Abstract
Background
Pediatric survivors of childhood cancer are at increased risk of poor quality of life and social-emotional outcomes following treatment. The relationship between parent psychological distress and child adjustment in pediatric cancer survivors has been well established. However, limited research has examined the factors that may buffer this association. The current study examined the associations between psychosocial family risk factors, parental psychological distress, and health-related quality of life (hrql) in pediatric cancer survivors.
Methods
Fifty-two pediatric cancer survivors (34 males, 18 females, mean age = 11.92) and their parents were recruited from a long-term cancer survivor clinic. Children and their parents who consented to participate completed the Pediatric Quality of Life Inventory 4.0. Parents completed a demographic information form, the Psychosocial Assessment Tool (pat 2.0) and the Brief Symptom Inventory (bsi). The Intensity of Treatment Rating (itr-3) was evaluated by the research team.
Results
Multiple regression analyses revealed that parental psychological distress negatively predicted parent-reported hrql, while treatment intensity, gender, and psychosocial risk negatively predicted parent and child-reported hrql. Psychosocial risk moderated the association between parent psychological distress and parent-reported child hrql (p = 0.03), whereby parents with high psychological distress but low levels of psychosocial risk reported their children to have higher hrql.
Conclusion
Low levels of family psychosocial risk buffer the impact of parent psychological distress on child hrql in pediatric cancer survivors. The findings highlight the importance of identifying parents and families with at-risk psychological distress and psychosocial risk in order to provide targeted support interventions to mitigate the impact on hrql.
Keywords: Pediatric, cancer, survivorship, health-related quality of life, psychosocial risk, parent distress
INTRODUCTION
Survival rates for childhood cancer have improved dramatically in recent years as a result of rapid advancements in treatments1,2. Given this growing population of survivors, attention has been increasingly placed on adjustment and health-related quality of life (hrql) as children move from treatment into survivorship. Health-related quality of life is a broad conceptual term that refers to the patient’s perception of the impact of their illness and treatment on their physical, psychological, and social well-being3. Although many survivors adapt quite well following cancer diagnosis and treatment1, there is evidence that the hrql of childhood cancer survivors suffers relative to that of other chronic disease groups and healthy controls4,5. Specifically, childhood cancer survivors are at risk of persistent physical, neurocognitive, and psychosocial late effects6–8. Ongoing psychosocial difficulties have also been reported in parents and siblings of pediatric cancer survivors, highlighting the long-term impact of cancer both on survivors and on their families9,10. Given that the broader social context is central to the adaptation of cancer survivors11, the examination of parent mental health as well as the broader family functioning are critical to understanding the adaptation of children previously treated for cancer.
Previous literature has demonstrated that parent psychological distress (i.e., difficulties coping with emotions and cognitions which may manifest as anxiety, worry, sadness, or depression) contributes to the hrql and well-being of pediatric cancer survivors12. Specifically, previous findings have shown that high levels of parent distress symptoms are positively associated with the distress symptoms of pediatric cancer survivors13. Indeed, the association between parent and child distress has been shown to become stronger over time following a child’s diagnosis, making this association particularly poignant as the child transitions to survivorship14,15. The mechanisms by which parent psychological distress impacts child hrql have been hypothesized to include biological susceptibility to emotional difficulties, interference with a parent’s ability to provide the emotional support necessary for successful child adaptation, and increased use of parent behaviours that are associated with child distress such as lowered sensitivity, increased hostility, and withdrawal11,15. One concern that has been noted in the literature with respect to the impact of parent psychological distress on the hrql of childhood cancer survivors is the reliance on parent-proxy reports of child hrql, which may result in reporting bias13,16. Although parent and child reports of hrql in the chronically ill have been shown to be quite high, there is greater agreement for observable functioning (e.g., physical hrql) than for non-observable functioning (e.g., emotional or social hrql)17, suggesting the need to consider both reports. The current study aims to provide clarity on these issues by examining the impact of parent psychological distress on both parent-proxy and child self-report of hrql.
While the association between parent psychological distress and child hrql is well established, limited research has examined factors that may exacerbate or buffer this association. One such factor is psychosocial family risk18, which refers to a group of risk factors that have been associated with poor adaptation in families with cancer. Psychosocial family risk has been conceptualized to include low levels of social support (e.g., lack of emotional support or availability of childcare), family problems (e.g., parental substance abuse problems, marital conflict, and child custody disputes), low availability of instrumental resources such as finances or transportation, sibling problems (e.g., sadness and withdrawal, learning difficulties, social difficulties), parental stress reactions (e.g., bad dreams and nightmares), child problems (e.g., mood difficulties, school difficulties, substance use), and negative beliefs about the future (e.g., negative thoughts about family closeness, ability to make treatment decisions, and likelihood child will beat cancer). Studies in the broader child development literature have demonstrated that family psychosocial functioning including social support, instrumental resources, and family cohesion can buffer the impact of stress on developmental outcomes19. Likewise, it has been hypothesized that the family context may be protective against the negative effects associated with cancer treatment11. There is evidence that lower levels of psychosocial family risk and lower levels of parent psychological distress independently predict better patient hrql (as reported by parent-proxy) in the first year following a cancer diagnosis16. However, the manner in which these two factors might interact to influence the outcomes of pediatric cancer survivors has not been explored, which is the goal of the current study.
It is possible that the transmission of parent psychological distress to child hrql may be modified by psychosocial family risk, whereby a positive family environment, high levels of social support and resources, and low levels of family problems may buffer the association between parent psychological distress and child hrql20. That is, for a parent who has high levels of psychological distress, low levels of psychosocial family risk (i.e., higher family functioning and higher levels of support) may mitigate the influence of these difficulties on the quality of life of the child survivor. Conversely, for a parent with high levels of psychological distress, the addition of high psychosocial family risk may exacerbate the association between parent psychological distress and child hrql. Identifying the variables that impact the association between parent psychological distress and child hrql will provide insight into developing potential targets for psychosocial interventions.
For the current study, we had two primary research aims: 1) examine whether parent psychological distress and psychosocial family risk (controlling for child age, gender, and treatment intensity) are associated with hrql in pediatric cancer survivors; and 2) examine whether psychosocial family risk factors moderate the relationship between parent psychological distress and hrql. In line with research question 1, it was hypothesized that higher levels of parent psychological distress and psychosocial family risk would be associated with lower parent- and child-reported hrql. With regard to research question 2, it was hypothesized that psychosocial family risk would moderate the association between parent psychological distress and hrql (child and parent proxy report), whereby low levels of psychosocial family risk would buffer the association between parent psychological distress and the hrql of pediatric cancer survivors. Finally, in line with previous research on hrql in pediatric cancer survivors21, it was hypothesized that older female survivors would have the lowest self-reported hrql and that treatment intensity would also be negatively associated with survivor hrql22.
METHODS
Procedure
Pediatric cancer survivors and one of their parents were recruited from Alberta Children’s Hospital Long Term Survivor Clinic over a 24-month period, as part of a larger study from February 2012 to November 2013. Both the survivors and the parent had to be fluent in English. Survivors met inclusion criteria if they were two or more years post-treatment at the time of recruitment and were no longer receiving active treatment. A research assistant approached families during their pediatric oncology follow-up appointment to assess interest in the study. Informed consent was obtained from a parent and children provided assent. After written consent was obtained from a parent, parents and survivors were provided with a questionnaire package, which was completed during clinic visits or completed at home and returned via mail in a pre-addressed, stamped envelope provided by the research assistant. The parent who attended the appointment, who typically was the primary caregiver, completed the questionnaire. Questionnaires were completed independently, and survivors received support from a research assistant as needed. For questionnaires completed at home, independent completion is not guaranteed and may have introduced bias. Parent-child dyads were reimbursed with a $10 gift card for their time. Ethics approval was obtained from the Conjoined Research Ethics Board at the University of Calgary.
Participants
Child and parent demographics can be found in Table I. Of 94 children with cancer and their parents invited to participate in the larger research study, 26 declined, 15 did not return the questionnaires, and one was not a cancer patient, leaving 52 parent-child dyads. Five of these 52 dyads (9.6%) had experienced recurrence of disease, whereas the majority were in remission. Using maximum likelihood estimation, we were able to include all 52 participants in the analysis despite missing data (further described in analytic plan below).
TABLE I.
Parent-child dyad demographic information
| Characteristic | Demographic Information |
|---|---|
| Child gender | Male 34 (64.2%), Female 18 (34.6%) |
| Child age (years) | M=11.92, SD=3.1, Range=6–17 |
| Time since diagnosis (years) | M=9.7, SD=2.84, Range=3.4–16.1 |
| Child ethnicity | Asian 5.8% |
| African-Canadian 1.9% | |
| White 76.9% | |
| Other 9.6% | |
| Missing 5.8% | |
| Cancer diagnosis | Brain tumour 6 (11.5%) |
| Acute lymphoblastic leukemia 14 (26.9%) | |
| Acute myeloid leukemia 2 (3.8%) | |
| Lymphoma 2 (3.8%) | |
| Fibrosarcoma 3 (5.8%) | |
| Hepatoblastoma/hepatocellular carcinoma 1 (1.9%) | |
| Wilms tumour 9 (17.3%) | |
| Rhabdomyosarcoma 1 (1.9%) | |
| Leydig cell tumour 1 (1.9%) | |
| Mesoblastic nephroma 2 (3.8%) | |
| Ewing sarcoma 1 (1.9%) | |
| Retinoblastoma 2 (3.8%) | |
| Germ cell tumour 2 (3.8%) | |
| Neuroblastoma 6 (11.5%) | |
| Parent gender | 90.4% mothers |
| 3.8% fathers | |
| 5.8% missing | |
| Parent age (years) | Fathers: M=44.27, SD=5.02 |
| Mothers: M=42.44, SD=5.92 | |
| Highest parent education | College 33.67% |
| University 22.45% | |
| Graduate or professional school 20.41% | |
| High school 23.47% | |
| Household family income | M=$126,803; SD=$55,221 |
SD = standard deviation.
Measures
Health-Related Quality of Life
Patient health-related quality of life was assessed using the Pediatric Quality of Life Inventory (PedsQL) 4.023. The PedsQL generic core module is a 23-item measure that assesses physical, social, emotional, and cognitive domains. Higher scores represent better quality of life. It has been used frequently and is well validated within pediatric oncology populations4. The PedsQL has demonstrated good psychometric properties across studies including Cronbach’s alphas that met or exceeded 0.70 and good construct validity in pediatric cancer samples24. A parent or caregiver completed a proxy report for their child. Child survivors completed the age-appropriate (8 to 12 years or 13 to 18 years) self-report version. Five children who were under the age of 8 years did not complete the questionnaire. In the current study, the survivor’s total score was used to operationalize self-reported hrql. The parent’s total score was used to operationalize the parent proxy-reported hrql.
Parent Psychological Distress
The Brief Symptom Inventory (bsi)25 was used to measure total parent psychological distress, which has excellent reliability and convergent and divergent validity25. The bsi is a 53-item measure based on the Symptom Checklist-90-Revised (scl-90-r) that has nine different symptom subscales and three global subscales. For the current study, we report on the Global Severity Index (gsi), which is an overall measure of an individual’s psychological distress. The adult nonpatient normative sample was used to obtain the T-scores.
Psychosocial Family Risk
Psychosocial family risk was assessed using the Psychosocial Assessment Tool (pat 2.0)26,27, which is a parent-report tool used to identify areas of psychosocial family risk following cancer diagnosis. The questionnaire consists of Likert ratings on 15 response sets that address the following subscales: family structure and resources, family social support, family problems, parent stress reactions, family beliefs, child problems, and sibling problems. An example item from the family social support subscale includes “who can you count on to provide the following: childcare/parenting, emotional support, financial support, information, help with everyday tasks?”. Higher scores on the pat 2.0 represent higher psychosocial risk (e.g., less support and more family problems). The pat 2.0 has high test re-test reliability and validity with an alpha level of 0.81 and test re-test reliability of 0.78 to 0.87 over a two-week period26,27.
Treatment Intensity
The intensity of treatment each child received was evaluated using the Intensity of Treatment Rating (itr-3) scale28. The itr-3 is used to categorize the intensity of pediatric cancer treatment from lowest through most intensive based on treatment modality and disease stage/risk level for the patient. The scale ranges from 1 to 4 with Level 1 being minimally intensive and Level 4 being the most intensive. The itr-3 was completed by two independent raters who reviewed the patient’s medical records. Inter-rater reliability for the current study was high and any discrepancies were resolved by consensus. The itr-3 has demonstrated excellent inter-rater reliability with agreements of 0.90 in other settings28.
Demographics
A demographics form was used to collect information about the patient’s gender and age, and family characteristics including parent’s age, education, and income. Date of diagnosis, tumour type, and treatment information were also retrieved from patient files.
Analytic Plan
Descriptive analyses were performed to identify child and parent characteristics. Analyses were conducted in Mplus Version 7.4 (Muthén & Muthén, Los Angeles, CA, USA) and SPSS Version 22.0 (IBM Corp., NY, USA). Analyses were performed with maximum likelihood, which allowed the inclusion of 52 parent-child dyads with missing data based on the missing at random assumption29. There were 5 children who were under the age of 8 years who did not complete questionnaires and 10 children who either were not given the questionnaire or only completed one side of a two-sided questionnaire. For treatment intensity, there was 7.7% missing data, for parent-reported hrql, there was 13.5% missing data, 3.8% on the pat, and 7.7% on the bsi. There were no significant differences based on child age or treatment intensity between children who did not complete questionnaires and those who did. With up to six predictors in some of the models, a power calculation was undertaken. In order to detect a large effect size, a sample size of N = 46 was required given a power level of 0.80 and a significance level of 0.05 for the multiple regression analyses. Adequate power was obtained for the current study.
To test our first hypothesis, zero-order correlational analyses were performed. To test our second hypothesis, that psychosocial family risk would moderate the association between parent psychological distress and child hrql, a multiple regression was conducted. The model included three covariates (child age, child gender, and treatment intensity) as these variables have been shown to be associated with hrql in pediatric cancer populations16,30,31. An interaction term was entered in the last step of the multiple regression. To help interpret the interaction, simple slope analyses were conducted, which allowed us to compute the strength of the association between parent psychological distress and child hrql for families with high (1 standard deviation [sd] deviation above the mean) and low (1 sd below the mean) family risk. All continuous variables were centred to minimize multicollinearity, and the interaction term was computed using the centred variables. Time since diagnosis was not included as a covariate given the number of predictor variables included in the current study and the fact that this variable has not been associated with hrql in previous studies16.
RESULTS
Research Question 1: Are parent psychological distress and psychosocial family risk associated with HRQL in pediatric cancer survivors?
Correlations among variables are presented in Table II. Parent psychological distress and psychosocial family risk were negatively associated with parent-reported child hrql. Psychosocial family risk, but not parent psychological distress, was negatively associated with child self-report of hrql. Parent psychological distress and psychosocial family risk were positively associated.
TABLE II.
Summary of intercorrelations, means, standard deviations, and ranges for variables
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Treatment intensity | 1 | — | — | — | — | — |
| 2. Child age | 0.01 (0.97) | 1 | — | — | — | — |
| 3. PedsQL-PR total | −0.22 (0.17) | 0.16 (0.29) | 1 | — | — | — |
| 4. PedsQL-CR total | −0.25 (0.16) | 0.28 (0.09) | 0.77 (0.00) | 1 | — | — |
| 5. bsi gsi | 0.06 (0.72) | −0.09 (0.56) | −0.48 (0.001) | −0.30 (.08) | 1 | — |
| 6. pat 2.0 total | −0.06 (.70) | −0.11 (0.43) | −0.55 (0.00) | −0.53 (0.00) | 0.44 (0.002) | 1 |
| N | 48 | 52 | 45 | 37 | 48 | 50 |
| Mean | 2.52 | 11.92 | 85.63 | 86.43 | 51.42 | 0.84 |
| sd | 0.95 | 3.11 | 13.90 | 13.18 | 11.62 | 0.74 |
| Range | 1–4 | 6–17 | 0–100 | 0–100 | 33–72 | 0–3 |
PedsQL-PR = pediatric quality of life inventory–parent-reported; PedsQL-CR = pediatric quality of life inventory–child-reported; bsi gsi = brief symptom inventory global severity index; pat = psychosocial assessment tool; sd = standard deviation.
P values are reported in parentheses. Bolded values indicate significant correlations.
Research Question 2: Does psychosocial family risk moderate the relationship between parent psychological distress and HRQL in pediatric cancer survivors?
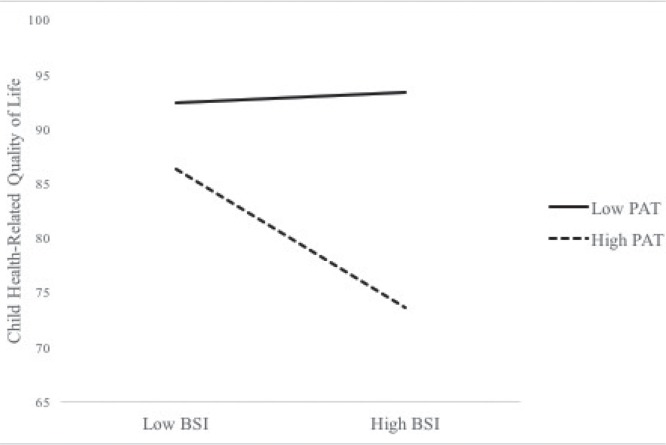
Results from the regression analyses can be found in Tables III and IV. Examining parent-reported child hrql, female gender and treatment intensity were negatively associated with parent-reported child hrql. Examining predictors, parent psychological distress and psychosocial family risk were negatively associated with the parent’s report of their child’s hrql. The interaction term between parent psychological distress and psychosocial family risk was significant, which indicated that the link between parent psychological distress and parent-reported child hrql varied as a function of psychosocial family risk. As seen in Figure 1, in order to facilitate the interpretation of the interaction, we plotted regression lines for relations between child hrql and parent psychological distress as moderated by psychosocial family risk (1 sd above and below the mean; two-way interaction). The slope for high psychosocial family risk scores is marginally significant (b = −0.59, p = 0.07), while the slope for low psychosocial family risk scores is not significant (b = 0.04, p = 0.87).
TABLE III.
Model 1: regression analysis examining the role of parent mental health in predicting parent-reported child hrql
| Variable | B | Standard error | p | 95% CI |
|---|---|---|---|---|
| Covariates | ||||
| Child age | −0.02 | 0.08 | 0.81 | −0.18, 0.14 |
| Gender | −0.38b | 0.10 | <0.001 | −0.57, −0.18 |
| Treatment intensity | −0.21a | 0.09 | <0.05 | −0.38, −0.03 |
| Predictors | ||||
| bsi | −0.29a | 0.12 | 0.02 | −0.53, −0.06 |
| pat | −0.35b | 0.01 | <0.001 | −0.55, −0.16 |
| Interaction | ||||
| bsi a pat | −0.21a | 0.10 | 0.04 | −0.41, −0.01 |
p < 0.05.
p < 0.01.
hrql = health-related quality of life; CI = confidence interval; bsi = brief symptom inventory; pat = psychosocial assessment tool. R2 = 0.60. N = 52.
TABLE IV.
Model 2: Regression analysis examining the role of parent mental health in predicting child-reported hrql
| Variable | B | Standard error | p | 95% CI |
|---|---|---|---|---|
| Covariates | ||||
| Child age | 0.18 | 0.10 | 0.12 | −0.05, 0.42 |
| Gender | −0.43b | 0.09 | <0.001 | −0.61, −0.25 |
| Treatment intensity | −0.36b | 0.10 | <0.001 | −0.56, −0.17 |
| Predictors | ||||
| bsi | −0.11 | 0.15 | 0.49 | −0.41, 0.20 |
| pat | −0.45b | 0.13 | <0.001 | −0.70, −0.20 |
| Interaction | ||||
| bsi a pat | −0.13 | 0.11 | 0.23 | −0.34, 0.08 |
p < 0.05.
p < 0.01.
hrql = health-related quality of life; CI = confidence interval; bsi = brief symptom inventory; pat = psychosocial assessment tool. R2 = 0.69. N = 52. Standardized regression coefficients presented.
FIGURE 1.
Parent-reported child health-related quality of life as a function of parent psychological distress and psychosocial family risk. Note: Regression lines for relations between child health-related quality of life and parent psychological distress as moderated by family psychosocial risk (1 sd above and below the mean; two-way interaction). Slope of high pat scores is marginally significant (b = −0.59, p = 0.07), while slope for low pat scores is not significant (b = 0.04, p = 0.87). sd = standard deviation; pat = psychosocial assessment tool; bsi = brief symptom inventory.
Turning to child self-reported hrql, female gender and treatment intensity both significantly negatively predicted child self-reported hrql, as did psychosocial family risk. The interaction term was not significant, indicating that psychosocial family risk did not moderate the association between parent psychological distress and child-reported hrql.
As a post-hoc analysis to better understand what components of family psychosocial risk were most predictive of parent-reported and child-reported hrql, we conducted Pearson correlations with the 7 subscales from the pat. The child problems subscale was negatively associated with child (r = −0.61, p < 0.001) and parent reports (r = −0.63, p < 0.001) of hrql. The parent stress reactions were also negatively associated with both parent (r = −0.38, p < 0.05) and child reports (r = −0.44, p < 0.05) of hrql. Family problems were negatively associated with parent-reported hrql (r = −0.30, p = 0.04).
DISCUSSION
As the number of pediatric cancer survivors has increased, greater focus has been placed on their hrql beyond diagnosis and treatment3. The primary aim of the current study was to investigate the impact of psychosocial family risk factors and parent psychological distress on the hrql of childhood cancer survivors. We hypothesized that increased levels of psychosocial family risk and parent psychological distress would be associated with lower parent-proxy and self-reported hrql. The current study makes a novel contribution to the literature by examining both parent and child reports of hrql as well as by examining family psychosocial risk as a moderator of the association between parent psychological distress and survivor hrql. Results partially confirmed our hypotheses whereby parent psychological distress symptoms were negatively associated with parent-proxy reported hrql, but not child self-reported hrql. In addition, psychosocial family risk was negatively associated with both parent-proxy and child self-reported hrql.
These findings are consistent with previous literature that has found that parent distress is associated with lower parent-reported child hrql in children on active treatment13,16. One possibility for the lack of relation between parental distress and child-reported hrql may be due to differences in parent and child perceptions of the child’s hrql. Specifically, parent psychological distress could have negatively influenced a parent’s perception, and subsequent report, of their child’s well-being, resulting in a more negative evaluation than expressed through the child’s self-report32. On the other hand, it may be that children and young adolescents have more insight into their internal states, while parents often need to rely on observable behaviours and information their child shares17,33. Our findings may also be attributed to the fact that the average age of survivors in the current study was 12 years of age, which is a time where children start to differentiate from their parents and may spend increasingly more time with peers and obtain support from peers, giving less of an opportunity for parent psychological distress to negatively impact their hrql. Nonetheless, given the small sample size in the current study, it may be that we were unable to detect the effect of parent psychological distress on child self-reported hrql.
Interestingly, psychosocial family risk was negatively associated with both parent and child report of hrql, despite being reported by parents. This indicates that psychosocial family risks as measured by the pat 2.0 are directly associated with child survivor hrql. This finding is consistent with previous work that has found family resources and socioeconomic status to be strongly associated with higher hrql in children with cancer34,35. To our knowledge, the current study is the first to demonstrate that psychosocial family risk is associated with hrql in survivors from the perspective of both parents and child survivors.
The current study also showed that psychosocial family risk moderates the association between parent psychological distress and parent-reported hrql, but not child self-report of hrql. For parents with high levels of psychological distress, having poorer family functioning (e.g., less social support, instrumental resources, and more family problems) was associated with lower reports of child hrql. It has been argued that parent perceptions of child functioning, such as hrql, are central to ongoing decisions about the child’s medical treatments, follow-up regimens, and overall health care usage1. Our findings suggest that interventions targeted at parents and families that focus on social support, reducing family conflict, and connecting families with instrumental resources may be beneficial in improving parent perceptions of their child’s hrql.
With regard to the covariates, child gender was a consistent predictor of survivors’ hrql, with parents and children reporting lower hrql for female survivors. This finding is consistent with previous research, in which female survivors of pediatric cancer report significantly worse hrql than male survivors31. When the subscales of the PedsQL were examined in the current study, females significantly differed from males on both parent report and child self-report of social functioning. Given that childhood cancer survivors are at increased risk of social difficulties36, it may be that females are more impacted. Treatment intensity also consistently predicted both parent and child self-reported hrql, which is in line with a recent review in which higher treatment intensity was associated with decreased quality of life in pediatric cancer patients37. Overall, the current study demonstrates that broader social factors are also important areas to consider.
The findings of the current study should be interpreted in the context of several limitations including the relatively small sample size, a diverse range of cancer diagnoses of survivors, and families of a relatively high socio-economic status. We cannot exclude the possibility of common method variance, whereby parent-reported independent variables were associated with parent-reported dependent variables. It is also possible that covariates (i.e., length of remission, type of cancer) that were not included in the current study may have influenced the results. For example, compared with other cancer survivors, children treated for brain tumours rate their hrql as lower, particularly on the social functioning subscales, as do their parents38. Future research should examine whether children who have experienced different cancers may be especially vulnerable to psychosocial family risk.
Our findings suggest that psychosocial risk within a family continues to impact the outcomes of children treated for cancer well into survivorship. These findings stress the importance of continuing to screen survivors for psychosocial difficulties across the cancer trajectory. Given clinical practice guidelines that recommend psychosocial screening for pediatric cancer survivors on an annual basis39, and that psychosocial follow-up in pediatric cancer survivors is one of 15 standards of care in pediatric psychosocial oncology1, both child and parent reports of child hrql should be obtained during routine screenings. Our findings indicate that particular attention should be paid to the hrql of survivors who are experiencing psychosocial family risks, female survivors, and for survivors who have experienced higher levels of treatment intensity, as these children are at the highest risk of poor hrql.
Furthermore, our findings indicate that psychosocial family risk strengthens the association between parent psychological distress and their report of lower levels of child hrql. We speculate that higher psychosocial family risk increases parenting stress and psychological distress, which leads to catastrophic parental perceptions with regard to their child’s functioning. From a clinical perspective, it is important to assess parental distress and overall family functioning during routine follow-up in pediatric survivor clinics in order to connect families with needed supports. According to our post-hoc analyses, parent stress reactions and larger family problems such as marital difficulties, anxiety and mood difficulties of adults in the home, parental substance use difficulties, or child custody disputes seem to be particularly associated with poor child hrql. Interventions that specifically target parent distress as well as family functioning may be particularly helpful in mitigating poor hrql in pediatric cancer survivors.
ACKNOWLEDGMENTS
This study was funded by the Alberta Children’s Hospital Foundation and the Alberta Children’s Hospital Research Institute (fellowship to Dr. Nicole Racine). Thank you to the families and children who participated in our research.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare that we have none.
REFERENCES
- 1.Lown EA, Phillips F, Schwartz LA, Rosenberg AR, Jones B. Psychosocial follow-up in survivorship as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62(suppl 5):S514–84. doi: 10.1002/pbc.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 5.Landolt MA, Vollrath M, Niggli FK, Gnehm HE, Sennhauser FH. Health-related quality of life in children with newly diagnosed cancer: a one year follow-up study. Health Qual Life Outcomes. 2006;4:63. doi: 10.1186/1477-7525-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr Blood Cancer. 2009;52(2):159–64. doi: 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- 7.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol. 2005;30(1):9–27. doi: 10.1093/jpepsy/jsi012. [DOI] [PubMed] [Google Scholar]
- 8.Speechley KN, Barrera M, Shaw AK, Morrison HI, Maunsell E. Health-related quality of life among child and adolescent survivors of childhood cancer. J Clin Oncol. 2006;24(16):2536–43. doi: 10.1200/JCO.2005.03.9628. [DOI] [PubMed] [Google Scholar]
- 9.Vrijmoet-Wiersma CM, van Klink JM, Kolk AM, Koopman HM, Ball LM, Maarten Egeler R. Assessment of parental psychological stress in pediatric cancer: a review. J Pediatr Psychol. 2008;33(7):694–706. doi: 10.1093/jpepsy/jsn007. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman L, Cernvall M, Gronqvist H, Ljotsson B, Ljungman G, von Essen L. Long-term positive and negative psychological late effects for parents of childhood cancer survivors: a systematic review. PLoS ONE. 2014;9(7):e103340. doi: 10.1371/journal.pone.0103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long KA, Marsland AL. Family adjustment to childhood cancer: a systematic review. Clin Child Fam Psychol Rev. 2011;14(1):57–88. doi: 10.1007/s10567-010-0082-z. [DOI] [PubMed] [Google Scholar]
- 12.Maurice-Stam H, Oort FJ, Last BF, Brons PP, Caron HN, Grootenhuis MA. Longitudinal assessment of health-related quality of life in preschool children with non-CNS cancer after the end of successful treatment. Pediatr Blood Cancer. 2008;50(5):1047–51. doi: 10.1002/pbc.21374. [DOI] [PubMed] [Google Scholar]
- 13.Malpert AV, Kimberg C, Luxton J, et al. Emotional distress in parents of long-term survivors of childhood acute lymphoblastic leukemia. Psychooncology. 2015;24(9):1116–23. doi: 10.1002/pon.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okado Y, Tillery R, Sharp KH, Long AM, Phipps S. Effects of time since diagnosis on the association between parent and child distress in families with pediatric cancer. Child Health Care. 2016;45(3):303–22. doi: 10.1080/02739615.2014.996883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okado Y, Long AM, Phipps S. Association between parent and child distress and the moderating effects of life events in families with and without a history of pediatric cancer. J Pediatr Psychol. 2014;39(9):1049–60. doi: 10.1093/jpepsy/jsu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce L, Hocking MC, Schwartz LA, Alderfer MA, Kazak AE, Barakat LP. Caregiver distress and patient health-related quality of life: psychosocial screening during pediatric cancer treatment. Psychooncology. 2016;26(10):1555–61. doi: 10.1002/pon.4171. [DOI] [PubMed] [Google Scholar]
- 17.Eiser C, Morse R. Can parents rate their child’s health-related quality of life? Results of a systematic review. Qual Life Res. 2001;10(4):347–57. doi: 10.1023/A:1012253723272. [DOI] [PubMed] [Google Scholar]
- 18.Kazak AE, Schneider S, Didonato S, Pai AL. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (pat) Acta Oncol. 2015;54(5):574–80. doi: 10.3109/0284186X.2014.995774. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong MI, Birnie-Lefcovitch S, Ungar MT. Pathways between social support, family well-being, quality of parenting, and child resilience: what we know. J Child Fam Stud. 2005;14(2):269–81. doi: 10.1007/s10826-005-5054-4. [DOI] [Google Scholar]
- 20.Cohen S, Wills T. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–57. doi: 10.1037/0033-2909.98.2.310. [DOI] [PubMed] [Google Scholar]
- 21.Yagci-Kupeli B, Akyuz C, Kupeli S, Buyukpamukcu M. Health-related quality of life in pediatric cancer survivors: a multifactorial assessment including parental factors. J Pediatr Hematol Oncol. 2012;34(3):194–9. doi: 10.1097/MPH.0b013e3182467f5f. [DOI] [PubMed] [Google Scholar]
- 22.Barakat LP, Li Y, Hobbie WL, et al. Health-related quality of life of adolescent and young adult survivors of childhood brain tumors. Psychooncology. 2015;24(7):804–11. doi: 10.1002/pon.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Limbers CA, Larson M. A systematic review of psychometric properties of the Pediatric Quality of Life Inventory 4.0 generic core scales: in pediatric cancer patients and survivors. Expert Review of Quality of Life in Cancer Care. 2016;(10):145–52. doi: 10.1080/23809000.2016.1163223. [DOI] [Google Scholar]
- 25.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. doi: 10.1017/S0033291700048017. [DOI] [PubMed] [Google Scholar]
- 26.Pai AL, Patino-Fernandez AM, McSherry M, et al. The Psychosocial Assessment Tool (pat2.0): psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. J Pediatr Psychol. 2008;33(1):50–62. doi: 10.1093/jpepsy/jsm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alderfer MA, Mougianis I, Barakat LP, et al. Family psychosocial risk, distress, and service utilization in pediatric cancer: predictive validity of the Psychosocial Assessment Tool. Cancer. 2009;115(18 suppl):4339–49. doi: 10.1002/cncr.24587. [DOI] [PubMed] [Google Scholar]
- 28.Kazak AE, Hocking MC, Ittenbach RF, et al. A revision of the intensity of treatment rating scale: classifying the intensity of pediatric cancer treatment. Pediatr Blood Cancer. 2012;59(1):96–9. doi: 10.1002/pbc.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham J. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 30.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–92. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 31.Lund LW, Schmiegelow K, Rechnitzer C, Johansen C. A systematic review of studies on psychosocial late effects of childhood cancer: structures of society and methodological pitfalls may challenge the conclusions. Pediatr Blood Cancer. 2011;56(4):532–43. doi: 10.1002/pbc.22883. [DOI] [PubMed] [Google Scholar]
- 32.Kearney JA, Salley CG, Muriel AC. Standards of psychosocial care for parents of children with cancer. Pediatr Blood Cancer. 2015;62(suppl 5):S632–83. doi: 10.1002/pbc.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulte F, Wurz A, Reynolds K, Strother D, Dewey D. Quality of life in survivors of pediatric cancer and their siblings: the consensus between parent-proxy and self-reports. Pediatr Blood Cancer. 2016;63(4):677–83. doi: 10.1002/pbc.25868. [DOI] [PubMed] [Google Scholar]
- 34.Barakat LP, Marmer PL, Schwartz LA. Quality of life of adolescents with cancer: family risks and resources. Health Qual Life Outcomes. 2010;8:63. doi: 10.1186/1477-7525-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penn A, Lowis SP, Hunt LP, et al. Health related quality of life in the first year after diagnosis in children with brain tumours compared with matched healthy controls; a prospective longitudinal study. Eur J Cancer. 2008;44(9):1243–52. doi: 10.1016/j.ejca.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25(24):3649–56. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 37.Momani TG, Hathaway DK, Mandrell BN. Factors affecting health-related quality of life in children undergoing curative treatment for cancer: a review of the literature. J Pediatr Oncol Nurs. 2016;33(3):228–40. doi: 10.1177/1043454215609585. [DOI] [PubMed] [Google Scholar]
- 38.Macartney G, Harrison MB, VanDenKerkhof E, Stacey D, McCarthy P. Quality of life and symptoms in pediatric brain tumor survivors: a systematic review. J Pediatr Oncol Nurs. 2014;31(2):65–77. doi: 10.1177/1043454213520191. [DOI] [PubMed] [Google Scholar]
- 39.Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, and related Health Links. Version 4. [Available online at: www.survivorshipguidelines.org]