Abstract
There is abundant evidence for formation of G protein-coupled receptor heteromers in heterologous expression systems, but little is known of the function of heteromers in native systems. Heteromers of δ and κ opioid receptors (DOR-KOR heteromers) have been identified in native systems. We previously reported that activation of DOR-KOR heteromers expressed by rat pain-sensing neurons (nociceptors) produces robust, peripherally mediated antinociception. Moreover, DOR agonist potency and efficacy is regulated by KOR antagonists via allosteric interactions within the DOR-KOR heteromer in a ligand-dependent manner. Here we assessed the reciprocal regulation of KOR agonist function by DOR antagonists in adult rat nociceptors in culture and in a behavioral assay of nociception. Naltrindole enhanced the potency of the KOR agonist 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-phenyl-2-pyrrolidin-1-ylethyl]acetamide (ICI-199441) 10- to 20-fold, but did not alter responses to 2-(3,4-dichlorophenyl)-N-methyl-N-[(1R,2R)-2-pyrrolidin-1-ylcyclohexyl]acetamide (U50488). By contrast, the potency of U50488 was enhanced 20-fold by 7-benzylidenenaltrexone. The efficacy of 6ʹ-guanidinonaltrindole (6ʹ-GNTI) to inhibit nociceptors was blocked by small interfering RNA knockdown of DOR or KOR. Replacing 6ʹ-GNTI occupancy of DOR with either naltrindole or 7-benzylidenenaltrexone abolished 6ʹ-GNTI efficacy. Further, peptides derived from DOR transmembrane segment 1 fused to the cell membrane–penetrating HIV transactivator of transcription peptide also blocked 6ʹ-GNTI–mediated responses ex vivo and in vivo, suggesting that 6ʹ-GNTI efficacy in nociceptors is due to its positive allosteric regulation of KOR via occupancy of DOR in a DOR-KOR heteromer. Together, these results provide evidence for the existence of functional DOR-KOR heteromers in rat peripheral sensory neurons and that reciprocal, ligand-dependent allosteric interactions occur between the DOR and KOR protomers.
Introduction
There is considerable evidence that G protein-coupled receptors (GPCRs) form heteromers (Gomes et al., 2016). However, compared with the large amount of data for heteromers obtained with heterologous expression systems, there is relatively little evidence for a role for heteromers in regulating function in physiologically relevant systems. In part, this may be due to limitations in the applicability of the techniques/approaches to study heteromers in native tissues. GPCR heteromers are of interest as drug targets because they can have unique pharmacologic properties and their restricted cellular/tissue distribution (heteromers can form only in cells that coexpress both protomers) provides for selectivity. Because heterologous systems do not always adequately model the cellular context of receptors expressed in native systems and cannot model the physiologic environment and complexity of integrated response systems that exist in vivo, it is important that more work be done to understand the role of heteromers in physiologically relevant systems.
Among the unique pharmacologic characteristics associated with heteromers are the allosteric interactions that can occur between the protomers. It has been shown that the presence of one protomer can alter membrane localization (Ellis et al., 2006; Rozenfeld et al., 2012), the affinity and/or efficacy of agonists (Martin and Prather, 2001; Wang et al., 2005; Rozenfeld et al., 2012), and signaling specificity (Charles et al., 2003; Rozenfeld et al., 2012) for the other promoter. Moreover, ligand binding to the orthosteric site of one promoter can change the pharmacologic properties of orthosteric ligands for the other protomer (Vischer et al., 2011; Ferré et al., 2016; Shivnaraine et al., 2016). For example, apelin binding to its receptor reduces the binding and efficacy of angiotensin II acting at the angiotensin II type 1 receptor via negative cooperativity between the protomers of the heteromer (Siddiquee et al., 2013). Guitart et al. (2014) showed synergy for dopamine D1 and D3 receptor agonists to stimulate extracellular signal-regulated kinase activation, but not for adenylyl cyclase activity, and this synergistic response was blocked by transmembrane peptides that blocked heteromer formation. Thus, orthosteric ligands for one protomer can act as allosteric regulators that influence affinity, efficacy, and signaling specificity of orthosteric ligands for the other protomer of a heteromer.
Several studies have shown that δ and κ opioid receptors (DOR and KOR, respectively) can form heteromers in heterologous expression systems (Jordan and Devi, 1999; Waldhoer et al., 2005; Xie et al., 2005), and there is also evidence for functional DOR-KOR heteromers in the spinal cord (Waldhoer et al., 2005; Ansonoff et al., 2010). Recently, we provided evidence for functional DOR-KOR heteromers in peripheral pain-sensing neurons (nociceptors) in primary cultures and in vivo (Berg et al., 2012). We found that KOR coimmunoprecipitated with DOR from cultures of adult rat sensory neurons from the trigeminal ganglia and that a DOR-KOR heteromer-selective antibody augmented the antinociceptive efficacy of the DOR agonist [D-Pen2,5]-enkephalin (DPDPE). Moreover, we found that antagonists selective for KOR altered the potency and/or efficacy of selective DOR agonists in a ligand-dependent manner both in sensory neuron cultures and in behavioral antinociception tests in vivo. Such ligand-dependent effects are a hallmark of allosterism (Smith and Milligan, 2010; Christopoulos et al., 2014). These results suggested a functional role for DOR-KOR heteromers in rat sensory neurons and that orthosteric KOR ligands regulated the function of orthosteric DOR ligands via intraheteromer allosteric interactions.
In addition to ligand dependence, another hallmark of allosterism is reciprocity (Smith and Milligan, 2010). Thus, it is expected that if the KOR antagonist regulation of DOR agonist function is due to intraheteromer allosteric regulation, then DOR ligands should regulate the function of KOR agonists. In this study, we examined the effects of DOR antagonists on KOR agonist-mediated inhibition of rat peripheral sensory neurons in culture and in vivo. We found that DOR antagonists altered the function of KOR agonists in a ligand-dependent manner. Moreover, we discovered that the putative DOR-KOR heteromer-selective activity of 6ʹ-guanidinonaltrindole (6ʹ-GNTI) in peripheral sensory neurons was due to allosteric enhancement of 6ʹ-GNTI efficacy at KOR via its occupancy of DOR and that this effect was blocked by DOR transmembrane peptides both in culture and in vivo. Taken together, these results provide strong evidence for a functional role of DOR-KOR heteromers in regulating the activity of rat peripheral pain-sensing neurons.
Materials and Methods
Materials.
We purchased 6ʹ-GNTI, 2-(3,4-dichlorophenyl)-N-methyl-N-[(1R,2R)-2-pyrrolidin-1-ylcyclohexyl]acetamide (U50488), and naltrindole (NTI), and rolipram from Sigma-Aldrich (St. Louis, MO). DPDPE, D-Ala2,N-MePhe4,Gly-ol5]-enkephalin (DAMGO), and bradykinin (BK) were purchased from Bachem Americas (Torrance, CA). ICI-199441 [2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-phenyl-2-pyrrolidin-1-ylethyl]acetamide], 7-benzylidenaltrexone (BNTX), and naltriben (NTB) were purchased from Tocris (Minneapolis, MN). Prostaglandin E2 (PGE2) was purchased from Cayman Chemicals (Ann Arbor, MI). 125I-cAMP was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Nerve growth factor was obtained from Harlan (Houston, TX), and collagenase from Worthington (Lakewood, NJ). ON-TARGET plus SMART pool rat small interfering RNA (siRNA) and Dharmafect transfection reagent were from GE Dharmacon (Pittsburgh, PA). Hank’s balanced salt solution, fetal bovine serum (FBS), and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Invitrogen (Carlsbad, CA). Dr. Philip S. Portoghese provided the bivalent KOR agonist/DOR antagonist ligand KDAN-18 (Daniels et al., 2005). All other drugs and chemical (reagent grade) were purchased from Sigma-Aldrich.
Animals.
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington MA) weighing 250–300 g were used in this study. The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to International Association for the Study of Pain and federal guidelines. Animals were housed for 1 week with food and water available ad libitum before behavioral testing or harvesting of sensory neurons of the trigeminal ganglia.
Behavioral Assay of Nociception.
Opioid agonist-mediated inhibition of PGE2-induced thermal allodynia was measured as described elsewhere (Rowan et al., 2009; Berg et al., 2011, 2012; Sullivan et al., 2015). Paw withdrawal latency (PWL) in response to application of a thermal stimulus to the ventral surface of the rat hindpaw was measured with a plantar test apparatus (Hargreaves et al., 1988). In brief, rats were placed in plastic boxes with a glass floor maintained at 30°C. After a 30-minute habituation period, the plantar surface of the hindpaw was exposed to a narrow beam (approximately 5 mm diameter) of radiant heat through the glass floor. The intensity of the thermal stimulus was adjusted so that baseline PWL values were 10 ± 2 seconds with a cutoff time of 25 seconds.
All drugs were dissolved in phosphate-buffered saline and administered via intraplantar injection (50 μl) into the rat hindpaw. To induce thermal allodynia, PGE2 (0.3 µg, intraplantar) was injected. We showed previously that this dose of PGE2 produces a mild (≈5-second reduction in PWL) and prolonged (>20 minutes) thermal allodynia (Rowan et al., 2009). Opioid agonists or vehicle were coinjected (intraplantar) with PGE2.
PWL measurements were taken in duplicate at least 30 seconds apart at 5-minute intervals before injections (baseline) and for 20 minutes after the last injection by observers blinded to the treatment allocation. As opioid receptors expressed by peripheral sensory neurons require an inflammatory stimulus for antinociceptive efficacy (Berg et al., 2007, 2011, 2012; Rowan et al., 2009; Stein and Lang, 2009; Stein and Zollner, 2009; Jamshidi et al., 2015; Sullivan et al., 2015), all experiments were done using pretreatment (15 minutes) with BK (25 µg, intraplantar) to induce opioid receptor functional competence. Injection of BK produces a transient allodynia that returns to baseline within 10 minutes (before administration of PGE2 ± opioid) (see Supplemental Figs. 4–6, 8, and 9). To verify that behavioral responses were due to local, peripherally restricted doses of a given agonist, PWL in the contralateral paw was measured after intraplantar injection into the ipsilateral paw (Rowan et al., 2009; Berg et al., 2011, 2012).
Primary Cultures of Peripheral Sensory Neurons.
Primary cultures derived from adult rat trigeminal ganglia were prepared as described elsewhere (Berg et al., 2007, 2012; Jamshidi et al., 2015). Cultures were re-fed with DMEM containing 10% FBS, 1% penicillin-streptomycin, 1% GlutaMAX I, 1% mitotic inhibitors (5-fluor-2-deoxyuridine, uridine) and 100 ng of nerve growth factor every 48 hours. Cultures were re-fed with DMEM without nerve growth factor or FBS (i.e., serum-free media) 24 hours before experiments performed between 6 and 8 days in culture.
siRNA Transfection.
Primary cultures of peripheral sensory neurons (3 days in culture) were transfected with ON-TARGET plus SMART pool rat siRNA (50 nM) directed against DOR (cat. no. L-080122-02), KOR (cat. no. L-090460-02) or nontargeting siRNA (cat. no. D-001810-10-05) using DharmaFECT 3 transfection reagent according to the manufacturers protocol. Cultures were re-fed with complete media 24 and 72 hours after transfection. Cells were re-fed with serum-free media 4 days after siRNA transfection, and experiments done 24 hours later.
Quantitative Real-Time Polymerase Chain Reaction Analysis of mRNA Levels.
The siRNA-mediated reduction in DOR or KOR mRNA levels was determined 48 hours and 5 days after siRNA transfection. Total RNA was isolated using the PureLink RNA Mini Kit (Life Technologies, Carlsbad, CA) and treated with DNAse (RNAse-free DNAse set; Qiagen, Valencia, CA). The RNA concentrations were measured using the Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA), and samples were normalized for equal RNA quantity.
Reverse transcription was performed following the manufacturer’s protocol using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Predesigned DOR (forward 5′-CCA GCT GGT ACT GGG ACA CT-3′; reverse 5′-CGA TGA CGA AGA TGT GGA TG-3′), KOR (forward 5′-TTG GCC TTT TGG AGA TGT TC-3′; reverse 5′-TGG TGC CTC CAA GGA CTA TC-3′), and the housekeeping gene and endogenous control actin (forward 5′-AGC CAT GTA CGT AGC CAT CC-3′; reverse 5′-ACC CTC ATA GAT GGG CAC AG-3′) primers were purchased from Invitrogen.
Reactions were performed in triplicate containing 2× SsoFast EvaGreenSupermix (Bio-Rad Laboratories, Hercules, CA), and quantitative real-time polymerase chain reaction was performed using the PE 9600 thermal cycler (PerkinElmer Life and Analytical Sciences). A “no template” control reaction was included in all assays. The relative expression of target genes to endogenous control was calculated using the formula 2−ΔΔCt, where ΔCt represents the magnitude of the difference between target and endogenous control cycle threshold (Ct) values. The values are expressed as the fold change relative to the control.
Measurement of Cellular cAMP Levels.
Opioid receptor-mediated inhibition of adenylyl cyclase activity was determined by measuring the amount of cAMP accumulated in cells treated with the Gs protein-coupled prostaglandin receptor agonist PGE2 and an opioid agonist in the presence of the phosphodiesterase inhibitor rolipram, as previously described elsewhere (Berg et al., 2007, 2012; Jamshidi et al., 2015). As described previously for the behavioral experiments, the cells were pretreated for 15 minutes with BK to induce opioid receptor functional competence. Cells were washed twice with Hanks’ balanced salt solution containing 20 mM HEPES, pH 7.4 (wash buffer), followed by preincubation in wash buffer (250 µl per well, 48-well plate) for 15 minutes at 37°C with BK (10 µM).
To assess opioid agonist-mediated responses, cells were incubated with rolipram (0.1 mM) along with a maximal concentration of PGE2 (1 µM) with opioid ligands or vehicle for 15 minutes at 37°C. Incubations were terminated by aspiration of the buffer and addition of 500 µl of ice-cold absolute ethanol. The ethanol extracts from individual wells were dried under a gentle air stream and reconstituted in 100 µl of 50 mM sodium acetate, pH 6.2. The cAMP content of each well was determined by radioimmunoassay.
DOR Transmembrane Peptides.
Peptides consisting of residues corresponding to the first transmembrane (TM) spanning domain of DOR (AITALYSAVCAVGLLGNVLVMFGI) fused to the cell membrane penetrating HIV transactivator of transcription (TAT) peptide (YGRKKRRQRRRPQ) (Brooks et al., 2005) were synthesized (95% purity) by Lieftein (Hillsborough, NJ). For insertion of the DOR transmembrane domain 1 (TM1) TAT peptide in the proper orientation (i.e., same direction as that of TM1 in native DOR), the TAT sequence was fused to the C terminus of the peptide. As a negative control, the TAT sequence was fused to the N terminus of the DOR TM1 peptide (TAT-TM1) so that membrane insertion would occur in the opposite direction of the native DOR TM1 (He et al., 2011). For behavioral experiments, TM-TAT peptides were dissolved in sterile phosphate-buffered saline (78.8 µg/ml), and the rats received 50 µl (3.94 µg) intraplantar 30 minutes before testing for opioid agonist-mediated inhibition of PGE2-stimulated thermal allodynia. BK was coinjected with the TM-TAT peptides. Based upon a paw volume of distribution of 1 ml, the approximate concentration of the peptides was 1 µM. For cell culture experiments, the peptides were dissolved in sterile Hanks’ balanced salt solution (10 µM) and used at a final concentration of 1 µM.
Data Analysis.
All statistical analyses were done using Prism software (version 6.0; GraphPad Software, San Diego, CA). For cell culture experiments, concentration–response data were fit to a logistic equation (eq. 1) using nonlinear regression analysis to provide estimates of maximal response (Emax), and potency (EC50).
| (1) |
Where R is the measured response at a given agonist concentration (A), Ro is the response in the absence of agonist, Ri is the response after maximal inhibition by the agonist, and EC50 is the concentration of agonist that produces a half-maximal response. The maximal effect of an agonist was calculated as Ro − Ri. Experiments were repeated at least 4 times in triplicate using cells obtained from different groups of rats. Statistical analysis of treatments on EC50 and Emax parameters, determined from individual curve fits, were done with a paired t test. When only a single drug concentration was used, statistical analysis was done with one-way analysis of variance (ANOVA) followed by Dunnett’s post test. P < 0.05 was considered statistically significant.
For behavior experiments, monotonic dose–response curve data were evaluated using nonlinear regression analysis (eq. 1). The significance of treatment effects on the mean fit values for ED50 and Emax parameters were determined with an F test. To determine statistical differences of U50488 U-shaped dose–response curves in the presence or absence of antagonists, data were analyzed by two-way ANOVA (two factors were dose and treatment) followed by Bonferroni’s post test. All time-course data were analyzed for statistical significance using two-way ANOVA (two factors were dose and time) followed by Bonferroni’s post test. Statistical analyses of PWL data 10 minutes after injection were done using one-way ANOVA followed by Dunnett’s post test. Unless otherwise stated, data are presented as mean ± S.E.M. of at least six rats per group. P <0.05 was considered statistically significant.
Results
Allosteric Regulation of KOR Agonist-Mediated Responses by DOR Antagonists in Peripheral Nociceptors Ex Vivo and In Vivo
Ligand-Dependent Effects of DOR Antagonists on KOR Agonist Responses.
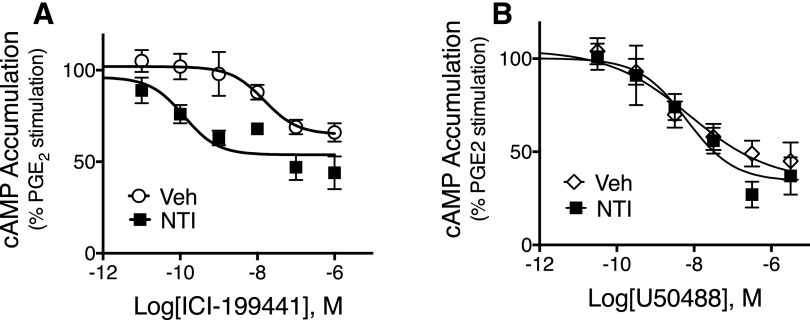
As shown in Fig. 1A, the concentration–response curve (CRC) for the KOR agonist ICI-199441 for inhibition of PGE2-stimulated cAMP accumulation in cultures of sensory neurons was shifted to the left approximately 20-fold with no change in the Emax by the DOR antagonist NTI (20 nM, 100 × Ki for DOR; Clark et al., 1997). The mean pEC50 was 7.94 ± 0.24 (11 nM) versus 9.27 ± 0.42 (0.5 nM), vehicle and NTI, respectively, mean ± S.E.M. of five individual curves (paired t test, P < 0.05). The mean maximal inhibition of PGE2-stimulated cAMP levels for ICI-199441 was 40% ± 3% versus 37% ± 2%, mean ± S.E.M. for vehicle and NTI, respectively, n = 5 (paired t test, P = 0.96). As we found previously (Berg et al., 2011; Jamshidi et al., 2015), neither basal nor PGE2-stimulated cAMP levels were altered by NTI alone (see legend of Fig. 1).
Fig. 1.
Ligand-dependent effects of the DOR antagonist naltrindole (NTI) on concentration–response curves for the KOR agonists (A) ICI-199441 and (B) U50488 for inhibition of PGE2-stimulated cAMP accumulation. Primary cultures of peripheral sensory neurons were pretreated with NTI (20 nM) or vehicle (Veh) for 15 minutes. After pretreatment, cells were treated with various concentrations of ICI-199441 or U50488, along with PGE2 (1 μM) and the phosphodiesterase inhibitor rolipram. Cellular cAMP levels were measured after 15 minutes. Data are expressed as the percentage of PGE2-stimulated cAMP levels and represent the mean ± S.E.M (n = 5). In the presence of NTI, the curve to ICI-199441 (A) was shifted to the left (F(1,56) = 21.65, P < 0.0001). By contrast, NTI did not alter the curve to U50488 (B) (F(1,56) = 0.217, P = 0.64). (A) Basal cAMP levels were 0.51 ± .07 pmol/well versus 0.45 ± 0.07 pmol/well, Veh and NTI, respectively, and PGE2-stimulated cAMP levels were 217% ± 45% above basal and 241% ± 22% above basal Veh and NTI, respectively (mean ± S.E.M., n = 5). (B) Basal cAMP levels were 0.80 ± 0.16 pmol/well versus 0.91 ± 0.19 pmol/well, Veh and NTI, respectively, and PGE2-stimulated cAMP levels were 186% ± 33% above basal and 130% ± 26% above basal, Veh and NTI, respectively (mean ± S.E.M., n = 5). As shown in Supplemental Fig. 1, NTI (20 nM) completely antagonized a maximal concentration of DPDPE (100 nM) for inhibition of cAMP accumulation, indicating full occupancy of DOR at this concentration.
To confirm effective receptor occupancy of DOR we measured the effects of NTI on inhibition of cAMP accumulation by maximal concentrations of the DOR agonist DPDPE (100 nM). As shown in Supplemental Fig. 1, the DPDPE-mediated response was blocked completely by 20 nM NTI.
To further probe the allosteric effect of the DOR antagonist NTI on the response to the KOR agonist ICI-199441, we examined the concentration dependence of NTI to enhance a subthreshold concentration of ICI-199441. As shown in Supplemental Fig. 2, incubation with 1 nM ICI-199441 had no effect on PGE2-stimulated cAMP accumulation alone. By contrast, in cells pretreated with NTI (0.2–100 nM), PGE2-stimulated cAMP levels were reduced by 1 nM ICI-199441 in an NTI concentration-dependent manner, with an EC50 value of 0.4 nM, which is consistent with the affinity reported for NTI (Clark et al., 1997).
Maximal enhancement of the response to 1 nM ICI-199441 occurred with 20 nM NTI, with no further enhancement with higher concentrations of NTI (100 nM), indicating that the concentration-dependent effect of the DOR antagonist was limited, a hallmark of an allosteric effect (Kenakin, 2009; Smith and Milligan, 2010; Christopoulos et al., 2014).
Importantly, in this experiment the inhibition of PGE2-stimulated cAMP accumulation by 1 nM ICI-199441 in the presence of maximally enhancing concentrations of NTI was less than that produced with a maximal concentration of ICI alone (1 µM), indicating that the plateau in the NTI-mediated enhancement was not due to a floor effect (see Supplemental Fig. 2). The magnitude of inhibition produced by 1 nM ICI-199441 in the presence of 20 nM NTI, relative to 1 µM ICI-199441, differed between experiments (Fig. 1A; Supplemental Fig. 2). Such variability may be attributable to differences in primary neuronal cultures (e.g., differences in receptor reserve) prepared at different times over the course of this study.
By contrast, NTI did not alter inhibition of PGE2-stimulated cAMP accumulation produced by the KOR agonist U50488 (Fig. 1B). The mean pEC50 values for U50488 were 8.74 ± 0.32 (2 nM) versus 8.46 ± 0.41 (3 nM) for vehicle versus NTI, respectively (paired t test, P = 0.71; n = 5). The mean maximal inhibition of PGE2-stimulated cAMP accumulation was 55% ± 5% versus 62% ± 1% for vehicle versus NTI, respectively (paired t test, P = 0.29; n = 5).
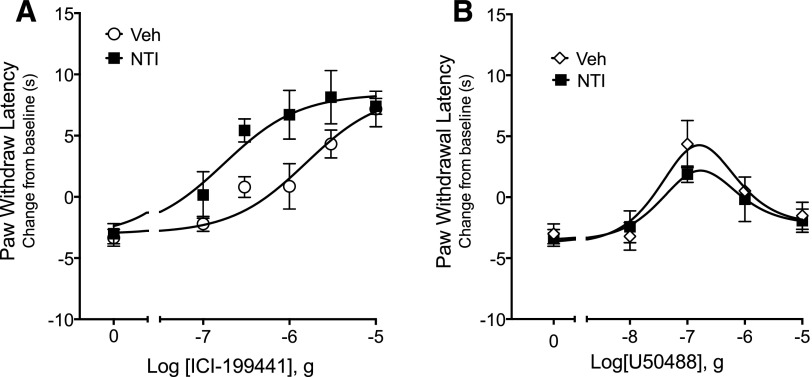
In the behavioral model of thermal nociception, the effect of NTI on KOR agonist-mediated antiallodynia was also dependent on the KOR agonist. As shown in Fig. 2A, local injection (intraplantar) of ICI-199441 dose dependently inhibited PGE2-evoked thermal allodynia (increased PWL). In the presence of NTI (40 μg, intraplantar), a dose that we have used before to block DOR agonist effects (Rowan et al., 2009; Berg et al., 2012) and see Supplemental Fig. 3), the ICI-199441 dose–response curve (DRC) was shifted to the left approximately 10-fold. The ED50 for ICI-199441 was 1.8 μg versus 0.14 μg for vehicle versus NTI, respectively (F(1,81) = 5.428, P = 0.02).
Fig. 2.
Ligand-dependent effects of the DOR antagonist naltrindole (NTI) on dose–response curves for the KOR agonists (A) ICI-199441 and (B) U50488 for inhibition of PGE2-stimulated thermal allodynia. Rats received intraplantar injection of NTI (40 μg) 15 minutes before intraplantar injection of PGE2 (0.3 μg) along with either ICI-199441 or U50488 at the indicated doses. Data are expressed as the change in seconds from individual baseline preinjection values 10 minutes after PGE2 ± opioid agonist administration and represent the mean ± S.E.M. of six to nine animals per group. In the presence of NTI, the ICI-199441 curve was shifted significantly to the left (F(1,75) = 9.849; P = 0.0024). By contrast, the curve for U50488 was not altered by NTI (F(1,59) = .8098; P = 0.37). Injection of NTI alone had no effect on baseline PWL (F(1,40) = 1.299, P = 0.27) or on PGE2–evoked thermal allodynia (F(1,52) = 0.109; P = 0.74). As shown in Supplemental Fig. 3, injection of 40 μg NTI completely blocked a maximal dose of DPDPE for inhibition of PGE2-induced thermal nociception. Full time-course curves are presented in Supplemental Figs. 4 and 5. Veh, vehicle.
We next tested the effect of NTI on the response to the KOR agonist U50488. We have previously reported that the DRC for U50488 for reduction of PGE2-evoked thermal allodynia has an inverted “U” shape (Berg et al., 2011) and that the descending limb is due to U50488-mediated activation of extracellular signal-regulated kinase, the mitogen-activated protein kinase (Jamshidi et al., 2015). KOR agonists that do not activate mitogen-activated protein kinase have a monotonic DRC (Jamshidi et al., 2015). Consistent with our previous findings, the DRC for U50488 was an inverted U shape (Fig. 2B). By contrast to the effect of ICI-199441, the DRC for U50488-mediated inhibition of PGE2-evoked thermal allodynia was not altered by NTI (Fig. 2B). In the presence of NTI, there was no change in either the ascending or descending limb of the U50488 DRC (Fig. 2B) (F(1,59) = 0.81, P = 0.37). As shown in Supplemental Fig. 3, injection of NTI produced complete blockade of a maximal dose of DPDPE for reduction of PGE2-evoked thermal allodynia.
We next tested the effects of a different DOR antagonist, BNTX, on the U50488–mediated response in the behavioral model of thermal nociception. Based on an estimated rat paw volume of distribution, a 1-ml injection of a 1-μg dose of BNTX would approximate 200 nM, a concentration of about 100 × Ki for DOR (Raynor et al., 1994; Neilan et al., 1999).
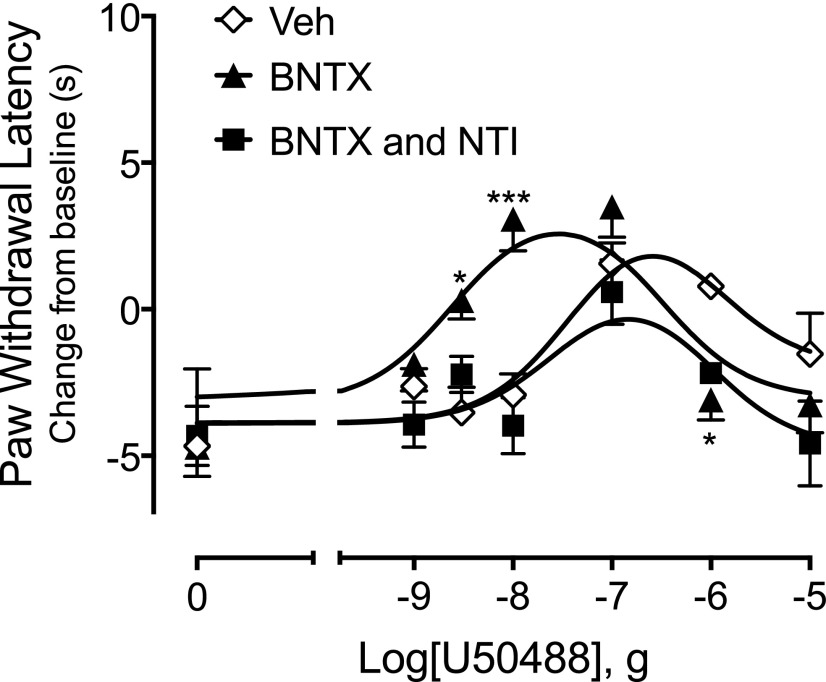
As shown in Supplemental Fig. 3, intraplantar injection of 1 µg BNTX blocked completely a maximal dose of DPDPE to inhibit PGE2-evoked thermal allodynia, indicating occupancy of DOR with this dose of BNTX. Injection of BNTX with U50488 shifted the U50488 DRC significantly to the left (Fig. 3) (F(1, 121) = 4.08, P = 0.04).
Fig. 3.
The DOR antagonist BNTX increases the potency of U50488 for reduction of PGE2-evoked thermal allodynia, and the BNTX effect is blocked by NTI. Rats were injected (intraplantar) with BK (25 μg) in combination with vehicle (Veh), BNTX (1 μg), or NTI (40 μg) plus BNTX (1 μg) 15 minutes before intraplantar injection with PGE2 (0.3 μg) with or without U50488 at the indicated doses. Paw withdrawal latencies are expressed as the change (seconds) from individual baseline values 10 minutes after PGE2/U50488 administration and represent the mean ± S.E.M. of 6–12 animals per group. Data were analyzed for statistical significance using two-way ANOVA. The U50488 curve in the presence of BNTX was significantly different from Veh (F(1, 121) = 4.08, P = 0.04). By contrast, the U50488 curve in the presence of BNTX and NTI in combination was not different from Veh (F(1, 89) = 3.177, P = 0.08). Full time-course curves are presented in Supplemental Fig. 6. *P < 0.05; ***P < 0.001 vs Veh.
Because there is only a 10-fold affinity difference of BNTX for DOR and MOR (Raynor et al., 1994), we tested whether the enhancing effect of BNTX was due to occupancy of DOR by determining if NTI was able to block the BNTX-mediated shift in the U50488 DRC. As shown in Fig. 3, coinjection of NTI (40 μg, i.pl) along with BNTX blocked completely the shift in the U50488 DRC (F(1,89) = 3.177, P = 0.08). As presented earlier, NTI did not alter the DRC for U50488-mediated reduction in thermal allodynia when tested alone (Fig. 2).
The DOR-KOR Heteromer Selectivity of 6ʹ-GNTI in Peripheral Sensory Neurons Is Due to Allosteric Interactions between the DOR and KOR Protomers
Agonist Efficacy of 6ʹ-GNTI Requires Expression of Both DOR and KOR.
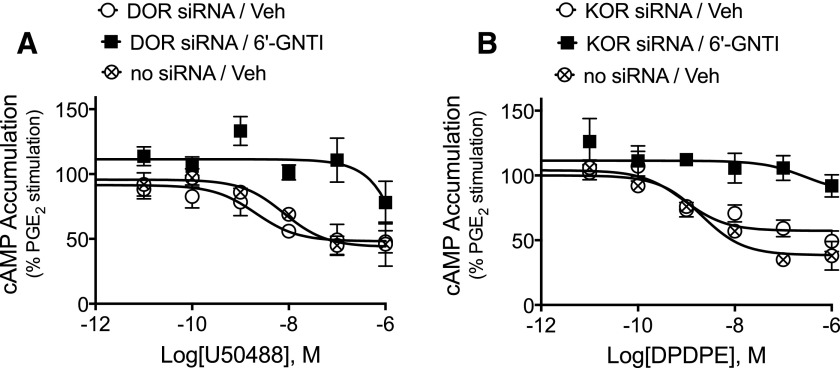
We have previously shown that 6ʹ-GNTI acts as an agonist for inhibition of PGE2-stimulated cAMP accumulation in primary cultures of peripheral sensory neurons as well as for reduction in PGE2-induced thermal allodynia in vivo (Berg et al., 2012). These actions of 6ʹ-GNTI are blocked by antagonists of either DOR or KOR (Berg et al., 2012). To confirm that agonism of 6ʹ-GNTI requires occupancy of both DOR and KOR, we tested the effect of knockdown of DOR or KOR using siRNA on 6ʹ-GNTI-mediated inhibition of PGE2 -stimulated cAMP accumulation.
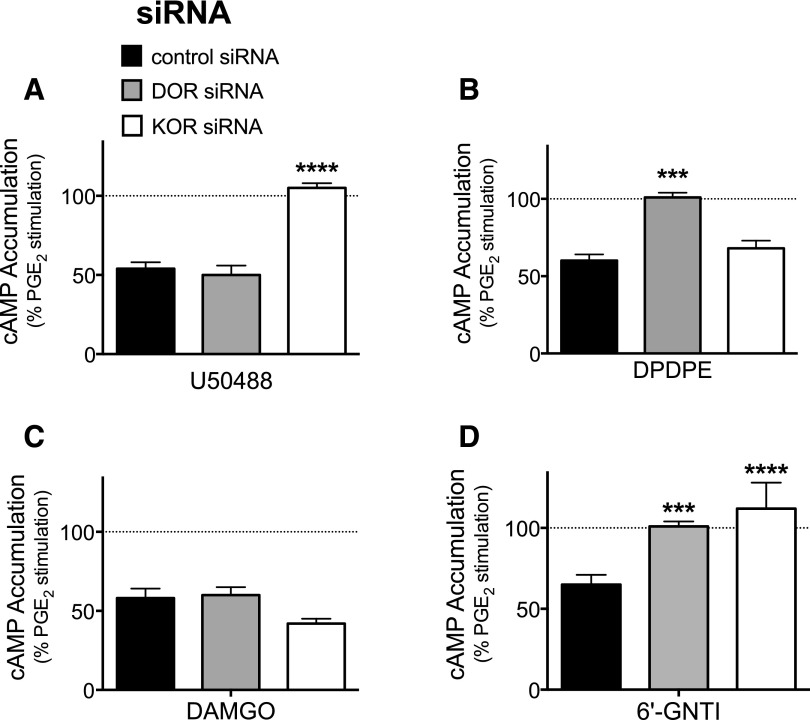
Transfection of peripheral sensory neuron cultures with siRNA directed against DOR (or nontargeting siRNA) resulted in a decrease in DOR mRNA (normalized to actin) by 1.5- and 2.2-fold measured 2 and 5 days later, respectively, compared with control as measured by quantitative real-time polymerase chain reaction. Similarly, KOR mRNA was decreased 1.65- and 1.92-fold versus control measured 2 and 5 days after siRNA transfection, respectively. As shown in Fig. 4A, 5 days after treatment with KOR siRNA the response to a maximal concentration of U50488 was abolished. By contrast, KOR siRNA treatment had no effect on the response to DPDPE (Fig. 4B). Similarly, 5 days after treatment with DOR siRNA (Fig. 4B) the response to DPDPE was abolished, but DOR siRNA treatment had no effect on the response to U50488 (Fig. 4A). Treatment with either DOR or KOR siRNA did not alter inhibition of adenylyl cyclase activity by a maximal concentration of the MOR agonist DAMGO (1 µM) (Fig. 4C). By contrast, 6ʹ-GNTI–mediated inhibition of PGE2-stimulated cAMP accumulation was abolished after treatment with either siRNA for DOR or siRNA for KOR (Fig. 4D).
Fig. 4.
6ʹ-GNTI agonist efficacy requires occupancy of both DOR and KOR in peripheral sensory neurons. Primary cultures of peripheral sensory neurons were transfected with siRNA directed against DOR (gray), KOR (white), or nontargeting siRNA as a control (black). Five days after transfection, inhibition of PGE2-stimulated cAMP accumulation in response to (A) U50488 (100 nM), (B) DPDPE (100 nM), (C) DAMGO (100 nM), or (D) 6ʹ-GNTI (100 nM) was measured. Data are expressed as the percentage of PGE2-stimulated cAMP levels and are mean ± S.E.M., n = 6. ***P < 0.001; ****P < 0.0001 versus off-target (control) siRNA, one-way ANOVA with Dunnett’s post test.
We also tested the effects of DOR and KOR siRNA treatment on inhibition of PGE2-stimulated cAMP accumulation by the putative DOR-KOR heteromer bivalent ligand KDAN-18, which is composed of the KOR agonist ICI-199441 linked to the DOR antagonist NTI (Daniels et al., 2005; Ansonoff et al., 2010). As shown in Supplemental Fig. 7, responsiveness to KDAN-18 was abolished after siRNA knockdown of either DOR or KOR.
6ʹ-GNTI Has Antagonist Properties at Both DOR and KOR in the Absence of Coexpression.
To determine whether 6ʹ-GNTI occupied DOR or KOR in the absence of the corresponding receptor, we measured the effect of 6ʹ-GNTI on CRCs to DPDPE or U50488 after siRNA-mediated knockdown of either DOR or KOR expression. In cells transfected with DOR siRNA, 6ʹ-GNTI antagonized U50488-mediated inhibition of PGE2-stimulated cAMP accumulation (Fig. 5A; F(1,42) = 44.23, P < 0.0001) demonstrating occupancy of KOR by 6ʹ-GNTI. Similarly, in cells transfected with KOR siRNA, 6ʹ-GNTI antagonized DPDPE-mediated inhibition of PGE2-stimulated cAMP accumulation (Fig. 5B; F(1,42) = 30.05, P < 0.0001). The CRC for U50488 was not altered by DOR siRNA treatment (Fig. 5A), nor was the CRC to DPDPE altered by KOR siRNA (Fig. 5B).
Fig. 5.
Antagonism of responses to (A) U50488 and (B) DPDPE by 6ʹ-GNTI after knockdown of (A) DOR or (B) KOR in peripheral sensory neurons. Primary cultures of peripheral sensory neurons were transfected with or without siRNA directed against either (A) DOR or (B) KOR. Five days after transfection, cells were treated with PGE2 (1 μM) along with various concentrations of (A) U50488 or (B) DPDPE with 6ʹ-GNTI (1 μM) or vehicle (Veh). Data are expressed as the percentage of PGE2-stimulated cAMP levels and are mean ± S.E.M., n = 4. Data were analyzed for statistical significance using two-way ANOVA. (A) In cells treated with DOR siRNA the U50488 curve in the presence 6ʹ-GNTI was significantly different from Veh (F(1,42) = 44.23, P < 0.0001). (B) In cells treated with KOR siRNA the U50488 curve in the presence 6ʹ-GNTI was significantly different from Veh (F(1,42) = 30.05, P < 0.0001).
DOR Antagonists NTI and BNTX Surmount Occupancy of DOR by 6ʹ-GNTI and Block 6ʹ-GNTI Agonism Ex Vivo and In Vivo.
The results described earlier suggest that antagonist occupancy of DOR allosterically regulates KOR agonist potency and/or efficacy. We hypothesized that the mechanism that underlies 6ʹ-GNTI agonist activity in peripheral nociceptors may be due to its occupancy of DOR producing positive allosteric regulation of its agonist efficacy at KOR within DOR-KOR heteromers. To test this hypothesis, we attempted first to surmount occupancy of DOR by 6ʹ-GNTI using other DOR antagonists.
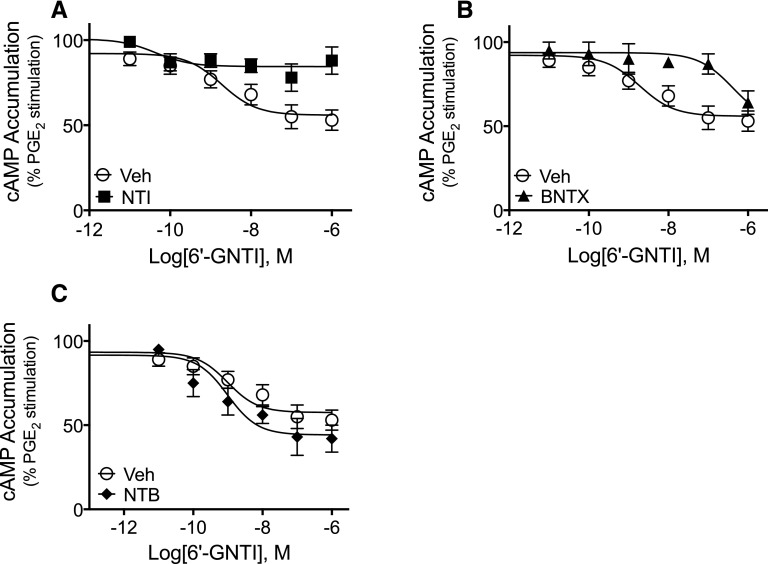
As shown in Supplemental Fig. 1, inhibition of cAMP accumulation by a maximal concentration of DPDPE (1 µM) was blocked by the DOR antagonists NTB, NTI, and BNTX, at concentrations of ∼100 × Ki for DOR (Raynor et al., 1994; Neilan et al., 1999). As shown in Fig. 6, both NTI and BNTX antagonized the effect of 6ʹ-GNTI (Fig. 6, A and B; F(1,42) = 19.41, P < 0.0001, and F(1,42) = 19.91, P < 0.0001 for NTI and BNTX, respectively). By contrast, NTB did not alter the CRC to 6ʹ-GNTI (Fig. 6C; F(1,42) = 1.25, P = 0.27). The mean pEC50 for 6ʹ-GNTI was 8.55 ± 0.23 (3 nM) and 8.83 ± 0.40 (1.5 nM), mean ± S.E.M., n = 4, for vehicle and NTB, respectively (paired t test, P = 0.67). The mean maximal inhibition of PGE2-mediated stimulation of cAMP by 6ʹ-GNTI was 52% ± 5% and 64% ± 10%, mean ± S.E.M., n = 4, for vehicle and NTB, respectively (paired t test, P = 0.10).
Fig. 6.
Differential effects of DOR occupancy with selective antagonists on 6ʹ-GNTI–mediated inhibition of PGE2-stimulated cAMP accumulation ex vivo. Primary cultures of peripheral sensory neurons were pretreated with vehicle (Veh), (A) NTI (20 nM), (B) BNTX (200 nM), or (C) NTB (1 nM) for 15 minutes. After pretreatment, cells were incubated for 15 minutes with PGE2 with or without the indicated concentrations of 6ʹ-GNTI. Data are expressed as the percentage of PGE2-stimulated cAMP levels and are mean ± S.E.M., n = 4 separate experiments. Data were analyzed for statistical significance using two-way ANOVA. The 6ʹ-GNTI curve in the presence of (A) NTI or (B) BNTX was significantly different from Veh (F(1,42) = 19.41, P < 0.0001, and F(1,42) = 19.91, P < 0.0001, for NTI and BNTX, respectively). (C) By contrast, the 6ʹ-GNTI curve in the presence of NTB was not different from Veh (F(1,42) = 1.25, P = 0.269). Incubation with antagonists did not alter either basal cAMP levels or PGE2-stimulated cAMP levels which were 1.34 pmol/well ± 0.23% and 220% above basal ± 47%, mean ± S.E.M., n = 12.
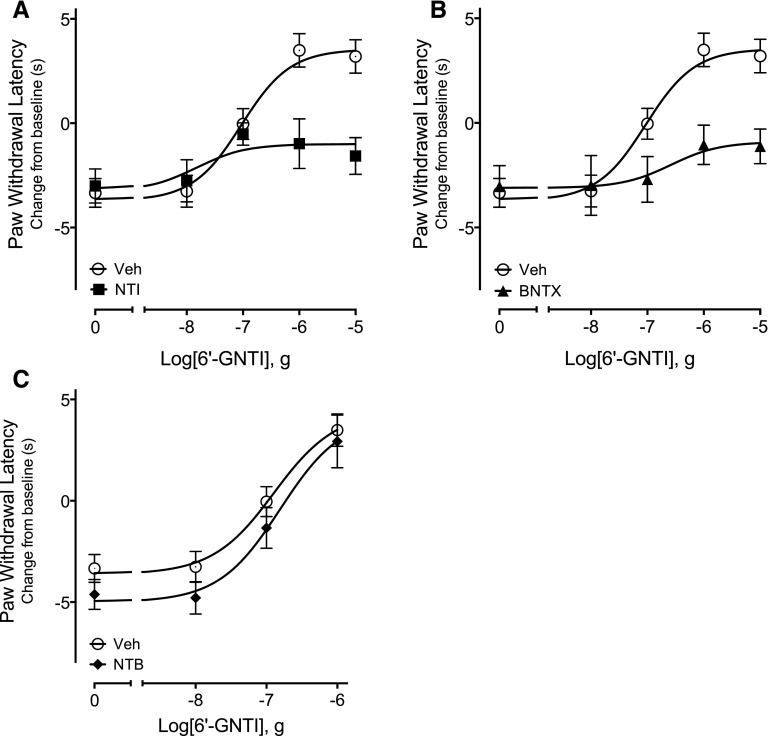
In the behavioral model of thermal nociception, 6ʹ-GNTI–mediated antiallodynic responses were also antagonized by NTI (F(1,62), 10.39, P = 0.002; Fig. 7A) and BNTX (F(1,59) = 13.37, P = 0.0005; Fig. 7B). By contrast, NTB at a dose that completely blocked maximal DPDPE-mediated antinociception (Supplemental Fig. 3) did not alter the DRC of 6ʹ-GNTI for reduction in PGE2-mediated thermal allodynia (Fig. 7C; F(1, 45) = 3.52, P = 0.07).
Fig. 7.
Differential effects of DOR occupancy with selective antagonists on 6ʹ-GNTI–mediated antinociception. Rats received intraplantar injection with (A) NTI (40 μg), (B) BNTX (1 μg), or (C) NTB (1 μg) 15 minutes before intraplantar injection with PGE2 (0.3 μg) in combination with either vehicle (Veh) or the indicated doses of 6ʹ-GNTI. Data are expressed as the change (seconds) from individual preinjection baseline values 10 minutes after PGE2 ± 6ʹ-GNTI injection and represent the mean ± S.E.M. of 6–12 animals per group. Data were analyzed for statistical significance using two-way ANOVA. The 6ʹ-GNTI curve in the presence of (A) NTI or (B) BNTX was significantly different from Veh (F(1,62) = 10.39, P = 0.002, and F(1,59) = 13.37, P = 0.0005, for NTI and BNTX, respectively). (C) NTB did not alter the 6ʹ-GNTI curve (F(1, 45) = 3.52, P = 0.07). As shown in Supplemental Fig. 3, none of the DOR antagonists altered baseline PWLs or PGE2-induced thermal allodynia. Full time-course curves are shown in Supplemental Fig. 8.
6ʹ-GNTI-Mediated Antinociceptive Signaling Is Blocked after Disruption of DOR-KOR Heteromers by a TAT-Fused Peptide Corresponding to the TM1 Domain of DOR.
If the efficacy of 6ʹ-GNTI is due to allosteric interactions between the DOR and KOR protomers of the heteromer, interference with the interaction between DOR and KOR would be expected to reduce the efficacy of 6ʹ-GNTI. To test this, we treated peripheral sensory neurons ex vivo and in vivo with a TM peptide that corresponds to TM1 of DOR. We fused the TM1 peptide at its C terminus with the cell-permeable HIV TAT sequence to confer membrane permeability and proper orientation of the TM domain within the membrane as described by He et al. (2011). The TM1 domain is often implicated as a dimer interface of opioid receptors (e.g., He et al., 2011; Shang and Filizola, 2015; Gahbauer and Bockmann, 2016). As a control, we also tested a DOR TM1 peptide with the TAT sequence on the N terminus (TAT-TM1 negative control), which would not insert into the membrane in the appropriate orientation.
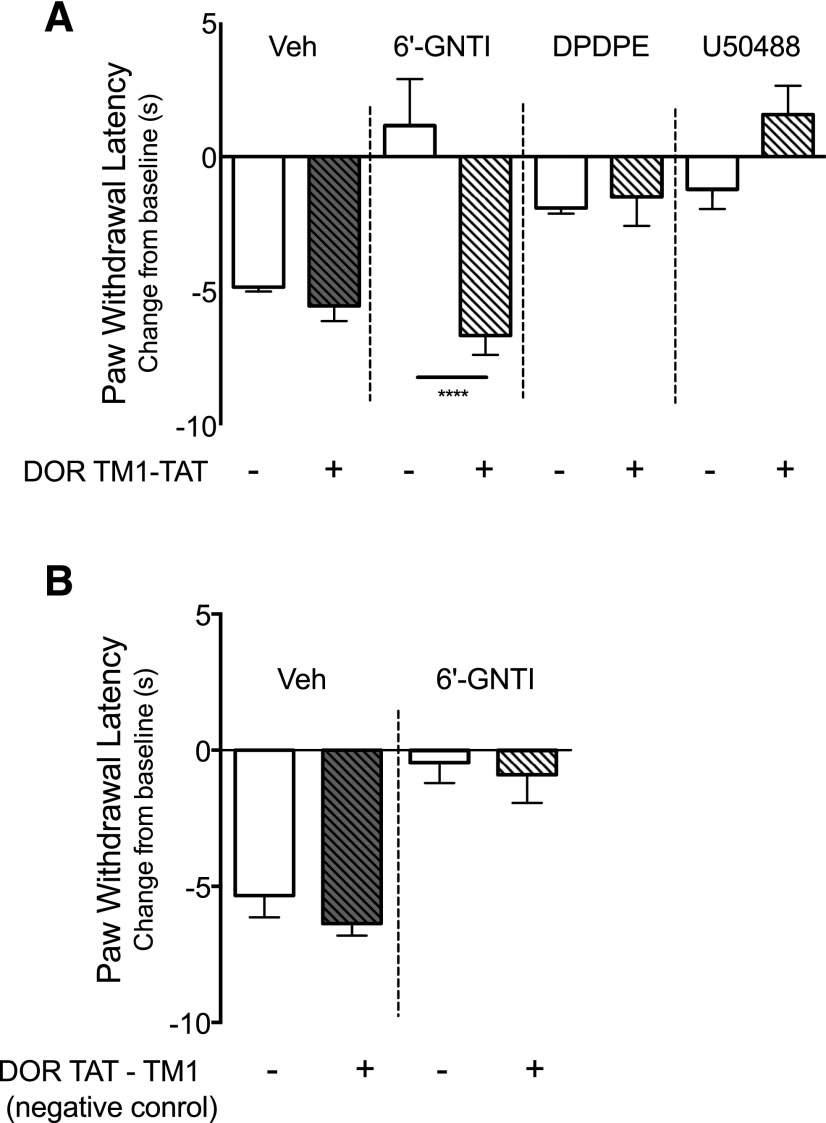
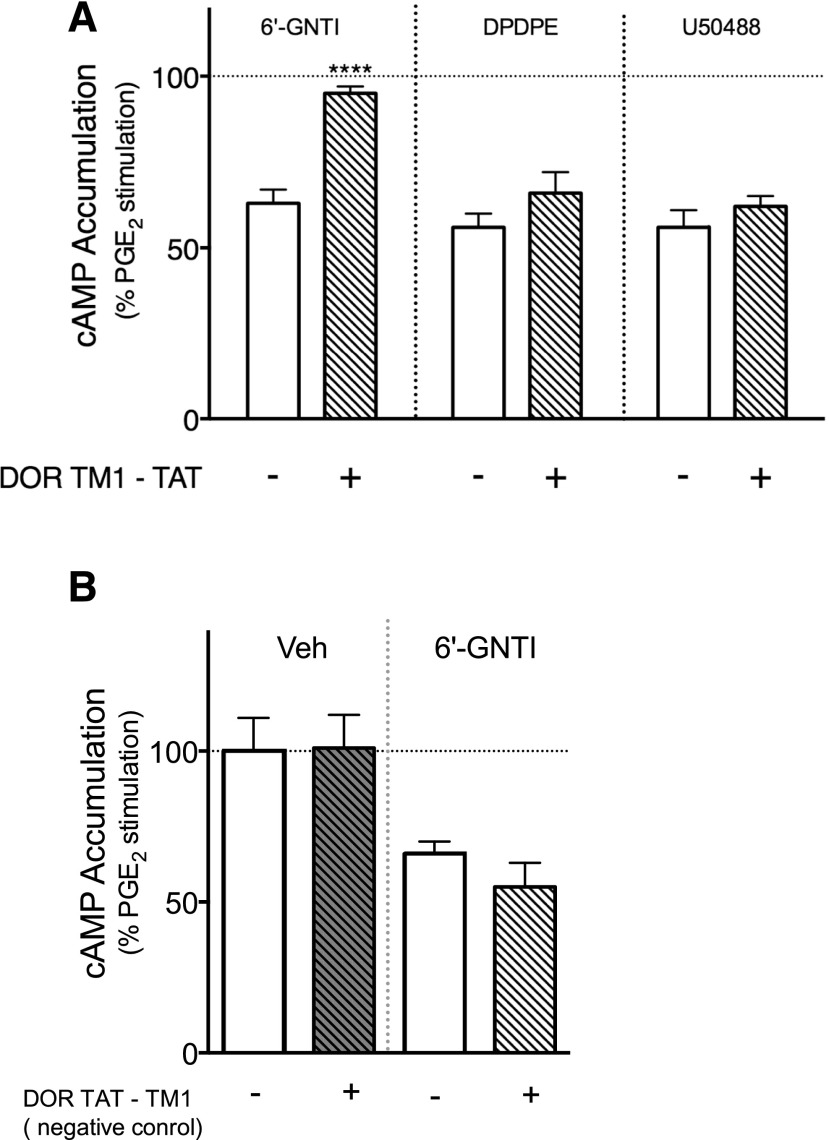
As shown in Fig. 8A, administration of the DOR TM1-TAT peptide did not alter the reduction of PGE2-evoked thermal nociception by DPDPE or by U50488. However, the antinociceptive response to 6ʹ-GNTI was abolished. By contrast, the DOR negative control TAT-TM1 peptide did not alter the antinociceptive response to 6ʹ-GNTI (Fig. 8B). Neither TAT-peptide altered BK- or PGE2-evoked responses (Supplemental Fig. 9A). In primary cultures of peripheral sensory neurons treated with DOR TM1-TAT, neither DPDPE- nor U50488-mediated inhibition of PGE2-stimulated cAMP accumulation was altered (Fig. 9A). However, 6ʹ-GNTI–mediated inhibition of PGE2-stimulated cAMP accumulation was eliminated completely. The TAT-TM1 negative control peptide did not alter the inhibition of adenylyl cyclase activity by 6ʹ-GNTI (Fig. 9B).
Fig. 8.
6ʹ-GNTI–mediated antinociception is blocked after disruption of DOR-KOR heteromers by DOR TM1-TAT peptides. Rats received an injection of vehicle (Veh), (A) DOR-TM1-TAT peptide (3.94 μg, intraplantar), or (B) TM-TAT negative control peptide (3.94 μg, intraplantar) followed 30 minutes later with a coinjection of PGE2 (0.3 μg) with Veh, DPDPE (20 µg intraplantar), U50488 (0.1 µg, intraplantar), or 6ʹ-GNTI (1 μg, intraplantar). Data are expressed as the change (seconds) from individual preinjection baseline values 10 minutes after PGE2 + agonist injection and represent the mean ± S.E.M. of six animals per group. Data were analyzed for statistical differences with one-way ANOVA followed by Bonferroni’s post test, ****P < 0.0001. Full time-course curves are shown in Supplemental Fig. 9.
Fig. 9.
6ʹ-GNTI–mediated inhibition of PGE2-stimulated cAMP accumulation is blocked after disruption of DOR-KOR heteromers by DOR TM1-TAT peptides. Primary cultures of peripheral sensory neurons were treated with vehicle, (A) the DOR TM1-TAT peptide (1 µM), or (B) the negative control TAT-TM1 peptide (1 µM) for 30 minutes. Cells were incubated with PGE2 (1 μM) and vehicle (Veh), 6ʹ-GNTI (100 nM), DPDPE (100 nM), or U50488 (100 nM) for an additional 15 minutes. Data are expressed as the percentage of PGE2-stimulated cAMP levels and are mean ± S.E.M., n = 4. ****P < 0.0001 versus vehicle (no TM peptide), one-way ANOVA with Dunnett’s post test.
Discussion
GPCR heteromers have been well studied in heterologous expression systems (Gomes et al., 2016), but much less is known of heteromer function in physiologically relevant systems due, in large part, to methodological limitations and lack of available heteromer-selective ligands. However, it may be possible to exploit allosteric interactions that can occur between the protomers of a heteromer to study heteromer function in native systems. In this regard, orthosteric ligands binding to one protomer can act as allosteric regulators of the activity of orthosteric ligands that bind to the other protomer within a heteromer (Fuxe et al., 2010; Smith and Milligan, 2010). A well-known hallmark of allosterism is that allosteric interactions are highly dependent upon the allosteric-orthosteric ligand pairs (Kenakin, 2009; Smith and Milligan, 2010; Christopoulos et al., 2014), so it would be expected that interprotomer allosteric effects of orthosteric ligands would be ligand-dependent as well.
Several studies have demonstrated allosteric interactions within DOR-KOR heteromers in heterologous expressions systems (Jordan and Devi, 1999; Waldhoer et al., 2005; Xie et al., 2005). In human embryonic kidney (HEK) cells coexpressing both DOR and KOR, but not in cells expressing either receptor alone, the affinity of the KOR agonist N-methyl-2-phenyl-N-[(5R,7S,8S)-7-pyrrolidin-1-yl-1-oxaspiro[4.5]decan-8-yl]acetamide (U69593) is increased by the DOR agonist DPDPE; reciprocally, the affinity of DPDPE is increased by U69593 (Jordan and Devi, 1999). Allosteric interactions have also been reported for DOR and KOR antagonists (Xie et al., 2005).
In peripheral sensory neurons both ex vivo and in vivo, we have shown that KOR orthosteric antagonists alter DOR orthosteric agonist-mediated responses in a ligand-dependent manner (Berg et al., 2012). Here, we report that interactions between DOR and KOR orthosteric ligands in peripheral sensory neurons are reciprocal: DOR antagonists regulated KOR agonist-mediated responses, and the nature of these effects was dependent on the DOR antagonist/KOR agonist pair. For example, the DOR antagonist NTI had no effect on responses mediated by the KOR agonist U50488; however, it enhanced responses to the KOR agonist ICI-199441 ex vivo and in vivo. Interestingly, the DOR-KOR heteromer selectivity of KDAN-18 (Daniels et al., 2005), which is composed of NTI and ICI-199441 linked together with an 18 carbon spacer, may be due to an allosteric effect of NTI via DOR to augment the KOR response to ICI-199441.
6ʹ-GNTI is a guanidino derivative of the selective DOR antagonist NTI. Addition of the guanidino group to the 5′ position of NTI converts the compound to a KOR selective antagonist (5′-GNTI), whereas movement of the guanidino group to the 6′ position changes the ligand to a low-efficacy KOR agonist (Sharma et al., 2001; Rives et al., 2012; Schmid et al., 2013). However, some studies have suggested that 6ʹ-GNTI activates DOR-KOR heteromers (Waldhoer et al., 2005; Ansonoff et al., 2010). For example, 6ʹ-GNTI has no efficacy at DOR and only weak efficacy at KOR when expressed individually in HEK cells, but when the receptors are coexpressed the agonist efficacy of 6ʹ-GNTI is greatly enhanced (Waldhoer et al., 2005). Although KOR is expressed in brain, 6ʹ-GNTI had no effect when administered intracerebroventricularly. However when administered intrathecally, 6ʹ-GNTI elicited antinociceptive responses that were blocked by either DOR or KOR antagonists (Waldhoer et al., 2005), or by knockout of either DOR or KOR (Ansonoff et al., 2010), indicating agonist efficacy in the spinal cord required both receptors.
Consistent with these studies, we have shown previously that 6ʹ-GNTI–mediated agonist responses in peripheral nociceptors are blocked by antagonists to either DOR or KOR (Berg et al., 2012). In our present study we found that siRNA knockdown of either DOR or KOR expression blocked 6ʹ-GNTI–mediated inhibition of adenylyl cyclase activity in cultured peripheral sensory neurons. Moreover, when DOR expression was reduced, 6ʹ-GNTI did not activate KOR but instead behaved as an antagonist. These data suggest that, compared with high expression that can occur in heterologous systems, KOR expression in the brain, spinal cord, and peripheral sensory neurons is too low for the low efficacy of 6ʹ-GNTI to be observed, but in the presence of DOR 6ʹ-GNTI efficacy is increased.
Given that efficacy of 6ʹ-GNTI in peripheral sensory neurons ex vivo and in vivo is dependent upon DOR and KOR, that 6ʹ-GNTI binds to both DOR and KOR, and that some orthosteric DOR antagonists could enhance KOR agonist responses, we considered that perhaps the efficacy of 6ʹ-GNTI was due to positive allosteric augmentation of efficacy at KOR by its occupancy of DOR. Using siRNA knockdown of KOR, we found that 6ʹ-GNTI functioned as a DOR antagonist, consistent with having affinity but no efficacy at DOR, as reported by Waldhoer et al. (2005). Furthermore, when the occupancy of 6ʹ-GNTI of DOR was surmounted with the other DOR antagonists NTI and BNTX, the agonist activity of 6ʹ-GNTI was reduced both ex vivo and in vivo. By contrast, NTB substituted for 6ʹ-GNTI at DOR, as 6ʹ-GNTI–mediated responses ex vivo and in vivo were not altered. The differential effects of DOR antagonists on 6ʹ-GNTI responsiveness support the hypothesis that in peripheral nociceptors 6ʹ-GNTI allosterically regulates its own agonist efficacy at KOR via occupancy of DOR. We suggest that this mechanism provides for selectivity of 6ʹ-GNTI for DOR-KOR heteromers in peripheral sensory neurons and perhaps in other areas such as the spinal cord.
If the DOR-dependent effect of 6ʹ-GNTI to augment efficacy at KOR is via allosteric interactions between the DOR and KOR protomers of DOR-KOR heteromers, then approaches that interfere with interactions between DOR and KOR should also interfere with the efficacy of 6ʹ-GNTI. To test this hypothesis, we measured the efficacy of 6ʹ-GNTI after delivery of peptides that correspond to the TM1 domain of DOR to peripheral sensory neurons ex vivo and in vivo. Following the method reported by He et al. (2011), we fused DOR TM1 domain peptides to the HIV TAT sequence to increase membrane permeability. Administration of DOR TM1 peptides with the TAT sequence fused to the C terminus completely prevented efficacy of 6ʹ-GNTI both for inhibition of adenylyl cyclase activity in peripheral sensory neurons in culture and for inhibition of thermal allodynia in behavioral tests. Control peptides with the TAT sequence fused to the N terminus, which would be expected to orient the TM1 sequence in the opposite direction in the membrane, were ineffective. Taken together, these results suggest that the efficacy of 6ʹ-GNTI is due to intraheteromer allosteric interactions between DOR occupied by 6ʹ-GNTI and KOR occupied by 6ʹ-GNTI. Furthermore, these results suggest that in peripheral sensory neurons 6ʹ-GNTI selectively activates DOR-KOR heteromers.
Interestingly, efficacy of 6ʹ-GNTI appears to be cell/tissue specific. In this regard, Kenakin and Beek (1980) showed over 30 years ago that the selective β1-adrenergic receptor ligand prenalterol is either a full agonist, a partial agonist, or an antagonist (i.e., has no efficacy) depending upon the tissue studied. Similarly, 6ʹ-GNTI has been shown to have KOR agonist activity in some cells/tissues but antagonist activity in others. In guinea pig ileum, 6ʹ-GNTI inhibits electrically stimulated smooth muscle contraction via KOR, but it is inactive in smooth muscle preparations from mouse vas deferens (Sharma et al., 2001). In KOR-expressing HEK cells, 6ʹ-GNTI is a partial agonist for inhibition of adenylyl cyclase activity, and its efficacy is not altered by coexpression of DOR (Rives et al., 2012). In Chinese hamster ovary cells expressing KOR, but not in striatal neurons endogenously expressing KOR, 6ʹ-GNTI is an agonist for activation of Gαi using the guanosine 5′-3-O-(thio)triphosphate (GTPγS) assay (Schmid et al., 2013).
Interestingly, although 6ʹ-GNTI did not increase GTPγS binding in striatal neurons, it did activate protein kinase B in a pertussis toxin–dependent and β-arrestin–independent manner. This latter finding highlights the importance of the sensitivity of the assay used to assess ligand efficacy. In our experiments, although 6ʹ-GNTI could bind to both DOR and KOR, efficacy of 6ʹ-GNTI for antinociception or to inhibit adenylyl cyclase activity required both DOR and KOR.
Taken together, it is likely that 6ʹ-GNTI is a low-efficacy agonist at KOR for activation of Gαi signaling. Depending upon the density of KOR expression (high in heterologous expression systems) and on the strength of receptor-effector coupling efficiency (e.g., the quantity and perhaps type of Gαi and accessory proteins expressed), a weak KOR activating signal of 6ʹ-GNTI may be amplified to a level where agonism is observed. However, in cells/tissues where KOR expression is low (as is typical of receptors expressed in native systems) or receptor-effector coupling is weak 6ʹ-GNTI may behave as a KOR antagonist, as we have found in peripheral sensory neurons.
In summary, in both ex vivo and in vivo models of peripheral sensory neuron function, DOR antagonists differentially regulated KOR agonist responses in a ligand-dependent manner. Taken together with our previous findings (Berg et al., 2012), these results support the existence of functional DOR-KOR heteromers in rat peripheral sensory neurons and are consistent with the notion that reciprocal, ligand-dependent, allosteric interactions occur between the DOR and KOR protomers. Because it is probable that homomers/monomers are expressed, we have likely underestimated the magnitude of the allosteric effects. Given the limitations of tools to study GPCR heteromer function in physiologically relevant systems and the paucity of selective ligands, perhaps capitalizing on allosteric effects of orthosteric ligands selective for the individual protomers may provide a viable approach to study heteromer function in native systems.
Acknowledgments
The authors thank Hudson Smith and Joshua Zamora for excellent technical assistance and Dr. Lakshmi Devi and Dr. Milena Girotti for helpful discussions.
Abbreviations
- ANOVA
analysis of variance
- BK
bradykinin
- BNTX
7-benzylidenaltrexone
- CRC
concentration–response curve
- Ct
cycle threshold
- DAMGO
[D-Ala2,N-MePhe4,Gly-ol5]-enkephalin
- DMEM
Dulbecco’s modified Eagle’s medium
- DOR
δ opioid receptor
- DPDPE
[D-Pen2,5]-enkephalin
- DRC
dose–response curve
- FBS
fetal bovine serum
- 6-GNTI
6ʹ-guanidinonaltrindole
- GPCR
G protein-coupled receptor
- GTPγS
guanosine 5′-3-O-(thio)triphosphate
- HEK
human embryonic kidney cells
- ICI-199441
2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-phenyl-2-pyrrolidin-1-ylethyl]acetamide
- KOR
κ opioid receptor
- NTB
naltriben
- NTI
naltrindole
- PGE2
prostaglandin E2
- PWL
paw withdrawal latency
- siRNA
small interfering RNA
- TAT
transactivator of transcription (peptide)
- TM1
transmembrane domain 1
- U50488
2-(3,4-dichlorophenyl)-N-methyl-N-[(1R,2R)-2-pyrrolidin-1-ylcyclohexyl]acetamide
- U69593
N-methyl-2-phenyl-N-[(5R,7S,8S)-7-pyrrolidin-1-yl-1-oxaspiro[4.5]decan-8-yl]acetamide.
Authorship Contributions
Participated in research design: Jacobs, Pando, Clarke, Berg.
Conducted experiments: Jacobs, Pando, Jennings, Chavera.
Performed data analysis: Jacobs, Pando, Jennings, Berg.
Wrote or contributed to the writing of the manuscript: Jacobs, Clarke, Berg.
Footnotes
This work was supported by U.S. Public Health Service grants from the National Institutes of Health National Institute of General Medical Sciences [Grant R01 GM 106035] and National Institute on Drug Abuse [Grant R21 DA 037572] (to W.P.C and K.A.B.); the National Institute of Dental and Craniofacial Research (COSTAR) [Training Grant T32 DE14318] (to B.A.J.); and the National Institute of Neurological Disorders and Stroke [Training Grant T32 NS082145] (to M.M.P.).
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Ansonoff MA, Portoghese PS, Pintar JE. (2010) Consequences of opioid receptor mutation on actions of univalent and bivalent kappa and delta ligands. Psychopharmacology (Berl) 210:161–168. [DOI] [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007) Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847. [DOI] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Gupta A, Sanchez TA, Silva M, Gomes I, McGuire BA, Portoghese PS, Hargreaves KM, Devi LA, et al. (2012) Allosteric interactions between δ and κ opioid receptors in peripheral sensory neurons. Mol Pharmacol 81:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Rowan MP, Sanchez TA, Silva M, Patwardhan AM, Milam SB, Hargreaves KM, Clarke WP. (2011) Regulation of κ-opioid receptor signaling in peripheral sensory neurons in vitro and in vivo. J Pharmacol Exp Ther 338:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks H, Lebleu B, Vivès E. (2005) Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev 57:559–577. [DOI] [PubMed] [Google Scholar]
- Charles AC, Mostovskaya N, Asas K, Evans CJ, Dankovich ML, Hales TG. (2003) Coexpression of delta-opioid receptors with micro receptors in GH3 cells changes the functional response to micro agonists from inhibitory to excitatory. Mol Pharmacol 63:89–95. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Changeux JP, Catterall WA, Fabbro D, Burris TP, Cidlowski JA, Olsen RW, Peters JA, Neubig RR, Pin JP, et al. (2014) International Union of Basic and Clinical Pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev 66:918–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MJ, Emmerson PJ, Mansour A, Akil H, Woods JH, Portoghese PS, Remmers AE, Medzihradsky F. (1997) Opioid efficacy in a C6 glioma cell line stably expressing the delta opioid receptor. J Pharmacol Exp Ther 283:501–510. [PubMed] [Google Scholar]
- Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. (2005) A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges δ2 and κ1 opioid receptor phenotypes. J Med Chem 48:1713–1716. [DOI] [PubMed] [Google Scholar]
- Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. (2006) Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem 281:38812–38824. [DOI] [PubMed] [Google Scholar]
- Ferré S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortés A, Lluís C, Casadó V, Volkow ND. (2016) Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer. Neuropharmacology 104:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Borroto-Escuela DO, Frankowska M, Ferraro L, Guidolin D, Ciruela F, Agnati LF. (2010) The changing world of G protein-coupled receptors: from monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. J Recept Signal Transduct Res 30:272–283. [DOI] [PubMed] [Google Scholar]
- Gahbauer S, Böckmann RA. (2016) Membrane-mediated oligomerization of G protein coupled receptors and its implications for GPCR function. Front Physiol 7:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KD, Devi LA. (2016) G protein-coupled receptor heteromers. Annu Rev Pharmacol Toxicol 56:403–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sánchez-Soto M, Kumar-Barodia S, Naidu YT, Mallol J, Cortés A, et al. (2014) Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol 86:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [DOI] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, et al. (2011) Facilitation of μ-opioid receptor activity by preventing δ-opioid receptor-mediated codegradation. Neuron 69:120–131. [DOI] [PubMed] [Google Scholar]
- Jamshidi RJ, Jacobs BA, Sullivan LC, Chavera TA, Saylor RM, Prisinzano TE, Clarke WP, Berg KA. (2015) Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J Pharmacol Exp Ther 355:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399:697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP. (2009) '7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol Sci 30:460–469. [DOI] [PubMed] [Google Scholar]
- Kenakin TP, Beek D. (1980) Is prenalterol (H133/80) really a selective beta 1 adrenoceptor agonist? Tissue selectivity resulting from differences in stimulus-response relationships. J Pharmacol Exp Ther 213:406–413. [PubMed] [Google Scholar]
- Martin NA, Prather PL. (2001) Interaction of co-expressed mu- and delta-opioid receptors in transfected rat pituitary GH(3) cells. Mol Pharmacol 59:774–783. [DOI] [PubMed] [Google Scholar]
- Neilan CL, Akil H, Woods JH, Traynor JR. (1999) Constitutive activity of the delta-opioid receptor expressed in C6 glioma cells: identification of non-peptide delta-inverse agonists. Br J Pharmacol 128:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. (1994) Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45:330–334. [PubMed] [Google Scholar]
- Rives ML, Rossillo M, Liu-Chen LY, Javitch JA. (2012) 6′-Guanidinonaltrindole (6′-GNTI) is a G protein-biased κ-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem 287:27050–27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. (2009) Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol 602:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Bushlin I, Gomes I, Tzavaras N, Gupta A, Neves S, Battini L, Gusella GL, Lachmann A, Ma’ayan A, et al. (2012) Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS One 7:e29239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. (2013) Functional selectivity of 6′-guanidinonaltrindole (6′-GNTI) at κ-opioid receptors in striatal neurons. J Biol Chem 288:22387–22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Filizola M. (2015) Opioid receptors: structural and mechanistic insights into pharmacology and signaling. Eur J Pharmacol 763(Pt B):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Jones RM, Metzger TG, Ferguson DM, Portoghese PS. (2001) Transformation of a kappa-opioid receptor antagonist to a kappa-agonist by transfer of a guanidinium group from the 5′- to 6′-position of naltrindole. J Med Chem 44:2073–2079. [DOI] [PubMed] [Google Scholar]
- Shivnaraine RV, Kelly B, Sankar KS, Redka DS, Han YR, Huang F, Elmslie G, Pinto D, Li Y, Rocheleau JV, et al. (2016) Allosteric modulation in monomers and oligomers of a G protein-coupled receptor. eLife 5:e11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquee K, Hampton J, McAnally D, May L, Smith L. (2013) The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br J Pharmacol 168:1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev 62:701–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Lang LJ. (2009) Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol 9:3–8. [DOI] [PubMed] [Google Scholar]
- Stein C, Zöllner C. (2009) Opioids and sensory nerves. Handb Exp Pharmacol (194):495–518. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Berg KA, Clarke WP. (2015) Dual regulation of δ-opioid receptor function by arachidonic acid metabolites in rat peripheral sensory neurons. J Pharmacol Exp Ther 353:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer HF, Watts AO, Nijmeijer S, Leurs R. (2011) G protein-coupled receptors: walking hand-in-hand, talking hand-in-hand? Br J Pharmacol 163:246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. (2005) A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA 102:9050–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun X, Bohn LM, Sadée W. (2005) Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol 67:2173–2184. [DOI] [PubMed] [Google Scholar]
- Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. (2005) Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol 68:1079–1086. [DOI] [PubMed] [Google Scholar]