Abstract
Background
Crizotinib has shown greater efficacy in clinical trials than chemotherapy in patients with anaplastic lymphoma kinase-positive (alk+) non-small cell lung cancer (nsclc), but little information is available on its use and outcomes in real-world settings. We therefore assessed treatment patterns and outcomes in alk+ nsclc patients treated with crizotinib in regular clinical practice.
Methods
A retrospective medical record review was conducted in North America for adults with alk+ nsclc treated with crizotinib as first- or later-line therapy for metastatic disease between 1 August 2011 and 31 March 2013 (for the United States) or 1 May 2012 and 31 March 2013 (for Canada). Crizotinib-related trial enrollees were excluded. Descriptive analyses were conducted to assess treatment patterns and objective response rate (orr). Progression-free survival (pfs) and overall survival (os) were descriptively analyzed using Kaplan-Meier methods.
Results
Data were extracted for 212 patients in the United States (n = 147) and Canada (n = 65). Mean (standard deviation [sd]) age was 58.9 (9.5) years, and 69% were male. Seventy-nine patients (37%) were deceased at record abstraction. Sixty-five percent (n = 137) initiated crizotinib as first-line therapy. Mean (sd) duration of crizotinib treatment was 8.7 (4.9) months. Objective response rate was 66% (69% for first-line recipients, 60% for second-/later-line). Median (95% ci) pfs and os from crizotinib initiation were 9.5 (8.7, 10.1) and 23.4 (19.5, −) months, respectively. One- and two-year survival probabilities were 82% and 49%, respectively.
Conclusions
Outcomes for crizotinib recipients in this study align with previous trials, with orr appearing more favourable in first-line recipients. Our findings indicate that crizotinib outcomes in clinical studies may translate to regular clinical practice.
Keywords: Non-small cell, lung cancer, crizotinib, outcome
INTRODUCTION
Lung cancer is the second-most commonly diagnosed malignancy in the United States, exceeded only by breast cancer, with an estimated 224,390 new cases projected to be diagnosed in 20161. Lung cancer is also the most common cause of cancer-related death in the United States, with a projected 158,080 deaths in 20161. For purposes of treatment planning, lung cancer has traditionally been classified based on histology, with the great majority (83%) of newly diagnosed cases classified as non-small cell lung cancer (nsclc)1. However, spurred by technological advances in recent years leading to an increased understanding of the molecular heterogeneity that drives oncogenesis and cancer cell survival and proliferation, nsclc is increasingly subclassified by the presence of specific oncogenic mutations2–4.
As a result of these advancements, targeted therapies have been developed to inhibit irregular oncogenic pathways in lung cancer5,6. Several epidermal growth factor receptor (egfr) and anaplastic lymphoma kinase (alk) inhibitors are now approved that have shown substantial response rates in patients with nsclc who have certain egfr mutations and alk rearrangements7–10. Crizotinib, for example, has been shown in clinical studies to be associated with tumour reduction or stabilization in 90% of alk+ patients, with response duration of up to 15 months11 and superior efficacy versus standard chemotherapy12,13. Based on these and other trials, crizotinib was approved in the United States, Canada, and various international markets for the treatment of patients with metastatic alk+ nsclc.
Data from the crizotinib clinical studies have been evolving, including other recent evaluations of its clinical effects after resumption of the therapy following progression of central nervous system metastases14. However, at this time, there are limited data describing the use of crizotinib and its outcomes in real-world practice settings outside the highly controlled environs of clinical trials. The objective of the current study was therefore to assess current treatment patterns and outcomes in patients with alk+ advanced nsclc who were treated with crizotinib in regular clinical practice.
MATERIALS AND METHODS
Study Design
The current study had a retrospective cohort design based on a review of medical records for patients with alk+ nsclc who received treatment with crizotinib in non-clinical trial settings. Physicians (primarily oncologists) treating nsclc patients were recruited for study participation from multiple treatment centres across the United States and Canada. For patients meeting the study inclusion criteria (described below), relevant medical record data were retrospectively abstracted by the participating physicians (or delegated clinical research staff) using a secure, web-based data collection form. All patient data were de-identified and anonymous. The study received ethics review and approval by federally authorized institutional review boards in both the United States and Canada.
Patient Selection
Patients meeting all of the following criteria were selected for study inclusion: (1) diagnosed with metastatic nsclc, with confirmation of alk rearrangement via diagnostic procedures used in each practice as recorded in the patient’s medical record; (2) 18 years of age or older at diagnosis of alk+ nsclc; (3) initiated treatment with crizotinib as first- or later-line of therapy for metastatic alk+ nsclc between 1 August 2011 and 31 March 2013 (for American patients, based on August 2011 approval of crizotinib in the United States) or between 1 April 2012 and 31 March 2013 (for Canada, based on April 2012 approval of crizotinib in Canada); and (4) complete medical record history from crizotinib initiation until at least three months after last recorded crizotinib dose, unless the patient died less than three months after last dose (if patient died less than three months after last dose, the patient record was still eligible). Patients who were treated with crizotinib as part of a clinical trial or patients who were ros1-positive were excluded from the study.
Study Variables and Endpoints
Various demographic and background clinical characteristics were documented for each patient, as were key characteristics of patients’ overall crizotinib treatment course, including time to crizotinib initiation after initial diagnosis of or progression to metastatic nsclc; setting of crizotinib initiation (first- or second-/later-line); initial crizotinib dose prescribed and subsequent dose changes; treatment duration and reasons for final discontinuation of crizotinib; and additional cancer-directed therapies received after crizotinib discontinuation.
Several clinical endpoints were also assessed. Objective response rate (orr) was defined as the proportion of patients achieving a best clinical response to crizotinib of either complete response or partial response, as recorded in the patient’s medical record. Progression-free survival (pfs) was defined as time from crizotinib initiation until the earlier of: (1) physician-documented clinical progression or death occurring during crizotinib treatment (up to and including two weeks after switch to/initiation of a new therapy, if a new therapy was initiated); or (2) death occurring between 2 and 14 weeks after crizotinib completion, if there was no initiation of a new therapy during this period. Patients without a progression event were censored at the earlier of initiation of a new therapy, death occurring more than 14 weeks after crizotinib completion, or last available medical record. Finally, overall survival (os) was defined as time from crizotinib initiation until death; patients still alive at the time of data collection were censored at the date of the last available medical record.
Statistical Analyses
All analyses were conducted using SAS statistical software (Version 9.3; Cary, NC). Analysis variables were summarized using univariate statistics and were stratified by the setting (first-line vs. second-/later-line) in which crizotinib was initiated for the treatment of metastatic nsclc. The statistical significance of descriptive differences in study variables and clinical outcomes between these groups was assessed using t-tests and chi-square tests, as appropriate, with corresponding p values reported. Progression-free survival and os were analyzed using the Kaplan-Meier method, with statistical significance of survival differences by line of crizotinib initiation assessed using a non-parametric log-rank test. All data were analyzed for the pooled study sample comprising patients from the United States and Canada combined. Objective response rate and os were additionally analyzed in a subgroup of patients with brain metastases present prior to crizotinib initiation.
RESULTS
A total of 107 physicians from the United States and 40 physicians from Canada participated in the medical record abstraction. Medical oncology and dual hematology/ oncology were the predominant specialties among these physicians. A total of 212 patients were identified for study inclusion (147 from the United States, 65 from Canada). Break-apart fluorescence in situ hybridization and immunohistochemistry were the most common diagnostic procedures used to confirm alk rearrangement (Table I). In the overall cohort, mean (standard deviation [sd]) age at diagnosis of metastatic alk+ nsclc was 58.9 (9.5) years, which did not vary substantially by line (first- or second-/later-line) of crizotinib initiation. The majority of the cohort was male (68.9%) and of white/Caucasian ethnicity (78.8%). A majority of patients (58.0%) were recorded as being former smokers at the time of diagnosis of metastatic alk+ nsclc, with 32.1% recorded as having never smoked; 8.5% were current smokers at the time of diagnosis. Thirty-three patients (15.6%) had brain metastases present at or prior to crizotinib initiation. More than half of patients (52.8%) received no other cancer-directed treatment prior to initiation of crizotinib; among patients receiving prior therapy, radiotherapy, and chemotherapy were the most common cancer-directed treatment modalities observed before crizotinib initiation. Median total observational duration, from crizotinib initiation until last available medical record, was 16.5 months.
TABLE I.
Demographic and clinical characteristics
| All Patients (n=212) | Setting of Crizotinib Initiation (n=210)a | |||
|---|---|---|---|---|
|
| ||||
| First-Line (n=137) | Second- or Later-Line (n=73) | P value | ||
| Age (years) at diagnosis,b mean (SD) | 58.9 (9.5) | 59.6 (9.0) | 57.6 (10.1) | 0.1343 |
| Male, n (%) | 146 (68.9) | 93 (67.9) | 51 (69.9) | 0.7685 |
| Ethnicity, n (%) | 0.3828 | |||
| White/Caucasian | 167 (78.8) | 103 (75.2) | 62 (84.9) | |
| African/black | 22 (10.4) | 16 (11.7) | 6 (8.2) | |
| Asian or Pacific Islander | 22 (10.4) | 17 (12.4) | 5 (6.8) | |
| Unknown | 1 (0.5) | 1 (0.7) | — | |
| Smoking status at diagnosis,b n (%) | 0.0875 | |||
| Current smoker | 18 (8.5) | 13 (9.5) | 5 (6.8) | |
| Former smoker | 123 (58.0) | 71 (51.8) | 51 (69.9) | |
| Never smoked | 68 (32.1) | 51 (37.2) | 16 (21.9) | |
| Unknown | 3 (1.4) | 2 (1.5) | 1 (1.4) | |
| ECOG performance status at diagnosis,b n (%) | 0.1076 | |||
| 0–1 | 159 (75.0) | 107 (78.1) | 51 (69.9) | |
| 2–4 | 53 (25.0) | 30 (21.9) | 22 (30.1) | |
| Brain metastases present at/prior to crizotinib initiation, n (%) | 33 (15.6) | 22 (16.1) | 11 (15.1) | 0.8646 |
| Diagnostic test(s) used to determine alk+ status,c n (%) | ||||
| Break-apart fluorescence in situ hybridization (FISH) | 143 (72.2) | 91 (71.7) | 50 (72.5) | 0.9040 |
| Reverse transcriptase-polymerase chain reaction (RT-PCR) | 44 (22.2) | 27 (21.3) | 16 (23.2) | 0.7553 |
| Quantitative polymerase chain reaction (qPCR) | 25 (12.6) | 15 (11.8) | 9 (13.0) | 0.8015 |
| Immunohistochemistry (IHC) | 82 (41.4) | 45 (35.4) | 36 (52.2) | 0.0230 |
| Another test | 3 (1.5) | 2 (1.6) | 1 (1.4) | 0.9455 |
| Symptoms present at diagnosis,b n (%) | ||||
| Chest pain/discomfort | 55 (25.9) | 38 (27.7) | 17 (23.3) | 0.4849 |
| Dyspnea | 117 (55.2) | 79 (57.7) | 38 (52.1) | 0.4358 |
| Cough | 150 (70.8) | 102 (74.5) | 48 (65.8) | 0.1839 |
| Fatigue | 137 (64.6) | 83 (60.6) | 54 (74.0) | 0.0524 |
| Other symptoms | 11 (5.2) | 4 (2.9) | 7 (9.6) | 0.0388 |
| Patient had no symptoms | 9 (4.2) | 6 (4.38) | 3 (4.1) | 0.9267 |
| Vital status at medical record abstraction, n (%) | 0.6998 | |||
| Alive | 124 (58.5) | 82 (59.9) | 40 (54.8) | |
| Deceased | 79 (37.3) | 50 (36.5) | 29 (39.7) | |
| Unknown | 9 (4.2) | 5 (3.6) | 4 (5.5) | |
| Other cancer-directed therapies administered prior to crizotinib initiation, n (%) | ||||
| None (supportive care only) | 112 (52.8) | 112 (81.8) | — | N/A |
| Surgery | 20 (9.4) | 8 (5.8) | 12 (16.4) | 0.0127 |
| Radiotherapy | 43 (20.3) | 16 (11.7) | 27 (37.0) | <0.0001 |
| Chemotherapy | 51 (24.1) | — | 51 (69.9) | N/A |
| Targeted therapyd | 13 (6.1) | — | 13 (17.8) | N/A |
| Duration (months) of observation, from crizotinib initiation until last available medical record, median | 16.5 | 16.7 | 16.5 | 0.2170 |
SD = standard deviation; ECOG = Eastern Cooperative Oncology Group; alk = anaplastic lymphoma kinase; N/A = not applicable; nsclc = non-small cell lung cancer; egfr = epidermal growth factor receptor; VEGF = vascular endothelial growth factor.
Line of crizotinib initiation was unknown for 2 patients.
“At diagnosis” refers, more specifically, to at first diagnosis of or progression to metastatic nsclc.
More than one diagnostic procedure may have been utilized per patient; test procedure types and associated proportions of patients receiving them therefore are not mutually exclusive.
Anti-egfr (afatinib, erlotinib, or gefitinib) or anti-VEGF (bevacizumab).
Median number of days to crizotinib initiation after initial metastatic nsclc diagnosis was 26 days (Table II). Line of crizotinib initiation after initial diagnosis of alk+ nsclc was documented for 210 of the 212 patients in the overall study sample. Among the 210 patients for whom line of crizotinib initiation was documented, 137 patients (65.2%) initiated crizotinib as first-line therapy, and 73 patients (34.8%) initiated crizotinib as second- or later-line therapy. In the overall cohort, 250 mg b.i.d. was the most common starting dose of crizotinib (79.7% of patients). Dose changes were infrequent once crizotinib was initiated: 89.6% of patients had no changes (escalation or reduction) in dose during their course of exposure. Disease progression following initial clinical response was the most commonly cited reason (59.4% of patients) for final crizotinib discontinuation; two patients died while on crizotinib treatment, both due to nsclc or related complications. Treatment-related toxicities or side effects were cited as a reason for final crizotinib discontinuation in 4.7% of patients. More than one-third of all patients (38.2%) received no other cancer-directed treatment (surgery, radiotherapy, chemotherapy, or targeted therapy) after discontinuation of crizotinib.
TABLE II.
Crizotinib treatment patterns
| All Patients (n=212) | Setting of Crizotinib Initiation (n=210)a | |||
|---|---|---|---|---|
|
| ||||
| First-Line (n=137) | Second- or Later-Line (n=73) | P value | ||
| Time (days) from initial diagnosisb to crizotinib initiation | ||||
| Mean (SD) | 85.4 (132.7) | 39.6 (64.5) | 169.1 (178.0) | <0.0001 |
| Median | 26 | 20 | 91 | |
| Range (minimum, maximum) | (0, 720) | (0, 420) | (0, 720) | |
| Initial crizotinib total daily dose prescribed, n (%) | 0.0452 | |||
| 200 mg b.i.d. | 32 (15.1) | 22 (16.2) | 8 (11.0) | |
| 250 mg b.i.d. | 169 (79.7) | 111 (81.6) | 58 (79.5) | |
| 200 mg q.d. | 8 (3.8) | 3 (2.2) | 4 (5.5) | |
| 250 mg q.d. | 3 (1.4) | — | 3 (4.1) | |
| Crizotinib dose changes, n (%) | ||||
| Had ≥1 dose reduction | 15 (7.1) | 7 (5.1) | 8 (11.0) | 0.1207 |
| Had ≥1 dose escalation | 6 (2.8) | 2 (1.5) | 4 (5.5) | 0.0980 |
| Had no dose changes | 190 (89.6) | 126 (92.6) | 61 (83.6) | 0.0413 |
| Unknown | 1 (0.5) | 2 (1.5) | — | 0.4329 |
| Duration (months) of crizotinib treatment, from initiation to last observed dose, median | 8.7 | 9.0 | 8.5 | 0.1008 |
| Reason(s) for final discontinuation of crizotinib, n (%) | ||||
| Death | 2 (3.1) | 1 (2.4) | 1 (4.3) | 0.6737 |
| Disease progression following initial response | 126 (59.4) | 90 (66.2) | 36 (49.3) | 0.0175 |
| Disease progression following no initial response | 29 (13.7) | 14 (10.3) | 14 (19.2) | 0.0722 |
| Treatment-related toxicity or side effects | 10 (4.7) | 2 (1.5) | 8 (11.0) | 0.0022 |
| Patient request | 41 (19.3) | 25 (18.4) | 15 (20.5) | 0.7044 |
| Other reason(s) | 8 (3.8) | 4 (2.9) | 4 (5.5) | 0.3619 |
| Unknown | 6 (2.8) | 3 (2.2) | 2 (2.7) | 0.7432 |
| Other treatments received after crizotinib discontinuation/completion, n (%) | ||||
| None | 81 (38.2) | 49 (35.8) | 32 (43.8) | 0.4258 |
| Surgery | 2 (0.5) | 1 (0.7) | — | 0.9455 |
| Radiotherapy | 38 (17.9) | 21 (15.3) | 17 (23.3) | 0.4884 |
| Chemotherapy | 75 (35.4) | 58 (42.3) | 17 (23.3) | 0.3274 |
| Other alk inhibitor in a clinical trial | 10 (4.7) | 5 (3.6) | 5 (6.8) | 0.3100 |
| Targeted therapy | 21 (9.9) | 16 (11.7) | 5 (6.8) | 0.4748 |
| Other | 2 (0.5) | 1 (0.7) | 2 (2.7) | 0.2252 |
SD = standard deviation; alk = anaplastic lymphoma kinase; nsclc = non-small cell lung cancer.
Line of crizotinib initiation was unknown for 2 patients.
“At diagnosis” refers, more specifically, to at diagnosis of metastatic alk+ nsclc.
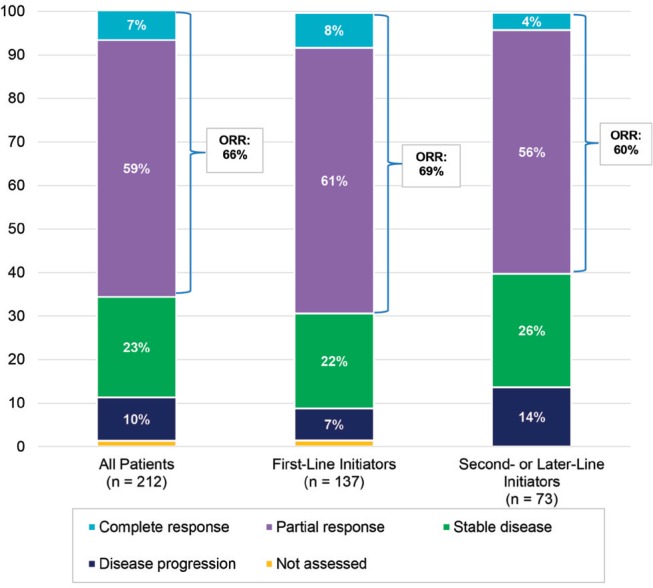
In the overall study sample, the orr for crizotinib treatment was 65.6%. Patients initiating crizotinib as first-line treatment appeared to respond somewhat better (69.3% orr) than patients initiating the drug in second/later-line therapy (60.3% orr). In the overall cohort, partial response during crizotinib treatment was the most common best clinical response recorded (59.0% of patients) (Figure 1). Stable disease was recorded as best response for 23.1% of patients and 6.6% of patients achieved complete response. Few patients (9.9%) experienced disease progression as their best clinical response during crizotinib treatment.
FIGURE 1.
Best clinical response during crizotinib treatment. orr = objective response rate. P value = 0.0457 for chi-square test for orr in first-line vs. second-/later-line initiators.
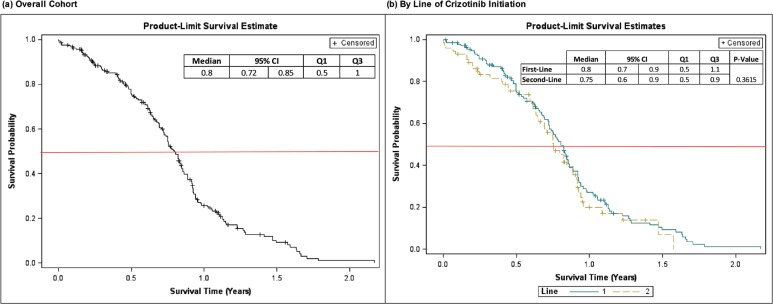
Table III presents tabular data from Kaplan-Meier analyses of pfs and os (scaled to months). Kaplan-Meier pfs and os curves (scaled to years) are shown in Figures 2 and 3. Median (95% confidence interval [ci]) pfs from crizotinib initiation was 9.5 (8.7, 10.1) months; by setting of crizotinib initiation, median pfs estimates were 9.6 (8.6, 10.2) and 9.0 (7.7, 10.6) months for first- and second-/later- line initiators, respectively (p = 0.3615).
TABLE III.
Kaplan-Meier point estimates of progression-free and overall survival
| Overall (n=212) | Setting of Crizotinib Initiation (n=210)a | ||
|---|---|---|---|
|
| |||
| First-Line (n=137) | Second- or Later-Line (n=73) | ||
| Progression-free survival | |||
| Time (months) to progression or death from crizotinib initiation | |||
| Mean (SE) | 9.8 (0.4) | 9.9 (0.5) | 9.2 (0.7) |
| Median (95% CI) | 9.5 (8.7, 10.1) | 9.6 (8.6, 10.2) | 9.0 (7.7, 10.6) |
| Q1, Q3 | 6, 12 | 6, 13 | 6, 11 |
| Overall survival | |||
| Time (months) to death from crizotinib initiation | |||
| Mean (SE) | 19.8 (0.5) | 20.0 (0.6) | 16.6 (0.7) |
| Median (95% CI) | 23.4 (19.5, —) | 23.4 (18.3, —) | — (15.1, —) |
| Q1, Q3 | 14, — | 15, — | 12, — |
| 1- and 2-year survival rates | |||
| Percent still alive at 1 year after crizotinib initiation (95% CI) | 81.9 (76.6–87.1) | 84.9 (78.8–91.0) | 75.9 (65.9– 85.9) |
| Percent still alive at 2 years after crizotinib initiation (95% CI) | 49.1 (39.2–59.0) | 47.2 (34.7–59.7) | 50.4 (35.1– 65.7) |
SE = standard error; CI = confidence interval; Q1 = 1st quartile; Q3 = 3rd quartile.
Line of crizotinib initiation was unknown for 2 patients
FIGURE 2.
Kaplan-Meier curves for progression-free survival from crizotinib initiation. CI = confidence interval; Q1 = 25th percentile; Q3 = 75th percentile. “Line” refers to line (1 = first-line, 2 = second-/later-line) of crizotinib initiation.
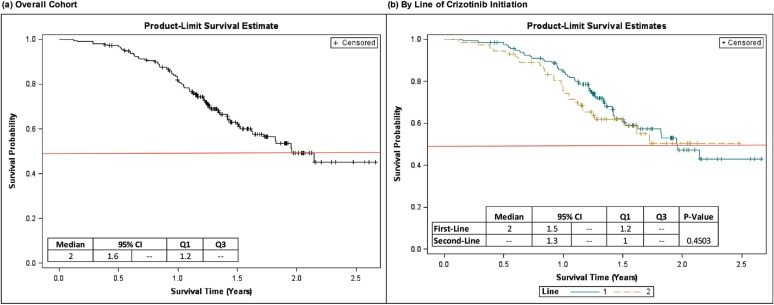
FIGURE 3.
Kaplan-Meier curves for overall survival from crizotinib initiation. CI = confidence interval; Q1 = 25th percentile; Q3 = 75th percentile. “Line” refers to line (1 = first-line, 2 = second-/later-line) of crizotinib initiation.
At the time of the medical record abstraction, 79 of the 212 patients included in the analysis were deceased, while 124 were still alive. Vital status at the medical record abstraction date could not be confirmed for 9 patients, but since no record of death was observed, these patients were censored at the record abstraction date for purposes of the os analysis. From crizotinib initiation, median (95% ci) os was 23.4 (19.5, —) months for the overall cohort. For patients initiating crizotinib as first-line treatment, median (95% ci) os from crizotinib initiation was also 23.4 (18.3, —) months. Median os was not reached in patients initiating crizotinib as second-line treatment. Kaplan-Meier estimates of 1-year and 2-year survival from crizotinib initiation were 81.9% (95% ci: 76.6 – 87.1%) and 49.1% (38.2 – 59.0%), respectively, for the overall cohort. Patients initiating crizotinib as first-line therapy had a numerically higher estimated one-year survival (84.9% [95% ci: 78.8 – 91.0%]) than patients initiating crizotinib as second-/later-line therapy (75.9% [95% ci: 65.9 – 85.9%]). Two-year survival, however, was similar in patients initiating crizotinib as first-line therapy (47.2% [95% ci: 34.7 – 59.7%]) versus second-/later-line therapy (50.4% [95% ci: [35.1 – 65.7%]).
In the current study, 33 patients were reported to have brain metastases at or prior to crizotinib initiation; 22 of these patients initiated crizotinib as first-line therapy for metastatic nsclc, and 11 initiated crizotinib as second-line therapy. In a subanalysis conducted in this small sample of patients, orr of the primary lung tumour for crizotinib was similar (61% among first-line crizotinib recipients, 60% among second-line recipients) to that observed in the overall study sample. One-year survival rates were also similar (81% in first-line patients, 77% in second-line patients) to the overall cohort.
DISCUSSION
There are currently few data available describing the use of crizotinib and its outcomes among alk+ metastatic nsclc patients in real-world practice settings. Before assessing the findings presented here within the context of the few available reports on crizotinib use in regular practice, our findings bear some comparison to available data from the key crizotinib trials. First, overall response rates to crizotinib seen in the current study (66% for the overall study sample, 69% for first-line crizotinib initiators and 60% for second-/later-line crizotinib initiators) were consistent with response rates reported in treatment-naïve patients (74%)12 and previously treated patients (65%)13 in the phase 3 crizotinib trials. In another study reporting phase 1 data, crizotinib recipients had an orr of 61%15. Similar to these clinical studies, objective responses in the current study comprised almost entirely partial responses. Given that crizotinib is a targeted agent requiring diagnostic testing, the high response rates observed here (as in the previous trials) provides evidence that providers are able to correctly diagnose the target treatment candidates in real-world settings.
Clinical benefits of crizotinib in alk+ nsclc patients with brain metastases have been documented in clinical trial data16,17 and in single-case reports18, but no study to our knowledge has yet made such an assessment in a broader real-world cohort. In the current study, we found robust response rates (orr > 60%, regardless of line of crizotinib initiation) for the primary tumour in the small subgroup of patients with brain metastases prior to crizotinib initiation. One-year survival in these patients was approximately 80% regardless of line of crizotinib initiation, which is notably higher than one-year survival in a recent trial-based report for alk+ nsclc patients with brain metastases (∼65%)17. These results support emerging literature on the possible clinical benefits of crizotinib in alk+ metastatic nsclc patients with brain metastases. Of related importance, we found a lower-than-expected rate of brain metastases (∼16%) at baseline (at/before crizotinib initiation) in the patients reviewed here as compared with other clinical and observational studies (ranging from 26 to 32%15,16,22).
Median pfs for treatment-naïve crizotinib recipients in the first-line clinical trial reported by Solomon et al. was 10.9 months12, which is consistent with the median pfs of 9.6 months estimated in our study for patients initiating crizotinib as first-line therapy for metastatic disease. In crizotinib recipients who received previous platinum-based chemotherapy, the second-line trial reported by Shaw et al. estimated a median pfs of 7.7 months13, which was numerically lower than, but still similar to, the median pfs of 9.0 months reported in the current study for patients receiving crizotinib as second- or later-line therapy for metastatic disease.
Overall survival was also consistent between the present study and previous clinical trials. Solomon et al.12, for example, reported a 1-year survival probability of 84% for treatment-naïve (i.e., first-line) crizotinib recipients, which is consistent with the 85% 1-year survival probability estimated here for patients who received crizotinib in the first-line. From the second-line trial, Shaw et al.13 reported a median os of 20.3 months based on interim, immature survival data in crizotinib recipients pre-treated with platinum-based chemotherapy; in a recently published update to this study, final median os was 21.7 months19. Median os for second-line crizotinib recipients was not reached in our study, but the second-line survival curve shown in Figure 3 indicates that median os was nearly reached and, in fact, approaches two years, which is concordant with final os from the second-line trial16. In our study, median os for first-line crizotinib recipients and for the overall study sample (regardless of line of crizotinib initiation) was 23.4 months, which is consistent with the second-line os estimates available in the Shaw et al. reports. Median os was not reached for first-line crizotinib recipients in the first-line trial reported by Solomon et al.12. Aside from the comparisons summarized above, which confirm similar survival of crizotinib-treated patients in regular practice as patients treated in clinical trials, our findings on os also highlight the potential benefit of real-world studies in providing confirmatory data or important interim evidence when clinical studies have not yet reached survival data maturity20.
To our knowledge, only two previous studies have been published that explore the topics of crizotinib use and its outcomes in alk+ nsclc patients in a retrospective, observational study design: a retrospective analysis by Shaw et al.21 of 82 crizotinib clinical trial enrollees in the United States, South Korea, and Australia, and a more recent medical record review by Guérin et al.22 of 119 alk+ nsclc patients treated with crizotinib in the United States. In both of these studies, as in our current study, all data were drawn from a timeframe prior to the approval of other alk inhibitors. Our study presents an additional stratification (setting of crizotinib initiation: first- vs. second-/later-line) and analysis (clinical response to crizotinib) that have not been previously reported in an observational study. Our study may therefore fill an important and previously unaddressed literature gap on crizotinib use and effectiveness in real-world settings.
To place the study population analyzed here into context with the populations analyzed in the retrospective studies by Shaw et al.21 and Guérin et al.22, mean age at crizotinib initiation in our pooled study was approximately 59 years, as compared with 51 years in the Shaw et al. study and 67 years in the Guérin et al. study; in Guérin et al., age was assessed at crizotinib discontinuation (the point at which follow-up began on downstream endpoints) rather than at crizotinib initiation. Our study sample was more disproportionately male (68.9%) as compared with Shaw et al. (54%)21 and Guérin et al. (47%)22. Finally, the distribution of smoking status in the study sample examined here conflicts with previous observations that nsclc patients with alk rearrangements are disproportionately “never smokers” (as opposed to current or former smokers) when compared with the general nsclc population. One previous study, for example, observed that 67% of a small sample (n = 27) of alk+ patients were “never smokers23,” which is consistent with the smoking status distributions reported in the clinical studies by Solomon et al.12 and Shaw et al.13,19. In our study, by contrast, only 32% of patients in were reported as “never smokers.” While it is unclear why the distribution of smoking status differed in our study from previous reports, it appears that this discrepancy was inconsequential with respect to clinical endpoints based on the consistency of orr and survival estimates in the present study with the previous crizotinib clinical studies.
Because the retrospective study by Shaw et al.21 assessed os from first crizotinib dose, whereas Guérin et al.22 assessed os from last dose, the Shaw et al. os estimates bear the most direct comparison with our study. In our cohort, Kaplan-Meier estimate of median os from crizotinib initiation was approximately 2 years, while 1- and 2-year survival from crizotinib initiation was 82% and 49%, respectively. In the Shaw et al. retrospective study21, median os was not reached, but 1- and 2-year survival rates were estimated at 74% and 54%, respectively, which is generally consistent with our findings. Shaw et al.21 additionally assessed os in a control group of alk+ patients who did not receive crizotinib, finding that in the absence of crizotinib, patients with alk+ nsclc have a prognosis similar to the general population of nsclc patients. Combined, results of our current study and the study by Shaw et al.21 provide evidence that crizotinib used in routine practice might substantially improve response rates and os in patients with advanced, alk+ nsclc. These findings also provide support for the concept of accelerated approval for treatments showing high response rates and improved survival in targeted patient populations.
While findings on os reported here align with the generally positive results available from previous crizotinib trials, interpretation of our results (as those of previous trials) should be made while considering the potential survival impact of alk rearrangement independent of treatment with alk inhibitors. While some previous research (Zhang et al.24, for example) has shown no survival difference in metastatic nsclc by alk status (after adjustments for initial disease stage, histology, and egfr/kras mutation), a majority of studies (including a recent review article by Kulig et al.25) have reported a poorer survival prognosis for patients with alk-mutated (vs. wild type) tumours independent of alk-inhibitor treatment. Further research is needed, however, to more fully clarify the role of alk rearrangement independent of treatments on the survival prognosis of patients with metastatic nsclc.
As in the retrospective study by Guérin et al.22, our study assessed reasons for final crizotinib discontinuation as well as additional treatments received in the post-crizotinib setting. Although categories of discontinuation reasons are not directly comparable between our study and the study by Guérin et al., disease progression, in some form, appears to be the most commonly cited reason for crizotinib discontinuation in both studies. Of note, patient request was cited as a reason for discontinuation in 19% of patients in our study, which was fairly consistent with the 12% discontinuation rate due to patient request reported by Guérin et al.22. However, in our study, approximately 90% of patients experienced no change in crizotinib dose while on the therapy, which combined with a 4.7% discontinuation rate due to toxicities, suggests a potentially favourable tolerability profile of the treatment in the patients studied. In a separate analysis of similarly-profiled crizotinib-treated alk+ nsclc patients in a large administrative claims database, Guérin et al.22 reported that approximately 72% of patients received no additional antineoplastic treatment after discontinuation of crizotinib; this estimate was consistent with our finding that 65% of patients received no additional systemic chemotherapy after crizotinib discontinuation. In the 75 patients in our study who initiated additional chemotherapy post-crizotinib, carboplatin + pemetrexed (17%), pemetrexed monotherapy (13%), and docetaxel monotherapy (13%) were the most common post-crizotinib chemotherapy regimens observed.
Our study was subject to several limitations inherent in retrospective medical record reviews. First, patients selected for study inclusion represented a “convenience” sample, in that the records were obtained from physicians who were willing to participate in the study. Therefore, study findings may not be generalizable to all alk+ nsclc patients treated with crizotinib or to all physicians who treat lung cancer in the countries we studied. Relatedly, selection bias cannot be excluded as an additional study limitation. Real-world studies in oncology typically include patients who are older and with poorer baseline performance status as compared with interventional trials. If such patients predisposed to better outcomes were more likely to be selected for the review, the study may present an overly optimistic estimate of the real-world effectiveness of crizotinib. However, based on the overall rarity of alk mutation in nsclc, evidence that alk-mutated nsclc tumours in real-world populations tend to occur in substantially younger patients26, and the relatively few exclusion criteria applied in previous crizotinib trials, it is reasonable to expect that the population selected for the current study would not systematically differ greatly from other alk+ nsclc populations in previous trials and observational studies. Second, information captured by the study’s data collection form was limited to information available in the patients’ medical records held by the physicians participating in the study. Potentially relevant information on healthcare services and events, occurring outside the physician’s care setting, including deaths occurring at non-local hospitals that were not reported back to the attending oncologist (which is expected to occur infrequently) was unavailable. Third, in retrospective studies, response criteria are not dictated by a protocol, and assessments (e.g., imaging studies) may not be done on a uniform schedule. Therefore, results regarding this endpoint may not be directly comparable to those observed in clinical trials. Similarly, assessments of tumour progression in routine practice may not be made as often or on a pre-defined, protocol-driven schedule, and therefore progression may be identified somewhat later in patients in routine practice as compared with clinical trials; estimates of pfs in retrospective studies may therefore have an upward bias. In addition, analytic choices (censoring progression events at initiation of a new therapy and censoring deaths occurring more than 14 weeks after crizotinib completion and in those with unknown vital status at the time of data collection) could exacerbate upward bias in pfs and os estimates. Fourth, data were entered into the data collection form directly by the treating physicians or delegated clinical staff and therefore may have been subject to entry errors and resulting inaccuracies in reporting. Responses to questions within the data collection form were not validated against the patients’ medical records by an independent reviewer. Finally, as noted earlier, all data for this study were drawn from a timeframe prior to the approval of other alk inhibitors. The impact of follow-up treatment with alternative alk inhibitors after crizotinib discontinuation therefore could not be assessed.
Despite these limitations, this study provides useful information on the use and outcomes of crizotinib in a real-world population of alk+ metastatic nsclc patients treated with crizotinib. The natural history of untreated advanced alk+ nsclc is generally uncertain15. However, 1-year survival for advanced nsclc, even within first-line clinical trials, is usually lower than 50%27. Results of the present study, as well as a few previous real-world and clinical trial-based studies, suggest that patients with alk+ metastatic nsclc patients treated with crizotinib have a 1-year survival rate of approximately 75% to 80%, as well as a substantial orr (> 60%). These data therefore support the potential of crizotinib to have a positive impact on outcomes in patients with alk+ nsclc and are in line with data previously reported in clinical studies.
CONFLICT OF INTERESTS DISCLOSURE
We have read and understood Current Oncology’s policy on conflicts of interest disclosure and declare the following interests: Keith Davis and James Kaye are employees of RTI Health Solutions, which received contract research funding from Pfizer Inc. in connection with the conduct of this study and the development of this manuscript; Elizabeth Masters and Shrividya Iyer are employees of Pfizer Inc., which conducts clinical research in and produces treatments, including crizotinib, for non-small cell lung cancer.
This study and preparation of this manuscript was funded by Pfizer, Inc. (study sponsor). Authors affiliated with the study sponsor (Elizabeth Masters, Shrividya Iyer) participated in the development of the study design, interpretation of the data, and critical review of this manuscript but had no involvement in analyses of the study data. Furthermore, no study author (including those affiliated with RTI Health Solutions: Keith Davis and James Kaye) was involved in the collection of the study data, nor did any study author have the ability to influence the selection of specific patient charts for inclusion in the review. As required to maintain patient anonymity, all patient selection (based on inclusion criteria set forth in the study protocol) and data entry was conducted solely by the participating clinicians and their staff. All study authors contributed equally to the decision to submit this manuscript for publication.
Portions of this research were previously presented in podium and poster format at the IASLC 16th World Conference on Lung Cancer, September 6–9, 2015, Denver, CO, USA.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Available at: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf; cited August 2016.] [Google Scholar]
- 2.Clinical Lung Cancer Genome Project Network Genomic Medicine (NGM): a genomics-based classification of human lung tumors. Sci Transl Med. 2013;5:209ra153. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–80. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2006;311:1998–2014. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non–small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33:1008–14. doi: 10.1200/JCO.2014.59.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husain H, Rudin CM. alk-targeted therapy for lung cancer: ready for prime time. Oncology. 2011;25(7):597–601. [PubMed] [Google Scholar]
- 7.Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21(L858R) substitution mutations. Oncologist. 2014;19:774–9. doi: 10.1634/theoncologist.2014-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dungo RT, Keating GM. Afatinib: first global approval. Drugs. 2013;73:1503–15. doi: 10.1007/s40265-013-0111-6. [DOI] [PubMed] [Google Scholar]
- 9.Malik SM, Maher VE, Bijwaard KE, et al. US Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014;20:2029–34. doi: 10.1158/1078-0432.CCR-13-3077. [DOI] [PubMed] [Google Scholar]
- 10.Chabner BA. Approval after phase I: ceritinib runs the three-minute mile. Oncologist. 2014;19:577–8. doi: 10.1634/theoncologist.2014-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadgeel SM, Bepler G. Crizotinib: an anaplastic lymphoma kinase inhibitor. Future Oncol. 2011;7(8):947–53. doi: 10.2217/fon.11.77. [DOI] [PubMed] [Google Scholar]
- 12.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in alk-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Kim D-W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced alk-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 14.Takeda M, Okamoto I, Nakagawa K. Clinical impact of continued crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for alk rearrangement. J Thorac Oncol. 2013;8(5):654–7. doi: 10.1097/JTO.0b013e31828c28e7. [DOI] [PubMed] [Google Scholar]
- 15.Camidge DR, Bang Y-J, Kwak EL, et al. Activity and safety of crizotinib in patients with alk-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced alk-positive non-small-cell lung cancer: results from PROFILE 1014. J Clin Oncol. 2016;34(24):2858–65. doi: 10.1200/JCO.2015.63.5888. [DOI] [PubMed] [Google Scholar]
- 17.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced alk-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–8. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita Y, Koga Y, Sakamoto A, Hidaka K. Long-lasting response to crizotinib in brain metastases due to eml4-alk-rearranged non-small-cell lung cancer. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-200867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw AT, Janne PA, Besse B, et al. Crizotinib vs chemotherapy in alk+ advanced non-small cell lung cancer (nsclc): final survival results from PROFILE 1007. J Clin Oncol. 2016;34(suppl) doi: 10.1200/jco.2016.34.26_suppl.27. abstr:9066. [DOI] [Google Scholar]
- 20.Sutter S. Pink sheet—real-world evidence may find a home on breakthrough pathway. [Available at: http://www.focr.org/news/pink-sheet-real-world-evidence-may-find-home-breakthrough-pathway; cited September 2016.]
- 21.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring alk gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guérin A, Sasane M, Wakelee H, et al. Treatment, overall survival, and costs in patients with alk+ non-small cell lung cancer after crizotinib monotherapy. Curr Med Res Opin. 2015;31(8):1587–97. doi: 10.1185/03007995.2015.1057115. [DOI] [PubMed] [Google Scholar]
- 23.Sequiest LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22(12):2616–24. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XC, Blanckmeister CA, Yang J, et al. Retrospective study of clinicopathologic factors associated with alk rearrangement and survival outcome in Chinese patients with nsclc. Cancer Res. 2012;72(8 suppl 1):4504. doi: 10.1158/1538-7445.AM2012-4504. [DOI] [Google Scholar]
- 25.Kulig K, Wang Y, Iyer S, Yang P. Predictive and prognostic value of alk gene rearrangement in non-small cell lung cancer. Epidemiol. 2014;4:146. [Google Scholar]
- 26.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-alk. J Clin Oncol. 2009;27(26):4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to nsclc histology: a review of two phase III studies. Oncologist. 2009;14:253–63. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]