Abstract
Background
Cisplatin (DDP)-based systemic chemotherapy has been widely used in the treatment of postoperative or advanced NSCLC patients, however, its effective rate is only 14~40%. HIF-2α can upregulate drug-resistant-related genes expression and lead to chemotherapy resistance in many tumors. However, little is known about the relationship between HIF-2α and chemotherapy resistance of lung cancer cells.
Material/Methods
In our study, the siRNA expression vectors targeting the HIF-2α gene were designed, constructed, and transfected into A549 cells. MTT assay and western blot analysis of P-glycoprotein 1 (P-gp) were used to explore the transfer influence of HIF-2α gene silencing on the A549 cells in the cisplatin-based chemotherapy resistance.
Results
After transfection with the siRNAHIF-2α into A549 cells, mRNA and protein expression of HIF-2α were downregulated. At the same time, expression of P-gp decreased significantly. Furthermore, the sensitivity to cisplatin significantly increased.
Conclusions
The constructed siRNA expression vectors can effectively suppress the expression of HIF-2α and P-gp, which then can reverse the chemotherapy resistance of A549 cells.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Cell Hypoxia; Cisplatin; Drug Therapy; P-Glycoprotein
Background
Lung cancer is one of the most common causes of human cancer-related death worldwide and its incidence is still rising [1,2]. Non-small cell lung cancer (NSCLC) which mainly contains adenocarcinoma, squamous cell carcinoma is the most common histological type of lung cancer [3]. Despite the diagnostic and treatment methods have undergone considerable advancements, the 5-year overall survival rate of NSCLC is still less than 15% [3,4]. Cisplatin (DDP)-based systemic chemotherapy is the main treatment of postoperative or advanced lung cancer [5], but mainly due to the resistance of lung cancer cells to DDP, its effective rate is only 14~40% [6]. The human multidrug resistance (Mdr1) gene, one of the most important drug-resistant-related genes, encodes for P-glycoprotein 1 (P-gp) and plays an important role in drug resistance of tumor cell lines [7,8].
The hypoxic microenvironment of solid tumors is closely related to its resistance to chemotherapy. Hypoxia induces hypoxia inducible factors (HIFs) which upregulate the expression of drug-resistant-related genes and eventually leads to the resistance of tumor to chemotherapy [9–11]. The function of HIFs is mainly determined by HIF-1α and HIF-2α [12]. Our previous studies showed that HIF-2α may play a more important role in tumorigenesis and tumor progression of human NSCLC [13-16]. In our present study, we inhibited the expression of HIF-2α gene in human lung adenocarcinoma (LUAD) A549 cells and observed the influence on the expression of Mdr1 gene and DDP chemotherapy resistance. We hoped that, by exploring the biological mechanism of HIF-2α in LUAD, we could provide new ideas and targets for reducing chemotherapy resistance of LUAD.
Material and Methods
Main materials
The pGPU6/GFP/Neo expression vector was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). The enzymes (T4 DNA ligase, BamH I, Bbs I, and Pst I enzymes), Escherichia coli JM109 cells, and Agarose Gel DNA Purification Kit Version 2.0 were purchased form Takara Biomedical Technology Co., Ltd. (Beijing, China). The plasmid kit was purchased form Sigma Co., LLC. (Shanghai, China). The Lipofectamine 2000 and TRIzol reagent were purchased from Invitrogen Applied Biosystems (Shanghai, China). The primary antibodies (HIF-2α and P-gp) and the goat anti-mouse HRP-conjugated secondary antibody were purchased from Abcam (Shanghai, China). MTT was purchased form Dojindo Laboratories (Kumamoto, Japan).
Small interfering RNA (siRNA) design and its recombined plasmid construction
To ensure that efficient siRNA sequences could be found, four siRNAs targeting HIF-2α gene (siRNAHIF-2α-1, siRNAHIF-2α-2, siRNAHIF-2α-3 and siRNAHIF-2α-4) (Table 1) and one nonsense siRNA (siRNANEG) referred to in the principles of siRNA design [17] were designed. The oligonucleotides were synthesized by the Sangon Biotech Company (Shanghai, China).
Table 1.
HIF-2α target sequence and single-strand oligonucleotide template.
| Denomination | Target sequence | Single-strand oligonucleotide template |
|---|---|---|
| siRNAHIF-2α-1 | 5′-GGTGGAGCTAA | Sense: 5′-CACCGGTGGAGCTAACAGGACATAGTTCAAGAGACTATGTCCTGTTAGCTCCACCTTTTTTG-3′ |
| CAGGACATAG-3′ | Antisense: 5′-GATCCAAAAAAGGTGGAGCTAACAGGACATAGTCTCTTGAACTATGTCCTGTTAGCTCCACC-3′ | |
| siRNAHIF-2α-2 | 5′-GCCGTACTGTC | Sense: 5′-CACCGCCGTACTGTCAACCTCAAGTTTCAAGAGAACTTGAGGTTGACAGTACGGCTTTTTTG-3′ |
| AACCTCAAGT-3′ | Antisense: 5′-GATCCAAAAAAGCCGTACTGTCAACCTCAAGTTCTCTTGAAACTTGAGGTTGACAGTACGGC-3′ | |
| siRNAHIF-2α-3 | 5′-GCCATCATCTC | Sense: 5′-CACCGCCATCATCTCTCTGGATTTCTTCAAGAGAGAAATCCAGAGAGATGATGGCTTTTTTG-3′ |
| TCTGGATTTC-3′ | Antisense: 5′-GATCCAAAAAAGCCATCATCTCTCTGGATTTCTCTCTTGAAGAAATCCAGAGAGATGATGGC-3′ | |
| siRNAHIF-2α-4 | 5′-GCTTCAGTGCC | Sense: 5′-CACCGCTTCAGTGCCATGACAAACATTCAAGAGATGTTTGTCATGGCACTGAAGCTTTTTTG-3′ |
| ATGACAAACA-3′ | Antisense: 5′-GATCCAAAAAAGCTTCAGTGCCATGACAAACATCTCTTGAATGTTTGTCATGGCACTGAAGC-3′ | |
| siRNANEG | 5′-TTCTCCGAACG | Sense: 5′-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3′ |
| TGTCACGT-3′ | Antisense: 5′-GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC-3′ |
The synthesized single-stranded oligonucleotides were dissolved in Tris-EDTA buffer (pH 8.0), diluted to 100 μmol/L and annealed to form a short hairpin RNA (shRNA) template (50 μL total volume: 5 μL annealing buffer, 5 μL sense strand oligo, 5 μL antisense strand oligo and 35 μL ddH2O (double distilled H2O)). By using a PCR instrument (Roto-Gene 3000, Corbett Research, Australia); the shRNA template was annealed as follows: 95°C for 5 minutes, 85°C for 5 minutes, 75°C for 5 minutes, 70°C for 5 minutes and stored at 4°C. Last, the generated shRNA (10 μmol/L) was diluted to 20 nmol/L for ligation. Restriction digestions were performed in 5 μL 10× buffer G. The solutions (5 μL 10× buffer G, 2 μL Bbs I, 2 μL BamH I, 2 μg pGPU6/GFP/Neo and ddH2O to a total volume of 50 μL) were mixed and digested at 37°C for one hour. Agarose Gel DNA Purification Kit Version2.0 was used to examine the product concentrations. Last, the product was diluted to 50 ng/μL. Then, ligations were performed. Briefly, 2 μL 10× T4 ligation buffer, 1 μL pGPU6/GFP/Neo (Bbs I + BamH I), 1 μL shRNA template, 1 μL T4 DNA ligase (5 U/μL) and 15 μL ddH2O were mixed and ligated at 22°C for one hour. Competent Escherichia coli JM109 cells were grown on the Kanamycin-containing lysis buffer medium plates at 37°C overnight. The colonies were picked and inoculated in 5 mL Kanamycin-containing lysis buffer medium on a shaker at 37°C overnight. Alkaline lysis was used to extract the plasmids (pGPU6/GFP/Neo-siRNA). Then, the plasmids were digested sequentially with BamH I and Pst I. The positive recombinant vectors could be digested by BamH I, but could not be digested by Pst I. Two clones of each vector were selected for sequencing (Invitrogen Applied Biosystems, Shanghai, China).
Drug, cell lines, and cell culture
DDP was purchased from Bristol-Myers Squibb (NY, USA) and stored as a 3.3 mmol/L stock solution in 0.9% NaCl in the dark at room temperature. A549 cells were obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBS, CAS, China) and cultured in DMEM media supplemented with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin at 37°C in a humidified incubator containing 5% CO2.
Plasmid transfection
We performed the transfection according to the transfection protocol for Lipofectamine 2000. Before transfection, 2 μg recombinant plasmid DNA and 10 μL liposomes were separately dissolved in 250 μL serum-free DMEM medium and mixed after standing. Then the mixed solution was added into 1.5 mL serum-free DMEM medium, well-mixed and tiled on the rinsed A549 cells. After cultured for six hours at 37°C, the medium was aspirated and replaced with fresh medium and then continuously cultured for 24 or 48 hours at 37°C in hypoxic environment (1% 02, approximately 5% CO2 and 95% N2).
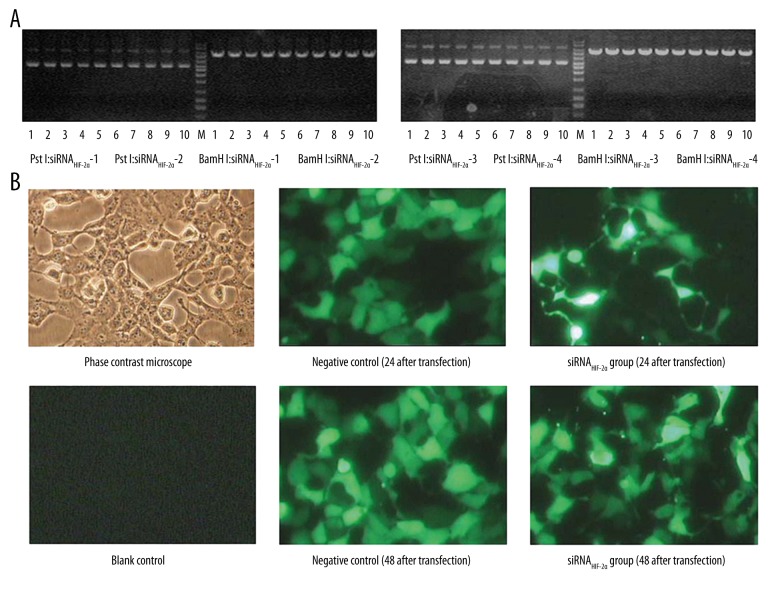
The cells were divided into the following three transfection groups: pGPU6/GFP/Neo-siRNAHIF-2α (siRNAHIF-2α group), pGPU6/GFP/Neo-siRNANEG (negative control group) and a transfection reagent control (blank control group). The pGPU6/GFP/Neo plasmid can express green fluorescent protein (GFP). We selected five visual fields and counted 100 cells under fluorescence microscopy (BX51, Olympus, Japan) at 24 or 48 hours after transfection and calculated the transfection efficiency, respectively. Then, all the cells were collected for further study.
RNA preparation and qRT-PCR
Cultured cells were collected and used to extract RNA by using TRIzol reagent. β-actin was used as an internal control. The qRT-PCR primer sequences for HIF-2α were: 5′-GAAAACGAGTCCGAAGCC-3′ (sense) and 5′-CCCAAAA CCAGAGCCATT-3′ (anti-sense). The primer sequences for β-actin were 5′-CTCTTCCAGCCTTCCTTCCTG-3′ (sense) and 5′-CAGCACTGTGTTGGCGTACAG-3′ (anti-sense). The qRT-PCR reaction included an initial denaturation step at 95°C for 90 seconds, followed by 40 cycles of 95°C for five seconds and 58°C for 30 seconds. The 2−ΔΔCT method was used to calculate the relative HIF-2α expression levels. All experiments were performed at least three times.
Western blot
All cells were homogenized and treated with lysis buffer on ice, and β-actin was used as an internal control. The proteins were separated in SDS-PAGE gels and then transferred onto a PVDF membrane. After incubated with blocking buffer (5% BSA) for one hour at room temperature, the membrane was probed with the primary antibodies diluted in blocking buffer (HIF-2α 1: 200, P-gp 1: 500 or β-actin 1: 400) overnight at 4°C. After washing, the membrane was incubated with goat anti-mouse HRP-conjugated secondary antibody (1: 5,000) for one hour at room temperature. Bands were visualized by ECL detection. All experiments were repeated at least three times.
MTT assay
Initially, cells have been seeded into 96-well plates (3-wells per group, 2×104 cells per well) and hence to make it possible for overnight adherence. Then, medium without DDP was added to the control and blank wells, and medium in other wells was removed and replaced by various concentrations (0.01, 0.1, 1, and 10 μg/mL) of the DDP. After 24, 48, or 72 hours incubation in hypoxic environment, the cell growth status was measured. Then 20 μL of MTT was added to each well and incubated for four hours. After aspiration of the culture medium, the resulting formazan was dissolved with 150 μL of dimethylsulfoxide (DMSO). After shaking for 10 minutes, optical density (OD) was determined by a microplate reader at the absorbance of each well at 490 nm wavelengths. All experiments were performed in triplicate, and the average of the results was calculated. Then, the IC50 was calculated.
Statistical analysis
All statistics are presented as means ±SD. Student’s t-test was used to analyze the data using SPSS 13.0 software (IBM, IL, USA). A value of p<0.05 was considered statistically significant. GraphPad Prism 6.02 software was used to make the graphs.
Results
Verification of the recombinant plasmid vectors and transfection efficiency
All recombinant plasmid vectors were tested with BamH I and Pst I. Successful digestion by BamH I, but not Pst I were observed in all recombinant plasmid vectors (Figure 1A). Sequencing verified that the siRNAs had been successfully cloned into the pGPU6/GFP/Neo vectors. Furthermore, the transfection efficiency was also evaluated. Figure 1B indicated that the recombinant plasmid vector was transferred into A549 cells with a high efficiency at 48 hours after transfection.
Figure 1.
Verification of the recombinant plasmid vectors and transfection efficiency. (A) Successful digestion by BamH I but not Pst I was observed in all recombinant plasmid vectors. (B) Recombinant plasmid vector was transferred into A549 cells with a high efficiency.
The inhibitive effect of siRNAHIF-2α-4 was the most obvious
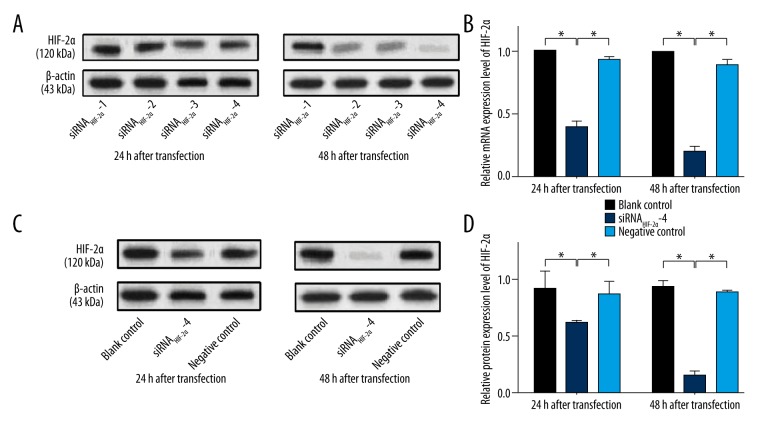
The relative expression level of HIF-2α mRNA in four siRNAHIF-2α subgroups and the inhibitory rate of the four siRNAHIF-2αs are shown in Table 2. We found that, in siRNAHIF-2α-4 subgroup, the relative expression level of HIF-2α mRNA was obvious decreased at both 24 and 48 hours after transfection compared with other three siRNAHIF-2α subgroups. Therefore, siRNAHIF-2α-4 showed a higher inhibitory rate on HIF-2α mRNA expression compared with other three siRNAHIF-2αs. Similar results were observed in HIF-2α protein expression. Table 3 and Figure 2A show that, compared with other three siRNAHIF-2α subgroups, the relative expression level of HIF-2α protein was obvious decreased at both 24 and 48 hours after transfection in siRNAHIF-2α-4 subgroup. Also, siRNAHIF-2α-4 showed a higher inhibitory rate on HIF-2α protein expression compared with other three siRNAHIF-2αs. That is to say, siRNAHIF-2α-4 showed the most obvious inhibitory rate on both HIF-2α mRNA and protein. So, siRNAHIF-2α-4 was chosen as the interfering RNA in the following study.
Table 2.
The relative expression of HIF-2α mRNA in four siRNAHIF-2α subgroups.
| Group | 24 h after transfection | 48 h after transfection | ||
|---|---|---|---|---|
| RE | IR (%) | RE | IR (%) | |
| siRNAHIF-2α-1 | 0.74±0.09 | 26.07±9.15 | 0.67±0.07 | 33.38±7.05 |
| siRNAHIF-2α-2 | 0.71±0.13 | 28.76±12.88 | 0.37±0.08 | 62.46±7.71 |
| siRNAHIF-2α-3 | 0.67±0.09 | 32.71±8.50 | 0.44±0.17 | 55.84±16.77 |
| siRNAHIF-2α-4 | 0.39±0.05 | 60.63±5.10 | 0.20±0.04 | 80.00±3.55 |
RE – relative expression; IR – inhibitory rate.
Table 3.
The relative expression of HIF-2α protein in four siRNAHIF-2α subgroups.
| Group | 24 h after transfection | 48 h after transfection | ||
|---|---|---|---|---|
| RE | IR (%) | RE | IR (%) | |
| siRNAHIF-2α-1 | 0.82±0.10 | 8.47±5.72 | 0.88±0.07 | 6.17±2.77 |
| siRNAHIF-2α-2 | 0.84±0.20 | 8.29±6.97 | 0.36±0.03 | 61.72±0.89 |
| siRNAHIF-2α-3 | 0.57±0.05 | 36.14±5.17 | 0.42±0.08 | 54.90±7.66 |
| siRNAHIF-2α-4 | 0.61±0.02 | 31.69±11.56 | 0.16±0.03 | 82.87±4.09 |
RE – relative expression; IR – inhibitory rate.
Figure 2.
Relative expression level of HIF-2α mRNA and protein. (A) The expression of HIF-2α protein in all four siRNAHIF-2α subgroups. (B) Compared with blank control and negative control group, the expression level of HIF-2α mRNA is significantly decreased in siRNAHIF-2α-4 subgroup at both 24 and 48 hours after transfection. (C) The expression of HIF-2α protein in blank control, siRNAHIF-2α and negative control group. (D) The expression level of HIF-2α protein is significantly decreased in siRNAHIF-2α-4 subgroup at both 24 and 48 hours after transfection when compared with blank control and negative control group. * p<0.05.
siRNAHIF-2α-4 significantly suppressed the expression of HIF-2α mRNA and protein
As shown in Figure 2B, compared with blank control group, the expression level of HIF-2α mRNA was significantly lower in siRNAHIF-2α-4 subgroup at both 24 and 48 hours after transfection (all p<0.05). Also, the expression level of HIF-2α mRNA in siRNAHIF-2α-4 subgroup was significantly lower than in negative control group at both 24 and 48 hours after transfection (all p<0.05) (Figure 2B). At either 24 or 48 hours after transfection, the expression level of HIF-2α mRNA in negative control group was observed slightly lower than the blank control group, however, there was no significantly difference between them (all p>0.05) (Figure 2B). Figure 2C shows the expression level of HIF-2α protein in blank control, siRNAHIF-2α-4 and negative control group. Similar to HIF-2α mRNA, the expression of HIF-2α protein was significantly lower in siRNAHIF-2α-4 subgroup at both 24 and 48 hours after transfection compared with blank control or negative control group (all p<0.05) (Figure 2D). However, the expression of HIF-2α protein in blank control group and negative control group showed no significantly difference at either 24 or 48 hours after transfection (all p>0.05) (Figure 2D). So, we concluded that siRNAHIF-2α-4 could significantly suppress the expression of HIF-2α mRNA and protein.
The sensitivity of A549 cells to DDP was reversed by siRNAHIF-2α-4
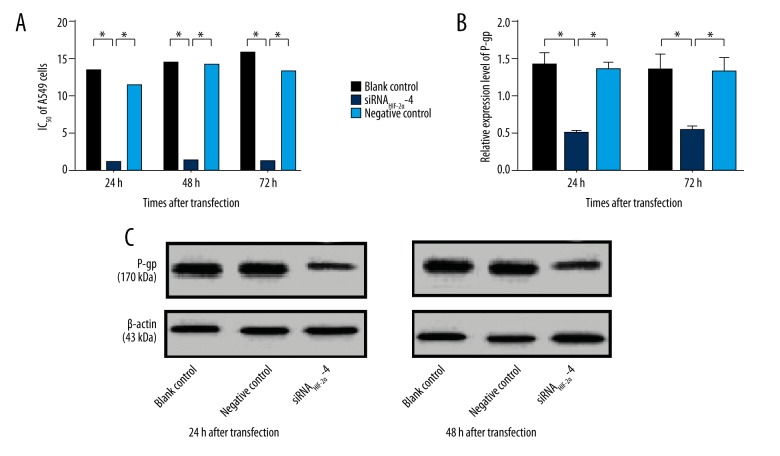
The experimental data showed that siRNAHIF-2α-4 significantly reduced IC50 of A549 cells at 24, 48 and 72 hours compared with blank control group (all p<0.05) (Figure 3A). And, when compared with negative control group, the similar results were observed in siRNAHIF-2α-4 subgroup (all p<0.05) (Figure 3A). However, compared with blank control group, the IC50 of A549 cells in negative control group showed no significant change (all p>0.05) (Figure 3A). Therefore, we believed that siRNAHIF-2α-4 could reverse the sensitivity of A549 cells to DDP.
Figure 3.
The chemosensitivity of A549 cells to DDP and expression level of P-gp. (A) siRNAHIF-2α-4 significantly reduced IC50 of A549 cells at 24, 48 or 72 hours compared with blank control and negative control group. (B) Compared with blank control and negative control group, the P-gp expression is significantly decreased in siRNAHIF-2α-4 group. (C) The expression of P-gp in blank control, siRNAHIF-2α and negative control group. * p<0.05.
siRNAHIF-2α-4 decreased the expression of P-gp
The P-gp expression in the three groups at 24 and 48 hours after transfection were shown in Figure 3C. Compared with the blank control group, siRNAHIF-2α-4 significantly decreased the P-gp expression at both 24 and 48 hours after transfection (all p<0.05) (Figure 3B). When compared with negative control group, the P-gp expression was also significantly decreased by siRNAHIF-2α-4 (all p<0.05) (Figure 3B). However, there was no significant difference of P-gp expression between the blank control group and negative control group (all p>0.05) (Figure 3B).
Discussion
As mentioned above, the hypoxic microenvironment of solid tumor is closely related to its resistance to chemotherapy. In the hypoxic area of solid tumors, due to the inefficient vascular supply, the effective plasma concentrations of chemotherapeutic drugs are insufficient and the efficacies of chemotherapeutic drugs are reduced [18]. Only in an aerobic environment, can many chemotherapeutic drugs fully play their roles [18]. In addition to more direct mechanisms, there are also indirect machineries involved in the development of therapeutic resistance. The hypoxia-driven proteome and genome changes and clonal selection which lead to resistance to anticancer chemotherapy are considered as the indirect machineries [19,20]. Tumor cells formed by hypoxic-driven clonal selection often have a stronger ability to proliferate, invade and metastasize [21,22]. HIFs play important roles in the development of therapeutic resistance. It has been reported that HIF-1α plays an important role in chemotherapy resistance of tumor cells [23–25]. In previous studies, we demonstrated that HIF-2α may play a more important role in tumorigenesis and tumor progression of human NSCLC [13–16]. However, little is known about the relationship between HIF-2α and chemotherapy resistance of tumor cells. In this study, the most efficient siRNAHIF-2α (siRNAHIF-2α-4) was constructed, screened out and transfected into A549 cells. Compared with the two control groups, in siRNAHIF-2α-4 subgroup, the expression of HIF-2α mRNA and protein were significantly downregulated and the sensitivity to cisplatin was significantly increased. Therefore, we considered that HIF-2α higher-expression leaded to the chemotherapy resistance of A549 cells to DDP, while downregulation of HIF-2α reverse the chemotherapy resistance of A549 cells to DDP. However, the major mechanism for why HIF-2α promotes the chemotherapy resistance of A549 cells to DDP is still not clear.
It has been reported that enhanced detoxification, decreased drug accumulation, and increased DNA repair efficiency were the major mechanisms of resistance to platinum [26–28]. Among these, decreased drug accumulation was the most universal mechanism [29–31]. It has been noted that in tumor cell lines, multidrug resistance is an important mechanism of drug resistance [7]. Gatti et al. reported that ATP-binding cassette transporters (ABC-transporters) can modulate drug resistance [32]. P-gp, belonging to ABC-transporters, is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs [33,34]. Thus, we investigated the relationship between HIF-2α and P-gp. In our study, we found that compared with the two control groups, the expression of P-gp in siRNAHIF-2α-4 subgroup was significantly downregulated. Combing the aforementioned experimental results, we speculated that the silencing of HIF-2α gene could suppress the expression of P-gp in a hypoxic environment, which is valuable for reversing the chemotherapy resistance in LUAD. In conclusion, we believe that specific siRNA can downregulate the expression of P-gp by targeting HIF-2α gene and can decrease the resistance of DDP towards tumor cells.
Conclusions
DDP-based chemotherapy plays an important role in the comprehensive treatment of NSCLC. However, the emergence of resistance to chemotherapy has seriously affected the effect of chemotherapy. Our study confirmed the important role HIF-2α played in chemotherapy resistance of LUAD and provided experimental evidence for reversing the chemotherapy resistance of LUAD by RNA interference. We hope our study may ultimately lead to a better utilization of DDP-based antitumor agents and thereby improve the chemotherapeutic efficacy in LUAD patients.
Footnotes
Source of support: This work was supported by Basic Research Project of Changzhou (Grant number. CJ20140041); High-Level Medical Talents Training Project (Grant number. 2016CZBJ042); Jiangsu Provincial Medical Youth Talent (Jiangsu Health Scientific Education (2017) No. 3); Postdoctoral Program of Nanjing Medical University
Conflicts of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. Cancer J Clin. 2011;61(2):91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 4.Subramaniam S, Thakur RK, Yadav VK, et al. Lung cancer biomarkers: State of the art. J Carcinog. 2013;12:3. doi: 10.4103/1477-3163.107958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18(2):317–23. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Dang NH, Skarin AT. Chemotherapy for advanced non-small cell lung cancer. Hematol Oncol Clin North Am. 1997;11(3):473–517. doi: 10.1016/s0889-8588(05)70445-7. [DOI] [PubMed] [Google Scholar]
- 7.Comerford KM, Wallace TJ, Karhausen J, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62(12):3387–94. [PubMed] [Google Scholar]
- 8.Li ZH, Zheng R, Chen JT, et al. The role of copper transporter ATP7A in platinum-resistance of esophageal squamous cell cancer (ESCC) J Cancer. 2016;7(14):2085–92. doi: 10.7150/jca.16117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O’Dwyer PJ. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 2013;19(11):2995–3007. doi: 10.1158/1078-0432.CCR-12-1542. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Bai X, Chen W, et al. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis. 2013;34(5):962–73. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B. 2015;16(1):32–43. doi: 10.1631/jzus.B1400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors – similar but not identical. Mol Cells. 2010;29(5):435–42. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 13.Yuan K, Zheng RH. [The correlation between the expression of HIF-2α and the proliferation of tumor and its impaction on the prognosis in human non-small cell lung cancer]. Chinese Journal of Clinical Medicine. 2009;16(2):194–97. [in Chinese] [Google Scholar]
- 14.Yuan K, Qian C. Expression of HIF-1α and HIF-2α in non-small cell lung cancer. Chin J Exp Surg. 2010;27(5):614–16. [Google Scholar]
- 15.Wu XH, Qian C, Yuan K. Correlations of hypoxia-inducible factor-1alpha/hypoxia-inducible factor-2alpha expression with angiogenesis factors expression and prognosis in non-small cell lung cancer. Chin Med J (Engl) 2011;124(1):11–18. [PubMed] [Google Scholar]
- 16.Yuan K, Wang Y, Tong JC, et al. [Effect of RNA interference targeting HIF-2α gene on invasion and proliferation of lung adenocarcinoma A549 cell line]. Chinese Journal of clinical Medicine. 2012;19(6):598–601. [in Chinese] [Google Scholar]
- 17.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26(2):199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 18.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29(4):297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 19.Brown JM. Exploiting the hypoxic cancer cell: Mechanisms and therapeutic strategies. Mol Med Today. 2000;6(4):157–62. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- 20.Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: Role of hypoxia and anemia. Med Oncol. 2001;18(4):243–59. doi: 10.1385/MO:18:4:243. [DOI] [PubMed] [Google Scholar]
- 21.Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 22.Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–54. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 23.Maher JC, Wangpaichitr M, Savaraj N, et al. Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-D-glucose. Mol Cancer Ther. 2007;6(2):732–41. doi: 10.1158/1535-7163.MCT-06-0407. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Sun L, Zhang H, et al. Hypoxia-mediated up-regulation of MGr1-Ag/37LRP in gastric cancers occurs via hypoxia-inducible-factor 1-dependent mechanism and contributes to drug resistance. Int J Cancer. 2009;124(7):1707–15. doi: 10.1002/ijc.24135. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan R, Pare GC, Frederiksen LJ, et al. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008;7(7):1961–73. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- 26.Samimi G, Varki NM, Wilczynski S, et al. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res. 2003;9(16 Pt 1):5853–59. [PubMed] [Google Scholar]
- 27.Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res. 2016;106:27–36. doi: 10.1016/j.phrs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: The role of DNA repair pathways. Clin Cancer Res. 2008;14(5):1291–95. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Li M, Yao Q, Chen C. Roles and mechanisms of copper transporting ATPases in cancer pathogenesis. Med Sci Monit. 2009;15(1):RA1–5. [PubMed] [Google Scholar]
- 30.Blair BG, Larson CA, Safaei R, Howell SB. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of Cisplatin and Carboplatin. Clin Cancer Res. 2009;15(13):4312–21. doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo MT, Chen HH, Song IS, et al. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26(1):71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 32.Gatti L, Beretta GL, Cossa G, et al. ABC transporters as potential targets for modulation of drug resistance. Mini Rev Med Chem. 2009;9(9):1102–12. doi: 10.2174/138955709788922656. [DOI] [PubMed] [Google Scholar]
- 33.Brambila-Tapia AJ. MDR1 (ABCB1) polymorphisms: functional effects and clinical implications. Rev Invest Clin. 2013;65(5):445–54. [PubMed] [Google Scholar]
- 34.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006;580(4):998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]