Abstract
Two classification systems are now at the forefront of clinical psychiatric research: (1) Diagnostic and Statistical Manual, Fifth Edition and (2) the National Institutes of Mental Health Research Domain Criteria. Herein, we propose that these two classification systems are complementary rather than mutually exclusive, and when combined provide important information for understanding aspects of the pathophysiology related to Generalized Anxiety Disorder (GAD). The neurobiological literature for GAD and one relevant research domain criteria component, sustained threat, are reviewed from multiple units of analysis (genetic, neuroimaging, neuroendocrine, and psychophysiological). It is hypothesized that generating a comprehensive, biologically based understanding of the relationship between GAD, sustained threat, and the measureable units of analysis will provide information critical to design the most effective treatments.
Keywords: brain imaging/neuroimaging, genetics, biological markers, anxiety, stress
Introduction
Two—not necessarily opposing—classification systems are now at the forefront of clinical research: (1) Diagnostic and Statistical Manual, Fifth Edition (DSM-51) and (2) the National Institutes of Mental Health (NIMH) Research Domain Criteria (RDoC2–4). RDoC provides a fresh perspective on new ways to approach anxiety research (e.g., through the construct of “potential threat”), but because of its clinical utility, the DSM-5 maintains its position as the diagnostic system that guides clinical practice (along with ICD-11). Yet, the biological and nosological characterization of DSM-5 anxiety disorders, particularly generalized anxiety disorder (GAD), and other comorbid disorders (e.g., major depressive disorder, MDD) are unclear and demonstrate high correlations and unreliability.5–7 It is hypothesized that an RDoC-based conceptualization and investigation may be able to provide answers to these shortcomings of the DSM-5 by developing a transdiagnostic, neurobiological model of anxiety that provides a more valid distinction from other psychiatric RDoC constructs (e.g., loss). Because of the primary clinical and research perspectives of the DSM-5 and RDoC classification systems, respectively, we provide a selective review of the neurobiological literature associated with GAD (DSM-5) and one relevant RDoC construct, chronic stress (“sustained threat”; RDoC). Notably, other RDoC constructs are also likely to be relevant to the neurobiology of GAD (e.g., potential threat, acute threat, arousal);8 however, sustained threat is used here to provide an illustration of how a connection between DSM-5 and RDoC classification systems can be made. The reader is referred to recent reviews of GAD neurobiology9–12 and RDoC8 for additional background information beyond the scope of this review.
Generalized Anxiety Disorder
GAD is one of the most common psychiatric disorders, occurring in up to 21% of adults in their lifetime.13 As defined in the DSM-5, GAD is characterized by excessive anxiety and worry about a number of events or activities (e.g., work, school performance), which an individual finds difficult to control. The worry is impairing across varied contexts (e.g., work, home, and social). Symptoms which are required for diagnosis include feeling restless, being easily fatigued, difficulty concentrating or mind going blank, irritability, muscle tension, and sleep disturbance (note: only one symptom needs to be present in children). GAD has high rates of comorbidity, particularly between GAD and MDD that ranges from 40% to 98% in treatment studies.14–18 In fact, the GAD/MDD comorbidity may occur more often than MDD or GAD alone.19 In addition to high comorbidity, DSM-5 field trials have highlighted the poor diagnostic reliability of GAD (kappa = 0.34).5 Other findings indicate differences between illness predictors and symptoms of MDD and GAD.20–23 GAD is often a precursor of MDD and if GAD is treated effectively, it lowers the risk for development of MDD and some individuals with MDD never develop GAD.24 Because of these mixed findings, cross-cutting and distinct symptoms of GAD need to be investigated in accordance with transdiagnostic and RDoC perspectives; however, diagnostic categories (e.g., GAD vs. MDD) should also be examined due to their clinical relevance and adherence to the current nosological system (DSM-5).
Given the clinical ambiguities, it is not surprising that there is a limited biological information regarding how similar or different GAD is from other disorders (e.g., MDD). At the biological level, studies that have pursued a biological conceptualization from one unit of analysis (e.g., brain data) have often highlighted similar findings in depression and anxiety.6,7,25 For example, the amygdala has demonstrated increased activity in both anxiety and depression groups when compared to healthy controls,6,25 and twin studies indicate high genetic correlations between MDD and GAD (r = .74–1.0).7,26,27 However, MDD and GAD can be differentiated with respect to biology,28–30 particularly with resting-state functional magnetic resonance imaging (R-fMRI).31
As the current literature lacks clarity regarding the clinical and biological differences between GAD and other disorders, such as MDD, both DSM-5 (categorical approaches) and RDoC (cross-cutting constructs) approaches offer critical information for understanding the differences and overlap between GAD and other disorders. Thus, the present literature review examines aspects of the neurobiological literature from both a categorical (GAD) and dimensional (chronic stress) perspective in order to build an initial understanding of the diagnostic differences versus dimensional intersections (e.g., chronic stress across disorders) related to GAD.
Chronic Stress
Chronic stress may be one cross-cutting construct or dimension (i.e., that occur across diagnostic categories defined by the DSM-5) related to GAD, as well as other diagnoses (such as highly related MDD). In general, a stress response may be adaptive in the short-term when faced with acute challenges.32,33 Over time, if an individual is subjected to short-term acute stressors that are repeatedly triggered, the resulting chronic maladaptive stress responses (i.e., chronic stress) may develop into interfering psychiatric difficulties or disorders.34–36 This resulting chronic stress impacts biological systems across multiple “units of analysis”—neuroendocrine, autonomic, and behavioral—when triggered by perceived or actual threat.37 Chronic stress can significantly impact the homeostatic biological system by increasing the allostatic load (i.e., effect of stress on the human body).38,39 Increased cumulative effects of stress on the human body (allostatic load) are linked to many adverse health consequences, including psychiatric illnesses such as GAD.
Individuals may be able to remain resilient (e.g., do not develop psychiatric disorders) to different amounts of cumulative stress based on their early-life experiences (e.g., trauma in early childhood40), hereditary factors, and stress history.41–44 For example, childhood trauma and life experiences increases the risk of onset and recurrence of depression and anxiety disorders.45 Childhood trauma, childhood life experiences, as well as genetic and environmental factors (e.g., stress in the family)46,47 may reduce an individual’s “tolerance” or resilience to increased allostatic load caused by repeated stressors; thus, increase the risk for the development of psychiatric disorders, including anxiety.
Early diagnosis (and treatment) of a psychiatric disorder also may contribute to an individual’s resilience and ability to cope with repeated stress. For example, carrying a diagnosis of depression or anxiety in childhood can increase risk of an individual being diagnosed with GAD in adulthood.48 Further, findings from a recent study found that more time in an episode of GAD (as well as MDD) was associated with the highest risk for experiencing suicidal thoughts and behaviors across adolescence and into adulthood.49 Not only was GAD diagnosis associated with the highest risk trajectory for suicidal thoughts and behaviors, broader anxiety symptoms were as well. Combined with the shocking 24% increase of suicide mortality in the past 15 years in the U.S.,50 these trajectory findings stress the impact of chronic stress and psychiatric diagnosis on future psychopathology.
The RDoC matrix51 breaks down cross-cutting constructs into five domains, including negative valence system domain, which includes constructs related to anxiety (e.g., potential threat and sustained threat). In the present review, we explore RDoC sustained threat as a highly relevant cross-cutting construct related to GAD and chronic stress. Although potential threat has been considered—and is highlighted in the RDoC matrix—as being related to anxiety, it is sustained threat (or the experience of chronic stress), whether potential or actual, that appears to have the most significant health consequences related to GAD (e.g., emergence of psychiatric disorders34–36). RDoC defines sustained threat as “an aversive emotional state caused by prolonged (i.e., weeks to months) exposure to internal and/or external condition(s), state(s), or stimuli that are adaptive to escape or avoid…” This description includes that the threat may be “actual or anticipated.” Alongside this description are the multilevel (molecules to behavior) neurobiological components hypothesized to be involved in the construct of sustained threat (see Table 1).
Table 1.
RDoC sustained threat.
| Molecules | Cells | Circuits | Physiology | Behavior |
|---|---|---|---|---|
| ACTH CRF HPA-axis hormones | Hippocampal Microglia Prefrontal | Attention network Dysregulation of amygdala reactivity Dysregulation of cingulate reactivity Habit systems Hypothalamic nuclei PVT Vigilance network | Dysregulated HPA Axis Error-related negativity | Anhedonia/decreased appetitive behavior Anxious arousal Attentional bias to threat Avoidance Decreased libido Helplessness behavior Increased conflict detection Increased perseverative behavior Memory retrieval deficits Punishment sensitivity |
Note. ACTH, andrenocorticotropic hormone; CRF, corticotropin releasing factor; HPA, hypothalamic-pituitary-adrenal; PVT, paraventricular thalamic nucleus.
Sustained Threat, GAD, Neurobiology: A Model
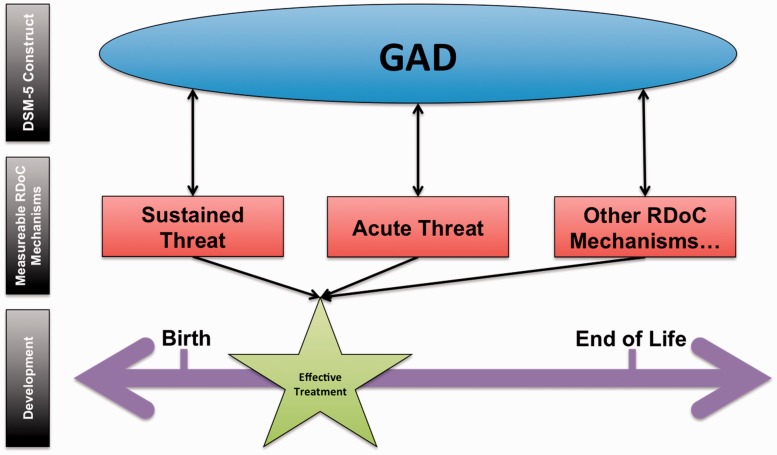
Here, we review the neurobiological literature associated with GAD and with the RDoC domain, sustained threat. As noted prior, this literature review is not intended to include an exhaustive review of all RDoC domains relevant to GAD (e.g., acute threat) but rather provide a demonstration of how both DSM-5 categorical perspectives and RDoC domains can be linked. Our proposed model (see Figure 1) includes three tiers: (1) DSM-5 construct (i.e., GAD), (2) RDoC mechanism, and (3) development. It is hypothesized that designing developmentally appropriate pharmacological and psychological treatments that target neurobiological mechanisms (e.g., bed nucleus of stria terminalis [BNST], elevated peripheral nervous system activity, 5-HTTLPR short-allele) related to sustained threat, or other measurable RDoC mechanisms, may generalize and target the chronic anxiety demonstrated in individuals with GAD. For example, implementing a pharmacogenomics or metabolomics protocol to inform medication selection may improve pharmacotherapy treatment outcomes. Not only can neurobiological markers provide insight for developing personalized medicine protocols and offer biological objective markers of treatment efficacy, the implementation of successful treatment strategies may be dependent on timing. There are critical neurobiological developmental windows in childhood that may be most ideal to target to produce the best outcomes when treating neuropsychiatric disorders, including GAD (see “effective treatment” star placed on developmental timeline).52
Figure 1.
RDoC sustained threat and GAD conceptual model.
Our conceptual model (Figure 1) organizes the relations between the relevant components discussed within the present review. GAD is intentionally placed within an oval to illustrate that the DSM-5 diagnoses are considered to be at the latent or at the construct level. Conversely, the RDoC mechanisms hypothesized to be related to GAD (as well as that GAD is related to these mechanisms; hence, bi-directional arrows between) are measurable (i.e., indicated by square shapes) through their specific units of analysis (i.e., genes, molecules, cells, circuits, physiology, behavior, self-reports, and paradigms). Our model highlights that it is the bi-directional relationship between DSM-5 constructs and RDoC mechanisms that will influence the development of effective treatments (not only one level or tier, such as an RDoC understanding only). Below, we review the DSM-5 (GAD) and one relevant RDoC dimension (sustained threat) to GAD in order to begin linking the levels in our model.
GAD and Neurobiology
Genetics and Epigenetics
It is estimated that genes contribute 30% to 50%53,54 to the development of an anxiety disorder. Conversely, development of an anxiety disorder due to non-genetic factors is approximately 50% to 70%. Environmental factors (e.g., stress, trauma, etc.) likely contribute to the development of anxiety disorders through epigenetic mechanisms that could impact the development of anxiety even beginning in utero. For example, mothers diagnosed with an anxiety disorder who did not receive medication for anxiety have demonstrated altered DNA methylation of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood and the genome55,56 and may increase risk of their child developing an anxiety disorder.
Understanding the genetic and epigenetic mechanisms of GAD is important for building more comprehensive neurobiological conceptualizations to develop the most effective preventative and treatment strategies. Despite the potential impact of understanding these mechanisms, few human studies of anxiety disorders, including GAD, exist relative to animal studies.12 One genome-wide association study of GAD symptoms found genome-wide significant association between a single-nucleotide polymorphism (rs78602344) intronic to thrombospondin 2 (THBS2) and GAD symptoms in a population-based sample of Hispanic/Latino adults (N = 12,282; aged 18–74).57 Although not specific to GAD, a study of anxious versus non-anxious adults (N = 47; M age = 32.89) found that DNA methylation indicated that global methylation levels were higher in anxious adults relative to non-anxious adults58 (anxiety determined by Hospital Anxiety and Depression Scale-Anxiety scores). Hospital Anxiety and Depression Scale-Anxiety scores in the anxious group were also significantly correlated with the expression of the DNA methyltransferases DNMT1/3A, such that greater anxiety severity was associated with more DNA expression. Notably, the generalizability of the findings of this DNA methylation study is limited due to the small sample size.
Neuroimaging
The disrupted coordination of brain activity has been proposed as one of the core neurobiological features of GAD,59–61 particularly reduced resting-state functional connectivity (RSFC) between the amygdala and prefrontal cortex (PFC) in both adults and adolescents with GAD.62–64 Yet, differences between GAD and healthy control groups, both in structural and functional neuroimaging, not only exists in frontolimbic areas but also in a downstream projection from the amygdala: the anterior cingulate cortex.61 Findings suggest that across adolescence and adulthood, decreased connectivity between the amygdala and PFC is associated with the diagnosis of GAD.62–64 The PFC is critical for the effective regulation of emotion, particularly ventromedial regions that appear to control negative emotion,65 such as anxiety. Fittingly, the ventrolateral PFC activity has been shown to increase (perhaps increasing regulation over limbic structures) following all pharmacological and psychological intervention in individuals with GAD.66
Frontolimbic structures not only impact anxiety-related responses in GAD but also have been demonstrated to differentially regulate autonomic mechanisms in individuals with GAD.59 Specifically, a recent neuroimaging study investigated the relationship between both central nervous system (PFC, amygdala) connectivity and autonomic (heart rate variability; HRV) in individuals with GAD pre- and post-perseverative cognition task.59 At baseline, individuals with GAD demonstrated less connectivity between the PFC and amygdala; however, after the perseverative cognition task, this PFC-amygdala connectivity increased in the GAD group whereas healthy controls decreased in this area (at a trend level). After the perseverative cognition task, HRV reduction (increase in autonomic activation) was predicted by decreased RSFC between the left amygdala-subgenual cingulate cortex and between the right amygdala-caudate nucleus. These findings provide initial evidence regarding specific neural modulation of differential psychophysiological responses between GAD and healthy control groups.
Psychophysiology
In general, the psychophysiological literature demonstrates that individuals with GAD are in a more physiologically dysregulated state (e.g., low HRV, high heart rate, higher skin conductance levels) at baseline relative to controls67,68 and individuals with other anxiety disorders.69 Yet, this hyperarousal psychophysiological pattern in GAD is not universally observed.70 It is possible that psychophysiological differences between GAD and controls are context specific. For example, increased sympathetic nervous system activity and reduced HRV was observed in high trait worry individuals.71,72 Additionally, worrying prior to fearful imagery exposure appears to diminish any associated cardiovascular reactivity, rather than relaxation or neutral stimuli prior to fearful exposure that exposes heightened cardiovascular reactivity during a fearful exposure in GAD participants.73,74 Thus, it appears that relative to controls, GAD participants demonstrate heightened physiological arousal at baseline (e.g., higher heart rate), and these individuals are likely to demonstrate the greatest cardiovascular reactivity to an anxiety-provoking stimulus when asked to relax or view neutral stimuli. This may suggest that individuals with GAD demonstrate chronic physiological arousal at baseline and demonstrate an exaggerated physiological reactivity to fearful stimuli. Similarly, when compared to other anxiety disorders (i.e., social anxiety and GAD with panic attacks), GAD is characterized by elevated baseline startle, suggesting the presence of anxious thoughts in the absence of threat.69 Overall, it appears that relative to healthy controls and individuals with other anxiety disorders, individuals with GAD may exhibit a unique psychophysiological signature that is characterized by hypervigilant physiological response at baseline, as well as greater reactivity to threat.
Summary: How findings fit into RDoC
Findings across genetics, neuroimaging, and psychophysiology indicate neurobiological signatures related to the diagnosis of GAD. When examined in more detail, findings appear more closely related to elevated baseline neurobiological states that are likely correlated with increased thoughts of worry/fear without the presence of an immediate threat. The durability of physiological and cognitive changes—whether related to actual or perceived threat—potentially contributes to the neurobiological differences highlighted above. This sustained threat (also the RDoC construct reviewed below) is one particularly relevant component in GAD. Please see Table 1 for the neurobiological and behavioral components hypothesized to be related to the RDoC construct of sustained threat.
Sustained Threat and Neurobiology
Genetics and Epigenetics
Prior findings suggest that serotonin (5-HT) regulation is related to emotional stability.75 Serotoninergic functioning and variability appear related to individual differences in responding to threat, particularly the variation of the serotonin transporter gene (5-HTTLPR), which is related to neural patterns associated with anxiety, including GAD. When shown fearful and angry faces, 5-HTTLPR short-allele carriers show bilateral amygdala hyperactivation compared to 5-HTTLPR long-allele homozygotes.76–78 Additionally, decreased connectivity between the amygdala and pregenual cingulate, as well as decreased gray matter volume in both brain structures, has been observed in 5-HTTLPR short-allele carriers.79
Though studies have demonstrated the relationship between amygdala hyperactivity in 5-HTTLPR short-allele carries (note: in healthy individuals with “normative” anxiety levels),76,77 some studies do not find the 5-HTTLPR × stress interaction to be significant.80,81 5-HTTLPR short-allele carriers appear to be sensitive to environmental cues, which may contribute to anxious thinking when stressors/threats are present.75 This highlights a propensity of these individuals to be sensitive to acute threat cues in the environment, which may contribute to a hypervigilance or sustained threat over time. In support of this notion, 5-HTTLPR short-allele carriers have been shown to have a positive correlation between life stress and resting activity of the amygdala and hippocampus,82 such that more lifetime stress is associated with more resting activity between the amygdala and hippocampus suggesting a sustained threat response.
Neuroendocrinology and Neuroimaging/Neural Circuits
As highlighted in Table 1, there are important molecular-level components hypothesized to be related to sustained threat (e.g., corticotropin-releasing factor (CRF); adrenocorticotropic hormone; paraventricular nucleus; hypothalamic-pituitary-adrenal axis). These sustained threat molecular components are also key parts of the neuroendocrine system, which regulates the stress response. It is hypothesized that dysregulation of the neuroendocrine system contributes to anxiety. In particular, when in a state of threat (or anxiety), CRF is released from the paraventricular nucleus of the hypothalamus into the primary capillary plexus of the hypothalamo-hypophyseal portal system to stimulate the anterior pituitary to synthesize proopiomelancortin into the blood. Adrenocorticotropic hormone in the blood activates the synthesis and release of cortisol in humans from the adrenal glands of the kidneys. The hypothalamic-pituitary-adrenal axis is then “shut down” by this release of cortisol, or corticosterone, through the influence of the hypothalamus, pituitary, and hippocampus.
Changes in the neuroendocrine system related to sustained threat (i.e., chronic activation of the system described above) have been demonstrated in both animal and human studies. For example, in one preclinical study, rats received intraventricular infusions of CRF and were placed in a sustained threat (sustained exposure to bright light) or acute threat condition. Results demonstrated that longer duration (sustained threat) threat responses were related to the BNST and could be differentiated from acute threat response (via central nucleus of the amygdala) by their sensitivity to CRF-RI receptor antagonists.83 Similarly, in humans, the BNST has been associated with sustained threat/anxiety.84 An fMRI study examined neural response to negative and neutral pictures at predictable and unpredictable intervals. The amygdala demonstrated reactivity to negative pictures that was not dependent on predictability, whereas the BNST showed a linear increase of activation across conditions as a function of anxiety;84 thus, identifying the BNST as related to sustained threat/anxiety versus acute stress (amygdala).
Psychophysiology
Psychophysiological responses related to sustained threat have been hypothesized to be measured by error-related negativity,85 which measures the fronto-centrally maximal negative deflection in the event-related potential, as individuals reporting high levels of worry have demonstrated enhanced error-related negativity during a stressor task (Stroop task).86 Further, peripheral nervous system literature highlights a heightened sensitivity to unpredictable threat in individuals with lower respiratory sinus arrhythmia (a measure of high frequency heart rate variability),87 which is consistent with the literature reviewed above that demonstrate that individuals with impairing levels of anxiety, as indicated by a diagnosis of GAD, demonstrate more sympathetic activity (e.g., higher heart rate, lower heart rate variability) at baseline and greater cardiovascular reactivity to unpredictable threat. Contrary to these cardiac findings, however, skin conductance responses to unpredictability appear to induce a physiological inhibition (e.g., possibly congruent with a “freezing” anxiety response) indicated by weaker skin conductance responses to cue (stimuli presented) and temporal (time intervals) unpredictability.88 In general, it appears that the unpredictability may maintain a sustained threat response that is correlated with a heightened baseline physiological state as well as elicit greater reactivity when a threat is presented.
Conclusions
Across GAD (DSM-5) and sustained threat (RDoC-based) studies, findings provide initial evidence of the biological system overlap (see Table 2 for summary of findings). Results indicate that there is the potential for 5-HTTLPR short allele carriers to be at-risk for development of GAD (likely over the course of repeated stress) and when anxiety/sustained threat responses reach a level of impairment, global methylation levels are increased (e.g., expression of DNMT1/3A). These genetic profiles or changes appear to influence RSFC, particularly involving amygdala. The chronicity of sustained threat—to the point of the development of GAD—appears to also affect baseline psychophysiological functioning (perhaps due to neural changes that result from genetic/epigenetic influences) and place an individual in a hypervigilant physiological state (high HR, lower HRV, higher SCR).
Table 2.
GAD and RDoC sustained threat (ST) literature review findings.
| Genetics/epigenetics |
Neuroimaging/neural circuits |
Psychophysiology |
|||
|---|---|---|---|---|---|
| GAD | ST | GAD | ST | GAD | ST |
| SNP rs78602344 related to GAD symptoms Global methylation higher in anxious vs. non-anxious (not diagnosed with GAD) Increased anxiety severity, increased expression of DNMT1/3A | 5-HTTLPR short allele show bilateral amygdala hyperactivity to fearful and angry faces vs. 5-HTTLPR long allele 5-HTTLPR short allele sensitive to environmental cues 5-HTTLPR short allele carriers show increased life stress with increased RSFC between amygdala and hippocampus | Decreased RFSC amygdala: PFC in adolescents and adults with GAD Decreased HRV predicts decreased RSFC between left amygdala: cingulate cortex and right amygdala: caudate nucleus during perseveration task | CRF infusion in rats during sustained threat activated BNST BNST linear increase in activation as function of anxiety (sustained threat) | Baseline GAD group: lower HRV, higher HR, higher SCR Increased cardiovascular reactivity during fearful exposure in GAD GAD has increased baseline startle response | Higher worry, greater event related negativity during stress Decreased HRV, increased sensitivity to unpredictable threat SCR weakened during threat (“freezing” response) |
Note. BNST, bed nucleus of the stria terminalis; CRF, corticotropin releasing factor; GAD, Generalized Anxiety Disorder; HR, heart rate; HRV, heart rate variability; PFC, prefrontal cortex; RDoC, Research Domain Criteria; RSFC, resting-state functional connectivity; SCR, skin conductance response; SNP, single nucleotide polymorphism.
Despite the compatibility of results across biological systems, many studies that we reviewed investigated one biological system (e.g., genetics, genome-wide association study57); however, two studies did investigate the link between genetics/epigenetics and neuroimaging82 as well as neuroimaging and psychophysiology.59 The investigation of both GAD and sustained threat primarily from one biological system highlights a significant gap in the literature. In fact, novel computational methods have recently been designed to examine the link between genetics, neuroimaging, and clinical data89 and could be expanded to include psychophysiology (e.g., measuring psychophysiological response during R-fMRI protocols). In line with RDoC goals, study of the integration of the biological systems, and their integration with clinical data, will provide an important understanding of the biological processes that relate not only to psychiatric disorders but also to the cross-cutting constructs, such as sustained threat, that are hypothesized to provide critical evidence for the development of new, targeted treatments.3
Future Directions
Going forward, it will be important to continue to seek a comprehensive, biologically based understanding of GAD, particularly in the context relevant RDoC anxiety constructs (e.g., sustained threat, acute threat). Further, treatments will need to be developed from a comprehensive (e.g., genes, brain, psychophysiology, behavior) neurobiological framework, and timing of treatment implementation will have to be empirically tested to understand the most impactful timing to produce the optimal treatment trajectory. This is particularly important for anxiety disorders, as they often emerge in school-age children when other key processes in neurodevelopment are occurring (e.g., synaptogenesis, myelination, and synaptic pruning).52 Other important considerations include the fluctuating nature of symptoms in clinical populations and inadequate power of many neuroimaging studies. These limitations stress the importance of study replication, which currently does not often occur across all of science.90 Data sharing (e.g., in line with priorities at NIMH), such as making study data sets publically available, can greatly aid investigators in their pursuit of study replication. Future directions in personalized medicine will not only need to take into account treatment targets and optimal timing (e.g., the developmental window from which to produce the quickest, most long-lasting, positive outcomes) but also carefully consider the limitations of this research that argue for replication and improved systems that encourage data sharing.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Mathew has received consulting fees from Acadia, Alkermes, Cerecor, Otsuka, and Valeant, and serves on an Advisory Board for VistaGen Therapeutics. He has received research support from Janssen Research & Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the U.S. Government.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Mathew receives support from the Johnson Family Chair for Research in Psychiatry at the Baylor College of Medicine, and support through the use of resources and facilities at the Michael E. Debakey VA Medical Center, Houston, Texas.
References
- 1.American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders (DSM-5), Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 2.Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am J Psychiatry 2014; 171(4): 395–397. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR, Collins PY, Hyman SE. Darkness invisible. Foreign Affairs 2015; 94(1): 18–19. [Google Scholar]
- 4.Insel TR, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167(7): 748. [DOI] [PubMed] [Google Scholar]
- 5.Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry 2013; 170(1): 59–70. [DOI] [PubMed] [Google Scholar]
- 6.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213(1): 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smoller JW. Disorders and borders: psychiatric genetics and nosology. Am J Med Genet B Neuropsychiatr Genet 2013; 162(7): 559–578. [DOI] [PubMed] [Google Scholar]
- 8.Lang PJ, McTeague LM, Bradley MM. RDoC, DSM, and the reflex physiology of fear: a biodimensional analysis of the anxiety disorders spectrum. Psychophysiology 2016; 53(3): 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nutt DJ. Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry 2001; 62: 22–27. [PubMed] [Google Scholar]
- 10.Ottaviani C, Watson DR, Meeten F, Makovac E, Garfinkel SN, Critchley HD. Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biol Psychol 2016; 119: 31–41. [DOI] [PubMed] [Google Scholar]
- 11.Lueken U, Zierhut KC, Hahn T, et al. Neurobiological markers predicting treatment response in anxiety disorders: a systematic review and implications for clinical application. Neurosci Biobehav Rev 2016; 66: 143–162. [DOI] [PubMed] [Google Scholar]
- 12.Nieto SJ, Patriquin MA, Nielsen DA, et al. Don’t worry; Be informed about the epigenetics of anxiety. Pharmacol Biochem Behav 2016; 146: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angst J, Paksarian D, Cui L, et al. The epidemiology of common mental disorders from age 20 to 50: results from the prospective Zurich cohort study. Epidemiol Psychiatr Sci 2016; 25(1): 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunderland M, Mewton L, Slade T, et al. Investigating differential symptom profiles in major depressive episode with and without generalized anxiety disorder: true co-morbidity or symptom similarity? Psychol Med 2010; 40(7): 1113–1123. [DOI] [PubMed] [Google Scholar]
- 15.Newman MG, Przeworski A, Fisher AJ, et al. Diagnostic comorbidity in adults with generalized anxiety disorder: impact of comorbidity on psychotherapy outcome and impact of psychotherapy on comorbid diagnoses. Behav Ther 2010; 41(1): 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown TA, Marten PA, Barlow DH. Discriminant validity of the symptoms constituting the DSM-III-R and DSM-IV associated symptom criterion of generalized anxiety disorder. J Anxiety Disord 1995; 9(4): 317–328. [Google Scholar]
- 17.Goisman RM, Goldenberg I, Vasile RG, et al. Comorbidity of anxiety disorders in a multicenter anxiety study. Compr Psychiatry 1995; 36(4): 303–311. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson WC, DiNardo PA, Rapee RM, et al. Syndrome comorbidity in patients diagnosed with a DSM-III—R anxiety disorder. J Abnorm Psychol 1990; 99(3): 308. [DOI] [PubMed] [Google Scholar]
- 19.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol 1998; 49(1): 377–412. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med 2008; 38(3): 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beesdo K, Pine DS, Lieb R, et al. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry 2010; 67(1): 47–57. [DOI] [PubMed] [Google Scholar]
- 22.Aldao A, Mennin D, Linardatos E, et al. Differential patterns of physical symptoms and subjective processes in generalized anxiety disorder and unipolar depression. J Anxiety Disord 2010; 24(2): 250–259. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence AE, Liverant GI, Rosellini AJ, et al. Generalized anxiety disorder within the course of major depressive disorder: examining the utility of the DSM-IV hierarchy rule. Depress Anxiety 2009; 26(10): 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittchen HU, Kessler R, Pfister H, et al. Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatr Scand 2000; 102(s406): 14–23. [PubMed] [Google Scholar]
- 25.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164(10): 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendler KS, Gardner CO, Gatz M, et al. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol Med 2007; 37(3): 453–462. [DOI] [PubMed] [Google Scholar]
- 27.Roy M-A, Neale M, Pedersen N, et al. A twin study of generalized anxiety disorder and major depression. Psychol Med 1995; 25(5): 1037–1049. [DOI] [PubMed] [Google Scholar]
- 28.MacNamara A, Kotov R, Hajcak G. Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: an event-related potential study. Cognit Ther Res 2015; 40(3): 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J Abnorm Psychol 2012; 121(4): 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann SG, Schulz SM, Heering S, et al. Psychophysiological correlates of generalized anxiety disorder with or without comorbid depression. Int J Psychophysiol 2010; 78(1): 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oathes DJ, Patenaude B, Schatzberg AF, et al. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry 2015; 77(4): 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 2004; 1032(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 33.Hüther G. The central adaptation syndrome: psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog Neurobiol 1996; 48(6): 569–612. [DOI] [PubMed] [Google Scholar]
- 34.Chrousos G. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord 2000; 24(S2): S50–S55. [DOI] [PubMed] [Google Scholar]
- 35.Mann SJ. Severe paroxysmal hypertension (pseudopheochromocytoma): understanding the cause and treatment. Arch Intern Med 1999; 159(7): 670–674. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry 2000; 48(8): 721–731. [DOI] [PubMed] [Google Scholar]
- 37.Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29(8): 1195–1200. [DOI] [PubMed] [Google Scholar]
- 38.Brindley DN, Rolland Y. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin Sci (Lond) 1989; 77(5): 453–461. [DOI] [PubMed] [Google Scholar]
- 39.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med 1993; 153(18): 2093–2101. [PubMed] [Google Scholar]
- 40.Patriquin MA, Wilson LC, Kelleher SA, et al. Psychophysiological reactivity to abuse-related stimuli in sexually revictimized women. J Aggress Maltreat Trauma 2012; 21(7): 758–775. [Google Scholar]
- 41.Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by α 1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34(6): 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claessens SE, et al. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology 2011; 214(1): 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 2004; 130(3): 355. [DOI] [PubMed] [Google Scholar]
- 44.Reiss D, Leve LD, Neiderhiser JM. How genes and the social environment moderate each other. Am J Public Health 2013; 103(S1): S111–S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hovens JG, Giltay EJ, Spinhoven P, et al. Impact of childhood life events and childhood trauma on the onset and recurrence of depressive and anxiety disorders. J Clin Psychiatry 2015; 76(7): 931–938. [DOI] [PubMed] [Google Scholar]
- 46.Eley TC, McAdams TA, Rijsdijk FV, et al. The intergenerational transmission of anxiety: a children-of-twins study. Am J Psychiatry 2015; 172(7): 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starr L, Conway C, Hammen C, et al. Transdiagnostic and disorder-specific models of intergenerational transmission of internalizing pathology. Psychol Med 2014; 44(1): 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moffitt TE, Caspi A, Harrington H, et al. Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychol Med 2007; 37(3): 441–452. [DOI] [PubMed] [Google Scholar]
- 49.Goldston DB, Erkanli A, Daniel SS, Heilbron N, Weller BE, Doyle O. Developmental trajectories of suicidal thoughts and behaviors from adolescence through adulthood. J Am Acad Child Adolesc Psychiatry 2016; 55(5): 400–407.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtin S, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014 (NCHS data brief, no 241), Hyattsville, MD: National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 51.Health, N.I.o.M. NIMH RDoC Matrix. https://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml. Accessed April 27, 2017.
- 52.Marín O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med 2016; 22(11): 1229–1238. [DOI] [PubMed] [Google Scholar]
- 53.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001; 158(10): 1568–1578. [DOI] [PubMed] [Google Scholar]
- 54.Smoller JW, Block SR, Young MM. Genetics of anxiety disorders: the complex road from DSM to DNA. Depress Anxiety 2009; 26(11): 965–975. [DOI] [PubMed] [Google Scholar]
- 55.Hompes T, Izzi B, Gellens E, et al. Investigating the influence of maternal cortiso and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J Psychiatr Res 2013; 47(7): 880–891. [DOI] [PubMed] [Google Scholar]
- 56.Non AL, Binder AM, Kubzansky LD, et al. Genome-wide DNA methylation in neonates exposed to maternal depression, anxiety, or SSRI medication during pregnancy. Epigenetics 2014; 9(7): 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunn EC, Sofer T, Gallo LC, et al. Genome-wide association study of generalized anxiety symptoms in the Hispanic Community Health Study/Study of Latinos. Am J Med Genet B Neuropsychiatr Genet 2016; 174(2): 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy TM, O'Donovan A, Mullins N, O'Farrelly C, McCann A, Malone K. Anxiety is associated with higher levels of global DNA methylation and altered expression of epigenetic and interleukin-6 genes. Psychiatr Genet 2015; 25(2): 71–78. [DOI] [PubMed] [Google Scholar]
- 59.Makovac E, Meeten F, Watson DR, et al. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol Psychiatry 2016; 80(10): 786–795. [DOI] [PubMed] [Google Scholar]
- 60.Fonzo GA, Etkin A. Brain connectivity reflects mental and physical states in generalized anxiety disorder. Biol. Psychiatry 2016; 80(10): 733–735. [DOI] [PubMed] [Google Scholar]
- 61.Hilbert K, Lueken U, Beesdo-Baum K. Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: a systematic review. J Affect Disord 2014; 158: 114–126. [DOI] [PubMed] [Google Scholar]
- 62.Etkin A, Prater KE, Schatzberg AF, et al. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 2009; 66(12): 1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monk C, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65(5): 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 2013; 52(3): 290–299.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diekhof EK, Geier K, Falkai P, et al. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 2011; 58(1): 275–285. [DOI] [PubMed] [Google Scholar]
- 66.Maslowsky J, Mogg K, Bradley BP, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol 2010; 20(2): 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pittig A, Arch JJ, Lam CW, et al. Heart rate and heart rate variability in panic, social anxiety, obsessive‚ Äìcompulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol 2013; 87(1): 19–27. [DOI] [PubMed] [Google Scholar]
- 68.Newman MG, Llera SJ, Erickson TM, et al. Worry and generalized anxiety disorder: a review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annu Rev Clin Psychol 2013; 9: 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grillon C, O’Connell K, Lieberman L, et al. Distinct responses to predictable and unpredictable threat in anxiety pathologies: effect of panic attack. Biol Psychiatry Cogn Neurosci Neuroimaging. In press. [DOI] [PMC free article] [PubMed]
- 70.Hoehn-Saric R, McLeod DR, Zimmerli WD. Somatic manifestations in women with generalized anxiety disorder: psychophysiological responses to psychological stress. Arch Gen Psychiatry 1989; 46(12): 1113–1119. [DOI] [PubMed] [Google Scholar]
- 71.Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. Int J Psychophysiol 2007; 63(1): 39–47. [DOI] [PubMed] [Google Scholar]
- 72.Pieper S, Brosschot JF, van der Leeden R, et al. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosom Med 2010; 72(6): 570–577. [DOI] [PubMed] [Google Scholar]
- 73.Llera SJ, Newman MG. Effects of worry on physiological and subjective reactivity to emotional stimuli in generalized anxiety disorder and nonanxious control participants. Emotion 2010; 10(5): 640. [DOI] [PubMed] [Google Scholar]
- 74.Stapinski LA, Abbott MJ, Rapee RM. Evaluating the cognitive avoidance model of generalised anxiety disorder: impact of worry on threat appraisal, perceived control and anxious arousal. Behav Res Ther 2010; 48(10): 1032–1040. [DOI] [PubMed] [Google Scholar]
- 75.Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, Pizzagalli DA. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety 2014; 31(3): 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 2005; 62(2): 146–152. [DOI] [PubMed] [Google Scholar]
- 77.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297(5580): 400–403. [DOI] [PubMed] [Google Scholar]
- 78.Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry 2008; 63(9): 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 2005; 8(6): 828–834. [DOI] [PubMed] [Google Scholar]
- 80.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 2009; 301(23): 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munafò MR, Durrant C, Lewis G, et al. Gene× environment interactions at the serotonin transporter locus. Biol Psychiatry 2009; 65(3): 211–219. [DOI] [PubMed] [Google Scholar]
- 82.Canli T, Qiu M, Omura K, et al. Neural correlates of epigenesis. Proc Natl Acad Sci 2006; 103(43): 16033–16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light-and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology 2009; 34(6): 1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex 2012; 23(1): 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinberg A, Meyer A, Hale-Rude E, et al. Error-related negativity (ERN) and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology 2016; 53(3): 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biol Psychol 2003; 64(1): 77–90. [DOI] [PubMed] [Google Scholar]
- 87.Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, Critchley HD. Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J Neurosci 2014; 34(19): 6573–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davies CD, Craske MG. Psychophysiological responses to unpredictable threat: effects of cue and temporal unpredictability. Emotion 2015; 15(2): 195. [DOI] [PubMed] [Google Scholar]
- 89.Baldwin PR, Curtis KN, Patriquin MA, et al. Identifying diagnostically-relevant resting state brain functional connectivity in the ventral posterior complex via genetic data mining in autism spectrum disorder. Autism Res 2016; 9(9): 553–562. [DOI] [PubMed] [Google Scholar]
- 90.Picho K, Maggio LA, Artino AR. Science: the slow march of accumulating evidence. Perspect Med Educ 2016; 5(6): 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]