Abstract
Cervical cancer rates are disproportionately high among women living with the human immunodeficiency virus (wlhiv). Cervical cancer is preventable through hpv screening, regular Pap tests, and early cancer detection. Evidence indicates that hpv and cervical cancer screening are suboptimal among wlhiv, who face a myriad of access barriers. Considering that screening is an effective first-line defense to cervical cancer, we conducted a scoping review with the aim of gaining a better understanding about: (1) the knowledge and perceptions of hpv and cervical cancer screening among wlhiv; and (2) the acceptability of self-sampling for hpv among wlhiv. We searched five electronic databases for peer-reviewed articles that were published in English within the last ten years, reported on studies with hiv-positive women who were aged 16 or older, and satisfied the topics of the review. A total of 621 articles were found. After accounting for duplicates and unmet criteria, 17 articles and 1 abstract, reporting on studies in the United States and Africa, were included in this review. The review highlighted that most wlhiv had inadequate knowledge of hpv transmission and cervical cancer prevention, which influenced their perceptions of risk and susceptibility. Screening barriers included misconceptions about Pap tests, fear of diagnosis of serious illness, perceived pain, embarrassment, bodily modesty, and limited access to female health care providers. This review also affirms that self-sampling is an acceptable and promising screening option for wlhiv. Implications for policy, research, and practice are discussed.
Keywords: Women living with hiv, hpv, cervical cancer, self-sampling, scoping review
INTRODUCTION
Women account for a growing proportion of hiv cases in Canada and elsewhere. Between 1985 and 2014, a cumulative total of 80,469 hiv cases were reported in Canada, with women accounting for 18.2% of all cases. Of the 2,044 hiv cases reported in 2014, women accounted for 24.6%. Further, over three-quarters of these new cases were identified as Aboriginal women (30.6%), Black women (35.6%), and other women of colour (9.6%). Heterosexual contact was identified as the key category of exposure1.
With the widespread use of highly active antiretroviral therapy (haart), the incidence of hiv-defining cancers such as Kaposi sarcoma and non-Hodgkin lymphoma have declined dramatically, but cervical cancer rates have remained high among women living with hiv (wlhiv)2. Cohort studies in Canada and the United States have consistently shown that the risk of invasive cervical cancer is higher among wlhiv than hiv-negative women3,4. Other studies have confirmed that the prevalence of high risk human papillomavirus (hrhpv) infection is also higher and more persistent in wlhiv5,6. There is general agreement that a long history of hiv infection and prolonged immunosuppression are associated with persistent hpv infection and invasive cervical cancer7,8.
Current Canadian guidelines on cervical cancer screening recommend that wlhiv receive Papanicolaou (Pap) tests at the initial assessment and at six months, with an annual follow-up for women with normal results9. However, cervical cancer screening remains suboptimal among wlhiv. Results of a Canadian retrospective population-based study of 2,661 wlhiv living in Ontario show that only up to half of the women had adhered to the cervical cancer screening guidelines10. Similarly, results of another retrospective cohort study of 218 wlhiv in Ottawa indicated that 42% of the participants did not undergo cervical cancer screening during the 3-year period even though 94% of them listed that they had primary care providers in their medical records11.
Studies in Canada have identified myriad barriers that deter wlhiv from accessing health care, including structural racism12,13, hiv-related stigma and discrimination within and outside of the health care system14,15, and criminalization of hiv non-disclosure16. Other studies show that the utilization of health services and the uptake of cancer screening among marginalized groups are associated with their health literacy17,18. These findings suggest that socially inclusive, innovative, and relevant strategies are needed to promote hpv and cervical cancer screening among wlhiv. Insights from studies on hiv self-testing19 suggest that hpv self-sampling is a potential strategy to effectively engage marginalized wlhiv.
Drawing on a critical health-literacy framework20, we conducted a scoping review to explore two questions: (1) What has been reported on the knowledge of hpv and/or cervical cancer screening among wlhiv in Canada and other countries? (2) What has been reported on the acceptability of hpv self-sampling among wlhiv in Canada and other countries?
METHODS
This scoping review is informed by the framework of Arksey and O’Malley21. We applied a multi-step approach that included the following: (1) identifying our search topics and questions; (2) searching for relevant studies; (3) selecting relevant studies based on the review questions; (4) charting the data collected; and (5) synthesizing the data collected into themes. Further, we adopted an additional step proposed by Levac and her colleagues22, that is, connecting the meaning of the findings to the overall study purpose and discussing the implications for future research, policy, and practice.
Search Strategies and Inclusion Criteria
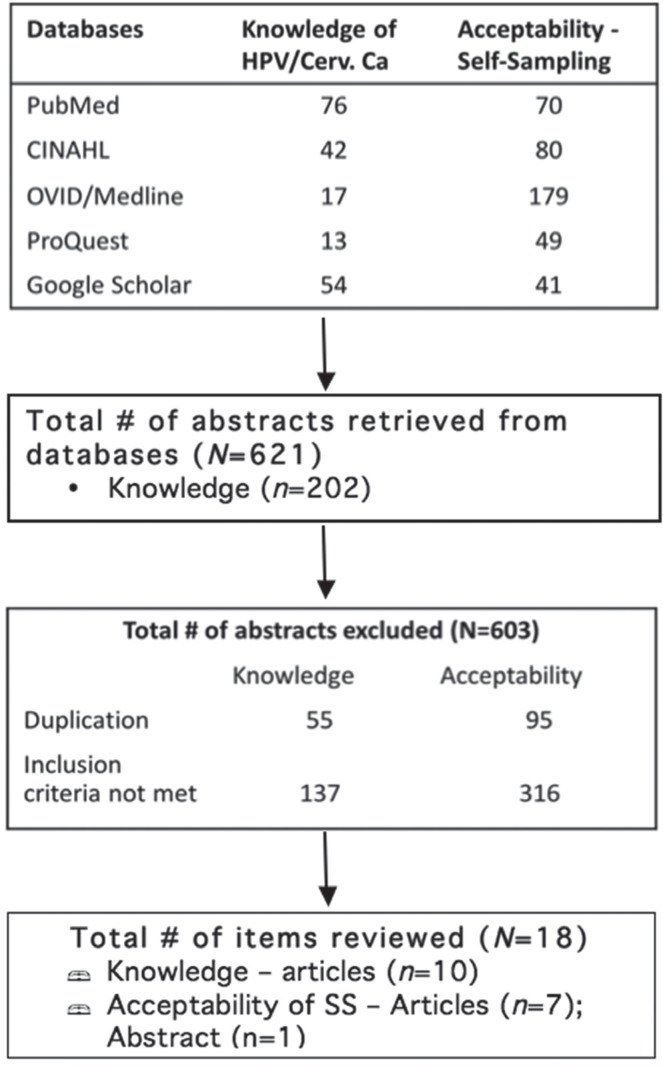
Our search focused on peer-reviewed articles that report on either primary or secondary sources of evidence. We searched five databases: PubMed, CINAHL, OVID/ Medline, ProQuest, and Google Scholar. The search terms included acceptability, attitudes, perceptions, knowledge, human papillomavirus/hpv, cervical cancer screening, self-sampling/self-collection/self-testing/self-screening, home-based collection, human immunodeficiency virus/hiv, wlh, wlhiv, and wlha. Inclusion criteria consisted of (1) peer-reviewed English language publications within the last ten years, (2) engaged women, aged 16 or older, living with hiv in Canada or elsewhere, and (3) the researched topics fitted with the two foci of this scoping, review, i.e., knowledge of hpv and cervical cancer, and the acceptability of hpv self-sampling. We limited our search to a ten-year period to coincide with the establishment of the Screening Performance Indicators Working Group (spiwg) in 2007. The spiwg defines core performance indicators for cervical cancer screening programs in Canada and facilitates inter-jurisdictional comparison23. A total of 621 articles were retrieved from the five databases. After accounting for duplicates (n=150) and unmet criteria (n=453), a total of 603 articles were excluded (see Figure 1). A table was used to identify pertinent study information on study authors, location of study, and study characteristics/population (see Table I). Each item included in this review was reviewed in its entirety, and relevant information was extracted to inform this synthesized review.
FIGURE 1.
Article selection process. HPV = human papillomavirus; ss = self-sampling.
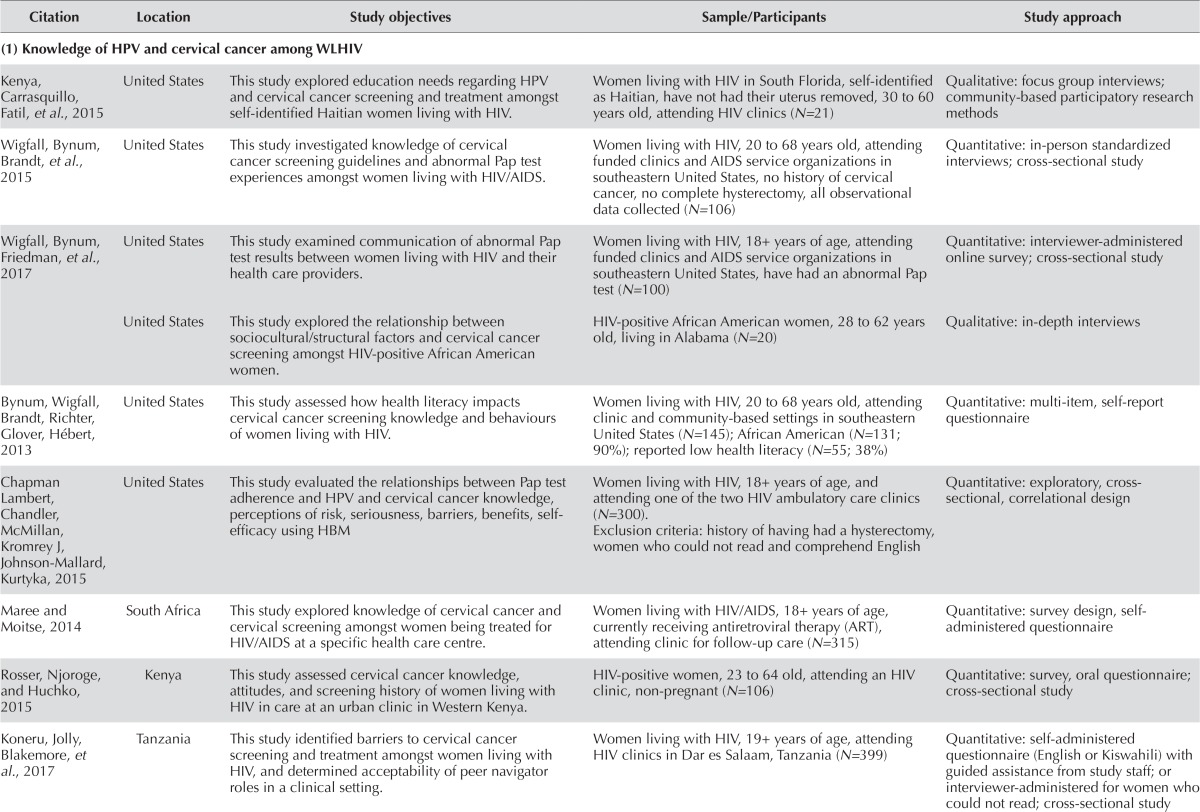
TABLE I.
Summaries of reviewed articles
| Citation | Location | Study objectives | Sample/Participants | Study approach |
|---|---|---|---|---|
| (1) Knowledge of hpv and cervical cancer among wlhiv | ||||
| Kenya, Carrasquillo, Fatil, et al., 2015 | United States | This study explored education needs regarding hpv and cervical cancer screening and treatment amongst self-identified Haitian women living with hiv. | Women living with hiv in South Florida, self-identified as Haitian, have not had their uterus removed, 30 to 60 years old, attending hiv clinics (N=21) | Qualitative: focus group interviews; community-based participatory research methods |
| Wigfall, Bynum, Brandt, et al., 2015 | United States | This study investigated knowledge of cervical cancer screening guidelines and abnormal Pap test experiences amongst women living with hiv/aids. | Women living with hiv, 20 to 68 years old, attending funded clinics and aids service organizations in southeastern United States, no history of cervical cancer, no complete hysterectomy, all observational data collected (N=106) | Quantitative: in-person standardized interviews; cross-sectional study |
| Wigfall, Bynum, Friedman, et al., 2017 | United States | This study examined communication of abnormal Pap test results between women living with hiv and their health care providers. | Women living with hiv, 18+ years of age, attending funded clinics and aids service organizations in southeastern United States, have had an abnormal Pap test (N=100) | Quantitative: interviewer-administered online survey; cross-sectional study |
| United States | This study explored the relationship between sociocultural/structural factors and cervical cancer screening amongst hiv-positive African American women. | hiv-positive African American women, 28 to 62 years old, living in Alabama (N=20) | Qualitative: in-depth interviews | |
| Bynum, Wigfall, Brandt, Richter, Glover, Hébert, 2013 | United States | This study assessed how health literacy impacts cervical cancer screening knowledge and behaviours of women living with hiv. | Women living with hiv, 20 to 68 years old, attending clinic and community-based settings in southeastern United States (N=145); African American (N=131; 90%); reported low health literacy (N=55; 38%) | Quantitative: multi-item, self-report questionnaire |
| Chapman Lambert, Chandler, McMillan, Kromrey J, Johnson-Mallard, Kurtyka, 2015 | United States | This study evaluated the relationships between Pap test adherence and hpv and cervical cancer knowledge, perceptions of risk, seriousness, barriers, benefits, self- efficacy using HBM | Women living with hiv, 18+ years of age, and attending one of the two hiv ambulatory care clinics (N=300). Exclusion criteria: history of having had a hysterectomy, women who could not read and comprehend English |
Quantitative: exploratory, cross- sectional, correlational design |
| Maree and Moitse, 2014 | South Africa | This study explored knowledge of cervical cancer and cervical screening amongst women being treated for hiv/aids at a specific health care centre. | Women living with hiv/aids, 18+ years of age, currently receiving antiretroviral therapy (ART), attending clinic for follow-up care (N=315) | Quantitative: survey design, self- administered questionnaire |
| Rosser, Njoroge, and Huchko, 2015 | Kenya | This study assessed cervical cancer knowledge, attitudes, and screening history of women living with hiv in care at an urban clinic in Western Kenya. | hiv -positive women, 23 to 64 old, attending an hiv clinic, non-pregnant (N=106) | Quantitative: survey, oral questionnaire; cross-sectional study |
| Koneru, Jolly, Blakemore, et al., 2017 | Tanzania | This study identified barriers to cervical cancer screening and treatment amongst women living with hiv, and determined acceptability of peer navigator roles in a clinical setting. | Women living with hiv, 19+ years of age, attending hiv clinics in Dar es Salaam, Tanzania (N=399) | Quantitative: self-administered questionnaire (English or Kiswahili) with guided assistance from study staff; or interviewer-administered for women who could not read; cross-sectional study |
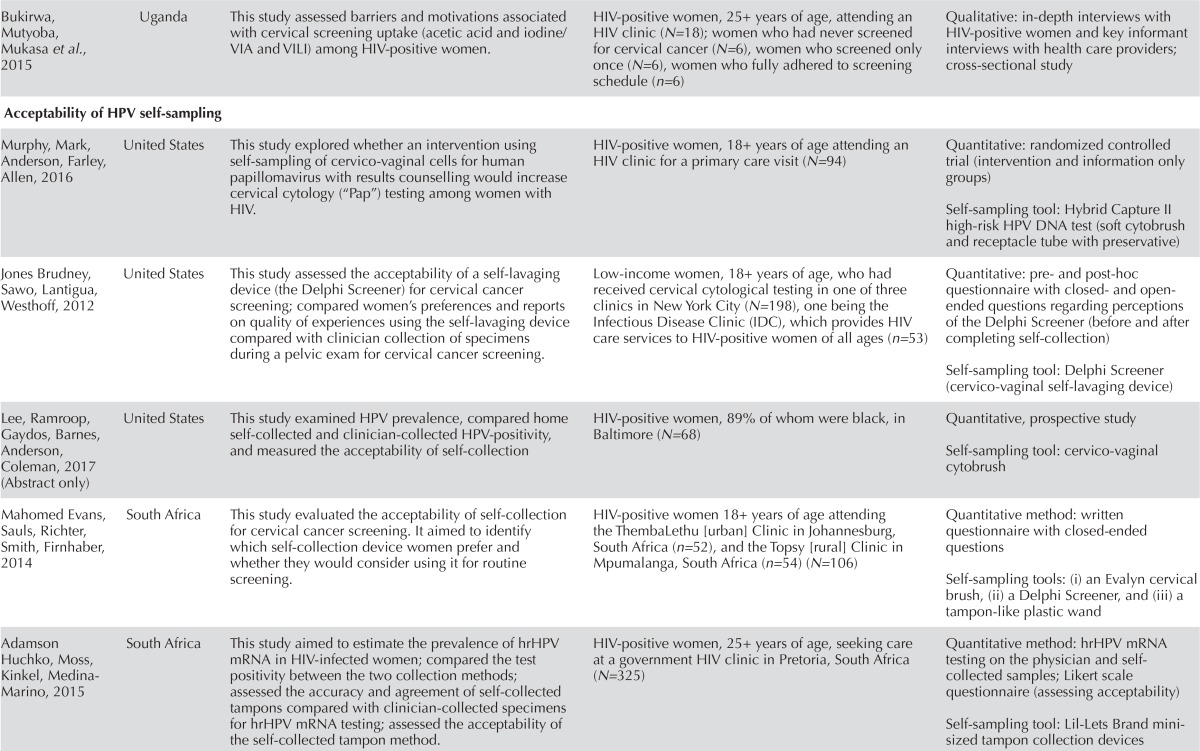
| Bukirwa, Mutyoba, Mukasa et al., 2015 | Uganda | This study assessed barriers and motivations associated with cervical screening uptake (acetic acid and iodine/VIA and VILI) among hiv-positive women. | hiv-positive women, 25+ years of age, attending an hiv clinic (N=18); women who had never screened for cervical cancer (N=6), women who screened only once (N=6), women who fully adhered to screening schedule (n=6) | Qualitative: in-depth interviews with hiv-positive women and key informant interviews with health care providers; cross-sectional study |
| Acceptability of hpv self-sampling | ||||
| Murphy, Mark, Anderson, Farley, Allen, 2016 | United States | This study explored whether an intervention using self-sampling of cervico-vaginal cells for human papillomavirus with results counselling would increase cervical cytology (“Pap”) testing among women with hiv. | hiv-positive women, 18+ years of age attending an hiv clinic for a primary care visit (N=94) | Quantitative: randomized controlled trial (intervention and information only groups) Self-sampling tool: Hybrid Capture II high-risk hpv DNA test (soft cytobrush and receptacle tube with preservative) |
| Jones Brudney, Sawo, Lantigua, Westhoff, 2012 | United States | This study assessed the acceptability of a self-lavaging device (the Delphi Screener) for cervical cancer screening; compared women’s preferences and reports on quality of experiences using the self-lavaging device compared with clinician collection of specimens during a pelvic exam for cervical cancer screening. | Low-income women, 18+ years of age, who had received cervical cytological testing in one of three clinics in New York City (N=198), one being the Infectious Disease Clinic (IDC), which provides hiv care services to hiv-positive women of all ages (n=53) | Quantitative: pre- and post-hoc questionnaire with closed- and open- ended questions regarding perceptions of the Delphi Screener (before and after completing self-collection) Self-sampling tool: Delphi Screener (cervico-vaginal self-lavaging device) |
| Lee, Ramroop, Gaydos, Barnes, Anderson, Coleman, 2017 (Abstract only) | United States | This study examined hpv prevalence, compared home self-collected and clinician-collected hpv-positivity, and measured the acceptability of self-collection | hiv-positive women, 89% of whom were black, in Baltimore (N=68) | Quantitative, prospective study Self-sampling tool: cervico-vaginal cytobrush |
| Mahomed Evans, Sauls, Richter, Smith, Firnhaber, 2014 | South Africa | This study evaluated the acceptability of self-collection for cervical cancer screening. It aimed to identify which self-collection device women prefer and whether they would consider using it for routine screening. | hiv-positive women 18+ years of age attending the ThembaLethu [urban] Clinic in Johannesburg, South Africa (n=52), and the Topsy [rural] Clinic in Mpumalanga, South Africa (n=54) (N=106) | Quantitative method: written questionnaire with closed-ended questions Self-sampling tools: (i) an Evalyn cervical brush, (ii) a Delphi Screener, and (iii) a tampon-like plastic wand |
| Adamson Huchko, Moss, Kinkel, Medina-Marino, 2015 | South Africa | This study aimed to estimate the prevalence of hr hpv mRNA in hiv-infected women; compared the test positivity between the two collection methods; assessed the accuracy and agreement of self-collected tampons compared with clinician-collected specimens for hr hpv mRNA testing; assessed the acceptability of the self-collected tampon method. | hiv-positive women, 25+ years of age, seeking care at a government hiv clinic in Pretoria, South Africa (N=325) | Quantitative method: hr hpv mRNA testing on the physician and self- collected samples; Likert scale questionnaire (assessing acceptability) Self-sampling tool: Lil-Lets Brand minisized tampon collection devices |
| Rositch, Gatuguta, Choi, et al., 2012 | Kenya | This study assessed adult women’s knowledge of hpv and cervical cancer and characterized their attitudes towards potential screening and prevention strategies (including routine Pap smears, self-sampling for hpv DNA testing, and hpv vaccination). | Women in hiv discordant couples (n=268 [65%] hiv-positive women) attending voluntary counselling and testing centres in Nairobi, Kenya (N=408) | Quantitative method: interviewer-administered questionnaire Self-sampling tool: no self-sampling device was assessed in this study |
| Mitchell, Pedersen, Eng Stime, et al., 2017 | Uganda | This study described knowledge and intentions of wlhiv towards hpv self-collection for cervical cancer screening; reported on factors related to hpv positivity among women who participated in testing. | hiv-positive women, 30 to 69 years old, attending the Kisenyi Health Unit (N=87) | Quantitative: cross-sectional validated survey (open-ended and closed-ended questions) Self-sampling tool: self-collected vaginal swab |
| Ndayisaba Verwijs, van Eeckhoudt, et al., 2013 | Rwanda | This study determined the feasibility and acceptability of the Delphi Screener (a novel cervico-vaginal lavage self-sampling device) among high-risk Rwandan women. | 60 Rwandan women, 22 to 35 years old (n=30 hiv-negative female sex workers [FSW] and n=30 hiv-positive women at an hiv clinic at Muhima Hospital) | Quantitative, prospective study: 7 study visits (clinician-observed self-sampling visits scheduled between visits 5 and 7, followed by interviews) Self-sampling tool: Delphi Screener (cervico-vaginal self-lavaging device) |
hpv = human papillomavirus; wlhiv = women living with hiv ; HBM = Health Belief Model; VIA = visual inspection with acetic acid; VILI = visual inspection with lugol’s iodine; hr hpv = high risk human papillomavirus.
RESULTS
The results of this review show that there is a paucity of research on wlhiv’s knowledge on hpv and cervical cancer screening, and their acceptability of self-sampling. A total of 17 articles and 1 abstract met the inclusion criteria. We included 1 published abstract of a relevant study that was informative but had not yet been published as an article or report. Ten of the articles focused on knowledge of hpv and cervical cancer screening among wlhiv, but 3 of these 10 articles were from the same research study reporting on different aspects of the study results. The remaining 7 articles and 1 abstract focus on acceptability of self-sampling. Together, we have identified a total of 16 studies in this review: United States (n=7); and African countries (n=9).
Part 1: Knowledge of WLHIV about HPV and cervical cancer screening
Of the eight studies (10 articles) included in the knowledge section of this review, three used qualitative methods of focus groups or individual interviews to explore knowledge and perceptions of hpv and cervical cancer screening24–26, and five used questionnaires to test knowledge, attitudes, perceptions, and screening behaviours27–33. A synthesized analysis of the data derived resulted in five major themes: (1) knowledge of hpv and cervical cancer screening; (2) factors influencing knowledge; (3) perceived risks and susceptibility; (4) misconceptions and barriers; and (5) screening compliance and adherence.
Knowledge of HPV and Cervical Cancer Screening
Our review results revealed that wlhiv across all regions had limited knowledge regarding hpv transmission and cervical cancer prevention. Most of them had not heard of hpv and did not know how hpv is transmitted24,29,33. Further, many wlhiv were unaware of the risk of cervical cancer and did not know that it is preventable25,31 and that hiv infection increases the risk of hpv and cervical cancer29.
Although some women were aware that their hiv-positive status and current sexual activities put them at risk of cervical cancer26, and that cervical cancer screening is important for wlhiv27,32,33, their knowledge about follow-up screening was mixed. Some recognized the need for regular Pap tests and repeated Pap tests after an abnormal test, but others thought that ‘doing nothing’ was one of the acceptable follow-up options27. Many were unaware of the screening recommendations for wlhiv25,26.
Factors Influencing Knowledge
Numerous individual and social factors shaped the knowledge of wlhiv regarding hpv and cervical cancer screening, including adequate and effective communication of health information from service providers28,31,33, media messages32, higher levels of education, previous history of cervical cancer screening, older age, being employed33. In the case of the United States and African countries, where health care access is not universal, access to hpv screening also increased wlhiv’s knowledge.33
Assessment of the relationship between health literacy and increased knowledge of hpv produced mixed results. One study showed that having high or low health literacy did not make any difference to the participants’ scores specific to knowledge of hpv, cervical cancer screening knowledge, and awareness of the hpv vaccine30. However, in a study of wlhiv with a history of abnormal Pap tests, participants with a high level of health literacy were 3.5 times more likely to read information about abnormal Pap tests given by health care providers and 4.7 times more likely to have understood the information compared with those with low health literacy28. Since these studies used quantitative methods, it was difficult to gain a better understanding about how the wlhiv’s lived experiences, social environments, and cultural contexts shaped their access to knowledge, as well as how they made sense of hpv and hpv-related messages or materials.
Perceived Risks and Susceptibility
Most of the wlhiv in this review lacked a clear understanding about the risks of cervical cancer associated with hiv and hpv co-infections24,25,29,31,32, which may explain their mixed and often contradictory perceptions about risk and susceptibility to cervical cancer. In an American study of 300 wlhiv, most participants identified having multiple sex partners and early onset of sexual intercourse as risks for cervical cancer, but few knew about hpv and other risks31. Women in another study conducted in the United States spoke about risks and susceptibility in the context of what they knew about hiv. Some felt that they were more susceptible to cervical cancer because they had “weak bodies” and they could catch “everything.” Other women in the same study rejected the idea that being hiv-positive increased their susceptibililty25. Further, while some women recognized increased susceptibility among wlhiv, they did not perceive themselves to be at risk until they experienced symptoms26,33. Others cited a lack of family history of cervical cancer and current sexual inactivity as reasons for not considering themselves at risk25.
Misconceptions and Barriers
This review uncovered many myths and misconceptions held by wlhiv, which might have inf luenced their participation in cervical cancer screening and follow-up behaviours. Over half of the wlhiv in a Kenya-based study believed that family planning increased the risks of cervical cancer and that vaginal washing would decrease the risks. Further, many considered cervical cancer as fate or the will of God and therefore felt nothing could prevent it33. Some participants in the Ugandan study did not engage in screening as they believed that service providers would remove their ovaries and uterus during the procedure26.
In terms of barriers to hpv and cervical cancer screening, wlhiv identified hiv stigma and discrimination from health care providers, family, and friends as a key barrier24,25,31. Other barriers to screening were related to psychological, sociocultural, and structural factors, including worries about finding out more health problems such as cervical cancer in addition to their hiv illnesses25, embarrassment, body modesty, and fear of painful procedures26,31,31. Many wlhiv indicated their need to have female primary care providers perform the cervical cancer screening due to their cultural and religious practices25,32. Others identified long wait time as a barrier to screening.
Screening Compliance and Adherence
This review also uncovered an interesting phenomenon. Many wlhiv who received instructions from health care providers to undergo follow-up screening tended to comply. For example, in a study based in the United States, participants had received a Pap test in the previous year even though none of them understood the purpose of a Pap test24. In a study of wlhiv with a history of abnormal Pap tests, close to half of the women indicated that they did not fully understand the information given by their primary care providers, but a majority of them complied with their recommendations of getting follow-up tests and procedures28. This shows that health care providers’ endorsement and recommendation for screening can influence women’s uptake.
Other studies suggest that screening adherence is associated with health literacy and social support. In the study of 399 women in an hiv clinic in Tanzania, only 9% of the participants had cervical cancer screening; 96% expressed the desire to learn more about cervical cancer screening and 87% prefer to have hiv-positive peer navigators to support them in learning and seeking screening services32.
Part 2: Acceptability of HPV Self-Sampling among Women Living with HIV
Literature on the acceptability of hpv self-sampling among wlhiv is also very limited; we included a total of eight studies (seven published articles and one abstract34) in this section. In synthesizing the evidence from these eight studies, we identified three major themes: (1) acceptability and preference for self-sampling vs. clinician-collected sampling; (2) concerns regarding self-sampling; and (3) feasibility and effectiveness of self-sampling devices.
Acceptability and Preference for Self-Sampling
With the exception of one questionnaire-based study35, a wide array of hpv and cervical cancer self-sampling devices were assessed for acceptability in the reviewed studies: cervico-vaginal self-lavaging devices36–38,, cervical brushes37, cytobrushes34,39, tampon-like self-collection devices37,40, and a vaginal swab41 (for descriptions of these devices, see Table II).
TABLE II.
Self-sampling devices used in reviewed studies
| Self-lavaging device37 (brand name: Delphi Screener) | A device that a woman inserts into her vagina. It contains a syringe in which 5 mm of buffered saline was plunged into the vagina, and the fluid specimen was retrieved by pulling back on the plunger. |
| Cytobrushes37 (brand name: Evalyn Brush) | A device consisting of an extended-tip spatula with an endocervical brush, which a woman inserts into her vagina and rotates the brush at the cervix five times to collect cells. |
| Tampon-like plastic wand device37 (brand name: Fournier) | A tampon-like plastic wand device with an electable tip which a woman inserts into her vagina and rotates the device to collect cells. |
| Mini-sized tampon40 (brand name-Lil-Lets) | A mini-size tampon that a woman inserts and leaves in her vagina for one to two hours before removing it and putting it in the specimen container. |
| Other devices39,41 | The brands and procedures for the self-sampling devices used in some studies were not specified. |
There was a universal acceptance among wlhiv across all studies in this review for self-sampling. Familiarity with the self-sampling device was a key factor associated with women’s willingness to use it. In one study of 325 wlhiv in South Africa40, the mini-tampon self-sampling device was used to assess the accuracy, agreement, and acceptability of self-sampled specimens versus clinician-collected specimens. The results showed that 90% of the participants reported no difficulties with self-sampling and that the instructions were clear and easy to understand, likely because over half of the women indicated familiarity in using tampons.
Other studies found that the self-lavaging device was highly accepted by participants at hiv clinics36,38. The women in these studies indicated that they would prefer self-lavaging for future cervical screening because this device felt more comfortable and less painful and was quick and easy to use. Some also identified increased privacy, decreased stress and embarrassment, and the possibility of performing the test on their own time as desirable36. Further, one-fifth of the women sampled in Rwanda preferred self-sampling over Pap tests mainly because of detesting the pelvic examination38.
While all the participants remained steadfast to self-sampling being the preferred option for cervical screening, they had varying preferences regarding the location for retrieval of their self-samples. Women in a study in Kenya preferred at-home collection35; women in the Ugandan study did not indicate any preference41. However, in a study in Pretoria, only one-tenth of the women preferred to perform self-sampling at home, while close to 65% preferred clinic-based self-collection, likely because they felt better cared for in the presence of a clinician during the collection procedure40.
Concerns Regarding Self-Sampling
Despite the wide acceptance of self-sampling among wlhiv, a small proportion had concerns regarding the devices and the self-sampling procedures. Some participants in an American study indicated that a pelvic exam performed by a physician would provide them with an increased sense of security; they felt that the physician would make minimal mistakes and could immediately intervene in case an abnormality was found36. Other women in the Rwandan and Kenyan studies expressed concerns about proper self-sample collection, pain, ability to insert the device, and not knowing how to interpret the results35,38. These concerns were in stark contrast to women in a study in Uganda, who expressed confidence and self-efficacy about self-sampling at home41. This contrast could possibly be related to the Advances in Screening and Prevention in Reproductive Cancers (aspire) project in Uganda, which has been established since 2013 to introduce self-sampling to this region and has likely helped to normalize cervical cancer self-sampling42.
Feasibility and Effectiveness of Self-Sampling Devices
In the context of this review, effectiveness referred to the similarity in testing scores between self-collected and physician-collected specimens, i.e., sensitivity and specificity, and feasibility was defined as the ability of participants to obtain the required amount of specimen during self-sampling without any self- or clinician-reported problems.
One study reported high specificity for cytobrush tests: the study found Pap tests were normal among 81.5% of negative home self-collected cytobrush tests34. Another study that utilized mini-tampons as a self-sampling device found no difference in sensitivity between the clinician-collected samples and the self-collected samples40.
Previous research reported that cytologic testing of hrhpv using specimens collected through self-lavaging and those collected by clinicians had equivalent sensitivity for detecting high-grade cervical intraepithelial neoplasia36. However, effectiveness of the self-lavaging devices was not assessed in any of the studies in this review. On the other hand, the self-lavaging device used with a cohort of 60 women in the Rwandan study showed moderate feasibility38.
DISCUSSION
We conducted this scoping review with the aim of obtaining an overall picture of wlhiv’s knowledge of hpv and cervical cancer screening, their acceptance of hpv self-sampling, and current gaps in research and knowledge. The results of this review showed both a concerning and a hopeful picture.
We found that wlhiv across all countries and regions had limited knowledge regarding hiv and hpv co-infection, cervical cancer prevention, and screening recommendations. There was also inadequate communication between wlhiv and their health care providers about screening for hpv and related cancers. Many wlhiv held misconceptions about cervical cancer screening. Although some women complied with cervical cancer screening and follow-up procedures based on their service providers’ recommendations, their lack of understanding about these tests impedes sustainable health behaviours. Further, their passive compliance reinforced their marginalization and disempowerment, which are contradictory to the principles of health promotion43. At the same time, wlhiv also reported a number of screening barriers in addition to their low hpv literacy. Women in the United States and African countries identified stigma as a barrier to access hpv and cervical cancer screening24,25,31. African-American wlhiv in one American study indicated that they avoid screening due to their fear of being diagnosed with cancer or serious illnesses25. These psychological challenges were likely unique to wlhiv, related to their experiences of being diagnosed hiv-positive44 and encountering everyday hiv-related stigma45. These women and women in the Tanzanian study also indicated their need for bodily modesty and having female primary care providers perform internal exams in order to maintain their cultural and religious practice25,32. Perhaps because of these barriers, hpv and cervical cancer self-sampling was found to be widely accepted by wlhiv in studies across countries and settings. Participants named many advantages, including flexibility and convenience in terms of time and location, privacy, and less discomfort and pain. However, some women expressed concerns about collecting proper self-samples and felt more secure about accessing tests at health care facilities.
Based on the results of this review, we offer a number of critical insights and recommendations. The concerns expressed by wlhiv have important policy and practice implications for primary health service delivery and health systems responses in Canada and across different countries. First, hiv and cancer have evolved over the past two decades into chronic health challenges as biomedical treatments have increased the survival rate of affected individuals. At the same time, we have also witnessed increased health disparities among individuals and groups who are simultaneously affected by multiple conditions, or what is known as synergistic epidemics (e.g., addiction and mental illness, diabetes and depression, hiv and Hepatitis C, hiv and hpv, etc.). It is important to note that synergistic epidemics (syndemics) are not merely concurrent physical diseases. Rather, they are health problems that are exacerbated by historical, sociocultural, economic, and political conditions, such as stigma and discrimination, access barriers to knowledge and services, uncoordinated health system responses, and lack of clear treatment guidelines or policies46. In the context of screening for hpv and related cancers among wlhiv, the adoption of a syndemic approach at the policy level will inform changes at the practice levels. For example, over the past few years, the Public Health Agency of Canada has adopted an integrated approach to address the syndemics of hiv, Hepatitis C, and other sexually transmitted and blood borne infections (stbbis) in Canada. This policy change was translated into the hiv and Hepatitis C Community Action Fund program, under which research and community service organizations were steered through funding incentives towards working more collaboratively at the local, regional and national levels, as well as expanding their program foci beyond hiv to address the co-infection of stbbi among priority (marginalized) populations47. However, judging by the organizations that had applied to this funding program, more deliberate efforts to promote cross-disciplinary collaboration among researchers and health care providers from the sectors of hiv and hpv-related cancer prevention is needed to develop effective responses.
Second, the framework of critical health literacy20 offers a potentially effective strategy to address the inadequate awareness about hiv-hpv co-infection and low screening for hpv and related cancers among wlhiv. Critical health literacy can be defined as the processes and outcomes that both increase people’s capacity to engage in a range of individual and collective actions that enhance their health, and also promote equitable access to health resources through policy change20. Critical health literacy is achieved and measured at three interconnected levels. In the context of this review, the first or basic level is measured by wlhiv’s ability to access, understand, critically appraise, and then apply health information on hiv-hpv co-infection to improve or maintain their health. To support wlhiv in achieving this basic level of health literacy, accurate, socioculturally inclusive, and accessible information on hiv-hpv co-infection (e.g., fact sheets, pamphlets, quizzes, videos, stories, posters, social marketing campaigns, etc.) must be made available. At the same time, hiv-hpv care competence must also be built among other stakeholders such as hiv specialists, primary care providers, and hiv/aids community service providers.
At the second or intermediate level, health literacy is measured by wlhiv’s ability to navigate the health care systems to access the screening they need. We postulate that when wlhiv acquire adequate knowledge about hiv-hpv co-infection, they are more likely to take part in screening. However, knowledge alone does not address other systemic and sociocultural barriers. To support wlhiv in developing confidence and effective communication with their health care providers, we recommend the use of peer empowerment programs, whereby wlhiv who are knowledgeable about hiv-hpv co-infection and have positive experiences in hpv screening are recruited and trained as peer leaders to support other wlhiv to navigate the hpv and related cancer screening systems32,48.
At the third and highest level, health literacy is measured by wlhiv’s ability to recognize the structural determinants of health and policy contexts of health care, and their engagement in social and political action that advance their individual and collective health. In Canada, under the Canadian Charter of Rights and Freedoms (1982), the Canadian Human Rights Act (1985), the Canadian Multiculturalism Act (1988), and various provincial human rights codes, health care providers and policy-makers are required to establish practice and policy guidelines that uphold and respect service users’ cultural and religious beliefs and practices49. Within the contexts of screening for hpv and related cancers among immigrant and racialized wlhiv, the availability of hpv self-sampling in home, community, or clinical settings offers a potential solution in addressing many wlhiv’s current unmet needs of time constraints, bodily privacy, or access to same-gender primary care providers. Currently, hpv self-sampling is not available in Canada. Engaging affected wlhiv in policy think-tanks is critical for the development of effective and inclusive responses on cervical cancer prevention.
Finally, we were not able to identify any Canadian research on our chosen topics during this scoping review. There are a number of plausible explanations. Within the field of cancer prevention research, research attentions have shifted to examining the clinical effectiveness and efficiency of hpv vaccines, vaccination as prevention, and the uptake of hpv vaccines among different vulnerable populations such as young women, young men, and men who have sex with men (msm). Within the hiv research field, the advent of haart has led to the assumptions that people living with hiv (plhiv) are no longer faced with serious health challenges. Research attentions have turned to hiv Pre-Exposure Prophylaxis (prep) and treatment as prevention, which, while they are important prevention strategies, do not address syndemic challenges such as disparities in hiv-hpv co-infection and hpv-related cancers among plhiv. Further, research does not take place in a vacuum. It is driven by available funding, human resources, and sociopolitical forces such as silos in education, research, and practice within and across disciplines. Research funding organizations and their review panels play an important role in shaping the topics and designs of research, as well as reinforcing or dismantling disciplinary silos in research. In Canada, it was only recently that the Canadian Institute of Health Research (cihr) began to address these silos by bringing different cihr institutes together to co-sponsor research funding opportunities. For example, in August 2017 cihr launched the Catalyst Grant: hpv Screening & Vaccination in Underserved Populations, sponsored by the Institute of Cancer Research in partnership with the Institute of Infection and Immunity in consultation with the Institute of Aboriginal Peoples’ Health. “Men who have sex with men (msm) and people living with hiv” were added to the original target populations (indigenous peoples, newcomers to Canada, sex workers, injection drug users and remote populations) to promote synergy50.
In conclusion, as Canada and other countries move forward with the 90-90-90 targets51 to achieve population hiv viral suppression and end the aids epidemic by 2030, policy-makers and service providers must not lose sight of the commitment to promote the health and quality of life of people who are living with hiv/aids. They must ensure that the health systems work in synergy to address hiv-related syndemics, including persistent hpv infections and hpv-related cancers46. Synergistic collaboration is also needed in the field of health education and health promotion to address the knowledge gap, among wlhiv and communities, about the complex interplay of hiv, hpv, and cervical cancer. Community empowerment and capacity building strategies52 are needed to address structural violence (stigma, discrimination, racism, cultural domination, etc.) that deters wlhiv from accessing hpv and cervical cancer screening and other health services. Despite a paucity of research, hpv self-sampling seems to offer the possibility of increasing screening among wlhiv. The adoption of hpv screening in Canada will open up dialogue about hiv-hpv syndemic challenges and increase awareness in the hiv/ aids communities. Building on the principles of greater and meaningful involvement of people living with hiv/aids (gipa/mipa), peer leadership models48,53 may enhance self-efficacy and confidence of wlhiv who engage in hpv self-sampling.
ACKNOWLEDGMENTS
This work was supported by the OHTN-CIHR New Investigator Award Priority Announcement in hiv/aids (Health Service/ Population Health Research) received by the first author (Funding Reference #129974).
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Public Health Agency of Canada. hiv and aids in Canada: Surveillance Report to December 31, 2013. Ottawa: Minister of Public Works and Government Services Canada; 2014. [Google Scholar]
- 2.Ghebre R, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in hiv-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101–8. doi: 10.1016/j.gore.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham AG, D’Souza G, Jing Y, et al. Invasive cervical cancer risk among hiv-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62(4):405–13. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with aids, 1980–2007. JAMA. 2011;305(14):1450–9. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-hiv cancer risk factors in persons living with hiv/aids: a meta-analysis. AIDS. 2016;30(2):273–91. doi: 10.1097/QAD.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in hiv-1-infected women. Clin Infect Dis. 2014;59(1):127–35. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobucci RNO, Lima PH, de Souza PC, et al. Assessing the impact of HAART on the incidence of defining and non-defining aids cancers among patients with hiv/aids: a systematic review. J Infect Public Health. 2015;8(1):1–10. doi: 10.1016/j.jiph.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with aids. J Natl Cancer Inst. 2009;101(16):1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Health Agency of Canada . Canadian guidelines on sexually transmitted infections. Ottawa, ON: Public Health Agency of Canada; 2006. [Web page]. [Available at: www.phac-aspc.gc.ca/std-mts/sti_2006/sti_intro2006_e.html; cited 25 July 2017] [Google Scholar]
- 10.Kendall CE, Taljaard M, Younger J, Hogg W, Glazier RH, Manuel DG. A population-based study comparing patterns of care delivery on the quality of care for persons living with hiv in Ontario. BMJ open. 2015;5(5):e007428. doi: 10.1136/bmjopen-2014-007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leece P, Kendall C, Touchie C, Pottie K, Angel JB, Jaffey J. Cervical cancer screening among hiv-positive women: retrospective cohort study from a tertiary care hiv clinic. Can Fam Physician. 2010;56(12):e425–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Carter A, Greene S, Nicholson V, et al. ‘It’s a very isolating world’: the journey to hiv care for women living with hiv in British Columbia, Canada. Gender Place Cult. 2016;23(7):941–54. doi: 10.1080/0966369X.2015.1073701. [DOI] [Google Scholar]
- 13.Kronfli N, Lacombe-Duncan A, Wang Y, et al. Access and engagement in hiv care among a national cohort of women living with hiv in Canada. aids Care. 2017;29(10):1235–42. doi: 10.1080/09540121.2017.1338658. [DOI] [PubMed] [Google Scholar]
- 14.Chen YYB, Li AT, Fung KP, Wong JP. Improving access to mental health services for racialized immigrants, refugees, and non-status people living with hiv/aids. J Health Care Poor Underserved. 2015;26(2):505–18. doi: 10.1353/hpu.2015.0049. [DOI] [PubMed] [Google Scholar]
- 15.Logie CH, James L, Tharao W, Loutfy MR. hiv, gender, race, sexual orientation, and sex work: a qualitative study of intersectional stigma experienced by hiv-positive women in Ontario, Canada. PLOS Med. 2011;8(11):e1001124. doi: 10.1371/journal.pmed.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson SE, Milloy M, Ogilvie G, et al. The impact of criminalization of hiv non-disclosure on the healthcare engagement of women living with hiv in Canada: a comprehensive review of the evidence. J Int aids Soc. 2015;18(1):20574–14. doi: 10.7448/IAS.18.1.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores BE, Acton GJ. Older Hispanic women, health literacy, and cervical cancer screening. Clin Nurs Res. 2013;22(4):402–15. doi: 10.1177/1054773813489309. [DOI] [PubMed] [Google Scholar]
- 18.Essink-Bot ML, Dekker E, Timmermans DRM, Uiters E, Fransen MP. Knowledge and informed decision-making about population-based colorectal cancer screening participation in groups with low and adequate health literacy. Gastroenterol Res Pract. 2016;7292369:1–8. doi: 10.1155/2016/7292369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of hiv self-testing: a systematic literature review. BMC Public Health. 2013;13(1):735. doi: 10.1186/1471-2458-13-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nutbeam D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259–67. doi: 10.1093/heapro/15.3.259. [DOI] [Google Scholar]
- 21.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 22.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the Methodology. Implement Sci. 2010;5(1):69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Government of Canada . Performance Monitoring for Cervical Cancer Screening Programs in Canada. Ottawa: Public Health Agency of Canada; [Web page] [Available at: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/cd-mc/cancer/pmccspc-srpdccuc/pdf/cervical-eng.pdf; cited 15 August 2017] [Google Scholar]
- 24.Kenya S, Carrasquillo O, Fatil M, et al. Human papilloma virus and cervical cancer education needs among hiv-positive Haitian women in Miami. Women Health Issues. 2015;25(3):262–6. doi: 10.1016/j.whi.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams M, Moneyham L, Kempf M-C, Chamot E, Scarinci I. Structural and sociocultural factors associated with cervical cancer screening among hiv-infected African American women in Alabama. AIDS Patient Care STDS. 2015;29(1):13–9. doi: 10.1089/apc.2014.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukirwa A, Mutyoba J, Mukasa B, et al. Motivations and barriers to cervical cancer screening among hiv-infected women in hiv care: a qualitative study. BMC Women’s Health. 2015;15:82. doi: 10.1186/s12905-015-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigfall L, Bynum S, Brandt H, et al. Cervical cancer prevention knowledge and abnormal pap test experiences among women living with hiv/aids. J Cancer Educ. 2015;30(2):213–9. doi: 10.1007/s13187-014-0688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wigfall L, Bynum S, Friedman D, et al. Patient-provider communication with hiv-positive women about abnormal Pap test results. Women Health. 2017;57(1):19–39. doi: 10.1080/03630242.2016.1150386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman Lambert C, Chandler R, McMillan S, Kromrey J, Johnson-Mallard V, Kurtyka D. Pap test adherence, cervical cancer perceptions, and hpv knowledge among hiv-infected women in a community health setting. J Assoc Nurses aids Care. 2015;26(3):271–80. doi: 10.1016/j.jana.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Bynum S, Wigfall L, Brandt H, Richter D, Glover S, Hébert J. Assessing the influence of health literacy on hiv-positive women’s cervical cancer prevention knowledge and behaviors. J Cancer Educ. 2013;28(2):352–6. doi: 10.1007/s13187-013-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maree J, Moitse K. Exploration of knowledge of cervical cancer and cervical cancer screening amongst hiv-positive women. Curationis. 2014;37(1):1209. doi: 10.4102/curationis.v37i1.1209. [DOI] [PubMed] [Google Scholar]
- 32.Koneru A, Jolly P, Blakemore S, et al. Acceptance of peer navigators to reduce barriers to cervical cancer screening and treatment among women with hiv infection in Tanzania. Int J Gynaecol Obstet. 2017;138(1):53–61. doi: 10.1002/ijgo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosser J, Njoroge B, Huchko M. Cervical cancer screening knowledge and behavior among women attending an urban hiv clinic in western Kenya. J Cancer Educ. 2015;30(3):567–72. doi: 10.1007/s13187-014-0787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K, Ramroop R, Gaydos CD, Barnes P, Anderson J, Coleman J. Home hpv self-collection in hiv-infected women: assessing acceptability and prevalence. Obstet Gynecol. 2017;129(suppl 1):135–6. doi: 10.1097/01.AOG.0000514684.83298.09. [abstract 13M] [DOI] [Google Scholar]
- 35.Rositch AF, Gatuguta A, Choi RY, et al. Knowledge and acceptability of Pap smears, self-sampling and hpv vaccination among adult women in Kenya. PLOS One. 2012;7:e40766. doi: 10.1371/journal.pone.0040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones HE, Brudney K, Sawo DJ, Lantigua R, Westhoff CL. The acceptability of a self-lavaging device compared to pelvic examination for cervical cancer screening among low-income women. J Women Health. 2012;21(12):1275–81. doi: 10.1089/jwh.2012.3512. [DOI] [PubMed] [Google Scholar]
- 37.Mahomed K, Evans D, Sauls C, Richter K, Smith J, Firnhaber C. Human papillomavirus (hpv) testing on self-collected specimens: perceptions among hiv positive women attending rural and urban clinics in South Africa. Pan Afr Med J. 2014;17:189. doi: 10.11604/pamj.2014.17.189.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndayisaba G, Verwijs MC, van Eeckhoudt S, et al. Feasibility and acceptability of a novel cervicovaginal lavage self-sampling device among women in Kigali, Rwanda. Sex Transm Dis. 2013;40(7):552–5. doi: 10.1097/OLQ.0b013e31828e5aa5. [DOI] [PubMed] [Google Scholar]
- 39.Murphy J, Mark H, Anderson J, Farley J, Allen J. A randomized trial of human papillomavirus self-sampling as an intervention to promote cervical cancer screening among women with hiv. J Low Genit Tract Dis. 2016;20(2):139–44. doi: 10.1097/LGT.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adamson PC, Huchko MJ, Moss AM, Kinkel HF, Medina-Marino A. Acceptability and accuracy of cervical cancer screening using a self-collected tampon for hpv messenger-RNA testing among hiv-infected women in South Africa. PLOS One. 2015;10:e0137299. doi: 10.1371/journal.pone.0137299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell SM, Pedersen HN, Eng Stime E, et al. Self-collection based hpv testing for cervical cancer screening among women living with hiv in Uganda: a descriptive analysis of knowledge, intentions to screen and factors associated with hpv positivity. BMC Women Health. 2017;17:4. doi: 10.1186/s12905-016-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Advances in Screening and Prevention in Reproductive Cancers . About the Project. Vancouver, BC: Women’s Health Research Institute at BC Women’s; 2013. [Web page] [Available at: http://whri.org/advanced-in-screening-and-prevention-in-reproductive-cancers-aspire/; cited 10 August 2017] [Google Scholar]
- 43.World Health Organization . Division of Health Promotion. Ottawa charter for health promotion. World Health Organization; 1986. [Web page] [Available at: http://www.who.int/healthpromotion/conferences/previous/ottawa/en/; cited 2 September 2017] [Google Scholar]
- 44.Stevens PE, Hildebrandt E. Life changing words: women’s responses to being diagnosed with hiv infection. ANS Adv Nurs Sci. 2006;29(3):207–21. doi: 10.1097/00012272-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Li AT, Wong JP, Cain R, Fung KP. Engaging African-Caribbean, Asian, and Latino community leaders to address hiv stigma in Toronto. Int J Migr Health Soc Care. 2016;12(4):288–300. doi: 10.1108/IJMHSC-07-2014-0029. [DOI] [Google Scholar]
- 46.Singer M, Bulled N, Ostrach B, Mendenhall ES. Syndemics and the biosocial conception of health. Lancet. 2017;389(10072):941–50. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 47.Government of Canada . hiv and Hepatitis C Community Action Fund. Public Health Agency of Canada; 2017. [Web page] [Available at: https://www.canada.ca/en/public-health/services/funding-opportunities/hiv-hepatitis-community-action-fund-next-steps.html#s2; cited 2 September 2017] [Google Scholar]
- 48.Li A, Jagwani R, Chuang DM, Wong K, Soje L, Wong JP. From self-empowerment to building community: evidence of positive impact of a cross cultural treatment literacy and peer support skill development program in Canada. The 23rd International AIDS Conference; Durban, South Africa. 2016; [abstract] [Available at: http://www.aids2016.org/Portals/0/File/AIDS2016_Abstracts_LOW.pdf?ver=2016-08-10-154247-087; cited 2 September 2017] [Google Scholar]
- 49.Seljak D. Protecting religious freedom in a multicultural Canada. Diversity Magazine. 2012;9(3) [Available at: http://www.ohrc.on.ca/en/creed-freedom-religion-and-human-rights-special-issue-diversity-magazine-volume-93-summer-2012/protecting-religious-freedom-multicultural-canada; cited 2 September 2017] [Google Scholar]
- 50.Canadian Institute of Health Research . Catalyst Grant: hpv Screening & Vaccination in Underserved Populations. Canadian Institute of Health Research (CIHR); 2017. [Web page] [Available at: https://researchnet.ca/rnr16/vwOpprtntyDtls.do?prog=2738&view=currentOpps&org=CIHR&type=EXACT&resultCount=25&sort=program&all=1&masterList=true cited 2 September 2017] [Google Scholar]
- 51.UNaids . 90-90-90 An ambitious treatment target to help end the aids epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 52.Romero L, Wallerstein N, Lucero J, Fredine HG, Keefe J, O’Connell J. Woman to woman: coming together for positive change—using empowerment and popular education to prevent hiv in women. aids Educ Prev. 2006;18(5):390–405. doi: 10.1521/aeap.2006.18.5.390. [DOI] [PubMed] [Google Scholar]
- 53.McCreary LL, Kaponda CP, Davis K, Kalengamaliro M, Norr KF. Empowering peer group leaders for hiv prevention in Malawi. J Nurs Scholarsh. 2013;45(3):288–97. doi: 10.1111/jnu.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]