Abstract
Background
Colorectal cancer (CRC) is a leading cause of death in the western world, and its incidence increases with patient age. It is also known that with age there occur changes in the levels of certain hormones, including an increase in the secretion of pituitary gonadotropins (PtGs) as a result of the loss of gonadal hormone feedback. We recently reported that functional PtG receptors are expressed in human lung cancer cells, rhabdomyosarcoma cells, and malignant hematopoietic stem cells.
Findings
Here we report for the first time that the receptors for follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are expressed in primary tumor samples isolated from CRC patients as well as in the established human CRC cell lines HTC116 and HTB37. Moreover, we also report that PtGs stimulate chemotaxis, adhesion, and proliferation of these cell lines.
Conclusions
Our results suggest that PtGs play an important and underappreciated role in CRC pathogenesis, and we call for further studies to better define their role in gastrointestinal malignancies and their direct effect on putative CRC cancer stem cells.
Introduction
Colorectal cancer (CRC) is one of the most common cancers in western countries. Current concepts concerning its pathogenesis revolve around stem cells (SCs) and innate immunity alterations [1,2], and numerous intrinsic and extrinsic factors have been proposed as contributing to the development of this malignancy [3,4]. The American Cancer Society suggests that the overall lifetime risk of developing CRC is about 1 in 20, with slightly lower risk in women than in men [5]. Currently more than 90% of CRCs occur in people in their sixth and seventh decade of life and older [6].
Importantly, pre-menopausal women have significantly lower risk of developing CRC than age-matched men [7,8], which is in contrast to older, post-menopausal females, who have a worse overall survival prognosis than their male counterparts of similar age [9,10]. As we previously hypothesized, this finding may reflect a higher level of PtGs, such as follicle-stimulating hormone (FSH), observed in postmenopausal women in response to a decrease in secretion of gonadal sex hormones and gonadal dysfunction [11].
Interestingly, it has been reported that the risk of CRC development and progression decreases in postmenopausal women with estrogen or combined estrogen-plus-progestin hormonal therapies [12,13]. This finding is potentially explained by negative feedback of these hormones upon release of pituitary glycoprotiens. To address this issue, we focused our research on the effect of PtGs and studied, in addition to FSH, the effects of luteinizing hormone (LH) and prolactin (PRL) on colorectal cancer (CRC) cell lines. All of these PtGs are potent mitogens, and their role has already been associated with other human malignancies, including prostate [14], breast [15], lung [16], and ovarian cancer [17] as well as certain sarcomas [18].
For example, it has been reported that the use of gonadotropin-based drugs to treat infertility is associated with increased occurrence of ovarian cancer in women, and, by contrast, the use of drugs lowering basal levels of gonadotropins reduces this risk [19]. Similarly, functional expression of FSH and LH receptors in established breast cancer cell lines has shown that sex hormones (SexHs) regulate breast cancer cell motility, adhesion, and invasion [20]. Moreover, functional receptors for pituitary gonadotropins and gonadal SexHs were identified on the surface of human lung cancer cells [16], rhabdomyosarcoma cells [21], and leukemia cells [22].
All of these observations prompted us to elucidate the role of PtGs in CRC, and to address this issue we performed studies with patient samples isolated from primary CRC tumors as well as established human CRC cell lines. Here we report that several SexH receptors are expressed by CRC cells isolated from patient colonic biopsies and the established human CRC cell lines HTC116 and HTB37. Both of these cell lines responded to stimulation by gonadal SexHs by increased adhesion and chemotaxis, resulting from activation of signaling pathways through the corresponding SexH receptors.
Our results may shed more light on the role of PtGs in CRC pathogenesis and open up new diagnostic and therapeutic avenues. The latter possibility will move closer to reality as new drugs with the potential to modulate PtG plasma levels become available [23].
Materials and methods
Patient samples
This study was approved by Pomeranian Medical University’s Bioethics Committee and was conducted according to the principles expressed in the Declaration of Helsinki. Frozen primary tumor colon cancer specimens (n = 7) were used to detect the expression of PtGs and gonadal SexH receptors. Tissue samples were obtained from patients during diagnostic colonoscopy after obtaining their written consent. All patients were newly diagnosed with colorectal adenocarcinoma G2. Total RNA was extracted from primary tumors using the RNeasy Mini kit (Qiagen Inc., Valencia CA, USA), including treatment with DNase I (Qiagen). All patients underwent subsequent treatment according to current oncological guidelines and remained under follow-up observation thereafter. The staging of the disease, according to the World Health Organization classification, and survival time were recorded.
Cell lines
Human CRC cell lines HTC116 (ATCC® CCL-247™) and HTB37 (ATCC HTB-37™) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 IU/ml penicillin and 10 μg/ml streptomycin with 10% heat-inactivated, non-charcoal-stripped FBS (Sigma-Aldrich, Cat. No. F9665). The cells were cultured in a humidified atmosphere at 37°C in 5% CO2.
Hormones
All hormones were prepared according to the manufacturer’s recommendations. Lyophilisates were dissolved in the substances recommended in the product specification [24–26] and based on previous experience [21, 22, 27] and then added to the culture medium:
FSH (R&D Systems, Cat. No. 5925-FS-010) and LH (R&D Systems, Cat. No. 8899-LH-010) were dissolved in sterile PBS containing bovine serum albumin (0.1% BSA) as a carrier protein.
Estrogen (Sigma-Aldrich, Cat. No. E2758), progesterone (Sigma-Aldrich, Cat. No. P8783), and danazol (androgen, Sigma-Aldrich, Cat. No. D8399) were dissolved in absolute ethanol (EtOH) and then in sterile culture medium. The final concentration of EtOH in medium with estrogen, progesterone, and danazol was 0.05%, 0.1%, and 0.16%, respectively. The final EtOH concentration in each well was ~0.1%, which is lower than the toxic level and was evaluated in comparison with vehicle controls.
Prolactin (Sigma-Aldrich, Cat. No. L4021) was dissolved in 4 mM HCl containing 0.1% BSA as a carrier protein. The final concentration of HCl in the medium with prolactin was 80 μM.
In order to minimize the potential toxicity related to hormone treatments and at the same time maximize hormone stimulation of cell migration, the optimal hormone doses used in experiments were established experimentally according to dose–response assays and toxicity tests. Based on these observations, we used the following doses in experiments: FSH (1 and 10 IU/ml), LH (1 and 10 IU/ml), estrogen (100 nM), progesterone (100 nM), danazol (80 μg/ml), and prolactin (0.5 μg/ml).
Chemotaxis assay
Chemotaxis assays were performed in a modified Boyden's chamber with 8-μm polycarbonate membrane inserts (Costar Transwell; Corning Incorporated Life Sciences, Lowell, MA, USA) as previously described. In brief, cells detached with 0.25% trypsin were seeded into the upper chambers of inserts at a density of 4 x 104 in 120 μl. The lower chambers were filled with pre-warmed culture medium containing hormones: FSH (10 IU/ml), LH (10 IU/ml), estrogen (100 nM), progesterone (100 nM), danazol (80 μg/ml), or prolactin (0.5 μg/ml). Medium supplemented with 0.5% BSA was used as a negative control. After 24 h, the inserts were removed from the Transwell supports. The cells that had not migrated were scraped off with cotton wool from the upper membrane, and the cells that had transmigrated to the lower side of the membrane were fixed and stained with Hema 3 fixative (Fisher Fisher Scientific) and counted on the lower side of the membrane using an inverted microscope.
Fibronectin adhesion assay
Ninety-six-well plates were coated with fibronectin (10 μg/ml) overnight at 4°C and blocked with 0.5% BSA for 1 h before the experiment. Cells were made quiescent for 3 h in medium with 0.5% BSA and then incubated for 5 min with hormones: FSH (10 IU/ml), LH (10 IU/ml), estrogen (100 nM), progesterone (100 nM), danazol (80 μg/ml), or prolactin (0.5 μg/ml). Subsequently, cell suspensions (2 x 103/100 μl) were added directly to 96-well plates coated with fibronectin and incubated. After 5 min at 37°C the plates were vigorously washed three times to remove non-adherent cells, and the adherent cells were counted using an inverted microscope.
Cell proliferation
Cells were grown in 24-well culture plates at an initial density of 1.0 x 104 cells/well. After 24 h the medium was changed to new medium supplemented only with hormones: FSH (1 or 10 IU/ml), LH (1 or 10 IU/ml), estrogen (100 nM), progesterone (100 nM), danazol (80 μg/ml), or prolactin (0.5 μg/ml), and clean medium without any supplements was used as a control. The cell number was gated and calculated at 24, 48, and 72 h by flow cytometry (FACS). In order to avoid a potential decrease in cell viability during 48- and 78-h incubations, we optimized the number of cells plated for proliferation assays as well as the assay conditions. For this purpose the medium was replaced every 24 h. Furthermore, in order to avoid serum-related hormone suppression, both treated cells and their respective controls were assayed in serum-free medium, which resulted in slower cell growth. All data obtained were related to the control sample at the 0-h time point (100%) and measure the proliferation of cancer cells up to 72 h. At the indicated time points, cancer cells were harvested from 24-well culture plates by trypsinization in trypsin-EDTA (0.25%) and scored by FACS analysis using a flow cytometer.
Conventional RT-PCR
Total RNA from various cells was isolated using the RNeasy Mini kit (Qiagen Inc., Valencia CA, USA), including treatment with DNase I (Qiagen). The RNA was reverse-transcribed with Taqman Reverse Transcription reagents (Applied Biosystems, Grand Island, NY, USA) using the necessary reagents for reverse transcriptase PCR (RT-PCR). Random hexamers, oligo d(T)16, and reverse primers were included for cDNA synthesis. The resulting cDNA fragments were amplified (1 cycle of 8 min at 95°C; 2 cycles of 2 min at 95°C, 1 min at 60°C, 1 min at 72°C; and subsequently 40 cycles of 30 sec at 95°C, 1 min at 60°C, 1 min at 72°C; and 1 cycle of 10 min at 72°C) using Amplitaq Gold polymerase with sequence-specific primers designed using the NCBI Primer-Blast program. One primer in each pair was designed to include an exon-–intron boundary: β-actin (F, GGATGCAGAAGGAGATCACTG and R, CGATCCACACGGAGTACTTG); hFSH-R (F, GCTTCTGAGATCTGTGGAGGTT and R, ACCTCAGTTCAATGGCATTCCT); hLH-R (F, GGGCCGCACTCAGAGG and R, AGGGAGGTAGGCAAGTGATAGTC); hESTRα-R (F, AGGTGCCCTACTACCTGGAG and R, CGGTCTTTTCGTATCCCACCT); hESTRβ-R (F, TTTTTGGACACCCACTCCCC and R, CACCTGTTGAGGAAAGCGAG); hANDR-R (F, CGACTTCACCGCACCTGATG and R, CTTCTGTTTCCCTTCAGCGG); hPROG-R (F, CGGACACCTTGCCTGAAGTT and R, AGTCCGCTGTCCTTTTCTGG); hPRL-R (F, GAGCTTCTTCTCACAGAGCCA and R, AAGTTCACTTCAGGGTTCATGTGG).
Signal transduction studies
Experiments were performed as previously described [22]. Briefly HTC116 cells were stimulated with either 0.5% BSA in Roswell Park Memorial Institute (RPMI) 1640 medium or SexHs for 5 min at 37°C followed by lysis with the RIPA Lysis Buffer System supplemented with protease and phosphatase inhibitors: Na-orthovanadate, PMSF, and PIC (Santa Cruz Biotechnology, Cat. No. sc-24948). The extracted proteins were separated on a 4–12% SDS-PAGE gel and transferred to a PVDF membrane. Phosphorylation of the intracellular kinase p42/44 mitogen-activated protein kinase (p42/44 MAPK) and AKT was detected by phosphospecific p42/44 MAPK (clone 9101) and phosphospecific AKT (Ser473; clone 9271) rabbit polyclonal antibodies (Cell Signaling), respectively. Horseradish peroxidase (HRP)-conjugated goat anti-rabit IgG was used as a secondary antibody (Santa Cruz Biotechnology). To ensure equal protein loading in all lanes, blots were stripped using stripping buffer (Thermo Scientific), and then reprobed with appropriate anti-rabbit p42/44 MAPK (clone 9102) and anti-rabbit AKT (clone 9272) monoclonal antibodies (Cell Signaling). All membranes were treated with enhanced chemiluminescence (ECL) reagent (Amersham Life Sciences), dried, and subsequently exposed to film (Hyperfilm, Amersham Life Sciences). For band visualization, an automatic film processor supplied with fresh warm developer and fixer solutions was used.
Fluorescence staining
HTC116 cells were fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X100 for 10 min, washed in PBS, pre-blocked with 2.5% BSA in PBS, and subsequently stained for 2 h with antibodies to follicle-stimulating hormone receptor (FSH-R, 1:200, rabbit polyclonal antibody; Cat. No. sc-13935, Santa Cruz Biotechnology), luteinizing hormone/choriogonadotropin receptor (LH-R, 1:200, rabbit polyclonal antibody; Cat. No. sc-25828, Santa Cruz Biotechnology), androgen receptor (Ab-2, 1:200, rabbit polyclonal antibody; Cat. No. RB-1358, Thermo Scientific), and estrogen receptor (ER, Ab-11, 1:200, mouse monoclonal IgG antibody; Cat. No. MS-354, Thermo Scientific). Antibodies were diluted in antibody diluents (DAKO). Appropriate secondary antibodies conjugated with Texas Red were used for 2 h at 37°C (1:400; Texas Red Goat Anti-Rabbit IgG, Cat. No. TI-1000; or Texas Red Horse Anti-Mouse IgG, Cat. No. TI-2000; Vector Labs). In control experiments, cells were stained with secondary antibodies only. The nuclei were labeled with DAPI, and the fluorescence images were collected with an FV1000 confocal laser-scanning microscope (Olympus).
Statistical analysis
All results are presented as mean ± SD. Statistical analysis of the data was done using Student's t-test for unpaired samples, with * p<0.05 and **p<0.005 considered a statistically significant result.
Results
Colon cancer HTC116 and HTB37 cell lines express mRNA for several pituitary and gonadal glycoproteins
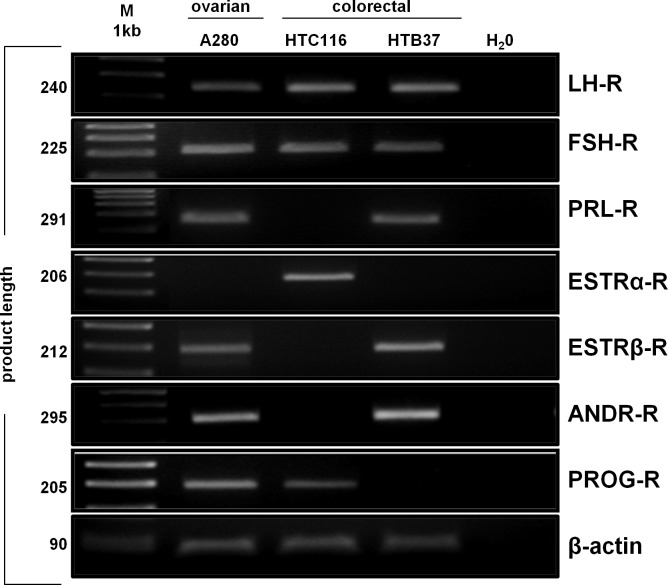
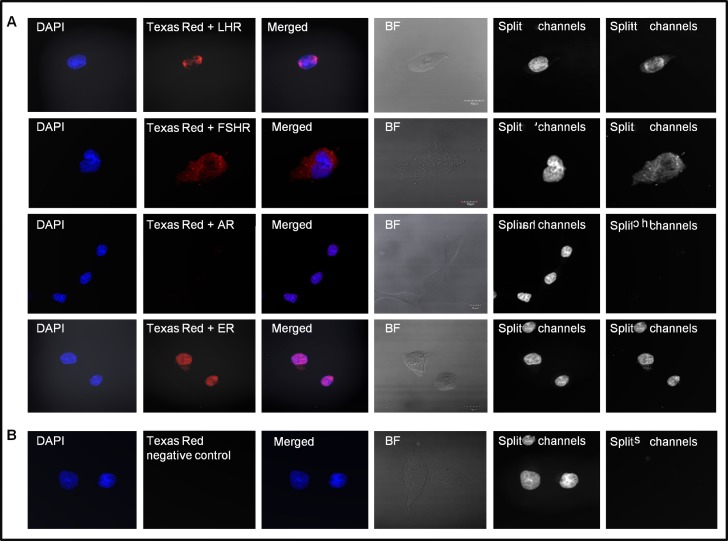
First, to evaluate mRNA levels of PtGs and gonadal SexHs in human colon cancer cell lines, we performed RT-PCR assays. As shown in Fig 1, we detected mRNA for several SexH receptors by conventional RT-PCR. The HTC116 and HTB37 cell lines evaluated in the present study expressed follicle-stimulating hormone receptor (FSH-R) and luteinizing hormone receptor (LH-R). Furthermore, we were able to detect expression of the progesterone receptor (PROG-R) and estrogen receptor alpha (ESTR-α) in HTC116 cells. However, we were not able to detect the mRNA of PROG-R and ESTR-α in HTB37 cells. By contrast, the HTB37 cell line was positive for the presence of mRNA for the prolactin (PRL-R) and androgen (ANDR-R) receptors as well as for estrogen receptor beta (ESTR-β-R). As a positive control, human ovarian A2780 (OV) cancer cells, which express all of the SexH receptors except ESTR-α, were used. Besides the detection of transmembrane receptor transcripts, we were also able to detect expression at the protein level by immunofluorescence staining. Fig 2 shows the expression of LH, FSH, and the nuclear androgen receptor (AR) and estrogen receptor (ESTR) in the established CRC cell line employed in our study.
Fig 1. Pituitary gonadotropins and gonadal sex hormone receptor transcripts are expressed in the colon cancer cell lines HTC116 and HTB37.
RT-PCR assays demonstrate the presence of PtGs and gonadal sex hormone RNA expression in selected colorectal cancer (CRC) cell lines. “H2O” indicates samples in which water was used instead of template. An ovarian cancer cell line (A280) served as positive control.
Fig 2. Human colon cancer cell line HTC116 expresses pituitary and gonadal glycoprotein receptors at the protein level.
(A) Immunofluorescence staining of the transmembrane luteinizing hormone receptor (LH-R) and follicle-stimulating hormone receptor (FSH-R) and the nuclear androgen receptor (AR) and estrogen receptor (ER) in HTC116 cells. (B) Negative control. BF, bright field. Scale of merged picture, 10 μM.
Pituitary gonadotropins induce cell motility and adhesion in colon cancer cell lines
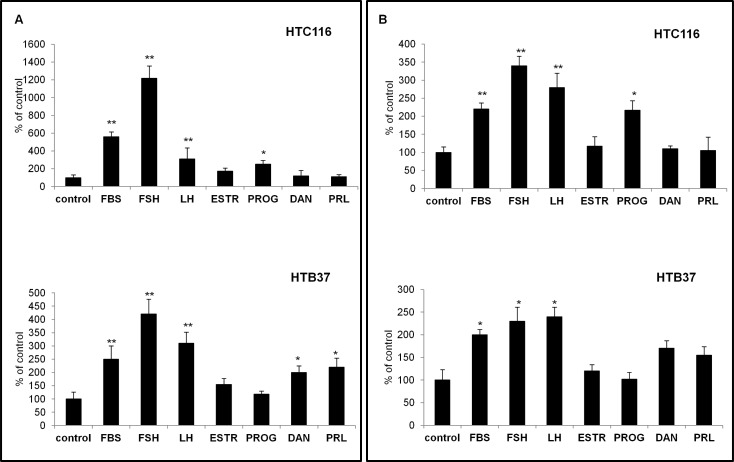
Next, we tested whether PtGs are functional in colon cancer cell lines by performing chemotaxis and cell adhesion assays. In Transwell chemotaxis assays, we employed PtGs at the indicated high doses to test their chemotactic effects against selected cell lines. We found that, of all the sex hormones tested, the strongest chemotactic activity was observed for FSH (10 IU/ml) and/or LH (10 IU/ml), but cells in established CRC cell lines also responded to stimulation by other hormones (Fig 3A). Moreover, as in the chemotaxis assays, the HTC116 and HTB37 human CRC cell lines responded to SexH stimulation in fibronectin adhesion assays (Fig 3B).
Fig 3. Effect of pituitary gonadotropins on migration and adhesion of CRC cell lines.
(A) Migration of HTC116 and HTB37 cells in response to FBS (10%), follicle-stimulating hormone (FSH, 10 IU/ml), luteinizing hormone (LH, 10 IU/ml), estrogen receptor (ESTR, 100 nM), progesterone (PROG, 100 nM), danazol (DAN, 80 μg/ml), or prolactin (PRL, 0.5 μg/ml). *p≤0.05, **p≤0,005. (B) Adhesion of HTC116 and HTB37 cells after treatment with FSH (10 IU/ml), LH (10 IU/ml), ESTR (100 nM), PROG (100 nM), DAN (80 μg/ml), or PRL (0.5 μg/ml) to fibronectin-coated plates. The hormones were tested in serum-free medium. Bars indicate standard deviations; *p≤0.05, **p≤0.005.
Pituitary gonadotropins may induce proliferation of colon cancer cells
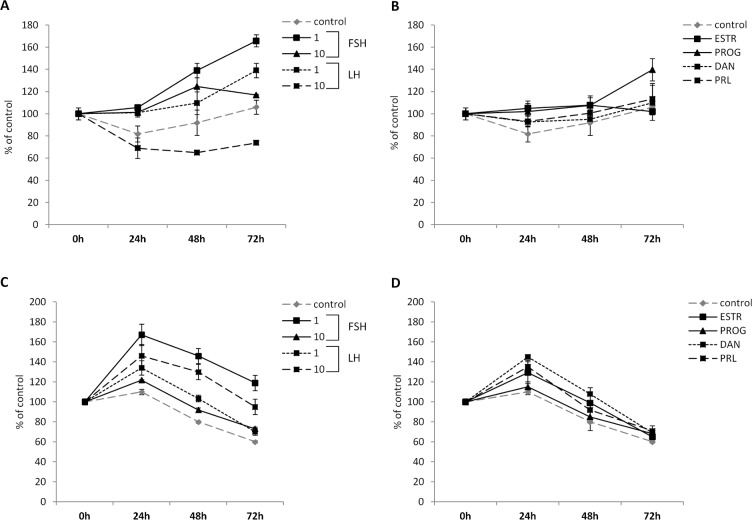
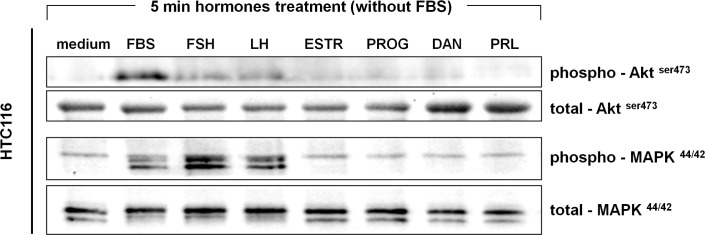
PtGs and gonadal SexHs have been reported to stimulate proliferation of both normal and malignant cells. To evaluate the effect of these hormones on colon cancer cell proliferation, we exposed HTC116 and HTB37 cells to all of the PtGs (Fig 4A and 4C) and gonadal SexHs (Fig 4B and 4D) evaluated in the present study. We observed that all of them stimulate proliferation of both CRC cell lines after a 24-h incubation. This stimulatory effect was further observed for up to 72 hours in the HTC116 but not in the HTB37 cell line. Furthermore, we found that the strongest proliferation activity of selected cells was observed for low doses of FSH (1 IU/ml) and/or LH (1 IU/ml). This effect seems to be direct, as the SexH receptors expressed by CRC cells responded to stimulation by phosporylation of MAPKp42/44 and AKTser473 (Fig 5).
Fig 4.
Effect of pituitary and gonadal glycoproteins on the proliferation of HTC116 (A, B) and HTB 37 (C, D) cells. Proliferation of HTC116 cells after treatment with (A) follicle-stimulating hormone (FSH, 1 and 10 IU/ml) or luteinizing hormone (LH, 1 and 10 IU/ml) or (B) estrogen receptor (ESTR, 100 nM), progesterone (PROG, 100 nM), danazol (DAN, 80 μg/ml), or prolactin (PRL, 0.5 μg/ml). Proliferation of HTB37 cells after treatment with (C) FSH (1 and 10 IU/ml) or LH (1 and 10 IU/ml) or (D) ESTR (100 nM), PROG (100 nM), DAN (80 μg/ml), or PRL (0.5 μg/ml).
Fig 5. Phosphorylation of the intracellular kinase p42/44 mitogen-activated protein kinase (p42/44 MAPK) and AKT in HTC116 cells.
The experiment was repeated twice with similar results, and representative data are shown. ll studied hormones were tested in serum-free medium (without FBS).
Detection of mRNA for pituitary gonadotropins and gonadal sex hormone receptors in patient primary colon cancer samples
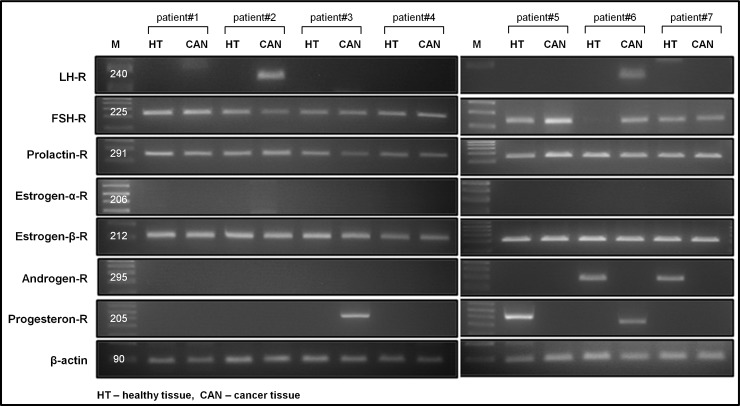
Finally, we evaluated the expression of pituitary and gonadal SexH receptors in seven (n = 7) primary patient colon tumor samples. We obtained colon samples from both healthy and cancerous regions to compare the results. All patients were diagnosed with CRC adenocarcinoma in various stages of disease. Detailed patient characteristics and clinical histories are presented in Table 1. We detected FSH-R, PRL-R, and ESTR-β-R expression in almost all samples. As an example of an analyzed sample obtained from patient #6, FSH-R expression was absent in healthy colon tissue (Fig 6). Of note, as loss of FSH-R expression was found in healthy colonic biopsies in 1 of 7 individuals studied, it is difficult to draw conclusions about the biological significance of this phenomenon. Furthermore, we were able to observe the expression of LH-R only in colon cancer tissues of two patients (n = 2, patients 2 and 6). These patients (male and female) had the shortest survival time, since they were diagnosed with advanced disease with distant metastases. Expression of ANDR-R was present only in healthy colon tissue (n = 2, patients 6 and 7) and not in colon cancer samples. Moreover, we did not detect the presence of estrogen receptor alpha (ESTR-α-R) mRNA in any of the patient samples tested. PROG-R RNA was detectable in cancerous samples of patients #3 and #6 and in the healthy tissue of patient #5. These results correlated with our results obtained from established CRC cell lines (Figs 1 and 2).
Table 1. Demographic data for patients diagnosed with colorectal cancer.
| CRC patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age | 35 | 68 | 66 | 68 | 66 | 74 | 82 |
| Gender | Male | Male | Male | Male | Male | Female | Female |
| BMI (kg/m2) | 29 | 24 | 25 | 30 | 23 | 27 | 20 |
| Histopatology* | G2 | G2, mucocellulare | G2 | G2 | G2 | G2 | G1 |
| Location (colon) | descending | cecum | rectum | sigmoid | descending | sigmoid | sigmoid |
| Staging (WHO) | pT3N0Mx | pT3N3M1 | pT3N0Mx | pT3N1Mx | pT3N1Mx | PT3N3M1 | pT1N0Mx |
| LVI | 0 | N/A | 0 | N/A | 1 | 0 | 0 |
| Co-morbidities | none | hypertension | none | none | hypertension | none | teratoma adultum (ovary) |
| Follow-up | ongoing | lost to death | ongoing | ongoing | ongoing | lost to death | ongoing |
| Survival since diagnosis | 15 month | 10 month | 14 month | 13 month | 11 month | 2 month | 17 month |
* Adenocarcinoma; BMI–body mass index, CRC–colorectal cancer, WHO–World Health Organisation; LVI–lymphovascular invasion; N/A–data not available
Fig 6. Pituitary gonadtropins and gonadal sex hormone receptors are expressed in colon cancer patient samples.
RT-PCR assays demonstrate SexH RNA expression in healthy human colon tissue (HT) and tumor colon tissue (CAN) in seven cases (n = 7). The assay was performed three times with similar results. A representative gel image is shown.
Discussion
The seminal observation of our work is that cells from established human CRC cell lines, as well as tissues samples from patients suffering from CRC, express several PtGs and gonadal SexH receptors. Moreover, we provide for the first time evidence that FSH and LH stimulate proliferation, adhesion, and chemotaxis of human CRC cells.
Epidemiological data suggests that female SexHs play an important protective role in the etiology and progression of CRC [12–13, 28–30]. In support of such a role, exogenous administration of estrogen and progestin during the development of CRC in postmenopausal woman has been reported in several studies [31], and this phenomenon could be explained, as we hypothesize here, by a negative feedback of these hormones on PtG release, including of FSH [32]. By contrast, older, post-menopausal females have a worse overall survival prognosis than their male counterparts of a similar age [9,10]. This may correlate with a higher level of FSH observed in postmenopausal women in response to age-related decrease in gonadal function and impaired secretion of estrogens [11].
Overall, evidence has accumulated that PtGs and gonadal SexHs play a role in several female and male reproductive tumors, including breast, ovarian, and prostate cancer [33,34], but also in other non-gonadal malignancies, such as stomach and lung cancers [35–37]. For example, an increased risk of gonadal tumors, but not for stomach or CRC cancers, has been associated with the use of postmenopausal hormone replacement therapy (HRT). By contrast, modulation of the pituitary–gonad axis by means of estrogen administration was correlated with a decreased risk of certain gastrointestinal tumors [37]. In our previous reports we demonstrated the presence of functional SexH receptors in human lung cancer [16], rhabdomyosarcoma [21], and leukemia cells [22].
In contrast to other malignancies, data on pituitary and gonadal hormone receptor expression in CRCs are scarce. Huang et al. demonstrated the presence of luteinizing hormone-releasing hormone (LH-RH) and its receptor immunoreactivity in the rat small and large intestinal epithelium [38]. Szepeshazi et al. [39] documented the presence of high-affinity binding sites for LH-RH in several established CRC cell lines and showed the potential of their functional inhibiton by LH-RH analogs.
In our study we report the novel observation that FSH, LH, but also PRL and DAN, are potent mitogens for human CRC cells. This observation is important and has clinical implications. Since the incidence of CRC worldwide is reported to be rising, the increase in levels of pituitary SexHs (FSH and LH) in patients due to age-related gonadal dysfunction requires more attention.
We demonstrate for the first time that PtGs stimulate migration, adhesion, and proliferation of established human CRC cell lines. In our experiments we observed the expression of PtGs and gonadal SexH receptors in established human CRC cell lines, not only at the mRNA level but also at the protein level. Moreover, PtGs mediate this effect by their specific receptors, which respond by the phosphorylation of intracellular pathways involved in cell proliferation and migration. Finally we detected SexH receptor expression in CRC patient samples and demonstrated SexH mRNA expression in healthy human colon tissue adjacent to the tumor (HT) and tumor colonic tissue (CAN).
Recently, tremendous effort has been made to better understand the mechanisms of CRC development and its metastasis. Current concepts revolve around stem cells [40, 41], the role of innate immunity [42,43], and alterations in the microbiome [44]. Our observation that SexH receptors are expressed not only in CRC cells but, what is more important, also in CRC colonic patient samples sheds new light on this phenomenon. Thus, pituitary and gonadal hormones may be involved in the egress of cancerous cells into blood or lymphatic vessels and their migration to distinct locations. In fact, we demonstrated the effects of SexHs on the migration, adhesion, and proliferation of CRC cells.
Moreover since the follicle-stimulating hormone receptor FSH-R has been shown to be expressed by the vascular endothelium in several human cancers [45] as well as in tumor metastases [46], including colon cancer [47], the detection of SexH receptors by RT-PCR may reflect the expression of SexH receptors on the surface of tumor-associated cells of the vasculature. In fact, Alam et al. [48] showed that binding of FSH to the FSH receptor in ovarian granulosa cells induced an increase in hypoxia-inducible factor 1α protein levels and led to up-regulation of vascular endothelial growth factor (VEGF). This and other observations provide evidence that FSH receptor expression can induce VEGF and VEGF receptor 2 (VEGFR-2) signaling in endothelial cells, promoting tumorigenic angiogenesis [49,50]. However, this effect could be local and limited to epithelium, as we did not observe differences in plasma VEGF concentrations between CRC patients and healthy controls in our previous reports [40]. In contrast to our previous study, we observed higher levels of hepatocyte growth factor (HGF) in the plasma of patients diagnosed with CRC [40]. Of interest, the reported action of FSH and LH on ovarian surface epithelium (OSE) growth could in part be mediated indirectly through an elevation in the expression of autocrine growth factors, such as HGF [51].
On the other hand, according to the hypothesis proposed by Virchow, Conheim, and Boll, some cancers originate from dormant embryonic or germ cells residing in adult tissues [52–54]. These cells could be activated locally or could be attracted from a population of circulating SCs [55]. However, this tempting 150-year-old hypothesis requires further experimental support to identify the cells that are the origin of these tumors. Potential candidate cells located in adult tissues could be very small embryonic-like stem cells (VSELs) [56,57], which have been shown to express several SexH receptors [27,32] Thus, it is important to explore the possibility that mutated VSELs can give rise to CRC stem cells. In support of a contribution by circulating SCs, Houghton et al. [58] observed that bone marrow-derived stem cells (VSELs?) repopulated the gastric epithelium in response to chronic inflammation and in some cases contributed to malignant transformation and the development of stomach cancer. Nevertheless, both of these hypotheses need to be validated for CRC by further experimentation.
We are aware, that the current study has limitations. First, we used only two CRC cell lines, and the colonic samples were obtained from only 7 patients. Second, it has yet to be confirmed whether the biological effects of FSH and LH are truly mediated by the FSH and LH receptors. Further studies with siRNA/shRNA knockdown or CRISPR-Cas9-mediated knockout of receptors in cell lines should be implemented. We are currently implementing functional studies to fully explore this issue and exclude possible non-canonical actions of gonadotropins in healthy and cancerous colonic tissues.
In conclusion, our results provide novel evidence supporting a link between PtGs and CRC. Since FSH levels increase with age due to loss of gonadal function, this evidence may explain the age-related increase in this and other malignancies. Our hypothesis is somewhat supported by epidemiological observations of lower CRC risk in women on long-term estrogen replacement therapy, which lowers secretion of FSH from the pituitary.
In this light, further studies that could lead to development of new therapeutic protocols, including compounds capable of balancing the effects of PtGs and gonadal SexH axes, are necessary. Similarly, further work is needed to better understand the regulation of pituitary and gonadal hormone receptors on the surface of cancer cells. While PtGs are well known to upregulate expression of estrogen receptors in several types of cells [59], the potential hormonal up-regulators of PtGs have not yet been identified.
Acknowledgments
This work was partially supported by Harmonia NCN grant UMO-2014/14/M/NZ3/00475 to MZR and Pomeranian Medical University, Department of Gastroenterology statutory funds. We acknowledge Dr. Paul Todd for expert English proof reading in his native language.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was partially supported by Harmonia NCN grant UMO-2014/14/M/NZ3/00475 to MZR and Pomeranian Medical University, Department of Gastroenterology statutory funds (TS).
References
- 1.Wang X, Yang Y, Huycke MM. Microbiome-driven carcinogenesis in colorectal cancer: Models and mechanisms. Free Radic Biol Med. 2017;105: 3–15. doi: 10.1016/j.freeradbiomed.2016.10.504 [DOI] [PubMed] [Google Scholar]
- 2.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347: 78–81. doi: 10.1126/science.1260825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529: 43–47. doi: 10.1038/nature16166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu S, Hannun Y. The importance of extrinsic factors in the development of cancers. Mol Cell Oncol. 2016;3: e1143079 doi: 10.1080/23723556.2016.1143079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64: 104–117. doi: 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 6.Brenner Herman KMPCP. Colorectal Cancer. Lancet. 2014;383: 1490–1502. doi: 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 7.Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA. 2011;306: 1352–1358. doi: 10.1001/jama.2011.1362 [DOI] [PubMed] [Google Scholar]
- 8.Regula J, Rupinski M, Kraszewska E Polkowski M, Pachlewski J, Orlowska J, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355: 1863–1872. doi: 10.1056/NEJMoa054967 [DOI] [PubMed] [Google Scholar]
- 9.Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic A, et al. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS ONE. 2013;8: e68077 doi: 10.1371/journal.pone.0068077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. JNCI: J Natl Cancer Inst. 2015; 107: pii: djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratajczak M. Why are hematopoietic stem cells so “sexy”? On a search for developmental explanation. Leukemia. 2017;31: 1671–1677. doi: 10.1038/leu.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11: 419–424. doi: 10.1016/j.cgh.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mørch LS, Lidegaard Ø, Keiding N, Løkkegaard E and Kjær SK: The influence of hormone therapies on colon and rectal cancer. Eur J Epidemiol. 2016;31: 481–489. doi: 10.1007/s10654-016-0116-z [DOI] [PubMed] [Google Scholar]
- 14.Sayyid RK, Evans A, Hersey K, Maloni R, Hurtado-Coll A, Kulkarni G, et al. A Phase II, Randomized, Open-Label Study of Neoadjuvant Degarelix versus LHRH Agonist in Prostate Cancer Patients Prior to Radical Prostatectomy. Clin Cancer Res. 2016;23: 1974–1980. doi: 10.1158/1078-0432.CCR-16-1790 [DOI] [PubMed] [Google Scholar]
- 15.Schernhammer ES, Sperati F, Razavi P, Agnoli C, Sieri S, Berrino F, et al. Endogenous sex steroids in premenopausal women and risk of breast cancer: the ORDET cohort. Breast Cancer Res. 2013;15: R46 doi: 10.1186/bcr3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelbaset-Ismail A, Pedziwiatr D, Schneider G, Niklinski J, Charkiewicz R, Moniuszko M, et al. Pituitary sex hormones enhance the pro-metastatic potential of human lung cancer cells by downregulating the intracellular expression of heme oxygenase-1. Int J Oncol. 2017;50: 317–328. doi: 10.3892/ijo.2016.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng D, Zhao T, Yan K, Liang H, Liang J, Zhou Y, et al. Gonadotropins promote human ovarian cancer cell migration and invasion via a cyclooxygenase 2-dependent pathway. Oncol Rep. 2017;38: 1091–1098. doi: 10.3892/or.2017.5784 [DOI] [PubMed] [Google Scholar]
- 18.Deivaraju C, Temple HT, Block N, Robinson P, Schally AV. LHRH receptor expression in sarcomas of bone and soft tissue. Horm Mol Biol Clin Investig. 2016;28: 105–111. doi: 10.1515/hmbci-2016-0001 [DOI] [PubMed] [Google Scholar]
- 19.Gharwan H, Bunch KP, Annunziata CM. The role of reproductive hormones in epithelial ovarian carcinogenesis. Endocr Relat Cancer. 2015;22: R339–R363. doi: 10.1530/ERC-14-0550 [DOI] [PubMed] [Google Scholar]
- 20.Sanchez AM, Flamini MI, Russo E, Casarosa E, Pacini S, Petrini M, et al. LH and FSH promote migration and invasion properties of a breast cancer cell line through regulatory actions on the actin cytoskeleton. Mol Cell Endocrinol. 2016;437: 22–34. doi: 10.1016/j.mce.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 21.Poniewierska-Baran A, Schneider G, Sun W, Abdelbaset-Ismail A, Barr FG, Ratajczak MZ. Human rhabdomyosarcoma cells express functional pituitary and gonadal sex hormone receptors: Therapeutic implications. Int J Oncol. 2016;48: 1815–1824. doi: 10.3892/ijo.2016.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelbaset-Ismail A, Borkowska S, Janowska-Wieczorek A, Tonn T, Rodriguez C, Moniuszko M, et al. Novel evidence that pituitary gonadotropins directly stimulate human leukemic cells-studies of myeloid cell lines and primary patient AML and CML cells. Oncotarget. 2016;7: 3033–3046. doi: 10.18632/oncotarget.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinton TN, Woldu SL, Raj GV. Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer. Expert Opin Pharmacother. 2017;18: 825–832. doi: 10.1080/14656566.2017.1328056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesario TC, Yousefi S, Carandang G, Sadati N, Le J, Vaziri N. Enhanced yields of gamma interferon in prolactin treated human peripheral bloo mononuclear cells. Proc Soc Exp Biol Med. 1994;205: 89–95. [DOI] [PubMed] [Google Scholar]
- 25.Bellone G, Geuna M, Carbone A, Silvestri S, Foa R, Emanuelli G, et al. Regulatory action of prolactin on the in vitro growth of CD34+ human hemopoietic progenitor cells. J Cell Physiol. 1995;163: 221–231. doi: 10.1002/jcp.1041630202 [DOI] [PubMed] [Google Scholar]
- 26.Forman S, Kás J, Fini F, Steinberg M, Ruml T.J The effect of different solvents on the ATP/ADP content and growth properties of HeLa cells. Biochem Mol Toxicol. 1999;13: 11–15 [DOI] [PubMed] [Google Scholar]
- 27.Mierzejewska K, Borkowska S, Suszynska E, Suszynska M, Poniewierska-Baran A, Maj M, et al. Hematopoietic stem/progenitor cells express several functional sex hormone receptors—novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells Dev. 2015;24: 927–937. doi: 10.1089/scd.2014.0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Segelman J, Nordgren A, Lindstrӧm L, Frisell J, Martling A. Increased risk of colorectal cancer in patients diagnosed with breast cancer in women. Cancer Epidemiol. 2016;41: 57–62. doi: 10.1016/j.canep.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 29.Rennert G. Reproductive factors, hormones and colorectal cancer—still unresolved., Br J Cancer. 2016;116: 1–3. doi: 10.1038/bjc.2016.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy N, Xu L, Zervoudakis A, Xue X, Kabat G, Rohan TE, et al. Reproductive and menstrual factors and colorectal cancer incidence in the Women’s Health Initiative Observational Study. Br J Cancer. 2017;116: 117–125. doi: 10.1038/bjc.2016.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chlebowski RT, Anderson GL. Menopausal hormone therapy and cancer: changing clinical observations of target site specificity. Steroids. 2014;90: 53–59. doi: 10.1016/j.steroids.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelbaset-Ismail A, Suszynska M, Borkowska S, Adamiak M, Ratajczak J, Kucia M, et al. Human haematopoietic stem/progenitor cells express several functional sex hormone receptors. J Cell Mol Med. 2016;20: 134–146. doi: 10.1111/jcmm.12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huhtaniemi I. Are gonadotrophins tumorigenic-a critical review of clinical and experimental data. Mol Cell Endocrinol. 2010;329: 56–61. doi: 10.1016/j.mce.2010.04.028 [DOI] [PubMed] [Google Scholar]
- 34.Rick FG, Schally AV. Bench-to-bedside development of agonists and antagonists of luteinizing hormone-releasing hormone for treatment of advanced prostate cancer. Urol Oncol. 2015;33: 270–274. doi: 10.1016/j.urolonc.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 35.Brusselaers N, Maret-Ouda J, Konings P, El-Serag HB, Lagergren J. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int J Cancer. 2017;140: 1693–1699. doi: 10.1002/ijc.30588 [DOI] [PubMed] [Google Scholar]
- 36.Ben Khedher S, Neri M, Papadopoulos A, Christiani DC, Diao N, Harris CC, et al. Menstrual and reproductive factors and lung cancer risk: A pooled analysis from the international lung cancer consortium. Int J Cancer. 2017;141: 309–323. doi: 10.1002/ijc.30750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis Cancer Epidemiol Biomarkers Prev. 2012;21: 20–38. doi: 10.1158/1055-9965.EPI-11-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W, Yao B, Sun L, Pu R, Wang L, Zhang R. Immunohistochemical and in situ hybridization studies of gonadotropin releasing hormone (GnRH) and its receptor in rat digestive tract. Life Sci. 2001;68: 1727–1734. [DOI] [PubMed] [Google Scholar]
- 39.Szepeshazi K, Schally AV, Halmos G. LH-RH receptors in human colorectal cancers: unexpected molecular targets for experimental therapy. Int J Oncol. 2007;30: 1485–1492. [PubMed] [Google Scholar]
- 40.Marlicz W, Sielatycka K, Serwin K, Kubis E, Tkacz M, Głuszko R, et al. Effect of colorectal cancer on the number of normal stem cells circulating in peripheral blood. Oncology Rep. 2016;36: 3635–3642. [DOI] [PubMed] [Google Scholar]
- 41.Szaryńska M, Olejniczak A, Kmieć Z. The role of cancer stem cells in pathogenesis of colorectal cancer. Postepy Hig Med Dosw. (Online) 2016;70: 1469–1482. [DOI] [PubMed] [Google Scholar]
- 42.Korbut E, Ptak-Belowska A, Brzozowski T. Mechanisms promoting physiological cells progression into tumorigenesis. J Physiol Pharmacol. 2012;63: 565–570. [PubMed] [Google Scholar]
- 43.Olechowska-Jarząb A, Ptak-Belowska A, Brzozowski T. Terapeutic importance of apoptosis pathways in pancreatic cancer. Folia Med Cracov. 2016;56: 61–70. [PubMed] [Google Scholar]
- 44.Gao R, Kong C, Huang L, Li H, Qu X, Liu Z, et al. Mucosa-associated microbiota signature in colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;doi: doi: 10.1007/s10096-017-3026-4 [DOI] [PubMed] [Google Scholar]
- 45.Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363: 1621–1630. doi: 10.1056/NEJMoa1001283 [DOI] [PubMed] [Google Scholar]
- 46.Siraj A, Desestret V, Antoine M, Fromont G, Huerre M, Sanson M, et al. Expression of follicle-stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer. 2013;13: 246 doi: 10.1186/1471-2407-13-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghiringhelli F, Isambert N, Ladoire S. Degarelix as a new antiangiogenic agent for metastatic colon cancer? World J Gastroenterol. 2013;19: 769–772. doi: 10.3748/wjg.v19.i5.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M et al. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology. 2009;150: 915–928. doi: 10.1210/en.2008-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raica M, Coculescu M, Cimpean AM, Ribatti D. Endocrine gland derived-VEGF is down-regulated in human pituitary adenoma. Anticancer Res. 2010;30: 3981–3986. [PubMed] [Google Scholar]
- 50.Sapoznik S, Cohen B, Tzuman Y, Meir G, Ben-Dor S, Harmelin A et al. Gonadotropin-regulated lymphangiogenesis in ovarian cancer is mediated by LEDGF-induced expression of VEGF-C. Cancer Res. 2009;69: 9306–9314. doi: 10.1158/0008-5472.CAN-09-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parrott JA, Doraiswamy V, Kim G, Mosher R, Skinner MK. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2001;172: 213–222. [DOI] [PubMed] [Google Scholar]
- 52.Virchow R. Editorial Archive fuer pathologische. Anatomie und Pysiologie fuer klinische Medizin 1855;8: 23–54. [Google Scholar]
- 53.Conheim J. Congenitales, quergestreiftes muskelsarkon der nireren. Virchows Arch. 1875;65: 64. [Google Scholar]
- 54.Soto AM and Sonnenschein C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays. 2004;26: 1097–1107. doi: 10.1002/bies.20087 [DOI] [PubMed] [Google Scholar]
- 55.Ratajczak MZ, Bujko K, Wojakowski W. Stem cells and clinical practice: new advances and challenges at the time of emerging problems with induced pluripotent stem cell therapies. Pol Arch Med Wewn. 2016;126: 879–890. doi: 10.20452/pamw.3644 [DOI] [PubMed] [Google Scholar]
- 56.Ratajczak MZ, Ratajczak J, Suszynska M, Miller DM, Kucia M, Shin D-M. A novel view of the adult stem cell compartment from the perspective of a quiescent population of very small embryonic-like stem cells. Circ Res. 2017;120: 166–178. doi: 10.1161/CIRCRESAHA.116.309362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratajczak MZ, Bartke A and Darzynkiewicz Z. Prolonged Growth Hormone/Insulin/Insulin-like Growth Factor Nutrient Response Signaling Pathway as a Silent Killer of Stem Cells and a Culprit in Aging. Stem Cell Rev 2017;13: 443–453. doi: 10.1007/s12015-017-9728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306: 1568–1571. doi: 10.1126/science.1099513 [DOI] [PubMed] [Google Scholar]
- 59.Tripathy M, Rai U. Temporal expression and gonadotropic regulation of aromatase and estrogen receptors in the ovary of wall lizard, Hemidactylus flaviviridis: Correlation with plasma estradiol and ovarian follicular development. Steroids. 2017;128: 23–31. doi: 10.1016/j.steroids.2017.10.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.