9 and aggrecan expression was generally greatest in hAEC and hAMSC groups (Figure 4A). hAEC sox-9 expression in basal medium was significantly greater at day 3 (P = .018), day 7 (P = .044), and day 14 (P = .003) compared with hADSCs at the same time points. Additionally, aggrecan expression was greater in hAECs and hAMSCs compared with hADSCs; these values were significant at day 3 (P = .0004) and day 14 (P = .005) for hAECs and at day 7 (P = .024) for hAMSCs (Figure 4B). After culture in chondrogenic differentiation medium, all amnion groups exhibited higher sox-9 transcript expression compared with ADSCs at day 3; this was significant (P = .02) for the mixed group (Figure 4C). As expected, peaks in sox-9 expression tended to precede peaks in aggrecan expression in mixed and hAMSC groups (Figure 4D). Aggrecan expression peaked earlier (day 7) and was statistically greater in hAMSC (P < .0001) and mixed cell groups (P = .0003) compared with hADSCs. Additionally, collagen type 2 expression was significantly greater in hAMSC (P = .044) and mixed cell groups (P = .026) compared with hADSCs at this time point (Figure 5C). Conversely, runx-2 gene transcript expression in hAECs, mixed, and hAMSC groups cultured in chondrogenic differentiation medium was significantly lower (P < .05) at day 7 and 14 compared with hADSCs (Figure 4E).
Figure 4.
Chondrogenic gene transcript expression of human amnion-derived mesenchymal stromal cells and epithelial cells (hAMSCs and hAECs) and human adipose-derived stromal cells (hADSCs). Transcript expression levels of (A) Sox-9 and (B) aggrecan in hAEC, mixed (hAEC + hAMSC co-cultured), and hAMSC groups relative to hADSCs (dotted line) cultured in basal medium. (C) Sox-9, (D) aggrecan, and (E) runx-2 expression of amnion mesenchymal stromal cell groups cultured in chondrogenic differentiation medium compared with respective expression levels in hADSCs cultured at the same time points (n = 4 per group per time point). Lines indicate statistical difference (P < .05) between connected groups. *Statistical differences compared with the hADSC group at the same time point (P < .05).
Figure 5.
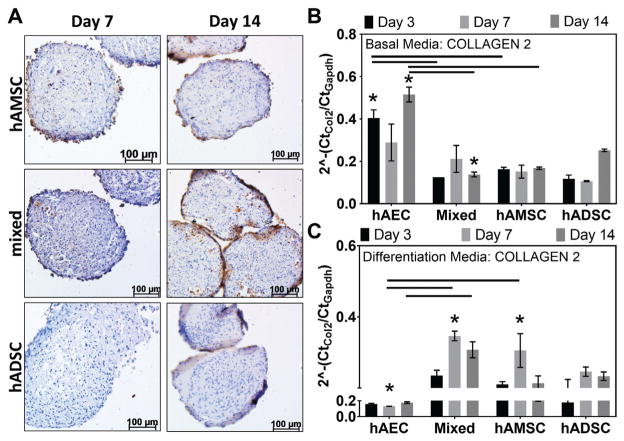
Collagen type 2 gene transcript and matrix deposition in human amnion-derived mesenchymal stromal cells and epithelial cells (hAMSCs and hAECs) and human adipose-derived stromal cells (hADSCs). (A) Immunohistochemical staining (brown = positive, blue = nuclei) for collagen type 2 in hAMSC, mixed (hAEC + hAMSC co-culture), and hADSC cell pellets cultured in chondrogenic differentiation medium. (B) Gene transcript expression levels of collagen type 2 in mixed (hAEC + hAMSC co-culture) and hAMSC groups relative to hADSCs (dotted line) cultured in basal medium. (C) Collagen type 2 expression of amnion-derived mesenchymal stromal cells cultured in chondrogenic differentiation medium compared with respective expression in hADSCs cultured at the same time points. Lines indicate statistical difference (P < .05) between connected groups. *Statistical differences compared with the hADSC group at the same time point (P < .05).
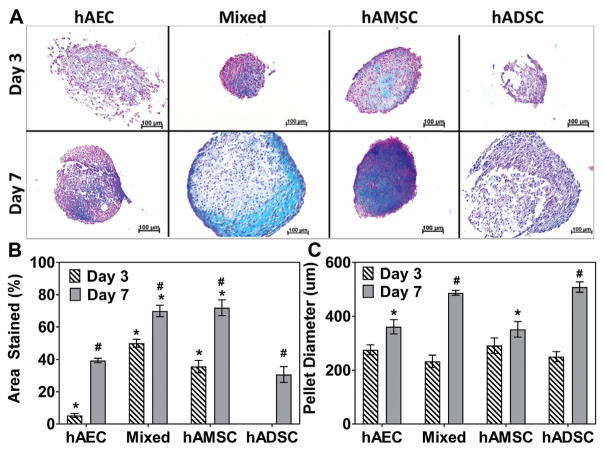
Immunohistochemical staining of cell pellets cultured in chondrogenic differentiation medium qualitatively illustrated that mixed and hAMSC groups produced collagen type 2 enriched ECM as early as day 7 (Figure 5A). This continued through day 14, at which time hADSC group exhibited minimal staining. A progressive increase in pellet diameter (ie, ECM production) with time in culture was observed in all groups (Figure 6, A and C). At day 7, pellet diameters in the hADSC group (508.71 ± 33.42 μm) were significantly greater than in the hAMSC (351.48 ± 49.95 μm) and hAEC (360.99 ± 45.65 μm) groups (P = .005 and P = .007, respectively). Semiquantitative Alcian Blue staining indicated that pellets formed by mixed and hAMSC cell groups at day 3 (50.02% ± 4.18% and 35.61% ± 6.58%, respectively) and day 7 (69.97% ± 6.13% and 71.96% ± 8.55%, respectively) had significantly (P < .001) increased areal staining for GAG-enriched ECM compared with hADSCs (Day 3, 0.00% ± 0.00%; Day 7, 30.69% ± 8.52%) (Figure 6B).
Figure 6.
Glycosaminoglycan (GAG) content in pellets of human amnion-derived mesenchymal stromal cells and epithelial cells (hAMSCs and hAECs) and human adipose-derived stromal cells (hADSCs). (A) Alcian Blue staining for glycosaminoglycan of hAEC, mixed (hAEC + hAMSC co-culture), hAMSC, and hADSC cell pellets (blue = positive staining) cultured in chondrogenic differentiation medium at day 3 and day 7. (B) Quantitative analysis of percentage area of the cell pellet stained positive for GAG and (C) comparison of hAEC, mixed (hAEC + hAMSC co-culture), hAMSC, and hADSC cell pellet diameter cultured in differentiation medium at day 3 and 7. *Statistical differences compared with the hADSC group at the same time point (P < .05). #Statistical difference (P < .05) within each cell group compared with its respective day 3 value.
DISCUSSION
Herein, a controlled comparative evaluation of the osteogenic and chondrogenic differentiation potential of MSCs derived from human amnion and adipose tissue was conducted. Human ADSCs were chosen as a comparator for these studies given their established osteogenic and chondrogenic differentiation capacity and their higher cell yields at harvest compared with BMSCs. Additionally, hADSC harvest typically uses enzymatic tissue dissociation and thus more closely resembles methods used for isolating stromal cells from the amnion, thereby minimizing the potential confounding effects due to isolation. Additionally, we evaluated measures of differentiation (ie, gene and ECM expression) at earlier time points compared with typical MSC differentiation studies to determine whether amnion- derived MSCs undergo this process more expeditiously. Our results confirm the identity of MSC populations within the term amnion through the use of well-established MSC surface markers9 and their significantly greater abundance at harvest compared with hADSCs. In comparison to the amnion MSC yields found herein, reports suggest that average hBMSC and hADSC yields at harvest are 1 × 103 and 3.4 × 104 per milliliter of tissue, respectively.18,25 Thus, MSC yields from the term amnion are 3500 and 100 times greater than those obtained from bone marrow and adipose tissue, respectively. From a clinical perspective, this could significantly reduce the need for in vitro expansion to obtain quantities of MSCs required for therapeutic benefit.
Additionally, we have uncovered several novel findings that may have significant clinical implications regarding the use of amnion-derived MSCs for orthopaedic applications. First, amnion-derived MSCs exhibited accelerated and enhanced osteogenic and chondrogenic differentiation, resulting in more robust mineralized and cartilaginous ECM generation compared with hADSCs. This was demonstrated at both the gene and protein level at relatively early time points compared with the 21 to 28 days typically required for differentiation of hADSCs and hBMSCs.12,19 For example, the expression of Sox-9, the key transcription factor governing chondrogenesis, peaked earlier in hAMSCs compared with hADSCs. Consequently, earlier expression of downstream genes and cartilage ECM was also observed. These findings are validated by the fact that Sox-9 drives the expression of aggrecan.11 Of note, in chondrogenic differentiation medium hADSCs exhibited significantly greater expression of Runx-2 (an osteogenic marker) compared with amnion-derived MSCs at day 7 and 14, potentially indicating their premature hypertrophy, and thus these cells may be less amenable for use in regenerative therapies targeting cartilage repair.15 Additionally, amnion-derived MSCs exhibited increased ECM mineralization, glycosaminoglycan, and collagen type 2 content compared with hADSCs after culture in differentiation medium. Taken together, the potential clinical implications for the observed earlier differentiation and enhanced ECM deposition by amnion-derived MSCs are significant as their use may support accelerated tissue formation and repair.
Due to the observed enhanced differentiation within amnion MSCs, we hypothesized that these results were due in part to increased expression of osteogenic and chondrogenic genes in amnion MSCs compared with hADSCs due to their innate gene expression. Thus, all cell types were cultured for the study duration in basal culture medium. Results from these experiments suggest a second novel finding: hAECs and hAMSCs tended to demonstrate greater expression of osteogenic and chondrogenic markers compared with hADSCs at early time points in basal culture medium. Additionally, enhanced differentiation could have been due in part to the age differences between the younger amnion and older adipose MSCs; donor age and health have been shown to affect MSC properties.3,4,6,22
Third, it was noted that the different MSC populations within the amnion demonstrate a propensity toward attaining a bone- or cartilage-forming phenotype. hAECs demonstrated the ability to produce calcified ECM in the absence of osteogenic induction, which was not noted in hAMSCs or hADSCs. This is of significant interest, and further study of this phenomenon is warranted. Alternatively, with respect to chondrogenesis, hAMSCs consistently demonstrated the highest aggrecan and collagen type 2 expression at the gene and ECM production levels compared with hAECs and hADSCs and thus may be more appropriate for cartilage repair applications.
Fourth, it was found that co-culture of hAECs and hAMSCs together did not enhance or diminish the ability of the cells to differentiate, as the expression patterns of co-cultures of hAECs and hAMSCs were typically between those of the respective enriched amnion MSC populations. Inclusion of a mixed amnion MSC study group was performed as therapeutic application of amnion allografts may likely contain both cell types. However, whether currently available commercial amnion allografts contain viable MSC remains questionable.
As with any study, limitations were noted. First, these studies were performed in monolayer or pellet cultures and thus results may not be extrapolated to 3-dimensional culture on scaffolds or within the environment of the human body. However, it is difficult to recapitulate the complexity of the in vivo healing environment by use of in vitro techniques. Second, it would have been beneficial to evaluate basal (innate) gene expression on freshly isolated MSC sources so as not to be influenced by in vitro expansion. While this would have been possible for our amnion MSC populations due to their high cell yields, we would have had difficulties procuring enough hADSCs at passage 0 to enable us to make direct comparisons between the cell types. An additional potential weakness is that we compared our own procured amnion MSC population yields and differentiation potential with values reported in literature, and the hADSCs used herein were purchased from a commercial vendor. While it may be possible that yield numbers and differentiation potential would have been different if we had harvested our own hADSCs, given the order of magnitude difference in MSC yield numbers and provided that the hADSCs were isolated from healthy donors, it is unlikely that such differences would drastically alter the observed results. In fact, commercial procurement of hADSCs may improve the generalizability of the study findings. Since the primary purpose of this paper was to compare the differentiation capacity of these 2 MSC populations, we felt this method gave the best chance of direct comparison of this capacity. Third, although enriched MSCs populations were isolated and pooled from 3 different donors for our studies, a larger sample size might better account for any patient to patient variability. Additionally, the mixed amnion MSC cell population used herein was derived from only one donor, and thus patient-to-patient variability may not be accurately portrayed by this study group. However, the primary outcome evaluated within the mixed study group was whether including both hAMSCs and hAECs together in culture would alter differentiation capacity, and therefore patient-to-patient variability was not as significant of a concern for this outcome measure. Although paracrine signaling by MSCs has been demonstrated to have an effect on tissue healing and regeneration, such effector functions were not compared within the studies herein but should be investigated as well.
In conclusion, hAECs and hAMSCs demonstrate significantly improved osteogenic and chondrogenic differentiation capacity compared with their adipose-derived counterparts. As differentiation capacity and ECM production are among the main determinants for bone and cartilage regeneration, these cells represent a potential advance in the treatment of lesions within these tissues.
Supplementary Material
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1.Abumaree MH, Al Jumah MA, Kalionis B, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 2013;9(5):620–641. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- 2.Barbati A, Grazia Mameli M, Sidoni A, Di Renzo GC. Amniotic membrane: separation of amniotic mesoderm from amniotic epithelium and isolation of their respective mesenchymal stromal and epithelial cells. Curr Protoc Stem Cell Biol. 2012;Chapter 1(Unit 1E.8) doi: 10.1002/9780470151808.sc01e08s20. [DOI] [PubMed] [Google Scholar]
- 3.Barboni B, Russo V, Curini V, Martelli A. Gestational stage affects amniotic epithelial cells phenotype, methylation status, immunomodulatory and stemness properties. Stem Cell Rev Reports. 2014;10:725–741. doi: 10.1007/s12015-014-9519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan AI. Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tiss Eng. 2005;11(7):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 5.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–353. [PubMed] [Google Scholar]
- 6.Coleman CM, Curtin C, Barry FP, Flatharta CO, Murphy JM. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010;1250:1239–1250. doi: 10.1089/hum.2010.138. [DOI] [PubMed] [Google Scholar]
- 7.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 8.Dean CS, Chahla J, Serra Cruz R, LaPrade RF. Fresh osteochondral allograft transplantation for treatment of articular cartilage defects of the knee. Arthrosc Tech. 2016;5(1):e157–161. doi: 10.1016/j.eats.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sport Traumatol Arthrosc. 2013;21(8):1717–1729. doi: 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 12.Im G-I, Shin Y-W, Lee K-B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13(10):845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 14.Keeley R, Topoluk N, Mercuri J. Tissues reborn: fetal membrane-derived matrices and stem cells in orthopedic regenerative medicine. Crit Rev Biomed Eng. 2014;42(3–4):249–270. doi: 10.1615/critrevbiomedeng.2014011591. [DOI] [PubMed] [Google Scholar]
- 15.Ko J-Y, Kim K-I, Park S, Im G-I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 2014;35(11):3571–3581. doi: 10.1016/j.biomaterials.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Lam J, Lu S, Lee EJ, et al. Osteochondral defect repair using bilayered hydrogels encapsulating both chondrogenically and osteogenically pre-differentiated mesenchymal stem cells in a rabbit model. Osteoarthritis Cartilage. 2014;22(9):1291–1300. doi: 10.1016/j.joca.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EH, Hui JHP. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006;88(7):841–851. doi: 10.1302/0301-620X.88B7.17305. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Wong WH-S, Chan S, et al. Factors affecting mesenchymal stromal cells yield from bone marrow aspiration. Chinese J Cancer Res. 2011;23(1):43–48. doi: 10.1007/s11670-011-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 20.Meng FG, Zhang ZQ, Huang GX, et al. Chondrogenesis of mesenchymal stem cells in a novel hyaluronate-collagen-tricalcium phosphate scaffolds for knee repair. Eur Cell Mater. 2016;31:79–94. doi: 10.22203/ecm.v031a06. [DOI] [PubMed] [Google Scholar]
- 21.Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25. doi: 10.1186/scrt66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 23.Nejadnik H, Hui JH, Feng Choong EP, Tai B-C, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 24.Nurkovic J, Dolicanin Z, Mustafic F, et al. Mesenchymal stem cells in regenerative rehabilitation. J Phys Ther Sci. 2016;28(6):1943–1948. doi: 10.1589/jpts.28.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8(2):166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 26.Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 27.Reyes R, Pec MK, Sánchez E, del Rosario C, Delgado A, Évora C. Comparative, osteochondral defect repair: stem cells versus chondrocytes versus bone morphogenetic protein-2, solely or in combination. Eur Cell Mater. 2013;25:351–365. doi: 10.22203/ecm.v025a25. [DOI] [PubMed] [Google Scholar]
- 28.Song K, Li L, Yan X, et al. Fabrication and development of artificial osteochondral constructs based on cancellous bone/hydrogel hybrid scaffold. J Mater Sci Mater Med. 2016;27(6):114. doi: 10.1007/s10856-016-5722-5. [DOI] [PubMed] [Google Scholar]
- 29.Tanavde V, Vaz C, Rao MS, Vemuri MC, Pochampally RR. Research using mesenchymal stem/stromal cells: quality metric towards developing a reference material. J Cytotherapy. 2015;17(9):1169–1177. doi: 10.1016/j.jcyt.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.