Abstract
Through a “virtual clinic,” we used the electronic medical record to identify and intervene upon patients with chronic lymphocytic leukemia (cll) who were not current for pneumococcal vaccines. Within 180 days, 100/160 patients (62%) received the recommended pneumococcal vaccine. A virtual clinic may improve vaccination rates among high-risk patient populations.
Keywords: 23-valent pneumococcal capsular polysaccharide vaccine, 13-valent pneumococcal vaccine, coordination of patient care, models of care, cancer care continuum
INTRODUCTION
Although pneumococcal immunizations are an important component of preventive health for adults, national immunization rates remain around 20%, well below the goal of 60% set by the United States Department of Health and Human Services1,2. For chronically ill adults who receive health care via specialty clinics, additional barriers may exist. First, physicians are both overly broad in applying contraindications and overestimate the proportion of patients in their panels who are current for adult vaccinations3. Second, specialty care clinics focus on specific medical problems and may overlook some aspects of preventive health, such as adult vaccinations. This may be due to competing priorities or because specialists believe that patients’ primary care physicians will address vaccines and other preventive health measures4. Third, health system barriers including incomplete or inaccessible documentation of previous vaccines may also contribute to missed opportunities for vaccination4.
The Advisory Committee on Immunization Practices (acip) recommends that adults with immunocompromising conditions, including chronic lymphocytic leukemia (cll), should receive one dose of the 13-valent pneumococcal conjugate vaccine (pcv13) and two doses, five years apart, of the 23-valent pneumococcal polysaccharide vaccine (ppsv23)5. Previously, we used a “virtual clinic” to improve rates of pneumococcal vaccine coverage among people with functional or anatomic asplenia6. Here, we describe using the virtual clinic to improve pneumococcal vaccinations among cll patients at a single Veteran Affairs (va) medical centre.
METHODS
Using structured language query (sql) to access our local Veterans Health Administration (vha) database, we employed International Classification of Diseases (icd) codes to identify a cohort of patients at our va medical centre with cll (icd9 codes 204.1 to 204.99 and icd10 codes C91.1, C91.90 and C91.Z). Inclusion criteria were a diagnosis of cll at the time of the study intervention and active in our regional medical system, defined as at least one in-person visit to a va clinic or hospital in our catchment area in the previous year. The exclusion criteria were a transfer of care to a va medical centre outside of our catchment area, a residential postal address outside of Ohio, actively receiving chemotherapy or hospice care, or a lack of clinic visits or medication refills in the prior year. Using both the vha database and the electronic medical record (emr), we further refined the cohort, verifying each patient’s pcv13 and ppsv23 immunization status, including the number and time intervals for ppsv23.
In August 2015, patients meeting our inclusion and exclusion criteria that were not current for either pcv13 and/or ppsv23 were enrolled into a “virtual clinic” as previously described6. The virtual clinic intervention had three components. First, virtual clinic providers reviewed the emr and placed an order for the appropriate pneumococcal vaccine that remained active for 90 days. Second, they wrote a note in the emr explaining the rationale for the vaccine and added as cosigners both the nurse and provider from the primary care patient-aligned care team (pact) working collaboratively to address the patients’ needs. For those patients receiving care for their cll from the va, the nurse and providers from the oncology team were also included as cosigners. Finally, the virtual clinic providers mailed a letter to patients recommending they receive a pneumococcal vaccine, explaining that they could receive the vaccine at any va facility, and that the appropriate vaccine was ordered. The letters explained risks of pneumococcal infection, the benefits of vaccination, and included the appropriate vaccine information statements for pcv13 or ppsv237,8.
The primary outcome measured after the above intervention was the administration of the recommended vaccine within 180 days following the placement of the pneumococcal vaccine order. Additional outcomes, assessed via chart review, included the type of clinic in which patients received the vaccine or reasons why patients were not vaccinated.
In May 2015, three months before this virtual clinic began, the va updated a national clinical reminder about pneumococcal vaccines to include recommendations for pcv13. To assess the effect of the virtual clinic on vaccination rates beyond that of the clinical reminder, we considered pcv13 vaccination rates before the clinical reminder (February – April 2015), after the introduction of the clinical reminder (May – July 2016), and after implementation of the virtual clinic (August – October 2016). For each of these periods, we considered the “at-risk” population to be active cll patients not yet vaccinated with pcv13. We used a log-rank test to assess differences in the pcv13 vaccination rates. All analyses were performed using R (R Version 3.1.1; Vienna, Austria)9. The Cleveland va Medical Center’s Institutional Review Board (IRB) approved the research protocol.
RESULTS
The initial sql query identified 336 patients with cll, 249 (74%) of whom were not current with their pneumococcal vaccines. After applying the exclusion criteria, 165 patients were enrolled in the virtual clinic. The majority were male (163; 99%) and self-identified as white (146; 88%); their mean age was 75 years (range 44 – 98). Most of the patients needed only pcv13 (154; 93%). For the minority that needed both pcv13 and ppsv23 (11, 7%), virtual clinic providers recommended pcv13 first, in accordance with acip recommendations10.
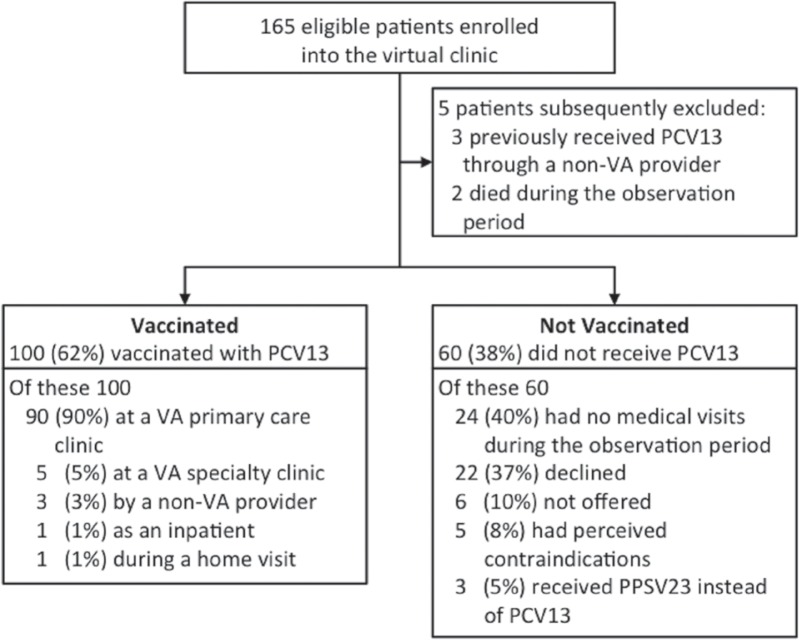
Figure 1 presents an overview of the outcomes. During the observation period, primary care providers determined that three patients had received pcv13 from a non-va provider prior to enrolment in the virtual clinic and added this information to those patients’ charts. Additionally, two patients died, leaving 160 patients in the cohort at the end of the observation period. A total of 100 patients (62%) in the virtual clinic received the recommended pneumococcal vaccine within 180 days of notification. Of these patients, 90 (90%) received their vaccine through their va primary care pact, while the remaining 10 patients received them from va specialty clinics, non-va providers, or during hospitalization or home visits. Of the 60 patients who did not receive the recommended pneumococcal vaccine, the most common reasons were infrequent visits to healthcare providers (24, 40%) and patients declining vaccination (22, 37%).
FIGURE 1.
Overview and outcomes of a virtual clinic for cll patients at a VA medical center. The primary outcome was vaccination with the recommended pneumococcal vaccine in the 180-day observation period following the virtual clinic intervention. CLL = chronic lymphocytic leukemia; PCV = pneumococcal conjugate vaccine; VA = veteran affairs.
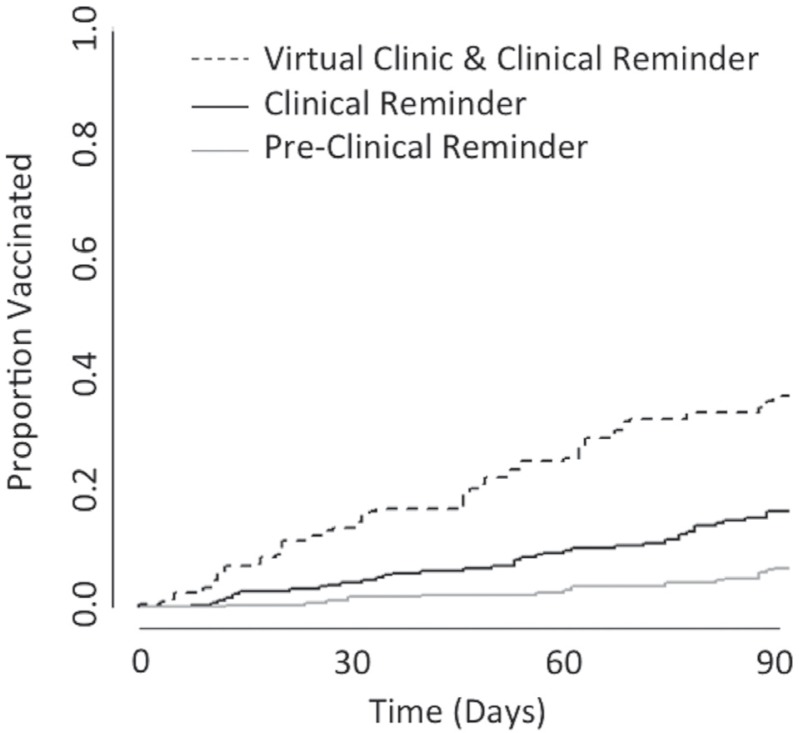
Figure 2 shows rates of pcv13 vaccination for previously unvaccinated cll patients at three consecutive three-month intervals. Using a log-rank test, we compared the time-to-event curves for the interval immediately following the introduction of the clinical reminder and following the virtual clinic intervention and found them to be significantly different (p < 0.01). These outcomes indicate that the virtual clinic increased pcv13 vaccination rates beyond that achieved by the clinical reminder alone.
FIGURE 2.
Rates of PCV13 vaccination among patients with CLL. Approximately three months before the virtual clinic intervention, the vha deployed a system-wide pneumococcal vaccine clinical reminder. To assess that influence compared with the virtual clinic, we compared the rates of PCV13 vaccination over 90-day intervals before the clinical reminder (January – March; grey line), following the deployment of the clinical reminder (May – July; black line), and after the virtual clinic intervention (August – November; dashed line). CLL = chronic lymphocytic leukemia; PCV = pneumococcal conjugate vaccine.
DISCUSSION
Our outcomes suggest that the virtual clinic strategy improves pneumococcal vaccination rates in a population that receives care through both primary and specialty care clinics. Furthermore, the increase in the vaccination rate following implementation of the virtual clinic indicates that this intervention augmented the clinical reminder in the emr.
Previous work by Lau et al. indicates that patient outreach, education, and clinical reminders are effective strategies to improve pneumococcal vaccination11. Our virtual clinic coupled outreach with education by sending letters notifying patients of their need for and the importance of pneumococcal vaccination. The virtual clinic also brought the need for vaccination to the attention of the patients’ primary care and oncology teams. Most patients in our cohort who received a pneumococcal vaccine did so via their primary care pact, which may reflect confidence in their providers, ease of access, and geographic proximity to their homes. Previous results indicated that notifying the pact nurses as well as the primary care provider improved vaccination rates6. In this intervention, some patients remained unvaccinated because they did not have medical visits during the observation period or declined the vaccine. Specific outreach from the primary care and/ or oncology teams to schedule a visit and provide education may have further increased vaccination rates.
Clinical reminders incorporated within the emr may increase the likelihood of pneumococcal vaccination among patients at vha medical centers12. Our regional emr had a pneumococcal clinical reminder for ppsv23 in place for several years, which helps to explain the relatively high compliance with this vaccine among our cohort. Updated to include pcv13 three months before the virtual clinic intervention, the pneumococcal clinical reminder prompts providers to discuss pneumococcal vaccines with patients, permits documentation of vaccine refusal or acceptance, and guides appropriate vaccine selection. The clinical reminder, however, still requires providers to prioritize vaccinations in the context of other clinical care, to discuss the vaccine with the patient, and to order the vaccine. An additional burden is “alert fatigue” as a result of the proliferation of clinical decision support notifications targeting various medical conditions and health measures13. The virtual clinic overcame one of these barriers by ordering the appropriate vaccine. Furthermore, notifying patients via letters mailed to their home may have made it easier for patients and providers to prioritize and discuss vaccination, in part by shifting some of this responsibility to the patient.
Our study has limitations. First, the intervention focused on a small group of patients within a single medical centre. Second, we did not systematically assess the reasons why patients did not receive the recommended vaccine, nor did we probe into reasons why patients refused vaccination. Third, the clinical reminder that preceded the virtual clinic intervention influenced pneumococcal vaccination rates, confounding the impact of the virtual clinic. Changes in the pcv13 vaccination rates following the virtual clinic intervention, however, suggest that it enhanced pcv13 vaccination rates beyond that of the clinical reminder alone.
The vha’s emr and patient-centered infrastructure were both highly conducive to this virtual clinic intervention, which can be implemented in vas nationwide. Several components may also be generalized to other healthcare settings that also have a robust emr accessible by primary and specialty care services. Specifically, these include using data queries to identify high-risk patients who need vaccinations, alerting providers about those patients, and sending letters to patients encouraging them to make an appointment and discuss vaccines with their healthcare team.
ACKNOWLEDGMENTS
This work was support by an educational grant from Pfizer (RJ, FP). This work was also supported in part by funds from the Cleveland Geriatric Research Education and Clinical Centers (GRECC) (FP, RB, BW, RJ) and from the National Institutes of Health (NIH), through the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH Roadmap for Medical Research (FP, RJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States Government.
FP and RJ designed and implemented the virtual clinic and also provided oversight for the project. RB, BW, and EC reviewed charts and analyzed data. EC wrote the initial manuscript draft, on which all authors subsequently commented.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Immunization and Infectious Diseases Healthy People 2020. [Available online at: http://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives#4658; cited 11 July, 2015.]
- 2.Williams WW. Surveillance of Vaccination Coverage Among Adult Populations — United States, 2014. MMWR Surveill Summ; 2016. [Available online at: http://www.cdc.gov/mmwr/volumes/65/ss/ss6501a1.htm; cited 25 February, 2017.] [DOI] [PubMed] [Google Scholar]
- 3.Donohoe MT. Comparing generalist and specialty care: discrepancies, deficiencies, and excesses. Arch Intern Med. 1998;158(15):1596–608. doi: 10.1001/archinte.158.15.1596. [DOI] [PubMed] [Google Scholar]
- 4.Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W, National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board Identifying barriers to adult pneumococcal vaccination: an nfid task force meeting. Postgrad Med. 2012;124(3):71–9. doi: 10.3810/pgm.2012.05.2550. [DOI] [PubMed] [Google Scholar]
- 5.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63(37):822–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Jump RL, Banks R, Wilson B, et al. A Virtual Clinic Improves Pneumococcal Vaccination for Asplenic Veterans at High Risk for Pneumococcal Disease. Open Forum Infect Dis; 2015. [Available online at: https://academic.oup.com/ofid/article/2/4/ofv165/2460559/A-Virtual-Clinic-Improves-Pneumococcal-Vaccination; cited 25 February, 2017.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccine Information Statement Pneumococcal Polysaccharide | VIS | CDC. [Available online at: http://www.cdc.gov/vaccines/hcp/vis/vis-statements/ppv.html; cited 11 July, 2015.]
- 8.Vaccine Information Statement Pneumococcal Conjugate | vis | cdc. [Available online at: http://www.cdc.gov/vaccines/hcp/vis/vis-statements/PCV13.html; cited 10 July, 2015.]
- 9.R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Available online at: URL http://www.R-project.org/]; cited 11 July, 2015. [Google Scholar]
- 10.Centers for Disease Control and Prevention (cdc) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (acip) MMWR Morb Mortal Wkly Rep. 2012;61(40):816–9. [PubMed] [Google Scholar]
- 11.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: a systematic review and meta-analysis. Ann Fam Med. 2012;10(6):538–46. doi: 10.1370/afm.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JS, Keyhani S, Keenan PS, et al. Use of recommended ambulatory care services: is the veterans affairs quality gap narrowing? Arch Intern Med. 2008;168(9):950–8. doi: 10.1001/archinte.168.9.950. [DOI] [PubMed] [Google Scholar]
- 13.McCullagh L, Mann D, Rosen L, Kannry J, McGinn T. Longitudinal adoption rates of complex decision support tools in primary care. Evid Based Med. 2014;19(6):204–9. doi: 10.1136/ebmed-2014-110054. [DOI] [PubMed] [Google Scholar]