Abstract
The study of mechanisms that govern feeding behaviour and its related disorders is a matter of global health interest. The roundworm Caenorhabditis elegans is becoming a model organism of choice to study these conserved pathways. C. elegans feeding depends on the contraction of the pharynx (pumping). Thanks to the worm transparency, pumping can be directly observed under a stereoscope. Therefore, C. elegans feeding has been historically investigated by counting pharyngeal pumping or by other indirect approaches. However, those methods are short-term, time-consuming and unsuitable for independent measurements of sizable numbers of individuals. Although some particular devices and long-term methods have been lately reported, they fail in the automated, scalable and/or continuous aspects. Here we present an automated bioluminescence-based method for the analysis and continuous monitoring of worm feeding in a multi-well format. We validate the method using genetic, environmental and pharmacological modulators of pharyngeal pumping. This flexible methodology allows studying food intake at specific time-points or during longer periods of time, in single worms or in populations at any developmental stage. Additionally, changes in feeding rates in response to differential metabolic status or external environmental cues can be monitored in real time, allowing accurate kinetic measurements.
Introduction
The regulation of food intake is a critical mechanism with major physiological impacts. On the one hand, the rate of food intake determines the rate of growth, and anomalous feeding behaviours are associated with an assortment of chronic diseases such as obesity, type 2 diabetes, cardiovascular diseases and some cancers1–3. On the other hand, dietary restriction has implications and beneficial effects on health and longevity4–6. Hence the growing interest and the increasing efforts to understand the processes and mechanisms that control feeding actions and its anomalies.
Despite their evolutionary distance, metabolism and central signalling pathways are highly conserved between mammals and Caenorhabditis elegans, and the regulation of feeding is not an exception7. Human and C. elegans genomes show a relevant orthology degree8. At least 50% of C. elegans genes have a human ortholog, and approximately 70% of genes related with human diseases have homologs in C. elegans9. Therefore, C. elegans has emerged as a powerful model to study food-related behaviours, shedding light in the underlying factors and mechanisms involved in the genetic regulation of feeding10–15.
C. elegans feeding occurs as a consequence of two motions: the cyclic contraction of the pharynx (pumping) and the isthmus peristalsis, being the former the better comprehended of the two16. These feeding motions can be modulated by the internal state of the worm and by environmental cues. Feeding rate is proportional to the worm pharyngeal pumping where bacteria are sucked from the environment and grinded up. Thanks to the transparency of C. elegans the grinder movement can be directly observed under a stereoscope. This allows the routine study of food intake by means of direct quantification of pumps per minute17. Alternatively, it can be indirectly measured using bacteria expressing fluorescent proteins or BODIPY dye18. While these methods are easily implemented, they are short-term and unsuitable for independent measurements of high numbers of animals. Therefore, it is challenging to find automated methods that allow continuous and long-term measurements to study food intake behaviour in a sizable number of individuals. Some methods based on food clearance assays, time-lapse imaging or electrophysiological readouts have been reported19–21. All these approaches cover some of these aspects; but automatic and continuous methods, sensitive enough to study food intake behaviour in both individual animals and populations, are essential and still lacking.
Here we present a high-throughput method to continuously measure food intake behaviour in single worms and in worm populations. This new approach is based on a method, previously described by some of us, that uses bioluminescence for continuous monitoring of development in C. elegans22. We measured bioluminescence in strains that constitutively and ubiquitously express luciferase (LUC)23,24. LUC catalyzes the conversion of the substrate luciferin into oxyluciferin, in a reaction that emits light25. The luminescence signal increases throughout development, but is abruptly interrupted coinciding with the lethargy characteristic of the molt stages22. The decrease in the signal is due to the specific depletion of ingested luciferin during the quiescent periods, what led us to hypothesise that the bioluminescence signal could report for food intake behaviours. Here, we validate the method for analysis of food intake using mutants with defective pharyngeal pumping, changes in environmental conditions, as well as drugs that increase or decrease pumping rates. As proof of principle we were able to detect a food intake defect in a mutant previously unsuspected of presenting this phenotype. This method is automated, can be performed in multi-well plates, and allows continuous monitoring of food intake and accurate kinetic measurements. The method is suitable for measuring both individual animals and small populations, making thus feasible screening a relatively large number of animals.
Results and Discussion
Luminescence assay for the analysis of food intake behaviour in C. elegans
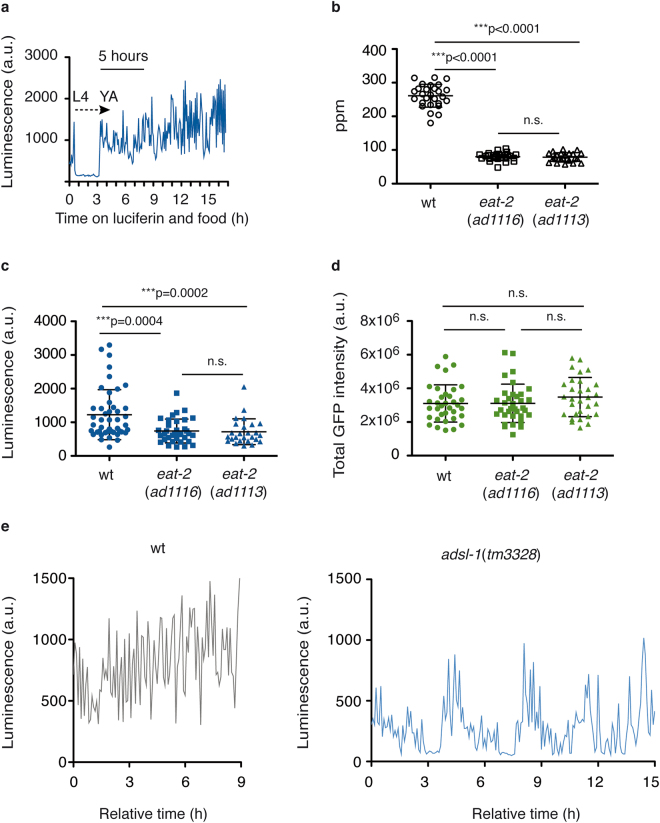
We measured luminescence from single animals that constitutively and ubiquitously express the luciferase protein fused to GFP (Psur-5::luc+::gfp)23,24, in 96-well plates. The expression of the LUC::GFP protein in this strain is driven by the regulatory region of the constitutively expressed sur-5 gene and LUC::GFP fusion protein is expressed in most cells of C. elegans throughout development23. We transferred L4 larvae from NGM agar plates to a 96-well plate, one worm per well, containing S-basal, food (E. coli) and luciferin (100 μM). We did not add compounds to increase the permeability of the cuticle, so that the luminescence signal comes primarily from the ingested luciferin. The L4 larvae ingest luciferin and emit light until they reach the fourth molt (L4 to adult). During molt stages, C. elegans forms a plug of extracellular material in the buccal cavity and they cease pharyngeal pumping26 and luminescence signal drops (Fig. 1a). After the quiescent period of the molt, worms resume feeding and luciferin uptake, as the animal enters the young-adult stage, provoking an increase in the luminescence signal. We took as reference value the mean of the luminescence signal within the first 5 hours of adulthood. However, shorter measurement periods are possible.
Figure 1.
Luminescence signal reports food intake of single animals. (a) Bioluminescence signal from a single LUC::GFP transgenic animal over 17 h from the end of the L4 larval stage (t = 0). The absence of substrate intake during the molt provokes a decrease in the bioluminescence signal that resumes when animals exit the fourth molt and enter the young-adult stage (YA). The first 5-hour period of adulthood, used as a reference to calculate the mean value of the luminescence signal, is shown. (b) Pharyngeal pumping per minute (ppm) of wild-type, eat-2(ad1116) and eat-2(ad1113) mutant animals transgenic for LUC::GFP, at the YA stage. The graph shows data from two experimental replicates. (c) Mean luminescence signal of wild-type, eat-2(ad1116) and eat-2(ad1113) mutant animals transgenic for LUC::GFP, at the YA stage. The graph shows data from four independent experimental replicates. (d) Total GFP fluorescence intensity from wild-type, eat-2(ad1116) and eat-2(ad1113) mutant animals transgenic for LUC::GFP, at the YA stage. The graph shows data from one representative experiment out of three biological replicates. (e) Bioluminescence signal of wild-type and adsl-1(tm3328) mutant animals transgenic for LUC::GFP. A time interval within the L4 stage is shown. adsl-1(tm3328) signal exhibits interruptions and reduction in the luminescence signal compared to wild-type. The graph shows data from a representative well out of 21 animals assayed in three experimental replicates. Error bars show s.d. All experiments were done using strains carrying the integrated transgene feIs4.
If luciferin is limiting the reaction, differential feeding rates, and so differential luciferin uptake, will result in different luminescence signals reflecting the amount of ingested food. We tested different luciferin concentrations (from 100 to 12.5 μM) and observed that decreasing luciferin resulted in a decrease of the luminescence signal (Supplementary Fig. S1). This indicated that luciferin was limiting the luminescence reaction, at least in the tested range. We established 100 μM as the default luciferin concentration for the rest of the experiments. We also tested that luciferin can last for at least one week to ten days under our experimental conditions.
Luminescence signal reports reduced feeding rates in mutants and detects alterations in feeding patterns
eat-2 encodes a nicotinic acetylcholine receptor subunit and functions post-synaptically in pharyngeal muscle regulating the rate of pharyngeal pumping27. Consequently, eat-2 mutants show a slow pumping phenotype, have a reduced feeding rate and are food restricted28,29. We crossed two pharyngeal pumping mutants, eat-2(ad1116) and eat-2(ad1113), with the LUC::GFP reporter strain. The resulting strains have reduced pharyngeal pumping rates, as expected (Fig. 1b). We therefore tested our method and measured luminescence from single animals (wild-type and eat-2 mutants) within the first five hours of adulthood. Mean luminescence signal emitted from single eat-2 mutants was reduced compared to wild-type animals (Fig. 1c), while no significant difference was detected between the two different eat-2 alleles. As it was mentioned above, the reporter has the advantage of harbouring the GFP protein fused to LUC. Therefore, levels of fluorescent protein can be easily investigated, allowing the evaluation of luciferase protein levels in any strain tested and under any experimental condition. We have noticed that some metabolically compromised worms show alterations in fusion protein levels (Supplementary Table S1). To discard that the reduced luminescence observed in eat-2 mutants could be a consequence of lower LUC::GFP fusion protein levels, we measure total GPF intensity under the same conditions of the luminescence assay. There were no significant differences in the fluorescence signals among the strains (Fig. 1d). Given that LUC catalyses the conversion of luciferin into oxyluciferin with ATP consumption25, it could be argued that reduction in the eat-2 signal is a consequence of ATP depletion. However, Houthoofd et al. established that ATP levels for wild-type worms and eat-2 mutants, raised on either agar plates or liquid cultures, were not affected at the young adult stage30. We hence concluded that the reduction in the luminescence signal emitted by eat-2 mutants results from a specific reduction of the ingested luciferin and hence reflects reduced food intake.
We tested whether this method can be useful to monitor feeding behaviour in small populations of worms. We measured luminescence signals from 20 synchronized L1 larvae per well, in a 96-well plate, throughout larval development. Age-specific luminescence signal from eat-2 mutant was reduced compared to the wild-type strain at all stages of development (Supplementary Fig. S2), validating the method to scrutinize feeding behaviour in populations of worms at any desired developmental stage, or through complete development. The mean luminescence signal emitted by eat-2 mutants and wild-type animals at the L1 stage was calculated as an example (Supplementary Fig. S2).
A method that continuously reports food intake, should be suitable to identify new mutants presenting anomalous food intake patterns. Indeed, in the course of our experiments we serendipitously detected a reduced and irregular luminescence pattern with interruptions of the bioluminescence signal in the adsl-1(tm3328) mutant (Fig. 1e), thus showing a food intake defect in this mutant. adsl-1 encodes for the enzyme adenylsuccinate lyase, involved in the purine biosynthesis pathway31. We confirmed this aberrant feeding behaviour by means of direct observation under the stereoscope. adsl-1(tm3328) animals showed an anomalous dynamic of pharyngeal pumping on food, with irregular rhythm of pumps, frequent long pauses and alternation between regular and slow pumping rates (in a range from 0 to approximately 140 pumps/30 sec in L4 animals; data not shown). This phenotype is difficult to characterize and quantify using short-term observation techniques and automated longitudinal technics will help the identification of irregular feeding patterns. This method represents an alternative methodology to shed light in feeding dynamics during long and continuous periods of time. We had no previous indication suggesting food intake defects in adsl-1(tm3328) mutants, confirming that our method allows the identification of new genes affecting food intake.
Luminescence signal reports the response to changes in food availability and quality
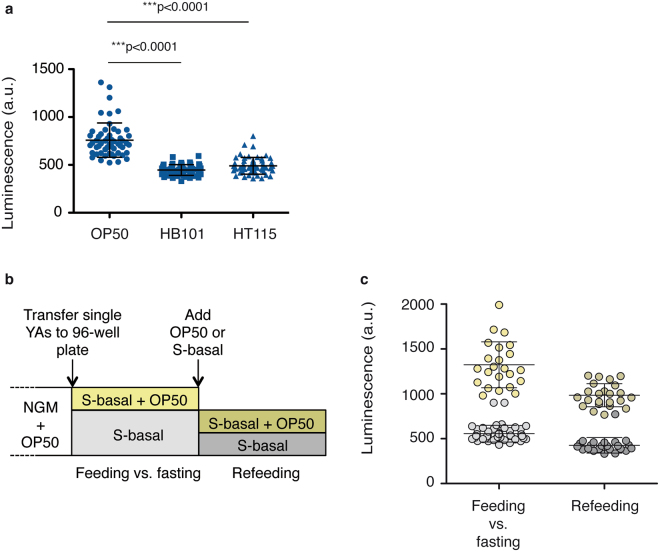
Environmental conditions, previous experience, food quality and the own energetic state of the animal, among others, modulate feeding behaviour in C. elegans32–35. Tipically, in laboratory conditions, C. elegans is fed on E. coli strain OP5036. However, it is documented that other bacterial strains are nutrient-richer food and have superior ability to support C. elegans growth than OP5037–39. Different diets induce particular behavioural responses. HB101 strain is high quality food and considered easier for worms to eat than other E. coli strains. Worms fed on HB101 grow faster and decrease pumping rate38. This feeding response is also observed in HT115, the E. coli strain used for RNAi assays40. We measured this differential modulation of food intake by distinctive food quality diets on the luminometry assay. That is, we tested if our method was able to detect the lower feeding rates reported on animals fed on HT115 or HB101 compared to OP50. Single L4 larvae, from NGM agar plates seeded with OP50, were transferred to a 96-well plate, one worm per well. Single worms were then simultaneously exposed to OP50, HB101, or HT115. Animals completed L4 larva stage on the specific diets, molted, and luminescence signal within the first five hours of adulthood was measured (Fig. 2a). The luminescence signal emitted by worms feeding on HB101 or HT115 was significantly lower than the one on OP50, as expected for lower feeding rates. Therefore, we concluded that our methodology is sensitive to detect the decreased food consumption already reported on C. elegans fed on HB101 and HT115.
Figure 2.
Luminescence signal reports responses to food availability and quality. (a) Mean bioluminescence signal from single LUC:GFP transgenic animals at young-adult stage (YA). Worms were fed on OP50, HB101, or HT115, as indicated. The graph shows data from two experimental replicates. (b) Description of the experiment of fasting and refeeding. YA animals are transferred from an NGM plate with OP50 to a well of a 96-well plate with 10 g/l OP50 in S-basal (Feeding). Alternatively, YA animals starved for 1 hour, are transferred to a well of a 96-well plate with S-basal (Fasting). After two hours of continuous measurement, the wells containing fasting animals are supplemented with either OP50 in S-basal (Refeeding) or S-basal alone, and are measured for another two hours. (c) Mean luminescence signal of two hours measurements for each condition. The graph shows data from one representative experiment of two independent replicates. The replicate is shown in Supplementary Fig. S3b. Error bars show s.d. All experiments were done using a strain carrying the integrated transgene sevIs1.
Animals also reduce their pumping rate when deprived of food and increase it again when they encounter food41–43. We measured the response of C. elegans to changes in food availability using luminometry. We transferred single YA worms from an NGM plate with OP50 to independent wells of a plate for luminometry (Fig. 2b). When there is only S-basal in the well, the animals show a reduced luminescence signal compared to the animals transferred to wells with 10 g/l OP50 in S-basal (Fig. 2c). Upon addition of food to the fasting animals, the signal increased again, unlike in the control situation when only S-basal is added to the fasted worms (Fig. 2c). The signal over the periods of fasting and refeeding can be followed if measurements are performed at short time intervals. During continuous feeding the signal increases slowly, probably due to the increase in size of the animal. When animals are fed after a period of fasting, the signal increases to reach a similar level to that of fed worms (Supplementary Fig. S3). This assay can therefore be used to study the modulation of food intake in response to changes in food quality or other environmental cues.
Serotonin and naloxone respectively increase and decrease the luminescence signal
To validate the suitability of the method for screening purposes or pharmacological tests, such as those on drugs that can potentially regulate feeding, we challenged our method with two compounds: serotonin and naloxone, that stimulate and inhibit feeding in wild-type worms, respectively.
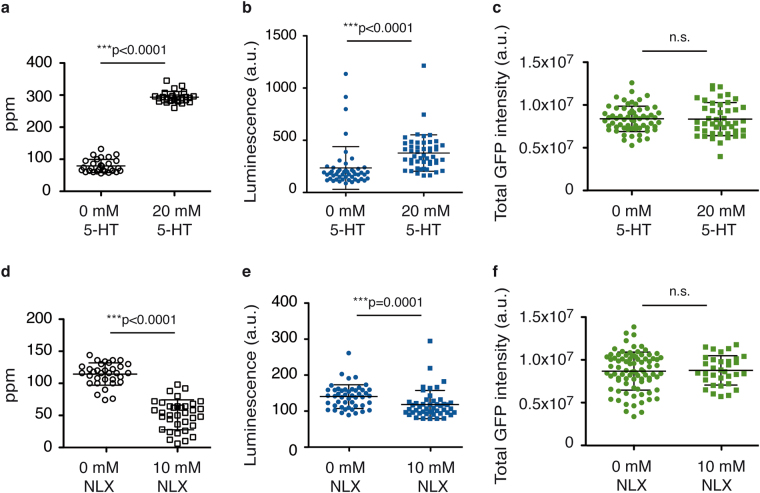
Serotonin is a biogenic amine neurotransmitter that modulates locomotion, egg laying and pharyngeal pumping in C. elegans44. Addition of external serotonin increases pharyngeal pumping frequency and consequently food intake45,46. We tested the serotonin response in our LUC::GFP reporter strain. Most studies use serotonin concentrations between 10 and 20 mM47–49. We set up serotonin 20 mM for all our assay conditions. The drug treatment increased pharyngeal pumping frequency of young-adult animals as predicted (Fig. 3a). We hypothesized that, if we add serotonin to the media, an increase in pharyngeal pumping would be also accompanied by an increase in the luciferin ingested and so in luminescence signal. We conducted luminescence assays in our system on serotonin treated worms. The luminescence signal of young-adult stage animals was higher in the presence of serotonin as compared to the control condition. Mean luminescence value within a 45 min period was taken as reference value (Fig. 3b). We noticed that the luminescence signal was serotonin-dose dependent and we did find that saturation of the effect was reached by the range 10–20 mM (Supplementary Fig. S4). Other authors find that this saturation was reached by 5 mM50. Differential experimental conditions from one study to another could explain this discrepancy. To rule out the hypothesis that serotonin was affecting protein expression levels of the LUC::GFP fusion protein, we measured total GPF intensity of serotonin-treated worms. We did not find significant differences in GFP intensity upon serotonin treatment (Fig. 3c).
Figure 3.
Luminescence signal respectively increases and decreases upon serotonin and naloxone treatment. (a) Mean pharyngeal pumping per minute (ppm) of single LUC::GFP transgenic animals, at the young-adult stage (YA), in the absence and presence of serotonin (5-HT). The graph shows data from two experimental replicates. (b) Mean luminescence signal from individual LUC::GFP transgenic animals, at the YA stage, in the absence and presence of serotonin (5-HT). The graph shows data from six experimental replicates. (c) Total fluorescence intensity from individual YA LUC::GFP transgenic animals in the absence and presence of serotonin (5-HT). The graph shows data from three experimental replicates. (d) Mean pharyngeal pumping per minute (ppm) of single LUC::GFP transgenic animals, in the absence and presence of naloxone (NLX), at the YA stage. The graph shows data from two independent biological replicates. (e) Mean luminescence signal from individual YA LUC::GFP transgenic animals in the absence or presence of naloxone (NLX). The graph shows data from six experimental replicates. (f) Total fluorescence intensity from individual LUC::GFP transgenic animals, in the absence or presence of naloxone (NLX), at the YA stage. The graph shows data from one representative experiment of two independent replicates. Error bars show s.d. All experiments were done using a strain carrying the integrated transgene sevIs1.
Naloxone is a morphine antagonist that inhibits food intake in starved worms51,52. We tested the naloxone response in LUC::GFP animals and confirmed that the drug treatment reduces pumping rate under our experimental conditions (Fig. 3d). We conducted luminescence assays on naloxone treated worms. The luminescence signal of young-adult animals was lower in the presence of naloxone. As above, mean value within a 45 min period was taken as reference value (Fig. 3e). We ruled out the possibility of naloxone affecting LUC::GFP fusion protein levels by measuring total GPF intensity of the worms under naloxone treatment and did not find significant differences (Fig. 3f). We conclude that the method presented here will be useful to unravel altered feeding phenotypes under different conditions or upon different drug treatments, as it is able to detect both increases and decreases in feeding rates.
Food intake behaviour can be detected in real time
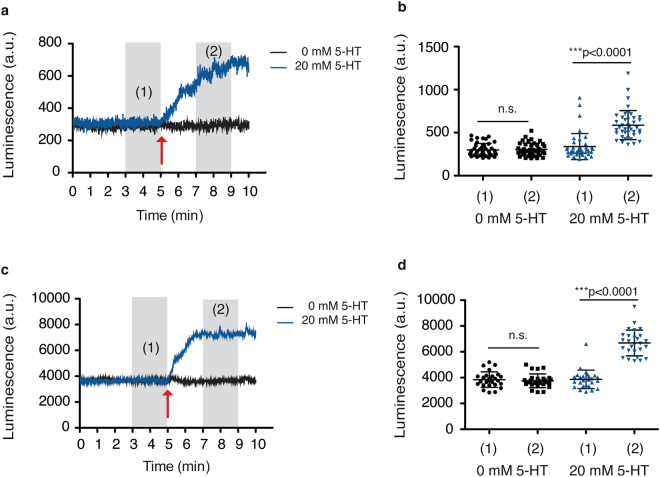
We have shown that this method allows the study of different feeding phenotypes, particularly during medium and long periods of time. Next, we determined whether this luminescence assay is sufficiently sensitive and precise to monitor changes in food intake behaviour in real time. With this purpose, we measured the luminescence emitted by single worms at the young-adult stage, in a 96-well plate, in a kinetic fashion as follows: Luminesce signal from individual worms was measured continuously for 5 min. Then, either serotonin dissolved in S-basal (20 mM final concentration) or S-basal alone were automatically injected into the well and measurements continued for another 5 min. The signal was constant within the very first 5 min, but the addition of the drug was accompanied by an immediate increase in the luminescence signal (Fig. 4a). Only two minutes after the addition of serotonin, the signal had increased by 83.43% ( ± 39.91 s.d.) relative to the basal value before serotonin was added (Fig. 4b). Also, if required, the response rate to the treatment could be inferred from the slope of the kinetic curve. In addition, we verified that this method was also suitable to measure real time changes in food intake of small populations. We performed the same type of kinetic assay in a population of 20 worms per well. Once again, the effect of the addition of the drug was observed by a rapid increase in the luminescence signal (Fig. 4c). The increment in luminescence signal in the worm population was in the same range as in single worms (74.99% ± 20.37 s.d.) (Fig. 4d).
Figure 4.
Luminescence signal reports the response to drugs on real time. (a) Kinetics of the luminescence signal before and after serotonin (5-HT) addition. A single LUC::GFP transgenic animal at the young-adult (YA) stage was measured. Red arrow points out the time point of 5-HT addition. (b) Mean luminescence signal before (1) and after (2) 5-HT addition, in single LUC::GFP transgenic animals, at the YA stage. The graph shows mean values over a 2 minutes period (grey shadows (1) and (2) shown in a) from three experimental replicates. (c) Kinetics of the luminescence signal before and after 5-HT addition, in a population of 20 LUC::GFP transgenic animals, at YA, per well. Red arrow indicates the time point of 5-HT addition. (d) Mean luminescence signal before (1) and after (2) 5-HT addition in a population of 20 LUC::GFP transgenic animals, at YA, per well. The graph shows data from two experimental replicates. Error bars show s.d. All experiments were done using a strain carrying the integrated transgene sevIs1.
In summary, the assay we describe here allows the automatic and continuous analysis of food intake in a multi-well format. This method permits the scrutiny of feeding behaviours at specific time-points, during long periods of time and in real time, being suitable for both, populations and single worms at any developmental stage. This methodology is a versatile tool that might prove useful to address other feeding behaviours and patterns, such as burst feeding and pauses, food seeking and avoidance, or satiety quiescence. The method described here adds an invaluable tool to investigate how internal and external cues modulate feeding behaviours.
Methods
Strains and experimental conditions
We used the reporter strains PE254 (fels4[Psur-5::luc+::gfp; rol-6 (su1006)]V)23,24, MRS290 (eat-2 (ad1116)II; fels4[Psur-5::luc+::gfp; rol-6(su1006)]V), MRS307 (eat-2 (ad1113)II; fels4[Psur-5::luc+::gfp; rol-6(su1006)]V), WBX130 (adsl-1(tm3328)/hT2 [bli-4(e937) let-? (q782) qls48]; fels4[Psur-5::luc+::gfp; rol-6 (su1006)]V), MRS382 (sgk-1(ok538)X; feIs4[Psur-5::luc + ::gfp; rol-6(su1006)]V); MRS423 (daf-16(mu86); sevIs1[Psur-5::luc + ::gfp]X); MRS433 (daf-2(e1370)III; sevIs1[Psur-5::luc + ::gfp]X); MOL43 (sgk-1(ft-15) X; sevls2[Psur-5::luc+::gfp]); MOL62 (age-1(mg305)II; sevIs1[Psur-5::luc + ::gfp]X). To generate MRS387 (sevls1[Psur-5::luc+::gfp]X), we X-irradiated the strain sevEx1[Psur-5::luc + ::gfp] to integrate the reporter array. The strain was outcrossed 10 times to the N2 strain. To generate MRS389 (sevls2[Psur-5::luc+::gfp]), we X-irradiated sevEx1[Psur-5::luc + ::gfp] to integrate the reporter array. The strain was outcrossed 10 times to the N2 strain. For stock animals we cultured the worms according to standard methods36. We routinely maintained the strains at 20 °C on nematode growth media (NGM) plates with a lawn of Escherichia coli OP50-1. Other Escherichia coli strains used in this work are HB101 [supE44, hsdS20(rB-mB-), recA13, ara-14, proA2, lacY1 galK2, rpsL20, xyl-5, mtl-1] and HT115(DE3) [F-, mcrA, mcrB, IN(rrnD-rrnE)1, rnc14::Tn10(DE3 lysogen: lavUV5 promoter -T7 polymerase]. All E. coli strains were obtained from the Caenorhabditis Genetics Center (CGC). Synchronized worms were obtained placing 15-20 young adults laying eggs for 2–3 hours at 20 °C on NGM plates seeded with E. coli. Adults were removed and plates were incubated until animals reached the L4 stage or young-adult stage, as desirable. To obtain synchronized L1 larvae, we collected eggs by hypochlorite treatment and allowed them to hatch and arrest by overnight incubation in M9.
Pharyngeal pumping assay
Mid-L4 larvae were transferred to fresh NGM plates seeded with E. coli, and incubated 5-6 hours until they reached the early young-adult stage. Young adults were then transferred to a new fresh NGM plate seeded with E. coli and allowed to crawl for 15 min. Pharyngeal pumping rate was determined by visual counting of the movement of the grinder under a dissecting microscope for 30 seconds.
Luminometry of animals in the presence of food
Luminometry assays were performed as previously described22 with some modifications. Briefly, mid-L4 larvae were transferred into a well of a white, flat bottom, 96-well plate by manual picking. Plate contained 100 μl of S-basal per well. Alternatively, arrested L1 larvae were transferred into a well by pipetting. Then animals were simultaneously exposed to food by adding 100 μl of S-basal with 20 g/litre of E. coli OP50-1 (wet weight) and 200 μM D-Luciferin per well. Each well finally contained 200 μl S-basal with 100 μM D-Luciferin and 10 g/litre of E. coli OP50-1. Plates were sealed with a gas-permeable cover (Breathe Easier, Diversified Biotech). We measured luminescence (Berthold Centro LB960 XS3 equipped with two injectors) for 1 s, typically at 5-min intervals. We performed all experiments at 20 °C inside temperature-controlled incubators (Panasonic MIR-154). The raw data from the luminometer were visualized using ChronoOSX 353. MS-Excel 2011 and GraphPad Prism 5 were used for all calculations and plotting of the data.
For feeding assays in presence of HB101 or HT115(DE3), well-fed mid-L4 larvae were transferred from plates seeded with OP50-1 to a fresh NGM plate without E. coli. Animals were allowed to crawl while incubated at 20 °C for 30 min. Then animals were transferred into a well of a white, flat bottom, 96-well plate by manual picking. Luminometer assay was performed as described above, but animals were simultaneously exposed to HB101 or HT115(DE3) as indicated. OP50-1 strain was used for control conditions.
Luminometry of animals in fasting/refeeding assay
For fasting condition, well-fed young-adult animals were transferred to a fresh NGM plate without E. coli and allowed to crawl while incubated at 20 °C for 1 hour. Single animals were then transferred into a well of a white, flat bottom, 96-well plate by manual picking. Each well contained 100 μl of S-basal with 100 μM D-Luciferin. Luminescence was read for 1 sec, at 5-min intervals, for a period of 2 hours. After this period, 100 μl of S-basal with 20 g/litre of E. coli OP50-1 and 100 μM D-Luciferin was added to the wells for the refeeding conditions. Each well finally contained 200 μl S-basal with 100 μM D-Luciferin and 10 g/litre of E. coli OP50-1. For control conditions 100 μl of S-basal with 100 μM D-Luciferin was added to the wells. Luminescence was read again for another period of 2 hours. Alternatively and in parallel, for feeding conditions, single well-fed young-adult animals were transferred into a well of a white, flat bottom, 96-well plate. Plate containing 100 μl of S-basal with 100 μM D-Luciferin and 10 g/litre of E. coli OP50-1 per well. Luminescence was read for 1 sec, at 5-min intervals, for a period of 4 hours.
Total fluorescence signal analysis
To measure total green fluorescence signal per worm, a young adult-stage population was washed from the plate and transferred to clear, flat bottom, 96-well plate. Worms were paralyzed with 10 mM levamisole (L9756, SIGMA-ALDRICH). Plates were sealed with a seal plate adhesive (SealMate System, Excel Scientific) and imaged in the automated microscope IN Cell Analyzer 2000 (GE Healthcare Life Science) using an objective Plan Apo 2X/0.1. Total GFP intensity was calculated with the Developer Toolbox (GE Healthcare Life Science) integrated software.
Drug treatment assays
Serotonin (5-HT) (H7752, SIGMA-ALDRICH) and Naloxone (PHR1802, SIGMA-ALDRICH) stocks were always freshly prepared in S-basal. To assess the effect of 5-HT, synchronized young adult-stage worms were washed out with S-basal from NGM plates seeded with OP50. Animals were transferred to fresh NGM plate, in the absence of food, and incubated at 20 °C for 1 hour. Starved worms were then transferred to NGM plates, without food, containing 5-HT (20 mM) and pharyngeal pumping was score after a 15-min acclimation period. For the luminescence assay, worms starved for 1 hour were transferred into a well of a 96-well plate containing 200 μl of S-basal and luciferin (100 μM), either with serotonin (20 mM) or without for control conditions. After a period of 15 min of acclimation, luminescence was read for 0.5 sec, typically at 20-sec intervals. To measure total green fluorescence signal, 1-hour starved worms were washed and transferred to 96-well plates, containing S-basal either with serotonin (20 mM) or without for control conditions. Fluorescence signal was analysed after 15 min of acclimation period. For Naloxone treatment (10 mM) we followed the same procedure but the acclimation period was 1 hour in all cases.
For real time assays, starved worms were transferred into a well of a 96-well plate containing 120 μl of S-basal and luciferin (100 μM). We measured luminescence for 0.5 sec, continuously, for a 5-min period. Then 80 μl of 5-HT stock solution (dissolved in S-basal and luciferin 100 μM) were automatically injected (final volume in the well: 200 μl, luciferin 100 μM, 5-HT 20 mM). For control conditions 80 μl of S-basal solution with luciferin (100 μM) was added. Luminescence measurement was resumed immediately after injection and continued for another 5 min.
Statistical analysis
Statistics were performed using GraphPad Prism 5 software. Before plotting the data, we removed the outliers detected using the Grubb’s test. Variations in luminescence, fluorescence signals and pumping rates were analysed with the nonparametric Mann-Whitney U test, two-tailed.
Electronic supplementary material
Acknowledgements
Strain PE254 was a gift from C. Lagido (University of Aberdeen). Strain age-1(mg305)II was kindly provided by Dr. Cathy Wolkow. We thank the help of Antonio Fernández-Yáñez, Alejandro Mata-Cabana and Fernando Rodríguez-Peris in the construction of reporter strains MRS382, MRS423, MRS433, and MOL62. We thank the Caenorhabditis Genetics Center for strains. Our work is supported by the European Research Council (ERC- 2011-StG-281691), the Spanish Ministerio de Economía y Competitividad (BFU2012-35509), the AFM-Téléthon (Trampoline grant 18313) and a Marie-Curie Intra-European Fellowship (FP7-PEOPLE-2013-IEF/GA Nr: 627263). M.A-S and M.O are supported by the Ramón y Cajal program of the Spanish Ministerio de Economía y Competitividad, RYC-2010-06167 and RYC-2014-15551, respectively. We thank the financial support of GENiE COST BM1408 for the visit of R.M. from J.-E. G to M. A-S. lab.
Author Contributions
M.-J.R.-P., A.L.-D., R.M. and M.O. carried out the experiments; M.-J.R.-P., J.-E.G., M.O. and M.A.-S., designed the experiments; M.-J.R.-P., A.L.-D., R.M., J.-E.G., M.O. and M.A.-S. interpreted results; and M.-J.R.-P., J.-E.G., M.O. and M.A.-S. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21964-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
María Olmedo, Email: mariaolmedo@us.es.
Marta Artal-Sanz, Email: martsan@upo.es.
References
- 1.Razzoli M, Pearson C, Crow S, Bartolomucci A. Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci Biobehav Rev. 2017;76:154–162. doi: 10.1016/j.neubiorev.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blancas-Velazquez A, Mendoza J, Garcia AN, la Fleur SE. Diet-Induced Obesity and Circadian Disruption of Feeding Behavior. Front Neurosci. 2017;11:23. doi: 10.3389/fnins.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Muniesa P, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 4.Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14:275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You YJ, Avery L. Appetite Control: worm’s-eye-view. Animal Cells Syst (Seoul) 2012;16:351–356. doi: 10.1080/19768354.2012.716791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaye DD, Greenwald I. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One. 2011;6:e20085. doi: 10.1371/journal.pone.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dexter PM, Caldwell KA, Caldwell GA. A predictable worm: application of Caenorhabditis elegans for mechanistic investigation of movement disorders. Neurotherapeutics. 2012;9:393–404. doi: 10.1007/s13311-012-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houthoofd K, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2005;60:1125–1131. doi: 10.1093/gerona/60.9.1125. [DOI] [PubMed] [Google Scholar]
- 11.Lee GD, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones KT, Ashrafi K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis Model Mech. 2009;2:224–229. doi: 10.1242/dmm.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemieux GA, Ashrafi K. Neural Regulatory Pathways of Feeding and Fat in Caenorhabditis elegans. Annu Rev Genet. 2015;49:413–438. doi: 10.1146/annurev-genet-120213-092244. [DOI] [PubMed] [Google Scholar]
- 14.Hyun M, et al. Fat Metabolism Regulates Satiety Behavior in C. elegans. Sci Rep. 2016;6:24841. doi: 10.1038/srep24841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis, K., Cheong, M. C., Park, J. S. & You, Y. J. In Appetite and Food Intake: Central Control (eds nd & R. B. S. Harris) 1–16 (2017).
- 16.Avery, L. & You, Y. In C. elegans feeding. The C. elegans Research Community, WormBook (ed WormBook) 10.1895/wormbook.1891.1150.1891, http://www.wormbook.org (May 21, 2012).
- 17.Raizen, D., Song, B., Trojanowski, N. & You, Y. In Methods for measuring pharyngeal behaviors. The C. elegans Research Community, WormBook (ed WormBook) 10.1895/wormbook.1891.1154.1891, http://www.wormbook.org (December 18, 2012). [DOI] [PMC free article] [PubMed]
- 18.You YJ, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockery SR, et al. A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab Chip. 2012;12:2211–2220. doi: 10.1039/c2lc00001f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Amaro RL, et al. Measuring Food Intake and Nutrient Absorption in Caenorhabditis elegans. Genetics. 2015;200:443–454. doi: 10.1534/genetics.115.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz M, Lynch DJ, Lee KS, Levine E, Biron D. A scalable method for automatically measuring pharyngeal pumping in C. elegans. J Neurosci Methods. 2016;274:172–178. doi: 10.1016/j.jneumeth.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Olmedo M, Geibel M, Artal-Sanz M, Merrow M. A High-Throughput Method for the Analysis of Larval Developmental Phenotypes in Caenorhabditis elegans. Genetics. 2015;201:443–448. doi: 10.1534/genetics.115.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagido C, Pettitt J, Flett A, Glover LA. Bridging the phenotypic gap: real-time assessment of mitochondrial function and metabolism of the nematode Caenorhabditis elegans. BMC Physiol. 2008;8:7. doi: 10.1186/1472-6793-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagido C, McLaggan D, Flett A, Pettitt J, Glover LA. Rapid sublethal toxicity assessment using bioluminescent Caenorhabditis elegans, a novel whole-animal metabolic biosensor. Toxicol Sci. 2009;109:88–95. doi: 10.1093/toxsci/kfp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/MCB.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George-Raizen JB, Shockley KR, Trojanowski NF, Lamb AL, Raizen DM. Dynamically-expressed prion-like proteins form a cuticle in the pharynx of Caenorhabditis elegans. Biol Open. 2014;3:1139–1149. doi: 10.1242/bio.20147500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay JP, Raizen DM, Gottschalk A, Schafer WR, Avery L. eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics. 2004;166:161–169. doi: 10.1534/genetics.166.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houthoofd K, et al. No reduction of metabolic rate in food restricted Caenorhabditis elegans. Exp Gerontol. 2002;37:1359–1369. doi: 10.1016/S0531-5565(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara PE, O’Neil N. The use of functional genomics in C. elegans for studying human development and disease. J Inherit Metab Dis. 2001;24:127–138. doi: 10.1023/A:1010306731764. [DOI] [PubMed] [Google Scholar]
- 32.Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yapici N, Zimmer M, Domingos AI. Cellular and molecular basis of decision-making. EMBO Rep. 2014;15:1023–1035. doi: 10.15252/embr.201438993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemieux GA, Ashrafi K. Investigating Connections between Metabolism, Longevity, and Behavior in Caenorhabditis elegans. Trends Endocrinol Metab. 2016;27:586–596. doi: 10.1016/j.tem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KS, et al. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat Commun. 2017;8:14221. doi: 10.1038/ncomms14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol. 2003;206:2441–2457. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.So S, Miyahara K, Ohshima Y. Control of body size in C. elegans dependent on food and insulin/IGF-1 signal. Genes Cells. 2011;16:639–651. doi: 10.1111/j.1365-2443.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 40.Xiao R, et al. RNAi Interrogation of Dietary Modulation of Development, Metabolism, Behavior, and Aging in C. elegans. Cell Rep. 2015;11:1123–1133. doi: 10.1016/j.celrep.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- 42.Lemieux GA, et al. Kynurenic acid is a nutritional cue that enables behavioral plasticity. Cell. 2015;160:119–131. doi: 10.1016/j.cell.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalliere N, et al. Multiple excitatory and inhibitory neural signals converge to fine-tune Caenorhabditis elegans feeding to food availability. FASEB J. 2016;30:836–848. doi: 10.1096/fj.15-279257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chase, D. L. & Koelle, M. R. In Biogenic amine neurotransmitters in C. elegans. The C. elegans Research Community, WormBook (ed WormBook) doi/10.1895/wormbook.1891.1132.1891, http://www.wormbook.org (February 20, 2007). [DOI] [PMC free article] [PubMed]
- 45.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 46.Niacaris T, Avery L. Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J Exp Biol. 2003;206:223–231. doi: 10.1242/jeb.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song BM, Avery L. Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans. J Neurosci. 2012;32:1920–1931. doi: 10.1523/JNEUROSCI.2064-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hobson RJ, et al. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics. 2006;172:159–169. doi: 10.1534/genetics.105.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Churgin, M. A., McCloskey, R. J., Peters, E. & Fang-Yen, C. Antagonistic serotonergic and octopaminergic neural circuits mediate food-dependent locomotory behavior in Caenorhabditis elegans. J Neurosci10.1523/JNEUROSCI.2636-16.2017 (2017). [DOI] [PMC free article] [PubMed]
- 50.Cunningham KA, et al. Loss of a neural AMP-activated kinase mimics the effects of elevated serotonin on fat, movement, and hormonal secretions. PLoS Genet. 2014;10:e1004394. doi: 10.1371/journal.pgen.1004394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheong, M. C., Artyukhin, A. B., You, Y. J. & Avery, L. An opioid-like system regulating feeding behavior in C. elegans. Elife410.7554/eLife.06683 (2015). [DOI] [PMC free article] [PubMed]
- 52.Mills H, et al. Opiates Modulate Noxious Chemical Nociception through a Complex Monoaminergic/Peptidergic Cascade. J Neurosci. 2016;36:5498–5508. doi: 10.1523/JNEUROSCI.4520-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roenneberg T, Taylor W. Automated recordings of bioluminescence with special reference to the analysis of circadian rhythms. Methods Enzymol. 2000;305:104–119. doi: 10.1016/S0076-6879(00)05481-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.