Abstract
A major diagnostic challenge for cardiologists is to distinguish cardiac syndrome X (CSX) from obstructive coronary artery disease in women with typical angina and a positive exercise tolerance test (ETT). We performed this study to develop a scoring system that more accurately predicts CSX in this patient population.
Data on 976 women with typical angina and a positive ETT who underwent coronary angiography at our center were randomly divided into derivation and validation datasets. We developed a backward stepwise logistic regression model that predicted the presence of CSX, and a scoring system was derived from it.
The derivation dataset (809 patients) was calibrated by uing a Hosmer-Lemeshow goodness-of-fit test (8 degrees of freedom; χ2=12.9; P=0.115), and the area under the curve was 0.758. The validation dataset (167 patients) was calibrated in the same way (8 degrees of freedom; χ2=9.0; P=0.339), and the area under the curve was 0.782. Independent predictors of CSX were age <55 years; negative histories of smoking, diabetes mellitus, hyperlipidemia, hypertension, or familial premature coronary artery disease; and highly positive ETTs. A total score >9.5 was the optimal cutoff point for differentiating CSX from obstructive coronary artery disease.
Our proposed scoring system is a simple, objective, and accurate system for distinguishing CSX from obstructive coronary artery disease in women with typical angina and positive ETTs. It may help determine which of these patients need invasive coronary angiograms or noninvasive tests like computed tomographic coronary angiography.
Keywords: Chi-square distribution, coronary angiography/utilization, exercise test, female, logistic models, microvascular angina/classification/epidemiology, predictive value of tests, referral and consultation, retrospective studies, ROC curve
Approximately 2 million coronary angiographic procedures are performed annually in Europe,1 and 1.7 million in the United States, for evaluation of angina-like chest pain.2 In 10% to 30% of these patients, the coronary arteries are categorized as “angiographically normal,”3–8 which typically means that no visible or nonobstructive disease is present. In 1973, Kemp9 described these patients as having “cardiac syndrome X” (CSX), defined as typical angina pectoris and normal or near-normal coronary arteries on coronary angiography (CA); later, the definition was modified to include ST-segment depression during an exercise stress test.10–13 Nearly 70% of patients with CSX are women.14 In a large patient cohort suspected to have myocardial ischemia and referred for CA, 41% of the women and only 8% of the men showed nonsignificant epicardial coronary artery disease (CAD).15
Cardiac syndrome X is associated with a wide range of clinical characteristics that may indicate differences in cause and determine patient outcome. However, a major diagnostic challenge for the cardiologist is finding a valid and reliable means of distinguishing patients with CSX from those with obstructive CAD on the basis of clinical characteristics and noninvasive evaluations. If a dependable means of correctly identifying patients with CSX were available, cardiologists could reduce the number of unnecessary CA procedures, which are associated with a small but definite risk to the patient. This, in turn, could lower healthcare costs and reduce waste of medical resources.2
With these goals in mind, we constructed a scoring system to better distinguish patients with CSX from those with obstructive CAD among women with typical angina and a positive exercise tolerance test (ETT). Our scoring system was also intended to identify those patients for whom either invasive or noninvasive evaluation was appropriate.
Patients and Methods
The data for our study were collected from the Tehran Heart Center's cardiac catheterization database, a computerized system implemented in 2004 that includes prospective data on more than 40,000 patients seen for cardiac catheterization. Trained research staff enter data daily, and the validity is checked periodically by comparing 5% of the computerized records against hard-copy records. The database includes information on about 200 variables, including demographic data, presenting symptoms, medical history, risk factors for ischemic heart disease, glucose and lipid profiles, and findings from CA, ETTs, and electrocardiographic, echocardiographic, and myocardial perfusion scans. All variables are consistent with American College of Cardiology (ACC) definitions.16
An ETT was considered positive when, during testing, the patient developed either 1) ischemic discomfort and a horizontal or downsloping ST-segment shift ≥1 mm (0.1 mV); or 2) a new horizontal or downsloping ST-segment shift ≥2 mm (0.2 mV), which is thought to indicate ischemia, even in the absence of discomfort. An ETT was interpreted as highly positive when one or more of the following occurred during testing: 1) ST-segment depression in 5 or more leads, 2) a maximal ST-segment depression >2 mm, 3) a positive test with a heart rate <120 beats/min, 4) hypotension, or 5) exercise capacity of <5 min.17
All patients who underwent elective diagnostic CA at our center's cardiac catheterization laboratory from October 2004 through October 2011 were evaluated for inclusion in our study, and those who had typical angina and a positive or highly positive ETT were selected for further evaluation. Patients with known ischemic heart disease, including those with a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting, were excluded. We also excluded those with a left ventricular ejection fraction <0.50 because, according to the American Heart Association (AHA)/ACC appropriateness guidelines for CA in patients with stable ischemic heart disease (class IIa), invasive CA was indicated for these patients.18 This left 2,436 patients (976 women; 1,460 men) for final analysis.
Before starting to construct our model, we evaluated the gender-specific frequency of normal-to-mild and obstructive CAD among the 2,436 patients with typical angina and a positive ETT. Obstructive CAD was defined as stenosis of ≥50% of the luminal diameter of at least one major epicardial vessel. Because the angiograms from 85.7% of the men revealed obstructive CAD, we concluded that performing invasive CA is appropriate in most men. In contrast, the angiograms from only 56.7% of the women revealed obstructive CAD. Therefore, we restricted our study to the 976 women.
Study Design and Statistical Analysis
The data were randomly assigned to 2 datasets: a derivation dataset (809 patient records) for constructing the scoring system, and a validation dataset (167 records) for testing and validating the model. The derivation and validation datasets were in an 80:20 ratio because that ratio was more likely to produce the optimal tradeoff between parameter estimate and performance statistic variances. In the derivation dataset, if >5% of data for any variable was missing, the variable was excluded from the analysis. Variables entered in the model were selected by using bivariate tests, χ2 tests for categorical covariates, and t tests or Wilcoxon rank sum tests for continuous covariates. Multivariate correlates for the presence of CSX were determined by stepwise backward logistic regression analysis and use of SPSS version 13.0 for Windows (SPSS Inc., an IBM company). To create a calibrated clinical model that could accurately predict risk for an individual patient according to her specific characteristics, we entered all variables significant at the P <0.2 level in univariate analysis into the model. A P value ≤0.05 was considered statistically significant. When all statistically insignificant variables had been eliminated from the model, goodness-of-fit testing (Hosmer-Lemeshow χ2) was used to evaluate how well the model was calibrated, and the area under the curve (AUC) was used to evaluate how well the model could distinguish patients who had CSX from those who had obstructive CAD.
A scoring system was developed from logistic regression analysis to simplify use of the data. We used a method of assigning risk scores to each predictive factor that has been described.19 Briefly, the weights attributed to each variable in the scoring system were obtained from the variables' odds ratios, which were related to logistic regression coefficients, and the risk scores were assigned by rounding up the corresponding odds ratios when the digit to the right of the least significant digit was ≥5 or rounding down when the digit to the right of the least significant digit was <5. The calibration and discriminative power of the model were then evaluated in the validation dataset.
To determine the optimal cutoff point for distinguishing patients with CSX from those with CAD, the point on the receiver operating characteristic (ROC) curve with the maximum Youden index (sensitivity – [1 – specificity]) and the point at the shortest distance from the point (0,1) ([1 – sensitivity]2 + [1 – specificity]2) were calculated.20 These are the 2 methods most often used for establishing an optimal cutoff point.21
The study was performed in accordance with the declaration of Helsinki and its subsequent modifications, and it was approved by the ethics committees of the Tehran University of Medical Sciences and the Tehran Heart Center.
Results
In the derivation dataset, 460 of 809 patients had obstructive CAD and 349 patients had CSX. Table I shows univariate comparisons of the clinical and biochemical characteristics of the patients in the CSX group versus those in the CAD group.
TABLE I.
Univariate Comparisons of Clinical and Biochemical Characteristics in Women with Cardiac Syndrome X and Those with Obstructive Coronary Artery Disease
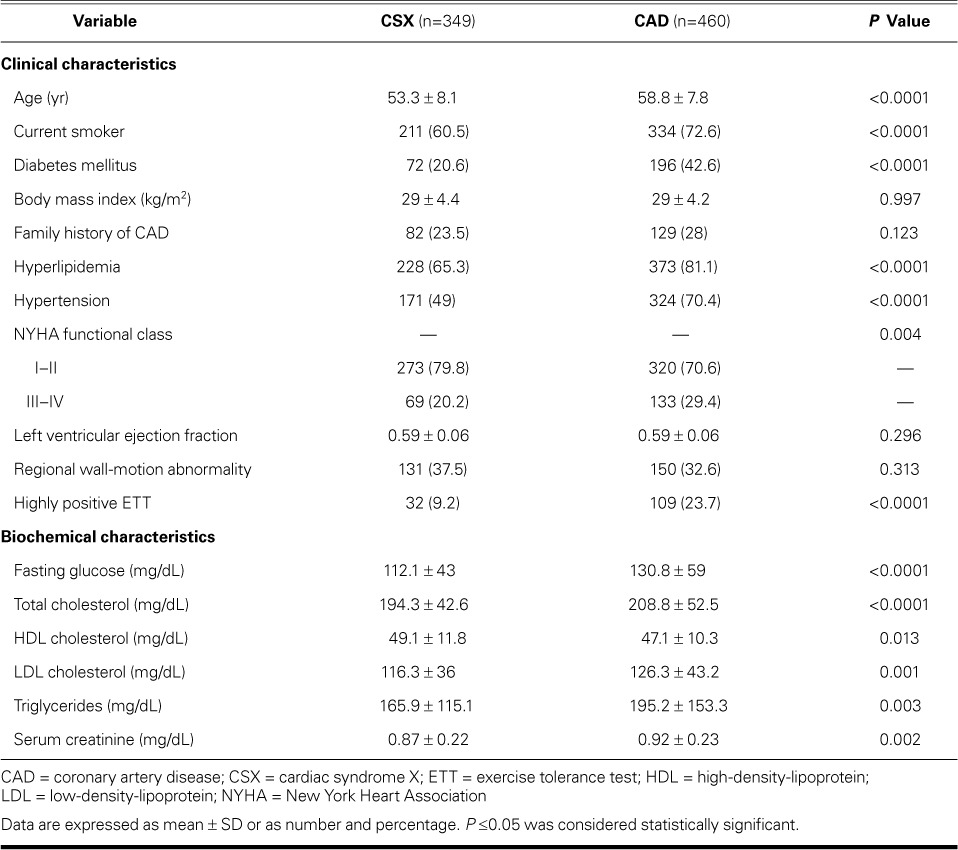
Results of a backward stepwise logistic regression analysis identified 7 independent predictors of CSX. Table II lists the predictors, regression coefficients, odds ratios, and weighted scores.22 The range of attained scores was 0 to 19.8 for the derivation dataset.
TABLE II.
Independent Predictors of Cardiac Syndrome X in Women with Typical Angina and a Positive ETT
Our calculations of the point on the ROC curve with the maximum Youden index and the point that was the shortest distance from the point (0,1) both indicated that 9.5 was the optimal cutoff value for distinguishing patients with CSX from those with CAD. A total score >9.5 had a sensitivity of 75.4%, a specificity of 74.6%, and a 70% positive predictive value for CSX in a woman with typical angina and a positive ETT.
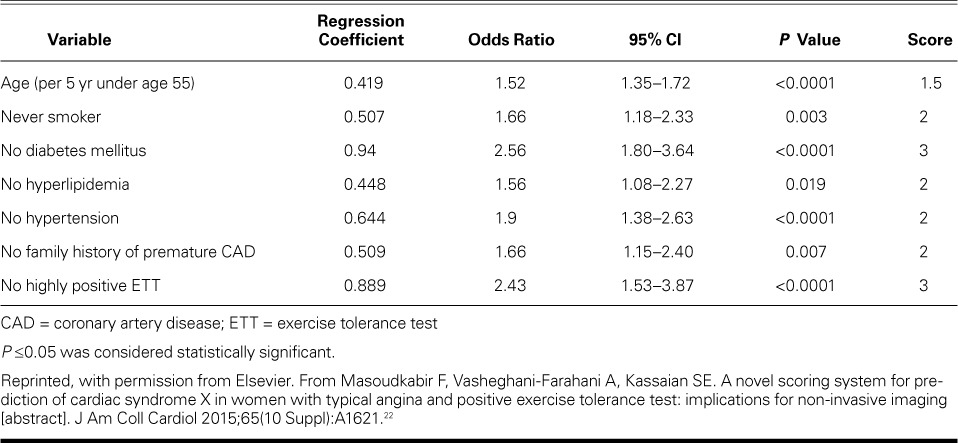
The AUC analysis showed the high power of our derivation dataset in distinguishing CSX from CAD (AUC=0.758; P <0.0001). We performed a validation study by calculating the total scores for subjects in the validation dataset, and the AUC analysis showed an even stronger discriminatory power for distinguishing CSX from CAD (AUC=0.782; P <0.0001). Table III shows the calibration and discriminative characteristics of the model in the derivation and validation datasets. Figure 1 shows the ROC curves for the discriminative power of the model in these datasets.
TABLE III.
Calibrative and Discriminatory Characteristics of the Model
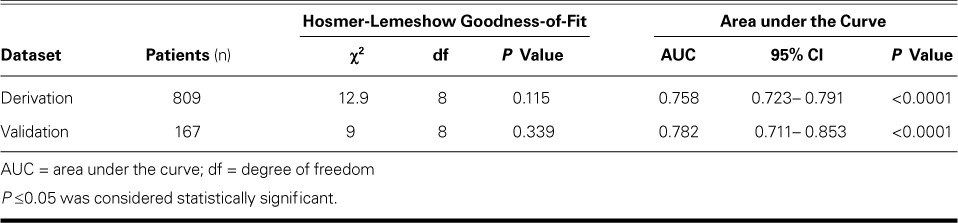
Fig. 1.
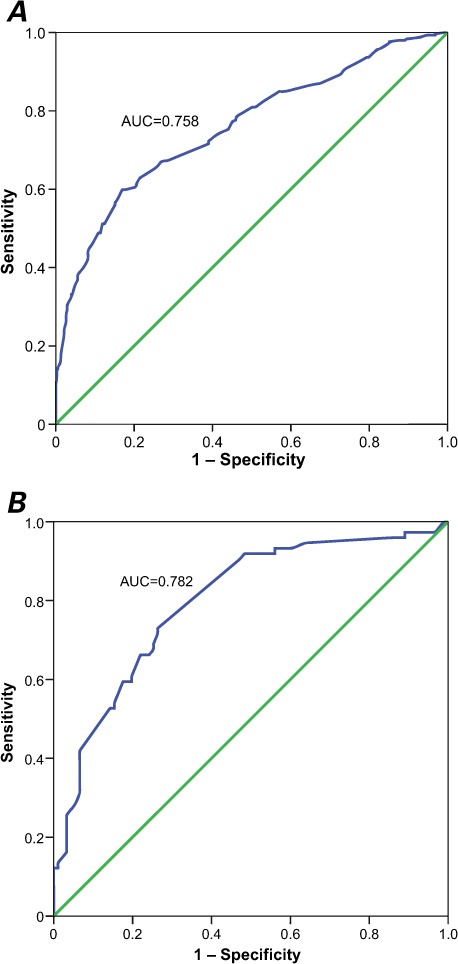
Receiver operating characteristic curve shows the predictive power of the proposed scoring system for cardiac syndrome X in women with typical angina and a positive exercise tolerance test in the A) derivation (n=809) and B) validation (n=167) datasets.
AUC = area under the curve
Discussion
The current AHA/ACC guidelines indicate that patients with stable ischemic heart disease should be referred for invasive CA if their clinical characteristics and results from noninvasive testing indicate a high likelihood of severe ischemic heart disease (class I), or if they have an unsatisfactory quality of life because of angina, have preserved left ventricular function with an ejection fraction >0.50, and have intermediate risk criteria upon noninvasive testing (class IIa).17 However, as has been reported,15 and as our study demonstrates, more than 40% of women with these characteristics have normal results on coronary angiograms. Therefore, more comprehensive criteria for determining which patients should undergo invasive CA or noninvasive imaging are needed. Our scoring system provides a tool for distinguishing women with typical angina and a positive ETT who are more likely to have CSX from those who probably have obstructive CAD.
Coronary computed tomographic angiography (CTA) has good sensitivity and negative predictive value for obstructive CAD.23–25 According to current AHA/ACC guidelines, it is considered a reasonable means of evaluating chest pain in patients who have a low-to-intermediate pretest probability of ischemic heart disease and in those with inconclusive results from prior exercise testing.16 It has also been shown that implementing a CTA program reduces the use of normal angiography.26 In contrast, use of CTA in patients who have a high likelihood of ischemic heart disease, such as those with a positive or highly positive ETT, is currently considered inappropriate.27 However, our results show that CTA may be useful in the clinic, specifically as a means for determining which women with typical angina and a positive ETT should undergo invasive CA or noninvasive imaging. Our scoring system may help guide physicians in selecting the appropriate diagnostic step.
To our knowledge, ours is the first study to propose this kind of scoring system for predicting CSX in women, and it has some strengths. First, we performed a derivation study in the process of creating the scoring system, and we then validated it in another randomly selected dataset. Second, we identified a cutoff point that can be used to differentiate between individuals with and without CSX. Finally, our scoring system includes 7 independent predictors of CSX, 6 of which are dichotomous.
Our scoring system showed high power for the prediction of outcomes in both the derivation and validation datasets (AUC, 0.758 and 0.782, respectively). However, a shortcoming is that our study included a relatively small sample size from a single center. Because the true test of such a system is in its widespread applicability, we invite physicians to test its accuracy, discriminative power, and cost in their hospitals, and to see whether it reduces the rate of normal CAs and complications among women with typical angina and a positive ETT. Meanwhile, we plan to update and maximize the power of our model by adding more cases and identifying more predictors of CSX.
References
- 1. Cook S, Walker A, Hugli O, Togni M, Meier B.. Percutaneous coronary interventions in Europe: prevalence, numerical estimates, and projections based on data up to 2004. Clin Res Cardiol 2007; 96( 6: 375– 82. [DOI] [PubMed] [Google Scholar]
- 2. Singh M, Singh S, Arora R, Khosla S.. Cardiac syndrome X: current concepts. Int J Cardiol 2010; 142( 2: 113– 9. [DOI] [PubMed] [Google Scholar]
- 3. Glaser R, Herrmann HC, Murphy SA, Demopoulos LA, Di-Battiste PM, Cannon CP, Braunwald E.. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 2002; 288( 24: 3124– 9. [DOI] [PubMed] [Google Scholar]
- 4. Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, . et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med 1999; 341( 4: 226– 32. [DOI] [PubMed] [Google Scholar]
- 5. Johnson LW, Krone R.. Cardiac catheterization 1991: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Cathet Cardiovasc Diagn 1993; 28( 3):: 219– 20. [DOI] [PubMed] [Google Scholar]
- 6. Lichtlen PR, Bargheer K, Wenzlaff P.. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol 1995; 25( 5: 1013– 8. [DOI] [PubMed] [Google Scholar]
- 7. Thompson CR, Humphries KH, Gao M, Galbraith PD, Norris C, Carere RG, . et al. Revascularization use and survival outcomes after cardiac catheterization in British Columbia and Alberta. Can J Cardiol 2004; 20( 14: 1417– 23. [PubMed] [Google Scholar]
- 8. Giannoglou GD, Antoniadis AP, Chatzizisis YS, Damvopoulou E, Parcharidis GE, Louridas GE.. Sex-related differences in the angiographic results of 14,500 cases referred for suspected coronary artery disease. Coron Artery Dis 2008; 19( 1: 9– 14. [DOI] [PubMed] [Google Scholar]
- 9. Kemp HG., Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol 1973; 32( 3: 375– 6. [DOI] [PubMed] [Google Scholar]
- 10. Gronke S, Schmidt M, Schwinger RH.. Typical angina pectoris complaints without coronary macroangiopathy [in German]. Dtsch Med Wochenschr 2005; 130( 15: 942– 5. [DOI] [PubMed] [Google Scholar]
- 11. Asbury EA, Collins P.. Cardiac syndrome X. Int J Clin Pract 2005; 59( 9: 1063– 9. [DOI] [PubMed] [Google Scholar]
- 12. Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart 2007; 93( 2: 159– 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermeltfoort IA, Raijmakers PG, Riphagen II, Odekerken DA, Kuijper AF, Zwijnenburg A, Teule GJ.. Definitions and incidence of cardiac syndrome X: review and analysis of clinical data. Clin Res Cardiol 2010; 99( 8: 475– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA.. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995; 25( 4: 807– 14. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan AK, Holdright DR, Wright CA, Sparrow JL, Cunningham D, Fox KM.. Chest pain in women: clinical, investigative, and prognostic features. BMJ 1994; 308( 6933: 883– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, . et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol 2001; 38( 7: 2114– 30. [DOI] [PubMed] [Google Scholar]
- 17. Gazzaruso C, Garzaniti A, Giordanetti S, Falcone C, Fratino P.. Silent coronary artery disease in type 2 diabetes mellitus: the role of lipoprotein(a), homocysteine and apo(a) polymorphism. Cardiovasc Diabetol 2002; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, . et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons [published erratum appears in Circulation 2014;129(16):e462]. Circulation 2012; 126( 25: 3097– 137. [DOI] [PubMed] [Google Scholar]
- 19. Paredes S, Matta-Coelho C, Monteiro AM, Bras A, Marques O, Alves M, Ribeiro L.. Cardiovascular safety of type 2 diabetes medications: review of existing literature and clinical implications. Hormones (Athens) 2016; 15( 2: 170– 85. [DOI] [PubMed] [Google Scholar]
- 20. Pepe M. The statistical evaluation of medical tests for classification and prediction. New York: Oxford University Press; 2003. [Google Scholar]
- 21. Perkins NJ, Schisterman EF.. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006; 163( 7: 670– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masoudkabir F, Vasheghani-Farahani A, Kassaian SE.. A novel scoring system for prediction of cardiac syndrome X in women with typical angina and positive exercise tolerance test: implications for non-invasive imaging [abstract]. J Am Coll Cardiol 2015; 65( 10 Suppl: A1621. [Google Scholar]
- 23. Beanlands RS, Chow BJ, Dick A, Friedrich MG, Gulenchyn KY, Kiess M, . et al. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease--executive summary. Can J Cardiol 2007; 23 2 107– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Carli MF, Hachamovitch R.. New technology for noninvasive evaluation of coronary artery disease. Circulation 2007; 115( 11: 1464– 80. [DOI] [PubMed] [Google Scholar]
- 25. Schuijf JD, Bax JJ, Shaw LJ, de Roos A, Lamb HJ, van der Wall EE, Wijns W.. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J 2006; 151( 2: 404– 11. [DOI] [PubMed] [Google Scholar]
- 26. Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, . et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2( 1: 16– 23. [DOI] [PubMed] [Google Scholar]
- 27. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, . et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr 2010; 4 6): 407.e1– 33. [DOI] [PubMed] [Google Scholar]


