Abstract
Background
Commencing 1 December 2017, Australia introduced human papillomavirus (hpv)-based cervical screening. As part of this Australian renewed National Cervical Screening Program (ncsp) women who are either never- or under-screened and who refuse a practitioner collected sample will be able to collect their own sample for cervical screening. The aim of this study is to examine the quantitative results of a pilot study into the acceptability of the self-collection alternative pathway.
Methods
Eligible participants were offered the opportunity to collect their own sample. Those who agreed were given a flocked swab and an instruction sheet and took their own sample in an area of the health care clinic that afforded them adequate privacy. These samples were then given to clinic staff who returned them to Victorian Cytology Service (vcs) Pathology for hpv nucleic acid testing.
Results
Of 98 eligible women, seventy-nine undertook self-collection for hpv-based cervical screening. Seventy-seven produced valid results, 14 were positive for oncogenic hpv, with 10 undertaking follow-up. Three women were found to have cervical squamous abnormalities with two of those being high-grade intraepithelial squamous lesions.
Conclusion
The pilot study for self-collection for cervical screening produced quantitative data that were similar to that already reported in the literature, but had a much higher rate of acceptance compared with self-collection programs based in the home.
Keywords: Human papillomavirus (hpv), self-collection, cervical cancer screening, diagnostic testing
INTRODUCTION
The renewed Australian National Cervical Screening Program (ncsp) began on 1 December 2017. The new program is based on the 2014 recommendations of the the Medical Services Advisory Committee (msac) which looked to include new advances in evidence and technology to improve cervical screening. The key recommendation was that the Pap test taken at two yearly intervals be replaced by a cervical screening test (cst) which would utilize a human papillomavirus (hpv) test as the primary screening test followed by reflex cytology of hpv-positive specimens1. Additional changes include the increase in screening entry age from 18 to 25 years, screening until the age of 69 with an exit test between 70 and 74 years, and to offer hpv self-collection, in a clinical setting, for under- and never-screened women2.
There is a wealth of evidence demonstrating the increased sensitivity of hpv-based screening compared with cytological screening3–5, and a population–based hpv primary cervical screening program began in the Netherlands in 2017.
One of the additional benefits of an hpv-based program is that the virus can be detected even if the sample is not taken from the cervix directly6. In countries with well-developed cervical screening programs, such as Australia, one of the most significant risk factors for developing cervical cancer is to be either never- or under-screened. In terms of the renewed ncsp, under-screened is defined as being more than two years overdue for screening and aged at least 30 years. In Victoria, over 90% of women diagnosed with cervical cancer were under-screened7.
There is a wide range of reasons why women might be under-screened, including a lack of access to appropriate health care services, a history of sexual assault, female genital mutilation, or a previous adverse experience. However, whilst extensive evidence is lacking2, it is generally acknowledged that certain groups are more likely to be under-screened including Aboriginal and Torres Strait Islander women8, women from culturally and linguistically diverse backgrounds, and economically disadvantaged women9.
A previous Australian study, the iPap trial, targeted women who were unscreened or under-screened, and sought to engage or re-engage them in the cervical screening program by offering them the option of self-collecting their own sample at home using a flocked swab10.
There has been a range of studies looking at hpv prevalence in the Australian population10–12 carried out as part of research-based surveillance programs using research use assays (to facilitate high definition of individual hpv types) rather than diagnostic assays. However, what is lacking are data examining the acceptability of self-sampling in a primary health care setting, and what cervical screening results can be expected from the alternative pathway of the renewed ncsp, which allows women who are under-screened and who refuse a practitioner-collected (speculum examination) cervical sample to self-collect in a clinical setting. This study examined how self-collection, using a cheap and commonly available collection device (a flocked swab) will perform using the pathway design utilized in the renewed ncsp.
METHODS
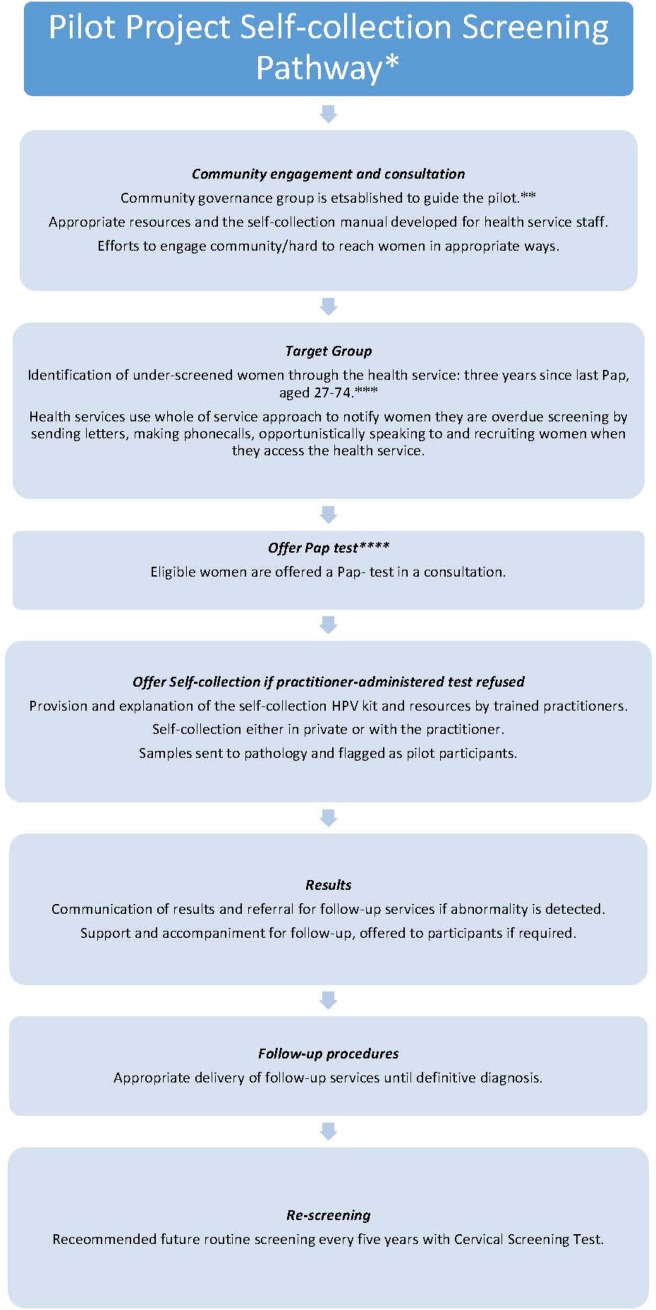
Participants were patients attending one of three participating practices who were overdue for cervical screening and had refused a conventional Pap smear, involving collection of a cellular sample from the cervix by a nurse or doctor. The three practices were chosen to target clinics with enriched populations of women who were more likely to be under-screened, specifically Aboriginal and Torres Strait Islander women, women from culturally and linguistically diverse backgrounds (including refugee women), homeless women, and other groups. Figure 1 demonstrates the Pilot Project Self-collection Screening Pathway. Briefly, all eligible women were offered the opportunity to collect their own sample for hpv testing. Those electing to take up this offer were given a dry flocked swab (FLOQSwab, Copan, Italy) and an instruction sheet and took their own sample in an area of the health care clinic that afforded them adequate privacy, often the clinic toilet. The dry flocked swabs were returned to their plastic sleeve and returned to clinic staff. Samples were then transported to the laboratory at vcs Pathology as part of usual practice.
FIGURE 1.
Outline of the self-collection screening pathway that was used in the pilot study. *This pilot was implemented before Renewal when the National Cervical Screening Program recommended Pap tests every 2 years for women aged 18 to 69 years. **Recommended however not necessarily implemented upon Renewal. This action was specifically taken to oversee the pilot project. ***Under-screened women in Renewal will be defined as aged 30 to 74 years and two years over the routine screening interval of five years. ****Under Renewal, a CST will be offered (a practitioner-administered hpv test). HPV = human papillomavirus; CST = cervical screening test.
As previously described13, when swabs arrived in the laboratory they were removed from their plastic sleeve and resuspended in 4 mL of PreservCyt media (Hologic, USA) in a 13 mL tube (Sarstedt, Germany). This tube was then loaded and run on the Roche cobas 4800 system using the cobas 4800 hpv test (Roche, United States). The Roche cobas 4800 hpv test examines 14 hpv types (referred to as ‘oncogenic’ in the Australian renewed ncsp). Three types of positive results can be produced; hpv16, hpv18, and hpv Other (hpv31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68). A sample without adequate cellular material or with the presence of inhibitory factors, such as blood or lubricant, can produce an ‘invalid’ result.
No further genotyping was undertaken as part of this study, as it was designed as a pilot program for the self-collection alternative pathway of the renewed ncsp rather than as an epidemiological study of hpv prevalence. It is estimated that more than 95% of testing in the renewed ncsp will be undertaken with hpv nucleic acid testing (nat) assays which produce only a hpv16, hpv18, and Other hpv result (e.g., Roche cobas 4800 hpv test, Roche cobas [6800] hpv test, and Abbott realtime High Risk hpv). Women who returned an oncogenic hpv-positive (non 16/18) result were invited to undertake a practitioner-collected sample (Figure 1).
Participating practitioners maintained a count of women offered self-sampling, in order to estimate the acceptability of self-sampling among eligible women.
The funding body, the Victorian Government Department of Health and Human Services, played no role in collection of data, its analysis and interpretation. The funding body did not have to approve publication of the finished manuscript.
RESULTS
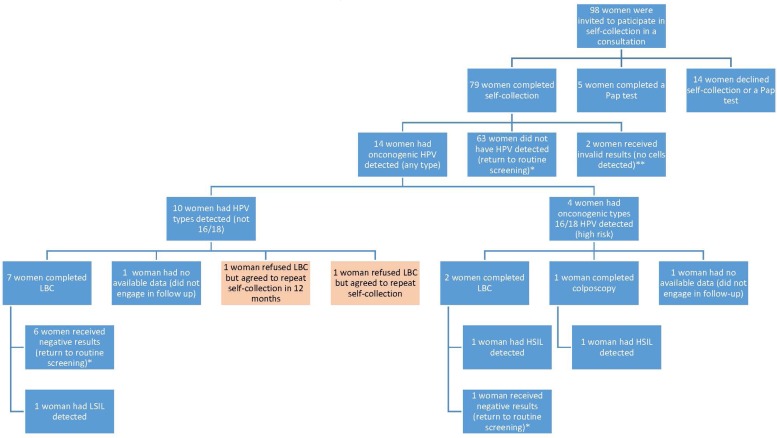
Ninety-eight women were offered the self-collection hpv test. Figure 2 demonstrates the clinical pathway that these women undertook. Seventy-nine women consented to collect their own sample. A further five women agreed to have a conventional Pap test after having originally declined one and later being offered the self-collection test. In total 84 of 98 women (85.7 %) underwent cervical screening as a result of being offered self-collection. Of the 79 women who had hpv tests, 2 (2.5%) of the samples returned invalid results. Of the 77 valid results, 14 (18.2%) were positive for oncogenic hpv and 65 were negative. Of the 14 positive, 4 (5.2%) were positive for hpv16 and 10 (13%) were positive for hpv Other. None were positive for hpv18 and there were no samples with multiple positive results.
FIGURE 2.
Participation rates, results and compliance with follow-up procedures. This figure shows the number of women who were invited to participate in self-collection, the results that were received and the numbers of participants who continued through follow-up procedures (LBC), colposcopy and repeat self-collection. *Recommended routine screening interval of five years with Cervical Screening Test. **Were recalled however could not be engaged in follow-up. ***Variation of the screening pathway as women were allowed to repeat self-collection rather than the recommended speculum examination (LBC). Legend: hpv = Human papillomavirus; LBC = Liquid Based Cytology – requires a speculum examination; Not 16/18 = hpv types that are either non-cancer causing, or much less likely to cause cancer; 16/18 = 70% of cervical cancer and pre-cancerous lesions are caused by hpv types 16 or 18; hsil = High-grade squamous intraepithelial lesion; LSIL = Low-grade squamous intraepithelial lesion; Negative = No cancerous cells detected.
Of the 14 participants with hpv-positive results, ten underwent follow-up, whilst four had not undertaken follow-up at the conclusion of the study. Nine participants had cytological samples taken, of whom seven returned normal results, one had a low-grade squamous intraepithelial lesion (lsil), and one had a high-grade squamous intraepithelial lesion (hsil). Two of these participants were hpv16-positive and had a practitioner-collected sample for cytological assessment after they refused to undergo a colposcopy. One of these patients then agreed to undergo a colposcopy based on the hsil result of this cytology test. One participant who was hpv16-positive underwent a colposcopy and biopsy which identified a histologically-confirmed hsil.
DISCUSSION
The primary aim of this study was to estimate the acceptability of self-sampling among unscreened and under-screened women who refused conventional cervical screening, as measured by the participation rate.
Whilst the number of women in this pilot study is too low for a robust statistical analysis of other measures, the rates of oncogenic hpv positivity, invalid samples, and follow-up abnormal cytology/histology can be compared with the broader screening program.
Acceptability of self-sampling among never- and under-screened women in Victoria
In Victoria, 59.2% of women aged 20 to 69 participated in the cervical screening program in a two-year period, which is the recommended interval for the current ncsp. This rises to 72.6% if participation in the program over a three-year period is examined, and this rate could be viewed pragmatically as the participation rate in the ncsp in Victoria. This means that 27.4% of women are either not participating in the program (never-screened) or are participating at a much longer interval than is recommended (under-screened). This could mean that as many as 200,000 women in Victoria alone would classify as under- or never-screened, and as such may benefit from the ability to undertake the alternative screening pathway involving self-collection. This pilot study demonstrated that offering self-collection in a primary care context could result in a higher acceptance rate (80%, with a further 5% choosing the practitioner-collected test). Previous studies which have utilized self-collection at home have had a ∼20% testing rate. We acknowledge that there are a number of factors which could contribute to this variation in hpv positivity, including the cohort, selection bias (which women return their home-collected samples), and other factors10,14. Additional information on the qualitative aspects of this study can be found in the companion paper by McLachlan and colleagues15.
hp v positivity
The samples collected as part of this study had an oncogenic hpv-positive rate of 18.2% (Figure 2). Recent data from our laboratory as part of the Compass Trial5 demonstrated that practitioner-collected samples from a population screening cohort had an hpv positivity rate of approximately 7%. These differences are likely to be explained by the increasing prevalence of oncogenic hpv as the interval since the last screen (if ever) increases, compared with well-screened women in the usual ncsp. Under-screened women are not at any higher risk of being exposed to hpv infections but they are less likely to have an hpv-positive lesion treated thereby removing the hpv infection, and as such, over time, an increase in hpv positivity is likely to occur.
The iPap study (described above) had an oncogenic hpv-positive rate of 12.4%10. A community-based study in Madagascar found an oncogenic hpv positivity rate of 32% using the cobas 4800 hpv test16. These two studies represent different facets of what the Australian self-collection pathway will encompass: firstly (iPap), it will be for under-screened women, and secondly (Madagascar), it will be clinic-based and likely to be utilized in resource-poor areas such as remote Australian indigenous communities. Taking all of this into account, the positivity rate observed in this study is closer to the iPap data and appears to be reasonable in this context. However, we will not really know how representative these data are until the self-collection pathway has been active in the Australian ncsp for several months.
Invalid results
There were two invalid results from pilot participants, giving an invalid rate of 2.5%. A further three invalid results were received, but were excluded from the pilot data as two were from women outside the pilot age group, and one was from a woman who was not under-screened. As a comparison, in the same laboratory that undertook testing for this study, the invalid rate for practitioner-collected samples used for hpv-based screening is less than 0.2%17.
It should be noted that the invalid rate for hpv screening samples varies widely, from 0.6% from the iPap trial10 to 9.8% from a study involving self-collection in Madagascar16, and down to 0.3% in the clinical validation of the Cepheid Xpert hpv assay18. There is a range of possible reasons for this variation, including differences in sample collection, or whether the women testing had previously refused a practitioner-collected sample10 or had never had access to cervical screening16.
In each of the invalid results, no cell content was detected. It is possible that these women returned the self-collection swabs without attempting to complete the test, which only requires a very small sample of cells (estimated at 80 cells in 400 μL of liquid-based cytology media). When contacted, these women failed to return to their health services to receive their results or complete follow-up examinations. As a comparator, the rate of invalid Pap tests was 2.7% in Victoria in 20147.
The risk of invalid results poses a reminder to health care providers that some women may agree to self-collection but then decide not to complete the test. It also supports the view that laboratories should only use hpv tests that include controls that will identify acellular samples, otherwise these samples would be reported as “hpv not detected,” falsely reassuring the practitioner about the woman’s risk. The Australian Requirements for Laboratories reporting tests for the National Cervical Screening Program19 state that all hpv NAT assays must contain controls for both cellularity and inhibition. The Australian requirements also state that all hpv nat assays used for the testing of self-collected samples must be a pcr-based test based on the evidence outlined in the meta-analysis by Arbyn and colleagues6.
Even if all invalid results are included (including women who were not eligible for the pilot study), there is only a 5% non-acceptance for taking a sample after agreeing to self-collect. To put this into context, it must be remembered that all of the women eligible for this study have refused a practitioner-collected sample and are overdue for cervical screening. However, from a clinical management point of view with the use of hpv tests with cellularity controls, the health practitioner will know that these women remain under-screened and can use that information as part of their approach to clinical care.
Cytology/histology
The most recent report from the Victorian Cervical Cytology Register7 identifies that approximately 6.5% of Pap tests presented as having a squamous abnormality, of which 1.5% were identified as having a high-grade squamous intraepithelial lesions (hsil). In this current study, three women who had an hpv-positive result (two hpv16 and one hpv Other) undertook follow-up which identified a squamous abnormality (3.9%), but two of these three (both hpv16 positive) had hsil (2.6%) (Figure 2). It is difficult to interpret these results due to the very small number of cases. However, what can be stated is that the ability to include women who are under-screened or never-screened in the renewed ncsp will almost certainly identify squamous abnormalities, a subset of which would be hsil, that will require investigation or treatment, but the detection and treatment of these lesions could prevent development of cervical cancer in these women.
Limitations
As this study examined a small number of participants from a select range of clinics as part of a clinical trial, it could be considered reasonable that the people involved were able to dedicate more resources than could be expected within a normal clinical setting. Additionally, no quantitative statistical analysis is able to be carried out due to the low number of (positive) samples. Finally, this study only looked at hpv nucleic acid testing in women who had refused a practitioner-collected cytology test, and it is therefore difficult to make any comparisons with cytology-based programs.
CONCLUSION
In this pilot study for the self-collection pathway of the renewed ncsp, 98 women were offered the self-collection hpv test. Seventy-nine women completed the self-collection test, with a further five women agreeing to having a conventional Pap test after having originally declined one and later being offered the self-collection test. Our data suggest that oncogenic hpv positivity, invalid rates, and squamous abnormality rates for the women who undertook the self-collection pathway were similar to those rates available in the literature. However, a more detailed analysis will have to be undertaken to compare self-collection and practitioner collection once the Renewed ncsp begins.
Ethical Approval
This study received approval from the Bellberry Human Research Ethics Committee (EC00419).
CONFLICTS OF INTEREST
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare the following interests: MS and DH are employees, and SA is a board member, of vcs Ltd, which has received support from Roche Molecular Systems for hpv testing as part of the Compass Trial, a large randomized trial of primary hpv screening. This project received funding from the Victorian Government Department of Health and Human Services.
REFERENCES
- 1.NPS . NPS MedicineWise. Special ed. NPS Radar; 2015. [Google Scholar]
- 2.Whop LJ, Cunningham J, Condon JR. How well is the National Cervical Screening Program performing for Indigenous Australian women? Why we don’t really know, and what we can and should do about it. Eur J Cancer Care. 2014;23:716–20. doi: 10.1111/ecc.12244. [DOI] [PubMed] [Google Scholar]
- 3.Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (hpv) type 16 or 18 and the possible utility of type-specific hpv testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 5.Canfell K, Caruana M, Gebski V, et al. Cervical screening with primary hpv testing or cytology in a population of women in which those aged 33 years or younger had previously been offered hpv vaccination: Results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi: 10.1371/journal.pmed.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15:172–83. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 7.Register vcs . Statistical Report 2014. Melbourne, Australia: 2014. [Google Scholar]
- 8.(AIHW) AIoHaW . Cancer Series. Canberra: AIHW; 2015. Cervical Screening in Australia 2012–2013. [Google Scholar]
- 9.Barbaro B, Brotherton JM, Gertig DM. Human papillomavirus vaccination and cervical cancer screening by socioeconomic status, Victoria. Med J Aust. 2012;196:445. doi: 10.5694/mja11.11360. [DOI] [PubMed] [Google Scholar]
- 10.Sultana F, English DR, Simpson JA, et al. Home-based hpv self-sampling improves participation by never-screened and under-screened women: Results from a large randomized trial (iPap) in Australia. Int J Cancer. 2016;139:281–90. doi: 10.1002/ijc.30031. [DOI] [PubMed] [Google Scholar]
- 11.Osborne SL, Tabrizi SN, Brotherton JM, et al. Assessing genital human papillomavirus genoprevalence in young Australian women following the introduction of a national vaccination program. Vaccine. 2015;33:201–8. doi: 10.1016/j.vaccine.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Brotherton JM, Condon JR, McIntyre PB, et al. Human papillomavirus prevalence to age 60 years among Australian women prevaccination. Sex Health. 2015;12:353–9. doi: 10.1071/SH15035. [DOI] [PubMed] [Google Scholar]
- 13.Sultana F, Gertig DM, Wrede CD, et al. A pilot study to compare dry cervical sample collection with standard practice of wet cervical samples for human papillomavirus testing. J Clin Virol. 2015;69:210–3. doi: 10.1016/j.jcv.2015.06.080. [DOI] [PubMed] [Google Scholar]
- 14.Lam JUH, Rebolj M, Ejegod DM, Pedersen H, Rygaard C, Lynge E, et al. Prevalence of human papillomavirus in self-taken samples from screening nonattenders. J Clin Microbiol. 2017;55(10):2913–23. doi: 10.1128/JCM.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLachlan E, Anderson S, Hawkes D, Saville M, Arabena K. Completing the cervical screening pathway: Factors that facilitate the increase of self-collection uptake among under-screened and never-screened women, an Australian pilot study. Curr Oncol. 25:e17–26. doi: 10.3747/co.25.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilakos P, Catarino R, Bougel S, et al. Use of swabs for dry collection of self-samples to detect human papillomavirus among Malagasy women. Infect Agent Cancer. 2016;11:13. doi: 10.1186/s13027-016-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkes D, Tan G, Caruana M, Knight B, Canfell K, Saville M. An analysis of invalid Human Papillomavirus DNA results as part of the Compass Trial on Cervical Screening. 31st International Papillomavirus Conference; Cape Town, South Africa. 2017. [Google Scholar]
- 18.Cuschieri K, Geraets D, Cuzick J, et al. Performance of a cartridge-based assay for detection of clinically significant human papillomavirus (hpv) infection: lessons from valgent (Validation of hpv Genotyping Tests) J Clin Microbiol. 2016;54:2337–42. doi: 10.1128/JCM.00897-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NPAAC . Requirements for Laboratories Reporting Tests for the National Cervical Screening Program. 1st ed. Canberra: Australian Government Department of Health; 2017. [Google Scholar]