Abstract
Objectives
To examine factors that enhance under-screened and never-screened women’s completion of the self-collection alternative pathway of the Renewed National Cervical Screening Program (ncsp) in Victoria, Australia.
Background
With the Australian ncsp changing, starting on 1 December 2017, the Medical Services Advisory Committee (msac) recommended implementing human papillomavirus (hpv) testing using a self-collected sample for under-screened and never-screened populations. In response, a multi-agency group implemented an hpv self-collection pilot project to trial self-collection screening pathways for eligible women.
Methods
Quantitative data were collected on participation rates and compliance rates with follow-up procedures across three primary health care settings. Forty women who self-collected were interviewed in a semi-structured format, and seven agency staff completed in-depth interviews. Qualitative data were used to identify and understand clinical and personal enablers that assisted women to complete self-collection cervical screening pathways successfully.
Results
Eighty-five per cent (10 women) of participants who tested positive for hpv successfully received their results and completed follow-up procedures as required. Two remaining participants also received hpv-positive results. However, agencies were unable to engage them in follow-up services and procedures. The overall participation rate in screening (self-collection or Pap test) was 85.7% (84 women), with 79 women self-collecting. Qualitative data indicated that clear explanations on self-collection, development of trusting, empathetic relationships with health professionals, and recognition of participants’ past experiences were critical to the successful completion of the self-collection pathway. When asked about possible inhibitors to screening and to following up on results and appointments, women cited poor physical and mental health, as well as financial and other structural barriers.
Conclusion
A well-implemented process, led by trusted, knowledgeable, and engaged health care professionals who can provide appropriate support and information, can assist under-screened and never-screened women to complete the hpv self-collection pathway successfully.
Keywords: hpv self-collection, cervical screening, indigenous, cultural and linguistically diverse, women
INTRODUCTION
Since its inception in 1991, the National Cervical Screening Program (ncsp) has seen a 50% reduction in both incidence of and mortality from cervical cancer in Australia1. In 2014, the Medical Services Advisory Committee (msac) recommended that Australia’s ncsp undergo Renewal to factor in new evidence and technological advances. The key recommendations of Renewal (as the renewed ncsp will be referred to) were as follows: to replace the Pap test with a cervical screening test (cst), which detects hpv2; to increase screening entry age from 18 to 25 years; to extend the routine screening interval from two to five years; and to offer hpv self-collection (also referred to as self-sampling) for under-screened and never-screened women, which would be facilitated by a screening provider also offering mainstream cervical screening1. Primary targets for the Renewal program are women who have been identified as under-screened (seven years or more since last screened) or never-screened (over 30 and have never screened) and who experience a disproportionately higher burden of cervical cancer1.
In the Australian State of Victoria, the site of the pilot study, 50% of the women diagnosed with invasive or micro-invasive cervical cancer in 2011 had no known screening history, and 28% had not been screened for at least 2.5 years prior to detection3. Under-screened and never-screened women tend to experience low-level engagement with health services for cervical cancer screening. They also experience health inequities that are complex and multifaceted, including factors such as social disadvantage, financial hardship, and linguistic and cultural diversities4. The data on Aboriginal and Torres Strait Islander women (hereafter referred to as Aboriginal women) are particularly concerning. Although there are limited data on the cervical screening participation of Aboriginal women1, it is likely that the higher incidence and mortality from cervical cancer that they experience is linked to under-screening and late detection of pre-cancerous lesions and infection with hpv4–7.
An in-clinic, self-collection approach has not yet been used in the ncsp. However, with the transition from Pap testing to cst testing, as part of Renewal, self-collection now represents a clinically validated screening alternative8 for women who decline a cervical screen by a health professional9. A recent Australian clinical study examined the uptake of home-based self-collection via postal delivery of the swab10. The study had participation rates of 11.5% for under-screened women and 20.3% for never-screened women. In an effort to support women to achieve higher participation rates, this pilot was the first study in Australia to examine self-collection participation in a clinical setting and to incorporate the development of all stages of implementation and follow-up procedures. Australia will become only the second country in the world, after the Netherlands, to implement self-collection in-clinic, a change that is predicted to reduce the incidence of, and mortality from, cervical cancer by up to 22%2.
Many studies have examined barriers to cervical screening for Aboriginal women, women with disabilities, those from lower socio-economic status (LSES) and culturally and linguistically diverse backgrounds11. Other studies have explored the uptake of self-collection and its acceptability in a range of countries and contexts, although few in clinical settings12–14. None, however, have focused on the implementation pathway (hereafter the “pathway”) and the determinants of successful completion. This article identifies the strategies used to support under-screened and never-screened women to complete the hpv self-collection pathway, as developed for the pilot study. It also identifies clinical and personal enablers and inhibitors to achieving successful service provision.
Developing a Sustainable Self-Collection Screening Pathway
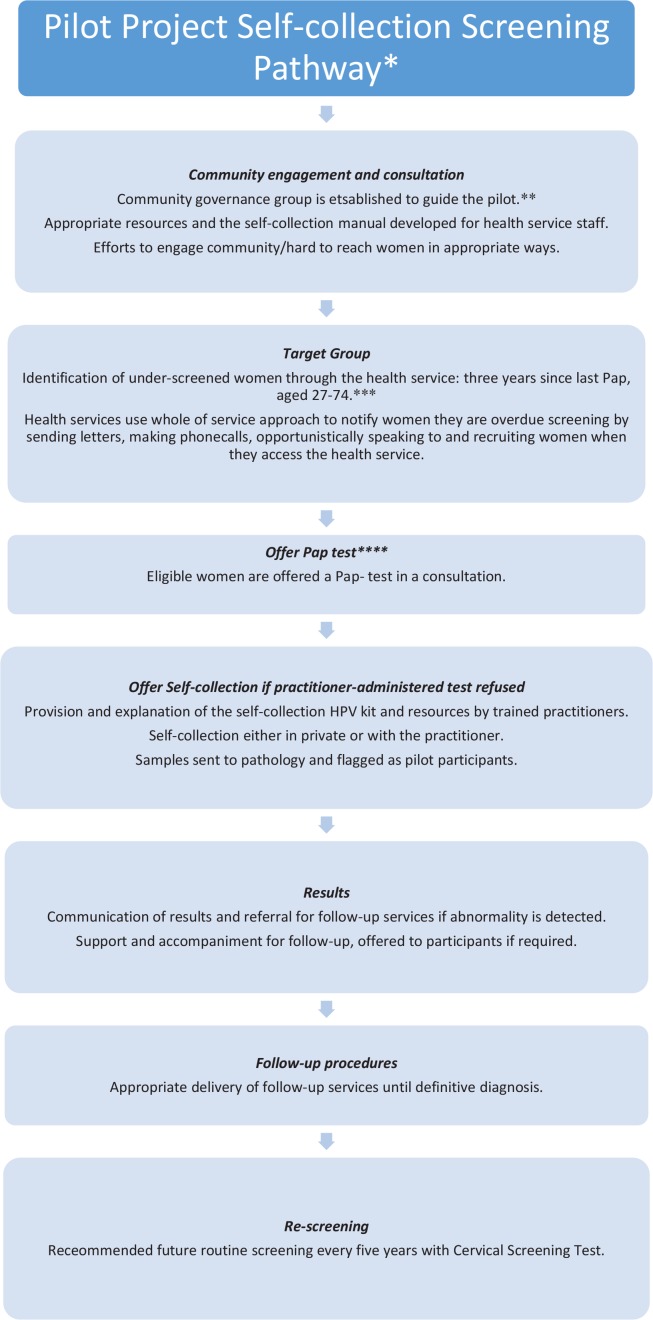
The pathway incorporated all steps—from identifying and recruiting women, to the provision of results and follow-up—and included women aged 27 to 74 years (Figure 1). For the sake of the pilot, women were considered under-screened if they were one year overdue for a Pap test (after the two-year recommended screening for cervical screening). The pilot was multi-institutional and collaborative, involving the community, policy, research, and primary and secondary health organisations that worked together, for the first time, to develop and implement the screening pathway. Data were simultaneously collected to assess the accessibility and effectiveness of the pilot. A steering group, made up of leads from each of these organisations, guided the pilot.
FIGURE 1.
Overview of the self-collection screening pathway. *This pilot was implemented before Renewal when the National Cervical Screening Program recommended Pap tests every two years for women aged 18 to 69 years. **Recommended but not necessarily implemented upon Renewal. This action was specifically taken to oversee the pilot project. ***Under-screened women in Renewal will be defined as aged 30 to 74 years and two years over the routine screening interval of five years. ****Under Renewal, a CST will be offered (a practitioner-administered hpv test). CST = cervical screening test; HPV = human papillomavirus
Phase 1 of the pilot was developed at an Aboriginal Controlled Community Organisation (acco), site 1, which provides a wide range of services to the Aboriginal community. The health arm of this acco—with its holistic approach to health, focus on preventive health care, and strong community engagement—was deemed an appropriate site for the pilot, as it had more than 950 Aboriginal clients. A community reference group was formed to guide the pilot, and included Aboriginal Elders and relevant community members who were able to monitor processes, address any unexpected adverse outcomes, and provide cultural guidance.
Phase 2 of the pilot was delivered at two community health organisations (sites 2 and 3). Site 2 was a large organisation which has more than 850 staff and 44 local services. Site 3 was a community health and crisis service. Both of these sites see high numbers of people living with illness and disabilities, children, Aboriginal people, refugees and asylum seekers, homeless individuals, sex workers, and people with alcohol or other drug dependencies.
The three sites initiated extensive community engagement strategies prior to and throughout the pilot. These included a launch to promote the pilot and consultation with the community in the development of communication resources. During Phase 2, the lead nurse working on the self-collection pilot engaged many community groups and under-screened women for consultation and promotion of the pilot. As part of this community engagement, two advisory groups and 12 women’s group meetings were organized, some using up to three interpreters.
Instructional resources on self-collecting for use by health professionals were developed with feedback from the reference group and the community. To support health professionals in discussing self-collection with women, two participant resources were also provided:
How to Take Your Own hpv Test – low-text diagram explaining the procedure
Explaining the hpv Test – plain English one-page document explaining the benefits and risks of self-collection
Other supports were also put in place to enable women to complete the screening pathway. This included offering accompaniment, transport, and financial support to follow-up procedures. All self-collection materials were translated into five languages and an interpreting service enlisted for use in the pilot.
Eligible women were those aged between 27 and 74 who were identified as under-screened or never-screened, were not pregnant, and had refused a Pap test; this followed the ncsp recommendation that self-collection should be offered only to women who are under-screened and reluctant to undergo a Pap test, as a practitioner-administered cst is preferable if an option. Women were identified using the quality audit data system PenCAT, which enables identification of participants by inserting the limits of age, sex, and year of last cervical screen. Women were then invited to participate in a Pap test via a variety of recruitment methods, including letters, phone calls, and opportunistic engagement in-clinic, that were tailored to the users of each health service. The women were initially offered a Pap test in a consultation and, if they refused this test, were then offered self-collection as an alternative.
At each site, a pilot lead carried out ongoing staff training, community engagement, coordination, and data tracking. To support staff’s existing knowledge of the community, they undertook extensive training on the self-collection pathway so as to more effectively engage with women in the program.
RESEARCH METHODS
Compliance with the Self-Collection Screening Pathway
As the pilot was implemented, Victorian Cytology Service (VCS) collected quantitative data on the number of completed self-collection tests, clinical results and outcomes (Figure 2). Each health service was responsible for monitoring data on the number both of invitations distributed and of self-collection consultations, along with participation in self-collection. This report uses quantitative data to assess participation and compliance with the screening pathway, displayed in Figure 2.
FIGURE 2.
Participation rates, results, and compliance with follow-up procedures. This figure shows the number of women who were invited to participate in self-collection, the results that were received, and the numbers of participants that continued through follow-up procedures (LBC, colposcopy, and repeat self-collection). *Recommended routine screening interval of five years with CST. ** Were recalled; however could not be engaged in follow-up. ***Variation of the screening pathway as women were allowed to repeat self-collection rather than the recommended speculum examination (LBC). CST = cervical screening test; HPV = human papillomavirus; LBC = liquid-based cytology; Not 16/18 = HPV types that are either non-cancer causing, or much less likely to cause cancer; 16/18 = 70% of cervical cancer and pre-cancerous lesions are caused by HPV types 16 or 18; LSIL = low-grade squamous intraepithelial lesion; HSIL = high-grade squamous intraepithelial lesion; Negative = no cancerous cells detected.
Understanding and Explaining Enablers and Inhibitors to the Self-Collection Pathway
All eligible women who agreed to self-collect were invited to participate in the qualitative study by the health provider during the self-collection consultation. Forty participants consented and completed the first interview post self-collection. Seventeen of these women went on to complete a second interview after receiving results and undertaking any follow-up care. The qualitative element of data collection was based on these interviews. Interviews were conducted by a researcher using open-ended questions in a semi-structured format. The women who did not complete the second interview either lost contact or did not complete follow-up interviews due to lack of time, poor health, or other constraints.
The interviews were intended to assess the acceptability of the self-collection hpv test; the acceptability and appropriateness of the service model; satisfaction with and intentions to screen and rescreen; and the positive and negative risk factors around participation. The focus of this paper is on results relevant to the implementation of the service pathway.
All research participants received a $25 gift card in recognition of their contribution to the pilot.
In-depth interviews conducted with seven health professionals focussed on the acceptability of the self-collection pathway; appropriateness of the resources and training provided; process issues particular to different health service settings; and suggestions for implementation of self-collection under Renewal.
Ethical Approval
This study received approval from the Bellberry Human Research Ethics Committee (EC00419) and was accepted by all participating parties.
RESULTS FROM THE QUANTITATIVE STUDY
As can be observed in Figure 2 and Table I, 98 under-screened women aged 27 to 74 years were invited to participate in the pilot within a clinical setting. Of the 98, 79 (80.6%) women completed a self-collection test. A further five women agreed to complete a Pap test, bringing the overall screening participation rate to 84 (85.7%). Although there was slight variation across sites, all achieved a participation rate above 84%.
TABLE I.
Overall participation rates
| Outcomes | Site 1 | Site 2 | Site 3 | Total |
|---|---|---|---|---|
| Self-collection consultations | 33 | 51 | 14 | 98 |
| Chose Pap test | 4 | 1 | 0 | 5 |
| Declined screening | 5 | 7 | 2 | 14 |
| Agreed to self-collection/Self-collection participation rate | 24 | 43 | 12 | 79 |
| Screening participation rate from women offered self-collection in a clinical setting | 28/33 | 44/51 | 12/14 | 84/98 |
Figure 2 demonstrates that, of the 14 women who had hpv detected, 12 (85.7%) had returned to their practitioner for follow-up by the time the study was completed, with 10 (71.4%) having completed either liquid-based cytology (lbc)9 or a colposcopy1.
Compliance with the screening pathway within 90 days of self-collection was 87.5%, and 91.6% within 180 days.
Of the 10 women who had hpv detected (not type 16/18), seven (70%) had follow-up cytology taken by their health practitioner. Two refused follow-up LBC, although one did agree to complete another self-collection hpv test in a further 12 months. There was one woman in this group who had not returned for follow-up testing.
Of the four women who had hpv detected (type 16/18), three had returned and had follow-up LBC but only one had completed a colposcopy at the time of writing.
Overall, there were two women (14.3%) who had hpv detected (any type) and who had not returned to their practitioner for follow-up by the time this article was written, and four (28.6%) who had not completed follow-up LBC and/or colposcopy.
Invalid Results
There were two invalid results from pilot participants, giving an invalid rate of 2.5%. In both of the invalid results, no cell content was detected. This suggests the women returned the self-collection swabs without attempting to complete the test, as only a very small sample of cells are required for detection. When contacted, these women failed to return to their health services to receive their results or to complete follow-up examinations.
Qualitative Results
Interviews were audio recorded by a researcher, with the exception of a small number of women who did not consent to recording. Qualitative data were transcribed by the researcher, then coded and grouped into themes using thematic content analysis techniques. This was repeated multiple times to ensure the coding was reflective of the data. These identified themes were then ranked in hierarchy per most common use. This follows a grounded theory approach qualitative research technique.
RESULTS FROM THE QUALITATIVE STUDY (40 PARTICIPANTS)
Participant demographics are given in Table II. Table III lists the themes relevant to the screening pathway and the percentage of women in the qualitative study that referred to these themes.
TABLE II.
Demographics of participants from the qualitative study
| Participant characteristics |
Site 1 n=12 (30%) |
Site 2 n=20 (50%) |
Site 3 n=8 (20%) |
Total n=40 |
||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| n | % | n | % | n | % | n | % | |
| Identifies as Aboriginala | 12 | 100.0 | 1 | 5.0 | 1 | 12.5 | 14 | 35.0 |
| Born in country other than Australia | 0 | 0.0 | 6 | 30.0 | 0 | 0.0 | 6 | 15.0 |
| Language other than English spoken at home | 2 | 17.0 | 5 | 25.0 | 1 | 12.5 | 8 | 20.0 |
| Responsible for children at home | 6 | 50.0 | 3 | 15.0 | 2 | 25.0 | 11 | 27.5 |
| Age | ||||||||
| 27–49 years | 8 | 66.7 | 8 | 40. | 7 | 87.5 | 23 | 57.5 |
| 50–74 years | 4 | 33.3 | 12 | 60. | 1 | 12.5 | 17 | 42.5 |
| Levels of education reached | ||||||||
| Year 10 or below | 2 | 16.7 | 7 | 35 | 6 | 75.0 | 15 | 37.5 |
| VCE or equivalent | 3 | 25.0 | 7 | 35 | 0 | 0 | 10 | 25.0 |
| Certificate | 5 | 41.7 | 1 | 5 | 1 | 12.5 | 7 | 17.5 |
| Diploma | 2 | 16.7 | 0 | 0 | 0 | 0.0 | 2 | 5.0 |
| Undergraduate degree | 0 | 0.0 | 4 | 20 | 0 | 0.0 | 4 | 10 |
| Postgraduate degree | 0 | 0.0 | 1 | 5 | 1 | 12.5 | 2 | 5.0 |
| Main source of income | ||||||||
| Centrelink (social welfare) | 6 | 50.0 | 17 | 85.0 | 8 | 100.0 | 31 | 77.5 |
| Centrelink and Home Duties | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 2.5 |
| Centrelink (student allowance) | 1 | 8.3 | 0 | 0.0 | 0 | 0.0 | 1 | 2.5 |
| Employed full-time | 3 | 25.0 | 1 | 5 | 0 | 0.0 | 4 | 10.0 |
| Employed part-time | 1 | 8.3 | 1 | 5 | 0 | 0.0 | 2 | 5.0 |
| Home Duties | 0 | 0.0 | 1 | 5 | 0 | 0.0 | 1 | 2.5 |
All women involved identified as Aboriginal, none as Torres Strait Islander
VCE = final year of secondary education.
TABLE III.
Qualitative themes
| Themes | Percentage |
|---|---|
| Women were experiencing multiple barriers to completing the pathway | |
| Low priority afforded to screening | 35 |
| Barriers due to health issues | 27.5 |
| Barriers due to mental health issues | 25 |
| Flexible pathway was required | |
| Would not complete follow-up speculum examination if required | 27 |
| Expressed a wish to take the test home | 10 |
| Expressed concerns regarding completing a speculum examination | 7.5 |
| Importance of information and support | |
| A well-informed and well-supported pathway | 35 |
| Fear of results | 22.5 |
| Concern over whether one could complete the test correctly | 22.5 |
| Experienced stress over the results received | 7.5 |
| Relationship with health care provider | |
| Importance of trust or rapport with health care professional | 47.5 |
Themes
Inhibitors to the Self-Collection Screening Pathway
Low Priority Afforded to Screening:
Previous research indicates that women of socio-economic disadvantage (low SES) groups, who belong to minority cultural groups, and who have disabilities are less likely to screen and therefore are at higher risk of cervical cancer15,16. Many of the women in the pilot had more immediate and pressing issues that they prioritized over cervical screening and accessing health care. As Table II shows, 27.5% of participants were responsible for children at home, and a majority were reliant on social welfare. Thirty-five per cent of women expressed that screening was not a priority for them. Reasons for this included a lack of time, financial barriers, prioritizing their families’ health, misuse of drugs and alcohol, and unstable housing. One woman had recently been homeless, and several described needing to put their families before themselves.
I’ve been homeless, so showering and going to the toilet was hard. I go to Anglicare for breakfast, Breezeway for lunch and soup van for tea if I had petrol [and] I could get there.
Fear of Results:
Many women also avoided screening due to a fear of results. This was driven by a number of scenarios: a fear of learning that their lives were threatened, having to leave children at home and in the care of others, and, predominantly for Aboriginal women, an anxiety surrounding treatment and the health system generally. For some of the women, positive results and treatment procedures would create major disruptions in their already chaotic lives.
I hate that hospital, I’m telling you, they are murderers as far as I’m concerned… I’ll go in for the day, I’ll go in for x-rays and all that, but you will not take me in, if I go in I might not come out.
Experiencing Stress over the Results Received:
There were three cases in which women experienced severe stress and anxiety over the results they had received. One woman was incorrectly informed by her practitioner that, although she was negative, she would still require a regular Pap test immediately. Others claimed their results had not been adequately explained.
She made me feel really uncomfortable. She didn’t explain it and she was really pushy. And when the results came back she didn’t explain.
Experiencing Barriers to Screening Due to Physical and Mental Health:
A high number of women were experiencing physical health issues, mental health issues, or both. Pressing health problems or impaired mental health inhibited participants’ ability to make rational decisions about their own health care. Consequently, screening was either not considered or not considered sufficiently important even when they were accessing a health service.
Yes, especially this time of year, I suffer depression, extreme winter blues. I’ve just gone back on anti-depressants for it, I try not to be on them, but sometimes I just know I go to black spaces and then I will hide inside, I won’t go out at all. I’m waiting on two knee replacements which makes it hard to go out and do any activity at all. I can walk around for maybe half a day and then for the next two days I can’t walk. And I self-medicate.
Enablers to the Self-Collection Screening Pathway
The Importance of Trust and the Relationship with a Health Care Provider:
For some women, their past experiences had involved one or a series of events that had caused them hurt and caused them to distrust others, whether it was sexual abuse, experiencing discrimination, or prejudice. Many of the women were victims of sexual abuse, and several had experienced traumatic previous Pap tests. A practitioner-administered Pap test triggered trauma for these women. For many of the women, the relationship with their health practitioner was revealed to be of high importance in their willingness to undergo screening and in seeking health advice more generally.
Forty-eight per cent of women reported on issues of trust when discussing barriers or enablers to cervical screening. It is quite possible that some of these women would not have followed through with self-collection had they not trusted the practitioner who had offered it to them. Several told stories of how their health provider had helped and supported them in the face of adversity.
Just what doctors are available, I’m very fussy with my doctors, because I’ve had bad experiences, therefore I’m very fussy. And I’ll only see my GP, and if he’s away I’ll only see one other person and if they’re away, I won’t see anyone. Basically, with my shoulder area, it was due to the hospital making mistakes. Therefore everyone worried about what I was doing legally rather than myself. One of the doctors called me a drug addict, pretty much to my face and told me I’d used needles before, which I never had, except for tests. And that was a doctor here. Yeah, pretty much all of that. And the only one that really supported me from the beginning until the end was [my doctor].
The Importance of Explanation on Self-Collection and Cervical Screening:
The majority of women were aware of the reasons for cervical screening. However, for many, this was the first time that a health practitioner had sat down to talk with them about cervical cancer or screening for many years. Thirty-five per cent of women spoke about the importance of being well informed and supported, and many commented that they had either not been given information about cervical screening before the pilot or had received information a very long time ago so had difficulty remembering it.
I don’t think I ever have until I come here and [the nurse] explained it to me. Well it was a group situation and I went to [the nurse] later on and asked her if she’d talk to me about it, because I didn’t want to say in front of everyone I didn’t know what a cervix is, because I’d feel stupid. But seriously, I don’t know much about the female body because I was in drug abuse from the time I was 13.
It is clear that a vague awareness of the dangers of cervical screening, and limited knowledge of the importance of early detection and treatment can be remedied by health practitioners engaging with women about these matters during meaningful discussion. While a desire to be informed was a theme, for many women, information about “the facts” was not a defining factor for why they accessed cervical screening. Instead, their hesitations with screening were due to concerns such as trauma and past experiences, which required much more multifaceted approaches to overcome.
Resources and Supports to Address Concern over Self-Collecting Correctly:
Participants’ major concern with self-collection was whether they would perform the test correctly. It was, therefore, important that the resources regarding the procedure were accessible, clear, and practical. Results demonstrate that the resources, in particular the diagram, were comprehensible, with most women finding the instructions easy to understand and self-collection easy to complete.
It was really good, got [the] pamphlet first and [the nurse] explained it all. Plus I went to the session and that was awesome. Everything was explained right down to if you didn’t get a clear reading you’d need a normal Pap. It was all well explained and discreet.
Flexible Clinical Approaches to Implementing the Self-Collection Pathway
The Importance of Appropriate Recruitment Methods
Staff interviews at two sites revealed that letters were an ineffective way to recruit women, whereas opportunistic recruitment, when women attended the clinic for another reason, was more effective and successful. This was due to many women being transient, which meant they did not have a registered address or were thought unlikely to respond to a letter.
Some of the women had only occasionally been offered a Pap test in the past, with more than 25% of the women reporting that they had been offered a test either ‘only once or twice’ or ‘never’ in their lives. One woman described only being offered a Pap test once in the last 15 years, and another woman said her doctor had never offered her a test in 12 to 15 years.
Apart from this one? Many, many years ago. Oh geez, no I can’t remember, no not that far back.
At times other issues were allocated a higher priority in consultations, whether by the practitioner or the women themselves. Furthermore, a health service experiencing a high volume of clients was considered by staff to affect the length of appointments and the chance of health professionals offering women self-collection.
Bringing self-collection to the regular attention of the women was important in increasing the likelihood of them presenting for screening. Often the health provider had previously identified under-screened clients and spoken about the possibility of self-collection with the women in the upcoming weeks. Some of the women needed time to consider the procedure prior to completion. One of the women who originally declined self-collection, later returned to complete it when she was feeling better. One woman described her uptake after she initially heard about self-collection at a community health meeting:
Yes, that’s when I first heard about it, and the next time I saw [my doctor] was just on a regular visit, because I needed Warfarin scripts and then she brought it up and we talked about it then.
Concerns with Follow-Up Procedures: Completing a Speculum Examination
Seventy-one percent of the women who had hpv types detected completed the required speculum examination. Twenty-seven per cent of the women who participated in the follow-up interview also reported that they would not complete a speculum examination in the future, which is the policy recommendation for routine screening. This is unsurprising, as many of the women were under-screened due to a range of common barriers to completing a practitioner-administered Pap test, such as embarrassment, pain, fear, or past trauma. Two women in the pilot who experienced very high stress levels were given permission by their provider to complete self-collection again rather than the recommended speculum examination.
I would do this again [self-collect]—but if I needed to do a Pap test with someone else involved I probably wouldn’t do it. I’d just surrender to my fate.
There are no available data on participation rates for women returning to complete a speculum examination at regular screening intervals following this test.
Priority Pathway to Specialist Gyne-Oncology Follow-Up
Staff found that it was important that women who self-collected could access colposcopy quickly and easily so that they could avoid long wait times and were not lost to follow-up. As these women were under-screened and, therefore, high-risk, there was more urgency for colposcopy and treatment. With waiting lists up to six months for a public colposcopy in Victoria, the reduced waiting time was believed to be vital to keeping women on the cervical screening pathway.
Difficulties? Yes unless there is some prioritized pathway, in particular given that, certainly the target groups for this pilot were further afield and isolated populations, so the other possibility is to have a specific female gyne-oncologist that goes out to these centres to do the colposcopies. So yes, there are a lot of question marks about all that.
DISCUSSION
Acceptability
Participation rates of over 80% demonstrated the acceptability of self-collection with women who were under-screened and otherwise reluctant to screen. However, to enable women to overcome barriers, prioritize screening, and complete the whole self-collection pathway, adequate and timely support was essential throughout.
Extensive Efforts to Monitor, Support, and Engage Women to Follow-Up
Additional support was beneficial to ensuring that women completed the entire pathway through follow-up. Many health service staff invested extra time and effort to engage under-screened women, to recall them to explain results and to track and follow up with the women if they required follow-up procedures. At two of the sites, participants were offered support in the form of transport or accompaniment, if needed, to attend follow-up appointments. Extra efforts included coordinating both with women’s admission dates into emergency accommodation and with case-workers and support plans.
As health service staff are often working at capacity, this poses a challenge to the adoption of an optimal pathway for offering self-collection to under-screened women. However, many health services that see high numbers of women likely to be under-screened (Aboriginal women, culturally and linguistically diverse (CALD) women, refugee and migrant populations) have in place supports and incentives to encourage women to access health checks and screening. This is currently dependent on the individual service. Practice change may be required for a service to adopt an optimal pathway that includes a workflow arrangement with sufficient time to identify under-screened women, longer consultations with women to sensitively discuss and offer self-collection, and enabling environments and support for follow-up care. A lead or champion in the health service for Renewal could be a model to achieve this. There is work underway in Australia to engage and support women experiencing vulnerabilities to screen.
Flexible Approaches
Flexible approaches were crucial at every stage of implementation, to enable participants to complete the screening pathway. Opportunistic testing was more time-efficient and involved less preparation than booking a specific appointment. For women who could not afford to prioritize cervical screening, it was far more likely that they would complete cervical screening if they were offered it opportunistically during an appointment. It was also vital that women be offered screening consistently and at appropriate times, as those experiencing multiple health barriers may need longer appointments to enable appropriate examinations. However, the current system in Australia encourages shorter appointment times, in particular for those who cannot afford to pay higher fees for longer appointments. The current health care system covers only 10 to 15 minutes per general practice appointment, meaning cervical screening is less likely to be done in a busy clinic. For women who have to be encouraged to engage with preventive health, a longer consultation that allows time for multiple procedures and tests would be more efficient and effective than trying to arrange multiple shorter appointments.
For women who find it difficult to attend appointments, there may be substantial advantage to being able to self-collect at home. This could also be the case for women who are experiencing extreme trauma or anxiety and do not wish to self-collect in a public setting. Similar to an Argentinian study17, it was recommended by one staff member that, in future, community/social workers be able to administer the test during home visits to women who do not otherwise access health services. As this was not in the scope of the pilot, further research is recommended to explore the acceptability, safety, and quality implications of this.
Further research could also provide insight into the cost and capacity requirements for maintaining the engagement of under-screened women to receive results and follow-up and would be beneficial for engaging hard-to- reach women in other screening and health care generally.
Completing the Pathway
Renewal is expected to reduce numbers of women referred to colposcopy in the long term, as the transition is made to five-year testing rather than two. In the short term, however, the already overburdened system will require a plan to enable under-screened women who self-collect to access colposcopy quickly and easily, particularly in rural and regional areas.
During the pilot, the prioritized pathway to follow-up assessment was highly supported by staff. However, it is not clear how this system could be replicated once Renewal is in place. While outside the scope of the pilot project, timely and supported access to follow-up assessment and treatment services is a critical enabler to an optimal screening pathway. Recommendations are that consultations be held with each of the public health providers of colposcopy; an urgent referral system be set up to prioritize colposcopies arising from self-collection (over regular referrals); a specialized gyne-oncologist position be created for women who self-collect and have hpv detected (16/18); and/or specialist gyne-oncologists be provided who are able to offer outreach services.
Loss to Follow-Up
Despite all the effort and support, three women in the pilot were still lost to follow-up. This signifies that even a sound, well-supported, alternative screening pathway cannot overcome all barriers for all women. The risk of invalid results poses a reminder to health providers that some women may agree to self-collection but then decide not to complete it, or not complete the test correctly.
Importance of the Relationship with the Practitioner
Cervical screening is a test that can, and has, invoked feelings of distrust, anxiety, and vulnerabilities for women18. The familiarity, trust, and support women had with their health practitioner was revealed as significant to women engaging in the pilot and obtaining follow-up care where needed (which involved a speculum examination). Simultaneously, women’s participation in this pilot cannot be viewed outside of the context of the established trusting relationships many of them had with their health professional, as this may have influenced their participation rates. It is likely that this will impact the number of women willing to self-collect once Renewal is in place, especially those already reluctant to screen.
One of the nurses suggested that self-collection was a good way to open a conversation on cervical screening and, in so doing, broach a topic that may be very sensitive for many women. Research on cervical screening for women with a history of sexual abuse also found that women wanted their practitioner to know their background, yet many would not bring it up themselves and the practitioners often failed to ask relevant questions18,19.
As many of the women had experienced trauma from previous screening experiences, they were particularly vulnerable when hearing results. It must be recognized that learning one has a potentially fatal condition puts anyone under extreme duress, and even more so when the person’s capacity to absorb information is limited. Notwithstanding this extreme issue, the pilot demonstrated the importance of a supportive, well-trained, and well-informed workforce that had the time to sit down and explain results and options sympathetically.
Overcoming Limitations
It is inevitable, upon Renewal, that not all under-screened women will have established relationships with their health provider. As such, cultural appropriateness, the sensitive treatment of women, and appreciation and empathy for their past experiences of cervical screening or sexual abuse are vital. This advice has been noted in self-collection resources. Recommendations are longer appointment times so that practitioners have time to speak to women about the screening and build a rapport with their patient, compulsory training on cervical screening and cultural competency for all staff, and raising awareness of the effects of health providers’ attitudes and relationships. Follow-up research into factors influencing women’s uptake of self-collection once Renewal is in place is also necessary.
Explanation, Information, and Training
The information provided to the women on screening and self-collection was accessible and well-explained. Practitioners, before offering the test, were instructed to sit down with women to explain the self-collection process, the possible results, and any follow-up procedures. However, despite training being provided to all practitioners involved in the pilot, the lead at each site was regularly required to remind practitioners of the correct process and pathway, especially at the larger sites. It is hoped that the need for reminders would become less acute as processes became common practice. All cervical screening providers have been given extensive information kits and resources on self-collection, complete with advice on women who are under-screened and vulnerable.
The acco also provided a culturally safe environment, with many Aboriginal staff and input from the local Aboriginal community. However, all staff at all sites were provided with cultural training, which was likely a factor in Aboriginal women’s willingness to screen. Studies echo the importance of cultural safety in health care and support20,21 and it is a recommendation from this study.
There is scope for evaluation research on self- collection as it is rolled out to explore ongoing strengths and limitations to implementation.
CONCLUSION
This article has focused on the process and implementation of an hpv self-collection pathway, which has been shown to facilitate an increase in hpv cervical screening rates for under-screened women in this study. A coordinated, well-implemented and flexible pathway enabled more than 85% of participants to complete the self-collection pathway through follow-up procedures. Dedicated and trusted health practitioners, who provided clear explanations of all processes, were vital to engaging women in the pilot and to maintaining their participation. In order that the results of this study be replicable in full-scale implementation, it is vital that evaluation and research be continued. Successful implementation is dependent on under-screened women who experience barriers being supported by trusting health providers, accessible pathways, financially viable treatment, and support to attend follow-up appointments and procedures.
ACKNOWLEDGMENTS
The authors wish to thank all the participants in the study, all health service staff involved in the project, Bernadette Suter and Paul Bourke, the Victorian Government, Department of Health and Human Services (DHHS), which provided funding to complete the study and organizational support throughout the pilot, Rachael Anderson, Bev Lewis, Nikki McGrath, and Ruvimbo Bako, (DHHS), and the reviewers and editor, Jane Yule.
CONFLICT OF INTERESTS DISCLOSURE
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare that we have none
REFERENCES
- 1.Whop LJ, Cunningham J, Condon JR. How well is the National Cervical Screening Program performing for Indigenous Australian women? Why we don’t really know, and what we can and should do about it. Eur J Cancer Care. 2014;23:716–20. doi: 10.1111/ecc.12244. [DOI] [PubMed] [Google Scholar]
- 2.NPS . NPS MedicineWise. Special ed. NPS Radar; 2015. [Available at: https://www.nps.org.au/radar/articles/changes-to-the-national-cervical-screening-program]. [Google Scholar]
- 3.Mullins R, Scalzo K, Sultana F. Self-sampling for cervical screening: could it overcome some of the barriers to the Pap test? J Med Screen. 2014;21:201–6. doi: 10.1177/0969141314555247. [DOI] [PubMed] [Google Scholar]
- 4.dos Santos Silva I, Beral V. Socioeconomic differences in reproductive behaviour. IARC Sci Publ. 1997:285–308. [PubMed] [Google Scholar]
- 5.Roder D. Comparative cancer incidence, mortality and survival in Indigenous and non-Indigenous residents of South Australia and the Northern Territory. Cancer Forum. 2005;29:7–9. [Google Scholar]
- 6.Cunningham J, Rumbold AR, Zhang X, Condon JR. Incidence, aetiology, and outcomes of cancer in Indigenous peoples in Australia. Lancet Oncol. 2008;9:585–95. doi: 10.1016/S1470-2045(08)70150-5. [DOI] [PubMed] [Google Scholar]
- 7.Garland SM, Brotherton JM, Condon JR, et al. Human papillomavirus prevalence among indigenous and non-indigenous Australian women prior to a national hpv vaccination program. BMC Med. 2011;9:104. doi: 10.1186/1741-7015-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–57. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172–83. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 10.Sultana F, English DR, Simpson JA, et al. Home-based hpv self-sampling improves participation by never-screened and under-screened women: results from a large randomized trial (iPap) in Australia. Int J Cancer. 2016;139:281–90. doi: 10.1002/ijc.30031. [DOI] [PubMed] [Google Scholar]
- 11.Day S, van Dort P, Tay Teo K. Identifying hard to reach groups: review of faactors (including barriers) associated with cancer screening. Carlton: Victorian Cytology Service; 2010. [Google Scholar]
- 12.Bais AG, van Kemenade FJ, Berkhof J, et al. Human papillomavirus testing on self-sampled cervicovaginal brushes: an effective alternative to protect nonresponders in cervical screening programs. Int J Cancer. 2007;120:1505–10. doi: 10.1002/ijc.22484. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen A, Nieminen P, Niironen M, Luostarinen T, Anttila A. Self-sampling experiences among non-attendees to cervical screening. Gynecol Oncol. 2014;135:487–94. doi: 10.1016/j.ygyno.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Mao C, Kulasingam SL, Whitham HK, Hawes SE, Lin J, Kiviat NB. Clinician and patient acceptability of self-collected human papillomavirus testing for cervical cancer screening. J Womens Health (Larchmt) 2017;26:609–15. doi: 10.1089/jwh.2016.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan L, McIlfatrick S. Exploring women’s knowledge, experiences and perceptions of cervical cancer screening in an area of social deprivation. Eur J Cancer Care (Engl) 2011;20:720–7. doi: 10.1111/j.1365-2354.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 16.Peters K. Politics and patriarchy: barriers to health screening for socially disadvantaged women. Contemp Nurse. 2012;42:190–7. doi: 10.5172/conu.2012.42.2.190. [DOI] [PubMed] [Google Scholar]
- 17.Arrossi S, Paolino M, Thouyaret L, Laudi R, Campanera A. Evaluation of scaling-up of hpv self-collection offered by community health workers at home visits to increase screening among socially vulnerable under-screened women in Jujuy Province, Argentina. Impl Sci. 2017;12:17. doi: 10.1186/s13012-017-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson K. Barriers to cervical screening experienced by victim/survivors of sexual assault: pilot study / Karen Carlson. Carlton, Vic: CASA House; 2002. [Google Scholar]
- 19.Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plan Reprod Health. 2012;38:214–20. doi: 10.1136/jfprhc-2012-100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelaher MA, Ferdinand AS, Paradies Y. Experiencing racism in health care: the mental health impacts for Victorian Aboriginal communities. Med J Aust. 2014;201:44–7. doi: 10.5694/mja13.10503. [DOI] [PubMed] [Google Scholar]
- 21.Shahid S, Finn LD, Thompson SC. Barriers to participation of Aboriginal people in cancer care: communication in the hospital setting. Med J Aust. 2009;190:574–9. doi: 10.5694/j.1326-5377.2009.tb02569.x. [DOI] [PubMed] [Google Scholar]