Abstract
Despite multiple examples of glucose lowering therapies affecting heart failure (HF) risk, ascertainment of HF data in cardiovascular outcome trials of these medications has not been systematically characterized. In this review, large (N >1,000) published phase III/IV cardiovascular outcome trials evaluating glucose lowering therapies through June 2017 were identified. Data were abstracted from publications, Food and Drug Administration (FDA) Advisory Committee records, and FDA labeling documents. Overall, 21 trials including 152,737 patients were evaluated. Rates and definitions of baseline HF and incident HF were inconsistently provided. Baseline ejection fraction data were provided in 3 studies but not specific to patients with HF. No trial reported functional class, ejection fraction, or HF therapy at time of incident HF diagnosis. HF hospitalization data were available in 15 trials, but only 2 included a HF-related event within the primary composite endpoint. This systematic review highlights gaps in HF data capture within cardiovascular outcome trials of glucose lowering therapies and outlines rationale and strategies for improving HF characterization.
Keywords: heart failure, diabetes mellitus, clinical trial, medication, outcomes
Cardiovascular (CV) death is the leading cause of death among patients with type 2 diabetes mellitus (DM) (1–4). While several studies show an association between hemoglobin A1c lowering and reduction in microvascular events, including retinopathy and nephropathy, benefits for macrovascular disease risk and CV mortality had not been seen until recently (1,5,6). Based partly on a meta-analysis of 42 clinical trials that showed elevated risk of myocardial infarction with rosiglitazone, in 2008, the United States (U.S.) Food and Drug Administration (FDA) issued an industry guidance regarding the routine evaluation of CV risk for new therapies to treat type 2 DM (7,8). This guidance recommended establishment of independent CV endpoints committees for DM trials to prospectively adjudicate all CV events occurring across the phase II and phase III registration program, which should encompass major adverse CV events (MACE), including CV death, non-fatal myocardial infarction, and non-fatal stroke.(7) Additional endpoints for consideration included hospitalization for acute coronary syndrome and urgent revascularization procedures.
The FDA guidance document did not specifically mention heart failure (HF) as a condition or endpoint in DM trials. In recent years, a close relationship between DM and HF has become increasingly apparent. In a large observational study of patients with DM, HF was the second most common initial presentation of CV disease, after peripheral arterial disease.(9) Among older patients with DM, >20% have HF with a high proportion experiencing HF-related death (10). Likewise, among patients hospitalized for HF, the prevalence of DM may exceed 40% (11,12). Concomitant presence of both conditions worsens prognosis and complicates treatment. Moreover, data suggest strict glycemic control may not meaningfully change risk of HF in patients with DM and multiple trials of glucose lowering therapies have now been associated with increased or decreased risk of HF events, compared with placebo (13–18).
Despite these findings confirming important pathophysiologic and clinical interactions between DM and HF, compared to atherothrombotic cardiovascular events, the rigor with which HF data are ascertained within DM trials remains modest. Although recent publications have emphasized the need for improved description of HF events in DM trials, HF data capture within such trials has not been systematically characterized (18–20). We present a systematic review of the ascertainment of baseline HF, incident HF, and reporting of HF-related clinical events within published clinical trials of glucose lowering therapies.
METHODS
Data Sources and Search Strategy
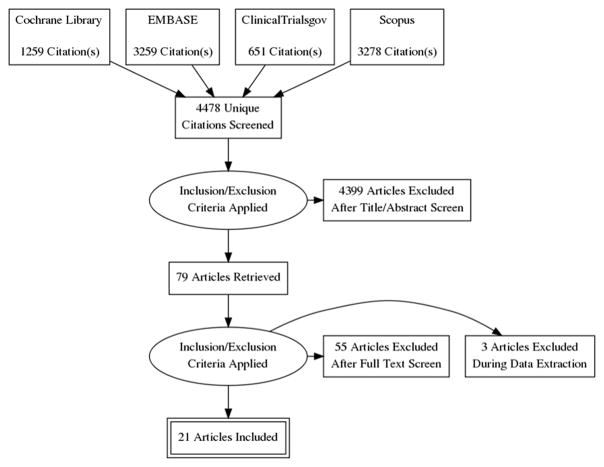
An extensive literature search was conducted using Cochrane library, EMBASE, PubMed, Scopus and ClinicalTrials.gov from the inception of these databases through June 2017 to identify publications from large (N >1,000) phase III or IV randomized clinical trials with primary clinical event endpoints evaluating glucose lowering therapies in adults >18 years of age. Medical subject headings and keywords used in the query included diabetes mellitus, glucose, sulfonylurea, metformin, glyburide, rosiglitazone, pioglitazone, glucose control, insulin, alogliptin, saxagliptin, sitagliptin, aleglitazar, sodium-glucose co-transporter 2 (SGLT-2) inhibitors, empagliflozin, canagliflozin, glucagon-like peptide 1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, liraglutide, semaglutide, lixisenatide, exenatide, and a combination of all these terms. Other data sources such as references of pertinent reviews and editorials from major medical journals were also searched. All publications indexed to a particular trial were screened, including the trial’s dedicated design papers, when available. The search strategy did not include language limits. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram summarizing the search strategy and selected studies is presented in Figure 1.
Figure 1. PRISMA Flow Diagram of Search Strategy and Study Selection.
Abbreviations: PRISMA= Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study Selection
Studies identified through the search strategy were transferred to Endnote X8 (Clarivate Analytics, Philadelphia, Pennsylvania) where duplicates were removed. All remaining articles underwent a rigorous manual screen by two independent reviewers. Large studies (N >1,000) including primary data from randomized clinical trials of glucose lowering therapies among patients with type 2 DM were included. Review articles and non-randomized studies were excluded. Discrepancies in study selection were resolved by consensus or, when necessary, by a third reviewer.
FDA Advisory Committee and Labeling Document Review 1996 to 2016
To best ensure inclusion of any available but unpublished data from any given clinical trial included in this review, for each study therapy, FDA Advisory Committee documents and FDA medical review documents were reviewed for relevant HF trial data. Specifically, we identified all Endocrinologic and Metabolic Drugs Advisory Committee meetings related to glucose lowering therapies from 1996 to 2016 available on the FDA website. We then systematically reviewed each published meeting transcript for the following terms: “heart failure”, “cardiomyopathy”, “pulmonary edema”, and “natriuretic peptide” (NP). Relevant meeting slides and minutes were also reviewed for corroborating information.
Data Extraction
All pertinent data were extracted from main manuscript texts, manuscript supplementary appendices and the aforementioned relevant FDA documents. All data were collected on a standardized form by two reviewers. The main outcome variables were the ascertainment and definitions of HF at baseline and during follow up. Specific data regarding the following elements were extracted:
Baseline HF assessment: Reporting of HF prevalence, definition for pre-existing HF, ejection fraction (EF), New York Heart Association (NYHA) class (data provided for patients or referenced in study selection criteria), NP level, and baseline HF therapy (including diuretic therapy).
Incident HF during follow-up: Ascertainment of new-onset HF, definition for new-onset HF, adjudication of new-onset HF, reporting of information at the time of new HF diagnosis (care setting of diagnosis, EF, NP level, HF therapies received), and clinical event reporting subsequent to new HF diagnosis.
Resource utilization and outcomes: Reporting of fatal HF events, HF hospitalizations, emergency department visits for HF, outpatient worsening HF, inclusion of a HF event within the primary composite trial outcome, and adjudication of reported HF events.
Data Analysis
As appropriate, descriptive analyses were performed, ranges were presented, and proportions were assessed. In circumstances where rates of baseline or incident HF for the overall study population were not provided, these rates were manually calculated from the raw trial data, when available. Analyses were performed using STATA version 14.0 (Stata Corporation, College Station, TX).
RESULTS
Studies
The initial query yielded a total of 8,447 potentially relevant abstracts, of which 4,478 remained after removing duplicates. Based on manual screen of each of the remaining articles, 4,457 articles did not meet the systematic review eligibility criteria and were excluded. The remaining 21 articles were included in the systematic review which included a total of 152,737 patients. Figure 1 shows a PRISMA flow chart outlining the search strategy. SGLT-2 inhibitors and DPP- 4 inhibitors were studied by 3 trials each, while peroxisome proliferator-activated receptor modulators and GLP-1 receptor agonists were studied by 4 trials each. Remaining trials evaluated other drug therapies (including insulin regimens) or the role of intensive glycemic control.
Baseline Heart Failure
Of the 21 trials, prevalence of baseline HF was reported in 14 (67%) studies (Table 1). One study, the ORIGIN (Outcome Reduction with an Initial Glargine Intervention) trial, listed HF as an exclusion criterion. LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) and SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes) included NYHA class II–III HF as trial eligibility criteria in patients above the age of 50 years.(21,22) Excluding ORIGIN, all trials not providing baseline HF prevalence were published prior to 2010. Among studies reporting baseline HF, prevalence ranged from 0.5% in the RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Combination Therapy for Type 2 Diabetes) trial to 27.9% in the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial (15,23).
Table 1.
Reporting of baseline heart failure among cardiovascular outcomes trials of glucose lowering medications
| Trial | Drug class / Intervention |
N | HF reported? |
Definition of baseline HF |
Proportion of HF |
Ejection fraction reported? |
NYHA class reported?* |
Natriuretic peptide level reported? |
HF treatment reported? |
|---|---|---|---|---|---|---|---|---|---|
| EXSCEL (2017)(26) | GLP-1 receptor agonists | 14,752 | Yes | not described | 16.2% | Yes | Yes | No | No |
| CANVAS (2017)(53) | SGLT-2 inhibitors | 4,330 | Yes | not described | 11.9% | No | Yes | No | No |
| CANVAS-R (2017)(53) | SGLT-2 inhibitors | 5,812 | Yes | not described | 16.3% | No | Yes | No | No |
| LEADER (2016)(21) | GLP-1 receptor agonists | 9,340 | Yes | not described | 17.8% | No | Yes | No | No |
| SUSTAIN 6 (2016)(22) | GLP-1 receptor agonists | 3,297 | Yes | not described | 23.6% | No | Yes | No | No |
| EMPA-REG OUTCOME (2015)(17,24) | SGLT-2 inhibitors | 7,020 | Yes | described | 10.1% | No | Yes | No | Yes† |
| ELIXA (2015)(31) | GLP-1 receptor agonists | 6,068 | Yes | not described | 22.4% | No | Yes | No | No |
| TECOS (2015)(65) | DPP-4 inhibitors | 14,671 | Yes | not described | 18.3% | No | Yes | No | No |
| AleCardio (2014)(30) | PPAR modulator | 7,226 | Yes | not described | 10.5% | No | Yes | Yes | No |
| EXAMINE (2013)(23,29) | DPP-4 inhibitors | 5,380 | Yes | not described | 27.9% | No | Yes | Yes | No |
| SAVOR-TIMI 53 (2013)(16,28) | DPP-4 inhibitors | 16,492 | Yes | not described | 12.8% | No | No | Yesc | No |
| ORIGIN (2012)(32) | Glargine insulin | 12,537 | HF listed as exclusion criterion | - | - | No | - | No | - |
| BARI 2D (2009)(27) | Oral glucose lowering therapy vs. insulin provision | 2,368 | Yes | not described | 6.7% | Yes | Yes | No | No |
| RECORD (2009)(15) | PPAR modulator | 4,447 | Treatment for HF listed as exclusion criterion but baseline HF was reported | not described | 0.5% | No | No | No | No |
| HEART2D (2009)(25) | Prandial vs. basal insulin | 1,115 | No | - | - | Yes | - | No | - |
| VADT (2009)(36,66,67) | Intensive glucose therapy | 1,791 | No | - | - | No | Yes | No | - |
| ACCORD (2008)(14) | Intensive glucose therapy | 10,251 | Yes | not described | 4.9% | No | Yes | No | No |
| ADVANCE (2008)(13) | Intensive glucose therapy | 11,140 | No | - | - | No | - | No | - |
| ADOPT (2006)(39) | Thiazolidinedione vs. Metformin vs. Sulfonylurea | 4,360 | HF listed as exclusion criterion | - | - | No | Yes | No | - |
| PROactive (2005)(37) | PPAR modulator | 5,238 | No | - | - | No | Yes | No | - |
| UKPDS (1998)(38) | Intensive glucose therapy | 5,102 | No | - | - | No | - | No | - |
Abbreviations: DPP = dipeptidyl peptidase; GLP = glucagon-like peptide; HF = heart failure; PPAR = peroxisome proliferator-activated receptor; SGLT = sodium-glucose co-transporter
Data provided or referenced in trial selection criteria
Although most HF patients were treated with renin-angiotensin-aldosterone-system inhibitors and beta-blockers, no data were available regarding doses of drugs, other therapies in the chronic HF armamentarium, or degree of optimization.
Baseline NT-proBNP data available for 12,301 patients
Of trials with baseline HF documentation, only 1 provided a definition of baseline HF (17). The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose) trial defined HF through a query of the Medical Dictionary for Regulatory Activities (17,24). No trial reported individual prevalence of HF with reduced versus preserved EF, incorporated NP data, or described therapy (and degree of optimization) among patients with baseline HF. Three studies provided EF data for the overall trial population (25–27). Baseline NP levels for the overall population were reported within the primary publication for 1 trial and secondary publications for 2 trials.(28–30) Data for NYHA class were inconsistently provided. Of publications where NYHA class was mentioned, most included only in the context of study selection criteria (e.g., exclusion of patents with NYHA class IV symptoms) and did not provide specific data on functional class of patients who were enrolled.
Incident Heart Failure
Of the 20 trials with published study results, 6 (30%) trials ascertained incident HF during study follow-up (Table 2). Rates of incident HF over follow-up ranged from 1.7% in the EXAMINE trial to 17.9% in the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial.(23,27,29) In BARI 2D, new onset HF was an adjudicated adverse event, but the exact definition used was not published (27). Aside from BARI 2D, incident HF was either not directly adjudicated or the adjudication status was unclear. Additionally, these 5 other studies included non-specific definitions of new onset HF with 4 studies providing data for hospitalization for HF among patients without prior history of HF (28,29,31,32). The remaining study (EMPA-REG OUTCOME) reported data on introduction of loop diuretics during follow-up (24). No trial reported data on clinical characteristics at the time of incident HF diagnosis, including EF, NP level, or HF therapy received. Two trials provided data on longitudinal NP levels during follow-up, but not at the time of new HF diagnosis (28,29). No trial accounted for potential new HF diagnoses made in the ambulatory setting or during urgent care or emergency department visits. No trial reported outcome data subsequent to an incident HF event.
Table 2.
Trials assessing incident heart failure during study follow-up
| Trial | Definition of new onset HF | Adjudicated results? | % new onset heart failure during trial follow-up | Specify inpatient vs. outpatient diagnosis of new HF? | Ejection fraction at HF diagnosis reported? | Natriuretic peptide at HF diagnosis reported? | Characterize HF treatment at time of new HF diagnosis? | Characterize clinical events subsequent to new HF diagnosis? |
|---|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME (2015)(17,24) | Data provided on introduction of loop diuretics | Unclear if introduction of loop diuretics adjudicated | 10.2% | No | No | No | No* | No |
| ELIXA (2015)(31) | Data provided on hospitalization for HF among patients without prior HF | HF hospitalization adjudicated | 2.4% | Inpatient | No | No | No | No |
| EXAMINE (2013)(23,29) | Data provided on hospitalization for HF among patients without prior HF | HF hospitalization adjudicated | 1.7% | Inpatient | No | No† | No‡ | No |
| SAVOR-TIMI 53 (2013)(16,28) | Data provided on hospitalization for HF among patients without prior HF | HF hospitalization adjudicated | 2.3% in saxagliptin arm; 1.7% in placebo arm§ | Inpatient | No | No | No|| | No |
| ORIGIN (2012)(32) | Data provided on hospitalization for HF in setting of baseline HF listed as trial exclusion criterion | HF hospitalization adjudicated | 5.2% | Inpatient | No | No | No | No |
| BARI 2D (2009)(27) | Not provided | Yes | 17.9% | No | No | No | No | No |
Abbreviations: HF = heart failure
Analyses compared adjudicated and investigator-reported HF events with new initiation of loop diuretics.
Longitudinal NT-proBNP data provided for trial patients with and without baseline HF, but not provided at time of new onset HF event.
Data regarding new initiation of loop diuretics by treatment arm in those with or without baseline HF were available.
Data represent 2-year study follow-up.
US Food and Drug Administration Advisory Committee records included information on symptoms and HF therapies at time of hospitalization for HF, but did not distinguish between event representing incident HF versus worsening of established HF.
Heart Failure Outcomes
Table 3 summarizes data on HF events reported during follow-up. Among trials reporting HF events, all utilized a blinded adjudication procedure. The RECORD trial used a separate prospective and post hoc adjudication committee for HF events (15,33,34). In the PROactive (Prospective Pioglitazone Clinical Trial in Macrovascular Events) trial, serious HF events were retrospectively reviewed in blinded fashion (35).
Table 3.
Heart failure outcome reporting among cardiovascular outcome trials of glucose lowering medications
| Trial | Reporting of fatal HF events? | Reporting of HF hospitalizations? | Reporting of HF emergency visit? | Reporting of outpatient worsening HF? | HF-event part of the primary composite endpoint? | Adjudication of reported HF events? |
|---|---|---|---|---|---|---|
| CANVAS (2017)(53) | No | Yes | No | No | No | Yes |
| CANVAS-R (2017)(53) | No | Yes | No | No | No | Yes |
| LEADER (2016)(21) | No | Yes | No | No | No | Yes |
| SUSTAIN 6 (2016)(22) | No | Yes | No | No | No | Yes |
| EMPA-REG OUTCOME (2015)(17,24) | Yes | Yes | No* | No | No | Yes |
| ELIXA (2015)(31) | No | Yes | No | No | No | Yes |
| TECOS (2015)(65) | No | Yes | No | No | No | Yes |
| AleCardio (2014)(30) | No | Yes | No | No | No | Yes |
| EXAMINE (2013)(23,29) | No | Yes | No | No | No | Yes |
| SAVOR-TIMI53 (2013)(16,28)† | Yes | Yes | No | No | No | Yes |
| ORIGIN (2012)(32) | No | Yes | No | No | Yes | Yes |
| BARI 2D (2009)(27) | No | No | No | No | No | - |
| RECORD (2009)(15,33,34) | Yes | Yes | No | No | No | Yes‡ |
| HEART2D (2009)(25)§ | No | No | No | No | No | - |
| VADT (2009)(36,66) | Yes | Yes|| | No | No | Yes | Yes |
| ACCORD (2008)(14) | Yes | Yes# | No | No | No | Yes |
| ADVANCE (2008)(13)** | No | No | No | No | No | - |
| ADOPT (2006) (39)†† | No | No | No | No | No | - |
| PROactive (2005)(37) | Yes | Yes | No | Yes | No | Yes‡‡ |
| UKPDS (1998)(38)§§ | No | No | No | No | No | - |
Abbreviations: HF = heart failure
The hospitalization for HF event definition was expanded during the trial to include overnight admissions, emergency room visits, and inpatient stays requiring changes in oral diuretics (not just intravenous diuretics), but data isolated to emergency room visits are not reported
Defined based on the standardized Clinical Data Interchange Standards Consortium event definitions
HF death was prospectively adjudicated. Subsequently, HF death and HF hospitalization events underwent a separate post-hoc adjudication process.
Reported rates of “congestive heart failure” as an adjudicated individual outcome during follow-up. Precise definition not reported.
Congestive heart failure defined by International Classification of Diseases, Ninth Revision code as “≥1 inpatient occurrence primary discharge.”
HF events were discussed during the Food and Drug Administration Advisory Committee meeting
Primary manuscript reports the rate of heart failure, defined as the composite of death due to heart failure, hospitalization for heart failure, or worsening New York Heart Association class. Data for individual endpoint components not provided.
“Congestive heart failure” data represented investigator-reported adverse event. Precise definition not reported.
Post-hoc retrospective adjudication of HF death and HF hospitalization.
Heart failure endpoint defined by clinical symptoms (not associated with myocardial infarction) confirmed by Kerley B lines, râles, raised jugular venous pressure, or third heart sound. No precise data reported for fatal HF events or HF hospitalization
Data were most frequently reported for hospitalizations for HF, with such information published for 15 (75%) trials. Six trials provided rates of HF death for both study arms (14–17,36,37). A single trial, the PROactive trial, provided data regarding outpatient worsening HF.(37) The EMPA-REG OUTCOME trial expanded the hospitalization for HF event definition to include emergency room visits and worsening HF (defined as presence of signs and symptoms of congestion requiring initiation or uptitration of HF therapies) after interim data unblinding to an independent monitoring team (17,24). No other trial had available data on rates of emergency department visits for HF. Three trials provided non-specific data on HF episodes during follow-up without detailing associated death, hospitalization, or need for escalated HF treatment (25,38,39). The ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial reported a composite of HF events, including HF death, hospitalization for HF, or worsening NYHA class, but did not publish data on specific components.(13) Two studies, the VADT (Veterans Affairs Diabetes Trial) trial and the ORIGIN (Outcome Reduction with an Initial Glargine Intervention) trial, included a HF-related event within a primary composite endpoint.(32,36)
DISCUSSION
This systematic review of 21 large CV outcomes trials testing a broad range of glucose lowering therapies highlights several key points:
There is limited characterization of the baseline prevalence, severity, or treatment optimization of HF.
Even in trials reporting baseline information, HF representation is variable and low, accounting for <15% of the total enrolled sample.
Only 30% of trials describe new-onset HF among patients without baseline HF and definitions of incident HF events were generally non-specific.
Only two trials include HF in a primary composite endpoint and few trials report adjudicated HF events outside of HF hospitalizations.
An Unmet and Compelling Need
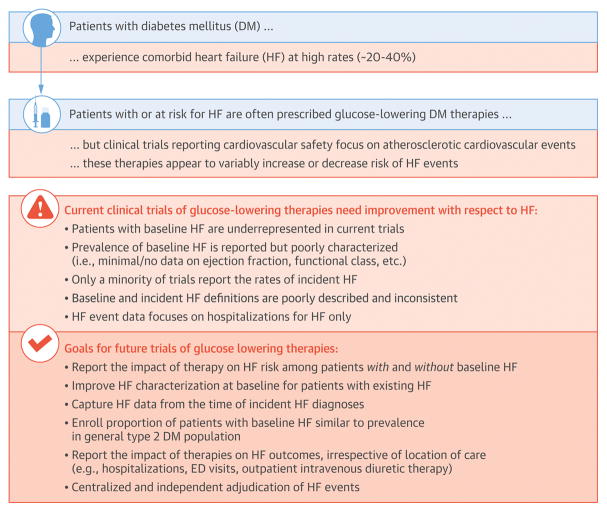
Accumulating data support an increasingly greater need to clearly appraise the potential benefits and harms of novel glucose lowering therapies in patients with or at risk for HF (Central Illustration). First, comorbid DM is present in a significant subset of patients with prevalent HF and is associated with heightened CV risk (10–12). The prevalence of HF is poised to increase given population trends towards increasing age, worsening comorbidity burden, and improved survival following myocardial infarction. Second, risk profiles of individuals with and without baseline HF may be markedly different. Judicious accounting and profiling of this HF subset in CV outcome trials of glucose lowering therapies will substantially influence the background risk of the trial cohort and the planned number of enrolled patients. Third, some glucose lowering therapies that improve overall CV outcomes in high-risk populations may not benefit patients with prevalent HF.(40) Fourth, HF-related events are frequently encountered in DM trials, and are perhaps more common than certain components of MACE, depending on the population studied. These events may preferentially drive treatment-related safety or efficacy. Unlike atherothrombotic events that may take time to accrue, mechanisms linking glucose lowering therapies with HF risk or benefit may operate on a shorter timescale (e.g., therapy induced changes in volume status influencing HF hospitalization risk), and may be more readily detectable during the course of typical CV outcome trials. Fifth, there is convincing CV benefit with select agents from at least 2 major glucose lowering therapeutic classes, one of which (i.e., SGLT-2 inhibitors) appears to have profound effects on risk of HF hospitalization.(17,21,22) Finally, the US FDA has broadened the indications for use of empagliflozin and liraglutide to specifically reduce CV risk, the first clinical outcomes indications for any glucose lowering therapy for type 2 DM. Indeed, there is growing appreciation and interest within the cardiology community for utilization of novel DM therapies to mitigate CV risk (41).
Central Illustration. Framework for Improving Characterization of Heart Failure in Cardiovascular Outcome Trials of Patients with Diabetes.
DM, diabetes mellitus; ED, emergency department; EF, ejection fraction; HF, heart failure; IV, intravenous; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Heart Failure Risks and Benefits of Novel Diabetes Therapies
Although designed to detect risk of MACE, CV outcome trials for glucose lowering therapies have found certain agents to increase HF risk.(37,42–44) Most recently, DPP-4 inhibitors have been shown to heighten risk of HF, but this does not appear to be a class effect, and may be specific to saxagliptin.(28,29,45,46) In the SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus–Thrombolysis In Myocardial Infarction) 53 trial, the risk of HF appeared to drive the safety hazard associated with the drug, and was more frequently observed than certain components of the primary endpoint (i.e., stroke) (47). As such, regulatory warnings have been issued and U.S. product labels modified to acknowledge potential HF risks with use of saxagliptin. The U.S. FDA now suggests judicious use of the DPP-4 inhibitor class in patients at risk for HF.
Despite the original purpose for confirming safety, outcome data from large, phase IV outcome trials has led to efficacy indications within 2 classes of glucose lowering therapies; GLP-1 receptor agonists and SGLT-2 inhibitors. However, although 2 large clinical trials studying the GLP-1 receptor agonists liraglutide and semaglutide showed reductions in the primary composite CV endpoint, no clear effects on risk of HF hospitalization were demonstrated.(21,22) Indeed, in 2 phase II studies, liraglutide did not improve clinical outcomes in patients with reduced EF and some have speculated that the GLP-1 receptor agonist mechanism may not be consistent with HF benefits (40,48,49).
Although the mechanism of therapeutic effects of SGLT-2 inhibitors requires further study, benefits appear at least partly mediated by effects on hemodynamic and congestive status.(50,51) The EMPA-REG OUTCOME trial found empagliflozin therapy to result in a pronounced and early lowering of risk of MACE, driven by reductions in CV death, along with reductions in HF events in patients with type 2 DM and established CV disease (10% of whom carried a history of HF at enrollment)(17,24). Similarly, the benefits of empagliflozin on CV death and HF hospitalization were consistent across the spectrum of low to high incident HF risk.(52) In the paired CANVAS (Canagliflozin Cardiovascular Assessment Study) trials of patients with type 2 DM at high CV risk (14% of whom had a baseline HF diagnosis), canagliflozin reduced the primary composite CV endpoint at the expense of heightened risk of lower extremity amputations.(53) In addition, canagliflozin demonstrated marked reductions in the risk of HF hospitalization and progression of renal disease (53).
Future Trials of Novel Glucose Lowering Therapies
Despite recent progress and converging lines of evidence regarding the general CV effects of novel glucose lowering therapies, the specific risk-benefit profile of these therapies with respect to HF remains uncertain. As such, a number of phase III clinical trials of SGLT-2 inhibitors in HF patients are currently ongoing, including EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction; NCT03057977), EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction; NCT03057951), and Dapa-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure; NCT03036124).(50,54)
Moving forward, we strongly recommend that CV outcome trials of glucose lowering therapies are viewed in the context of 2 distinct but important goals for purposes of HF: a) evaluation of downstream incident HF among those without HF at baseline, and b) evaluation of safety and/or efficacy signals among those with prevalent HF at baseline. As such, subgroup analysis by presence or absence of baseline HF should be standard and adequate enrollment of both subgroups should be ensured to allow for meaningful analysis. Although dedicated trials of glucose lowering therapies among patients with established HF will likely remain necessary for purposes of drug labeling and changing HF guidelines, we believe that accurately defining and characterizing HF within CV outcome trials is critical for successful application of safety and efficacy findings to routine clinical practice. Moreover, and perhaps underappreciated, we believe that CV outcome trials may have the important potential to inform HF prevention strategies and potentially change prevention guidelines (although dedicated trials would likely be required for class I recommendations). Acknowledgement of these 2 discrete objectives sets a framework for specific strategies aimed at improving HF characterization in future CV outcome trials of glucose lowering therapies. To define the CV profile of these agents more comprehensively, we propose a modified approach in emerging CV outcome trials of glucose lowering therapies focused on the following elements:
Population Enrolled: CV outcome trials should routinely enroll sufficient number of patients with baseline HF to allow meaningful evaluation of therapeutic safety in this subgroup. The proportion of patients enrolled with manifest HF in EMPA-REG OUTCOME and CANVAS were <15%.(17,53) Likewise, despite specifying NYHA class II–III HF as a means of meeting trial inclusion criteria, LEADER only enrolled ~18% patients with HF at baseline. Given the substantial overlap between HF and DM in routine practice, robust representation of HF patients in CV outcome trials must be a priority to adequately evaluate safety. Moreover, although the threshold for declaring therapeutic efficacy must remain higher than that for raising safety concerns, improved enrollment of HF patients should be strongly considered to generate a subgroup with sample size sufficient for exploring potential HF efficacy signals worthy of further dedicated study.
Improved Data Collection: Routine assessment of cardiac imaging and biomarkers parameters would add significant additional cost and complexity to CV outcome trials. Thus, collection of these data should be guided largely by the presence or absence of baseline HF. For example, when assessing HF prevention in patients without baseline HF, prospective routine collection of these tests cannot be recommended for cost and logistical reasons. However, for such patients who develop new-onset HF during study follow-up, the majority likely undergo such testing locally as part of routine clinical care at the time of the suspected incident HF event (e.g., echocardiogram, NP level testing); acquisition of these data would be invaluable in characterizing potential treatment-related adverse HF effects (e.g., incident HF with reduced versus preserved EF, HF severity at diagnosis) and would carry only modest incremental cost to the trial. In contrast, for patients with an existing diagnosis of HF at trial enrollment, we believe added prospective data collection at baseline is imperative to best explore the impact of therapy in various HF subsets based on EF, baseline HF therapy, and severity of disease. More granular data from patients with prevalent HF would better inform a) the application of trial findings to the general DM population with concomitant HF, and b) design of dedicated HF trials should a therapeutic indication for the treatment of HF be pursued.
Standardized Event Ascertainment: HF events should be prospectively and clearly defined with objective criteria. Indeed, evolution in the definition of HF hospitalization (with liberalization to include emergency room visits and worsening HF) during the EMPA-REG OUTCOME trial, together with incomplete baseline HF profiling, contributed to the FDA considering the HF-based results exploratory and in need of further confirmation. In and of themselves, clinical signs and symptoms for HF may be non-specific and may therefore preclude accurate endpoint assessment. However, documentation of escalation of HF care for such signs and symptoms, such as treatment with intravenous diuretics, would more definitively confirm a HF event by linking a subjective clinician assessment with an objective therapeutic decision. The multidisciplinary Clinical Data Interchange Standards Consortium (CDISC) task force has provided guidance regarding the specific ascertainment of HF events, as employed in SAVOR-TIMI 53.(16,55,56) In addition, the Cardiovascular Safety Research Consortium has designed comprehensive examples of HF case report forms that may be adapted for use in trials of glucose lowering therapies.(57) In this systematic review, we found that no trial reported data for CV biomarkers, such as NPs, to contextualize the HF event definition. Assessment of NP level is inexpensive, widely utilized, and may improve the diagnostic accuracy of HF-related events. When available, trials should collect local NP level and other relevant data (e.g., cardiac imaging) from the time of a suspected HF event. Although differences in local laboratory assays could impede interpretation of NP data collected at the time of HF events, data reflecting levels in relation to local upper reference limits could be considered. We believe standardized definitions are critical for evaluating the impact of HF events on other clinical outcomes. Indeed, although worsening HF events have been generally associated with poor subsequent prognosis, whether this relationship is consistent in the settings of specific glucose lowering therapies needs further study.(58)
Centralized and Independent Endpoint Adjudication: Despite potential incremental costs, we recommend incident and worsening HF events be adjudicated by an independent clinical events committee. The exact handling of these events within the trial design and statistical analysis plan can be debated and may vary by situation, but potential options include incorporation of a HF endpoint within a single extended MACE definition, co-primary endpoints with traditional and extended MACE endpoints, or a separate worsening HF composite or hierarchical endpoint.(40) Regardless, given variation in global trial conduct, biomarker ascertainment, and thresholds for patient and clinician hospitalization decisions, adjudication of HF events within DM trials should be strongly considered and is consistent with recommendations applied towards other types of outcome trials.(59) In future trials, we propose a worsening HF event be defined as worsening signs and symptoms of HF with confirmation of elevated NP level and requiring urgent or emergent treatment (e.g., intravenous diuretic administration). Consistent with evolving practice in HF clinical trials and accumulating data suggesting similarly poor prognosis for worsening HF patients in the inpatient and outpatient care settings, we suggest CV outcome trials consider capture of worsening HF events (according to pre-specified criteria) irrespective of the location of care (including ambulatory clinics, urgent care facilities, emergency departments, and hospitals).(58,60–62) Although such a procedure may carry a modest increase in trial cost and complexity, inclusion of the spectrum of worsening HF events may improve power for detecting therapeutic signals and would be congruent with trends among healthcare systems towards increasing emphasis on outpatient management of worsening HF to decrease costs associated with hospitalization.(62,63)
Limitations
We recognize that a more comprehensive approach towards HF ascertainment in future trials has its limitations. Increasing the number of enrolled HF patients and HF-related data collection increases the cost and complexity of trial programs and may conflict with recent emphasis on pragmatic and streamlined trial design.(64) Additionally, biomarkers to aid in HF event ascertainment or adjudication are subject to significant variation with respect to rigor in collection and cut-offs employed. Furthermore, any transition from a traditional 3-component MACE to an extended MACE with a HF endpoint could dilute a signal for atherothrombotic CV events. Such concerns could be mitigated with incorporation of HF events as a co-primary or secondary endpoint. Regarding limitations specific to the present systematic review, although we carefully reviewed trial publications, FDA labeling documents, and FDA Advisory Committee records, it is possible that such retrospective data extraction was incomplete and we cannot rule out presence of additional collected, but unpublished, data by study sponsors or investigators. This limitation further underscores the need for prospective and complete publication of HF-related data from trials of glucose lowering therapies going forward.
Conclusions
Although others have called for greater focus on HF events in clinical trials of novel glucose lowering therapies, we present a comprehensive systematic review examining the ascertainment of HF data.(19,20) Even recently completed large CV outcome trials of novel glucose lowering agents lack sufficient details to fully appraise treatment effects on a HF endpoint or relative safety in patients with prevalent HF. Given increasing attention towards the variable risk of HF events with various glucose lowering therapies and drug classes, we believe limitations in these pre- and post-marketing trial experiences have hindered thorough understanding of the utility of novel glucose lowering therapies with respect to HF prevention, safety, and treatment. We strongly suggest that future CV outcome trials of glucose lowering therapies enroll a proportion of patients with baseline HF similar to the prevalence of HF in the general type 2 DM population. Improved data collection within such trials should include detailed profiling of patients with baseline HF and a rigorous assessment of downstream incident and worsening HF events using pre-specified and adjudicated endpoints. We believe these added efforts towards improved HF characterization within CV outcome studies of glucose lowering therapies have the important potential to a) inform HF prevention strategies b) better define the safety profile of glucose lowering therapies among the general type 2 DM population with respect to HF, and c) better inform the utility and design of dedicated trials evaluating the efficacy of glucose lowering therapies as potential treatments specifically for HF.
Acknowledgments
Funding Sources: None
This manuscript does not represent the views of the United States Food and Drug Administration, nor does this manuscript represent regulatory guidance.
ABBREVIATIONS
- CV
cardiovascular
- DM
diabetes mellitus
- DPP-4
dipeptidyl peptidase-4
- EF
ejection fraction
- FDA
U.S. Food and Drug Administration
- GLP-1
glucagon-like peptide 1
- HF
heart failure
- MACE
major adverse cardiovascular events
- NP
natriuretic peptide
- SGLT-2
sodium-glucose co-transporter 2
Footnotes
Disclosures: Dr. Greene is supported by the NHLBI T32 postdoctoral training grant (T32HL069749-14) and a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis. Dr. Vaduganathan is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). Dr. Bakris has been a principal investigator on FIDELIO (Bayer), a steering committee member for Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) (Janssen) and Study Of Diabetic Nephropathy With Atrasentan (SONAR) (AbbVie), and a consultant for Merck, Relypsa, Vascular Dynamics, GlaxoSmithKline, Bayer, Janssen, and AbbVie. Dr. Sattar reports consulting fees for Amgen, Sanofi, AstraZeneca, Merck, Eli Lilly, and Boehringer Ingelheim and grant support through his institution from AstraZeneca and Amgen. Dr. McGuire has received personal fees from for clinical trials leadership from AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, Esperion; and personal consulting fees from AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk. Dr. Januzzi has received research support from Janssen, Boehringer Ingelheim, Novartis, Roche Diagnostics, Siemens, Prevencio, Singulex, and Amgen; has served as a consultant for Janssen, Abbott, Boehringer Ingelheim, Novartis, Roche Diagnostics, and Philips; and has served on clinical endpoints adjudication committees for AbbVie and Pfizer. Dr. Butler is a principal investigator of the EMPEROR program (Boehringer Ingelheim); has received research support from the NIH and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Novartis, Relypsa, ZS Pharma, Medtronic, Merck, and CVRx. All other authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan SS, Butler J, Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311:2379–80. doi: 10.1001/jama.2014.4115. [DOI] [PubMed] [Google Scholar]
- 2.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–9. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3:136–45. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 7.Administration FaD. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type. 2008;2 [Google Scholar]
- 8.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 9.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1. 9 million people. Lancet Diabetes Endocrinol. 2015;3:105–13. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 11.Maggioni AP, Greene SJ, Fonarow GC, et al. Effect of aliskiren on post-discharge outcomes among diabetic and non-diabetic patients hospitalized for heart failure: insights from the ASTRONAUT trial. Eur Heart J. 2013;34:3117–27. doi: 10.1093/eurheartj/eht342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 14.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 16.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 17.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 18.Standl E, Schnell O, McGuire DK. Heart Failure Considerations of Antihyperglycemic Medications for Type 2 Diabetes. Circ Res. 2016;118:1830–43. doi: 10.1161/CIRCRESAHA.116.306924. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Bhatt DL, Calvo G, Brown NJ, Zannad F, Mentz RJ. Heart failure event definitions in drug trials in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4:294–6. doi: 10.1016/S2213-8587(16)00049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–51. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 21.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 23.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 24.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37:1526–34. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32:381–6. doi: 10.2337/dc08-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mentz RJ, Bethel MA, Gustavson S, et al. Baseline characteristics of patients enrolled in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) Am Heart J. 2017;187:1–9. doi: 10.1016/j.ahj.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Group BDS, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 29.Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–76. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 30.Lincoff AM, Tardif JC, Schwartz GG, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311:1515–25. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 32.Investigators OT. Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 33.Lopes RD, Dickerson S, Hafley G, et al. Methodology of a reevaluation of cardiovascular outcomes in the RECORD trial: study design and conduct. Am Heart J. 2013;166:208–216e28. doi: 10.1016/j.ahj.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Mahaffey KW, Hafley G, Dickerson S, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J. 2013;166:240–249. e1. doi: 10.1016/j.ahj.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Ryden L, Thrainsdottir I, Swedberg K. Adjudication of serious heart failure in patients from PROactive. Lancet. 2007;369:189–90. doi: 10.1016/S0140-6736(07)60106-8. [DOI] [PubMed] [Google Scholar]
- 36.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 37.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 38.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 39.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 40.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;316:500–8. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattar N, Petrie MC, Zinman B, Januzzi JL., Jr Novel Diabetes Drugs and the Cardiovascular Specialist. J Am Coll Cardiol. 2017;69:2646–2656. doi: 10.1016/j.jacc.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011;11:115–28. doi: 10.2165/11587580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08) Diabetes Care. 2007;30:2773–8. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res. 2013;10:289–301. doi: 10.1177/1479164112475102. [DOI] [PubMed] [Google Scholar]
- 45.Rehman MB, Tudrej BV, Soustre J, et al. Efficacy and safety of DPP-4 inhibitors in patients with type 2 diabetes: Meta-analysis of placebo-controlled randomized clinical trials. Diabetes Metab. 2017;43:48–58. doi: 10.1016/j.diabet.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 46.McGuire DK, Van de Werf F, Armstrong PW, et al. Association Between Sitagliptin Use and Heart Failure Hospitalization and Related Outcomes in Type 2 Diabetes Mellitus: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016;1:126–35. doi: 10.1001/jamacardio.2016.0103. [DOI] [PubMed] [Google Scholar]
- 47.Scirica BM, Braunwald E, Raz I, et al. Heart Failure, Saxagliptin, and Diabetes Mellitus: Observations from the SAVOR-TIMI 53 Randomized Trial. Circulation. 2015;132:e198. doi: 10.1161/CIR.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 48.Packer M. Will long-acting glucagon-like peptide-1 analogues recapitulate our agonizing experience with cyclic AMP-dependent positive inotropic agents in heart failure? Eur J Heart Fail. 2017 Oct 30; doi: 10.1002/ejhf.1047. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 50.Butler J, Hamo CE, Filippatos G, et al. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 51.Januzzi JL, Jr, Butler J, Jarolim P, et al. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults With Type 2 Diabetes. J Am Coll Cardiol. 2017;70:704–712. doi: 10.1016/j.jacc.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2017 Aug 28; doi: 10.1093/eurheartj/ehx511. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 54.Butler J, Anker SD. The Ethics of Conducting Clinical Trials With Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure: Is Placebo Assignment Justified in Patients With Comorbid Diabetes Mellitus and Heart Failure? Circulation. 2017;136:1459–1461. doi: 10.1161/CIRCULATIONAHA.117.029284. [DOI] [PubMed] [Google Scholar]
- 55.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) Circulation. 2015;132:302–61. doi: 10.1161/CIR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 56.CDISC Glossary. [Accessed August 26, 2017]; Available at: http://cdisc.org/therapeutic.
- 57.Cardiac Safety Research Consortium. [Accessed September 7, 2017];Noncardiovascular Clinical Trials CRF. Available at: http://cardiac-safety.org/cardiovascular-case-report-forms-for-non-cardiovascular-clinical-trials.
- 58.Okumura N, Jhund PS, Gong J, et al. Importance of Clinical Worsening of Heart Failure Treated in the Outpatient Setting: Evidence From the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF) Circulation. 2016;133:2254–62. doi: 10.1161/CIRCULATIONAHA.115.020729. [DOI] [PubMed] [Google Scholar]
- 59.Seltzer JH, Heise T, Carson P, et al. Use of endpoint adjudication to improve the quality and validity of endpoint assessment for medical device development and post marketing evaluation: Rationale and best practices. A report from the cardiac safety research consortium. Am Heart J. 2017;190:76–85. doi: 10.1016/j.ahj.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Butler J, Braunwald E, Gheorghiade M. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA. 2014;312:789–90. doi: 10.1001/jama.2014.6643. [DOI] [PubMed] [Google Scholar]
- 61.Skali H, Dwyer EM, Goldstein R, et al. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT-CRT. Eur J Heart Fail. 2014;16:560–5. doi: 10.1002/ejhf.71. [DOI] [PubMed] [Google Scholar]
- 62.Greene SJ, Mentz RJ, Felker GM. Outpatient Worsening Heart Failure as a Target for Therapy: A Review. JAMA Cardiol. 2018:3. doi: 10.1001/jamacardio.2017.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen L, Jhund PS, Mogensen UM, et al. Re-Examination of the BEST Trial Using Composite Outcomes, Including Emergency Department Visits. JACC Heart Fail. 2017;5:591–599. doi: 10.1016/j.jchf.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Eapen ZJ, Lauer MS, Temple RJ. The imperative of overcoming barriers to the conduct of large, simple trials. JAMA. 2014;311:1397–8. doi: 10.1001/jama.2014.1030. [DOI] [PubMed] [Google Scholar]
- 65.Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 66.Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 67.Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17:314–22. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]