Abstract
Background/Aims
Changes in lipid profiles in patients infected with hepatitis C virus (HCV) during direct-acting antiviral therapy have been reported in recent years. However, the clinical aspects of disturbed lipid metabolism in chronic HCV infection have not been fully elucidated.
Methods
Dynamic changes in serum total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol and apolipoprotein levels in patients infected with HCV genotype 1b were examined during combination therapy with daclatasvir (DCV) and asunaprevir (ASV).
Results
Total, LDL−, and HDL-cholesterol levels increased rapidly and persistently after week 4. Apolipoprotein (apo) A-I, apo B, apo C-II, and apo C-III levels were significantly higher at week 4 than at week 0. In contrast, apo A-II and apo E levels were significantly lower. The differences in LDL− and HDL-cholesterol levels were positively correlated with those of apo B and apo A-I, respectively. Interestingly, in patients with non-sustained virological response, these cholesterol levels decreased rapidly after viral breakthrough or viral relapse. Furthermore, similar changes were observed for apo A-I, apo B and apo C-III levels.
Conclusions
Clearance of HCV using combination therapy with DCV and ASV results in rapid changes in serum lipid profiles, suggesting an influence of HCV infection on disturbed lipid metabolism.
Keywords: Hepatitis C virus infection, Disturbed lipid metabolism, Apolipoproteins
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a primary cause of chronic hepatitis, which often progresses to liver cirrhosis and hepatocellular carcinoma (HCC). Moreover, chronic HCV infection disturbs lipid metabolism, including hypocholesterolemia and hypobetalipoproteinemia.1–3 HCV infection and replication are known to rely on host lipid metabolisms. Briefly, HCV virion form complexes with triglyceride-rich lipoproteins known as lipoviral particles (LVPs), including very-low-density lipoprotein (VLDL) and low-density lipoprotein (LDL), which circulate in the bloodstream.4,5 HCV-LVPs enter hepatocytes from LDL receptors via multiple steps, including attachment with CD81, scavenger receptor class B type 1 (SR-B1), claudin-1, and occludin.6,7 HCV activates sterol regulatory element binding proteins, which regulate cholesterol and fatty acid biosynthesis,8 and reduce β-oxidation by downregulating peroxisome proliferation-activated receptor α9,10 to enhance lipid biogenesis for replication of HCV in hepatocytes. HCV also reduces VLDL secretion from hepatocytes by inhibiting microsomal triglyceride transfer protein activity.11 In addition, several apolipoproteins are crucial for HCV-LVPs formation12 and entry into hepatocytes.6,7 HCV infection is strongly associated with characteristic apolipoprotein levels.13–15
Interferon (IFN)-based therapy was the most common form of treatment for chronic HCV infection for more than two decades. Triple therapy with pegylated-IFN, ribavirin (RBV), and HCV protease inhibitors achieved high rates of overall sustained virological response (SVR) in patients infected with HCV genotype 1.16–18 Successful IFN-based therapy for chronic HCV infection was shown to improve liver function, and to reduce the incidence of HCC and liver-related mortality.19–21 Furthermore, posttreatment increases in serum total and LDL-cholesterol levels were observed in HCV patients with SVR.14,22 However, studies with IFN-based therapy were limited in terms of evaluating dynamic changes in serum biochemical parameters of lipid metabolism during treatment, as administration of exogenous IFN-α/β in the setting of treatment for chronic HCV infection and other conditions can reduce LDL-cholesterol levels and raise triglyceride levels.23–25
Treatment for chronic HCV infection is rapidly evolving from IFN-based to IFN-free therapy consisting of direct acting antivirals (DAAs). Combination therapy with daclatasvir (DCV) and asunaprevir (ASV) has been approved as the first IFN-free regimen, with oral combinations of DAAs used for patients infected with HCV genotype 1 in Japan. In a phase 3 study evaluating combination therapy with DCV and ASV, SVR at 24 weeks after treatment (SVR24) was achieved by approximately 85% of the patients, including those that were ineligible, intolerant, or non-responsive to IFN-based therapy.26 However, the effects of DAAs on the risks of developing HCC, liver-related mortality, and extrahepatic manifestations, including disturbed lipid metabolism, have not been fully clarified. Increase in serum total, LDL-, and HDL-cholesterol levels in patients infected with HCV during DAAs were demonstrated in recent years.27,28 In addition, changes in apolipoprotein levels during combination therapy with sofosbuvir (SOF) and ledipasvir (LDV) were reported.29 However, the link between these cholesterol and apolipoprotein levels during DAAs therapy has not been elucidated. Thus, the present study was performed to determine the serial changes in serum total, LDL−, and high-density lipoprotein (HDL)-cholesterol, and apolipoprotein levels during combination therapy with DCV and ASV in patients infected with HCV genotype 1b.
MATERIALS AND METHODS
1. Patient population
This was a retrospective cohort study of patients infected with HCV genotype 1b receiving DCV at a dose of 60 mg once daily, and ASV at a dose of 100 mg twice daily for 24 weeks at Hamamatsu University School of Medicine and Hamamatsu City Idaira Clinic. Exclusion criteria included being administered a lipid-lowering agent before or during this study, and having coinfection with hepatitis B virus. Eighty-seven patients were started treatment of combination therapy with DCV and ASV from 2014 to 2016. According to exclusion criteria, data from 70 patients were analyzed for this study. All patients provided written informed consent and the study was approved by the Ethics Committee of Hamamatsu University School of Medicine (IRB number: EG14-098). The study protocols conformed to the ethical guidelines of the Declaration of Helsinki.
2. Laboratory tests
Hematological and biochemical parameters, including total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, cholinesterase, total cholesterol, LDL-cholesterol, and HDL-cholesterol, were measured in our clinical laboratory using standard techniques. Data for serum triglyceride analysis were excluded because patients were not instructed to fast before blood sampling. Apolipoproteins (apo A-I, apo A-II, apo B, apo C-II, apo C-III, and apo E) were measured by immunoturbidimetric assay using serum stored at −80°C and later thawed for analysis. These measurements were performed by a contract laboratory (LSI Medience Corp., Tokyo, Japan). Serum HCV-RNA titers were measured using the Roche COBAS TaqMan test or AccuGene m-HCV (Abbott Japan, Tokyo, Japan). HCV NS5A variants of Y93 and L31 before combination therapy with DCV and ASV were determined by direct sequencing and cycleave PCR (SRL Inc., Tokyo, Japan), and some of NS5A-Y93H mutation positive patients did not start receiving this combination therapy.
3. Statistical analysis
All statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). The data were presented as means±standard deviation and were evaluated with Wilcoxon’s paired signed-rank test, the Mann-Whitney U test, one-way analysis of variance and the Kruskal-Wallis test with Dunn’s multiple comparison test applied as appropriate. Correlation coefficients were calculated by linear correlation analysis.
RESULTS
1. Baseline characteristics
Baseline characteristics, including gender, age, HCV-RNA titer, liver enzymes, liver functions, and lipid parameters at week 0 of treatment are shown in Table 1. Mean AST and ALT levels were 51±30 IU/L and 45±27 IU/L, respectively. Mean albumin and cholinesterase levels were 3.9±0.5 g/dL and 239±87 IU/L, respectively. Mean HCV-RNA titer was 6.1±0.7 log10 IU/mL.
Table 1.
Clinical Characteristics of 70 Patients Infected with HCV Genotype 1b before Combination Therapy with Daclatasvir and Asunaprevir
| Characteristic | Value |
|---|---|
| Sex, male/female | 28/42 |
| Age, yr | 71±9 |
| HCV-RNA, log10 IU/mL | 6.1±0.7 |
| NS5A-Y93H mutation | 5 (7) |
| Platelet, /μL | 12.7±6.1 |
| Total bilirubin, mg/dL | 0.8±0.3 |
| AST, IU/L | 51±30 |
| ALT, IU/L | 45±27 |
| Albumin, g/dL | 3.9±0.5 |
| Cholinesterase, IU/L* | 239±87 |
| Total cholesterol, mg/dL | 160±31 |
| LDL-cholesterol, mg/dL* | 83±27 |
| HDL-cholesterol, mg/dL* | 50±16 |
| Apo A-I, μg/mL† | 141±24 |
| Apo A-II, μg/mL† | 26±5 |
| Apo B, μg/mL† | 70±16 |
| Apo C-II, μg/mL† | 3.0±1.0 |
| Apo C-III, μg/mL† | 6.6±2.0 |
| Apo E, μg/mL† | 3.3±0.8 |
Data are presented as mean±SD or number (%).
HCV, hepatitis C virus; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Apo, apolipoprotein.
n=64;
n=44.
2. Biochemical and viral responses
Serum AST levels normalized in 51 (73%), 45 (64%), and 45 (64%) of 70 patients at 4, 12, and 24 weeks after initiation of treatment, respectively. Sixty-two (89%), 53 (76%), and 55 (79%) patients had normalization of serum ALT levels at weeks 4, 12, and 24 during treatment, respectively. Thirty-eight (54%) and 65 (93%) of 70 patients had undetectable serum HCV-RNA titers following 4 and 12 weeks of treatment, respectively. At week 24, i.e., at the end of treatment, 68 patients (97%) had undetectable serum HCV-RNA titers. Two patients experienced viral breakthrough (VBT) during treatment, and one patient had viral relapse (VR) at 4 weeks following the end of treatment. Overall, 67 of 70 patients (96%) achieved SVR at posttreatment follow-up week 12 (SVR12).
3. Changes in serum lipid parameters
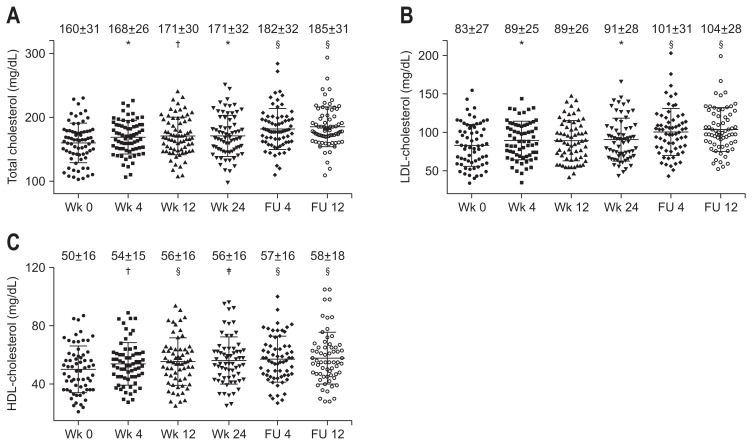
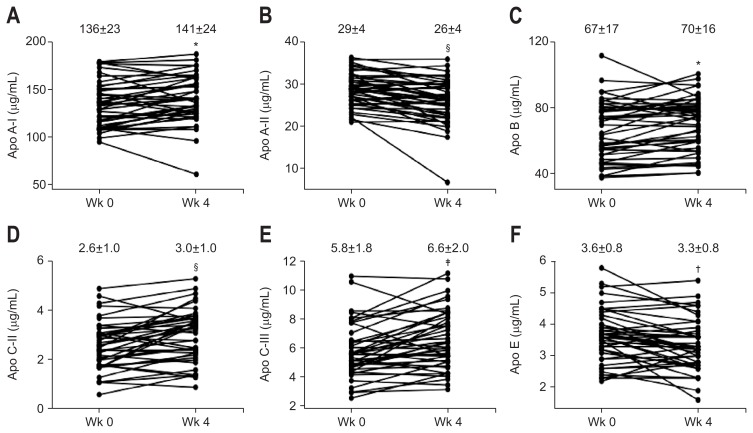
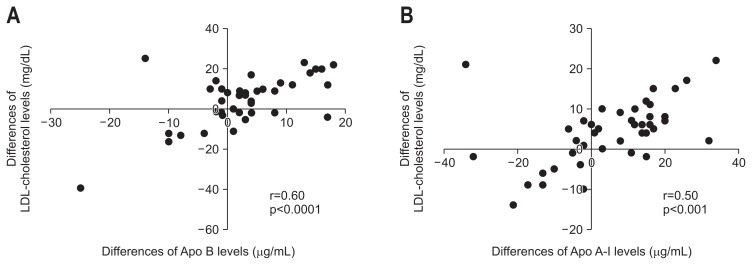
Serum total, LDL−, and HDL-cholesterol levels were significantly elevated at week 4 of treatment, and these increases were sustained until the end of the follow-up period (Fig. 1). Serum apo A-I, apo B, apo C-II, and apo C-III levels were significantly higher at week 4 than at week 0 (Fig. 2A, C–E). In contrast, serum apo A-II and apo E levels were significantly lower at week 4 than at week 0 (Fig. 2B and F). Furthermore, the differences of total cholesterol levels were positively correlated with those of apo A-I (r=0.51, p<0.001), apo B (r=0.69, p<0.0001), apo C-II (r=0.52, p<0.001), and apo C-III (r=0.59, p<0.001) levels, but not with those of apo A-II and apo E levels for the initial 4 weeks of treatment. As shown in Fig. 3, the differences of LDL-cholesterol levels and those of apo B levels showed a positive correlation (r=0.60, p<0.001) (Fig. 3A). There was a significant positive correlation between the differences of HDL-cholesterol levels and those of apo A-I levels (r=0.50, p<0.001) (Fig. 3B). Total, LDL-, HDL-cholesterol or apolipoprotein levels in undetectable serum HCV-RNA titer group were no different compared to these levels in detectable serum HCV-RNA titer group at week 4 of treatment. Furthermore, there was no correlation between the differences of serum HCV-RNA titers and those of serum total, LDL−, HDL-cholesterol or apolipoprotein levels for the initial 4 weeks of treatment.
Fig. 1.
Serial changes in serum total (A), low-density lipoprotein (LDL)-cholesterol (B), and high-density lipoprotein (HDL)-cholesterol (C) levels after the initiation of combination therapy with daclatasvir and asunaprevir in hepatitis C virus patients. p-values compared to week 0 of treatment.
FU, follow-up. *p<0.05, †p<0.01, ‡p<0.001, §p<0.0001, and determined using the Kruskal-Wallis test followed by Dunn’s multiple comparison test.
Fig. 2.
Changes in apolipoprotein (apo) A-I (A), apo A-II (B), apo B (C), apo C-II (D), apo C-III (E), and apo E (F) levels from week 0 to week 4 during combination therapy with daclatasvir (DCV) and asunaprevir (ASV) in 44 patients. *p<0.05, †p<0.01, ‡p<0.001, §p<0.0001, and determined using Wilcoxon’s paired sign-rank test.
Fig. 3.
Correlation between the differences of serum low-density lipoprotein (LDL)-cholesterol (A) and high-density lipoprotein (HDL)-cholesterol levels (B) with those of serum apolipoprotein (apo) B and apo A-I levels, respectively, from week 0 to week 4 during combination therapy with daclatasvir (DCV) and asunaprevir (ASV) in 44 patients. Correlation coefficients were calculated using linear correlation analysis.
4. Changes in lipid parameters and apolipoprotein levels after VBT or VR
To understand the impact of HCV infection on host metabolism, we examined serum total, LDL−, HDL-cholesterol and apolipoprotein levels before and after VBT or VR in patients with non-SVR. Based on the sample availability at 4 weeks before virological failure, and at the time when the virological failure was recognized for the first time, two of three patients with non-SVR were selected. One patient experienced VBT was not evaluated because the left sample was not enough to measure apolipoprotein levels. As shown in Table 2, total, LDL−, and HDL-cholesterol levels decreased after VBT or VR. Similar changes were observed for apo A-I, apo B, and apo C-III levels. There were no changes in apo A-II, apo C-II, or apo E levels.
Table 2.
Serum Lipid Profile and HCV-RNA Titer before and after VBT or VR
| Characteristics | VBT | VR |
|---|---|---|
|
| ||
| Recognition of HCV-RNA (before/after) | ||
| Total cholesterol, mg/dL | 176/155 | 209/177 |
| LDL-cholesterol, mg/dL | 77/65 | 145/121 |
| HDL-cholesterol, mg/dL | 79/73 | 33/30 |
| Apo A-I, mg/dL | 171/158 | 104/97 |
| Apo A-II, mg/dL | 33.5/31.0 | 22.0/21.3 |
| Apo B, mg/dL | 54/48 | 103/88 |
| Apo C-II, mg/dL | 2.1/2.2 | 3.3/3.3 |
| Apo C-III, mg/dL | 6.4/4.1 | 10.8/8.4 |
| Apo E, mg/dL | 2.7/2.6 | 4.3/3.5 |
| HCV-RNA, log10 IU/mL | 0/2.4 | 0/1.7 |
HCV, hepatitis C virus; VBT, viral breakthrough; VR, viral relapse; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Apo, apolipoprotein.
5. Improvement of liver synthetic function
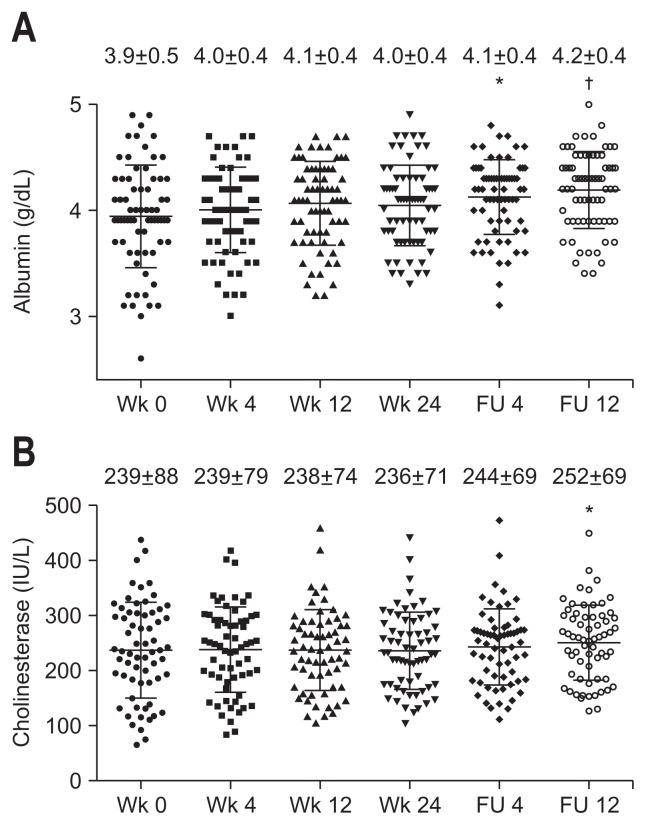
Serum albumin levels were significantly increased at posttreatment follow-up week 4, and the increase was sustained until the end of the follow-up period (Fig. 4A). Cholinesterase levels were increased significantly at posttreatment follow-up week 12 (Fig. 4B).
Fig. 4.
Serial changes in serum albumin (A) and cholinesterase levels (B) after the initiation of combination therapy with daclatasvir and asunaprevir in hepatitis C virus patients. p-values compared to week 0 of treatment.
FU, follow-up. *p<0.01 and †p<0.0001 determined using the Kruskal-Wallis test followed by Dunn’s multiple comparison test.
DISCUSSION
HCV life cycle is tightly linked to host lipid metabolism, and experimental evidence suggests that HCV utilizes hepatocyte lipid metabolism pathways for cellular entry, replication, assembly, and secretion.1,7 Disturbed lipid metabolism is encountered in patients with chronic HCV infection. Successful IFN-based therapy is known to ameliorate disturbed lipid metabolism in chronic HCV infection patients,14,22 but IFN itself affects the regulation of serum lipid concentrations, directly and indirectly modulating lipid metabolism in hepatocytes, adipocytes, and enterocytes.30,31 Accordingly, changes in serum lipid profiles during or immediately following the eradication of HCV infection remain poorly understood. There has been a great deal of progress in IFN-free DAAs therapies as antiviral therapy for patients with chronic HCV infection in recent years. Therefore, we examined serial changes in serum total, LDL−, and HDL-cholesterol, and apolipoprotein levels in patients infected with HCV genotype 1b during combination therapy with DCV and ASV, which is one of the DAAs treatment regimens. Our main findings can be summarized as follows: (1) serum total, LDL−, and HDL-cholesterol levels were rapidly and persistently increased after the initiation of treatment; (2) serum apo A-I, apo B, apo C-II, and apo C-III levels were upregulated early during therapy, while serum apo A-II and apo E expressions were downregulated concomitantly; and (3) reverse changes of serum lipid profiles were observed after VR or VBT. We have recognized that the limitations of our study include that there is a small number of patients with non-SVR and there is a lack of long-term follow-up following combination therapy with DCV and ASV for analysis in lipid profiles.
This study showed rapid increases in serum total, LDL−, and HDL-cholesterol levels during combination therapy with DCV and ASV in patients infected with HCV genotype 1b. There was no correlation between the differences of HCV-RNA titers and those of total, LDL−, and HDL-cholesterol levels for the initial 4 weeks of treatment. However, increase in serum lipid parameters was not reported as major adverse effect of combination therapy with DCV and ASV. Similar changes were recently reported during combination therapy with SOF and RBV or SOF and LDV, other IFN-free DAAs treatment regimens for chronic HCV infection.27,28 There was positive correlation between the differences of HCV core antigen and those of LDL-cholesterol levels for the initial 4 weeks of DAAs treatments.28 Altered expressions of lipid metabolism associated genes were confirmed in paired liver biopsy samples at pre and end of combination therapy with SOF and RBV.27 Furthermore, serum total, LDL−, and HDL-cholesterol levels were decreased after VBT or VR in this study. Accordingly, rapid changes of serum lipid profiles were presumed to have the association with the decline of HCV-RNA. The direct mechanisms likely involve the removal of the host lipid metabolism perturbation induced by HCV.
Apolipoproteins play a key role in lipid metabolism. Therefore, monitoring apolipoproteins is beneficial for understanding disturbed lipid metabolism in chronic HCV infection. This study showed significant increases in serum apo A-I, apo B, apo C-II, and apo C-III levels with concomitant reductions in serum apo A-II and apo E levels after the initiation of combination therapy with DCV and ASV. Recently, similar changes in serum apo A-II, apo E, and apo C-II levels were reported during combination therapy with SOF and LDV.29 Several apolipoproteins, such as apo A, apo B, apo C, and apo E, participate in the formation and stabilization of infectious HCV particles, and are closely involved in HCV entry, replication, and virions production.1,7 Disturbed serum apolipoprotein profiles include downregulated apo C-II and apo C-III, and upregulated apo A-II and apo E in HCV patients.14 Furthermore, expressions of apo B are possible to be regulated transcriptionally or posttranscriptionally due to noncoding RNA induced by HCV.32 Therefore, it is plausible that the eradication of HCV by antiviral therapy can influence the serum apolipoprotein profiles. In addition, this study showed significant correlations between the increases in serum total cholesterol levels and apo A-1, apo B, apo C-II, or apo C-III levels. Especially, the increases in HDL− and LDL-cholesterol levels were also found to be correlated with those in apo A-1 and apo B levels, respectively. Apo A-1, a major apolipoprotein of HDL particles, is able to interact with SR-B1 and participate with activation of lecithin acyl cholesterol acyltransferase. Apo B is classified two isoforms, apo B 100 and apo B 48. Apo B 100 is an especially major apolipoprotein of LDL particles and involved in VLDL assembly in hepatocytes.33
In this study, the small number of HCV patients with non-SVR precluded the ability to determine whether serum cholesterol level can become a predictor of treatment outcome in combination therapy with DCV and ASV. Lower concentrations of LDL-cholesterol level at week 0 are known to be less sensitive to IFN-based therapy.34–39 Recently, a similar association of serum LDL-cholesterol level with treatment outcome was reported in combination therapy with SOF and RBV.27 Considering the associations between IL28B genotype and host serum LDL-cholesterol levels,40 and between IL28B genotype and virological response for some IFN-free DAAs treatment regimens,41,42 further larger-scale studies are needed to clarify the association between serum cholesterol level and treatment outcome of combination therapy with DCV and ASV.
In conclusion, clearance of HCV using combination therapy with DCV and ASV, an IFN-free antiviral regimen resulted in rapid changes in serum lipid profiles including apolipoproteins, suggesting an influence of HCV infection on disturbed lipid metabolism.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Felmlee DJ, Hafirassou ML, Lefevre M, Baumert TF, Schuster C. Hepatitis C virus, cholesterol and lipoproteins: impact for the viral life cycle and pathogenesis of liver disease. Viruses. 2013;5:1292–1324. doi: 10.3390/v5051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André P, Komurian-Pradel F, Deforges S, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz O, Delers F, Maynard M, et al. Preferential association of hepatitis C virus with apolipoprotein B48-containing lipoproteins. J Gen Virol. 2006;87(Pt 10):2983–2991. doi: 10.1099/vir.0.82033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassendine MF, Sheridan DA, Bridge SH, Felmlee DJ, Neely RD. Lipids and HCV. Semin Immunopathol. 2013;35:87–100. doi: 10.1007/s00281-012-0356-2. [DOI] [PubMed] [Google Scholar]
- 7.Aizawa Y, Seki N, Nagano T, Abe H. Chronic hepatitis C virus infection and lipoprotein metabolism. World J Gastroenterol. 2015;21:10299–10313. doi: 10.3748/wjg.v21.i36.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waris G, Felmlee DJ, Negro F, Siddiqui A. Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J Virol. 2007;81:8122–8130. doi: 10.1128/JVI.00125-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Dharancy S, Malapel M, Perlemuter G, et al. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology. 2005;128:334–342. doi: 10.1053/j.gastro.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi A, Tazuma S, Nishioka T, et al. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig Dis Sci. 2005;50:1361–1371. doi: 10.1007/s10620-005-2788-1. [DOI] [PubMed] [Google Scholar]
- 11.Perlemuter G, Sabile A, Letteron P, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 12.Fukuhara T, Ono C, Puig-Basagoiti F, Matsuura Y. Roles of lipoproteins and apolipoproteins in particle formation of hepatitis C virus. Trends Microbiol. 2015;23:618–629. doi: 10.1016/j.tim.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Rowell J, Thompson AJ, Guyton JR, et al. Serum apolipoprotein C-III is independently associated with chronic hepatitis C infection and advanced fibrosis. Hepatol Int. 2012;6:475–481. doi: 10.1007/s12072-011-9291-x. [DOI] [PubMed] [Google Scholar]
- 14.Seki N, Sugita T, Aida Y, et al. Assessment of the features of serum apolipoprotein profiles in chronic HCV infection: difference between HCV genotypes 1b and 2. Hepatol Int. 2014;8:550–559. doi: 10.1007/s12072-014-9572-2. [DOI] [PubMed] [Google Scholar]
- 15.Moriya K, Shintani Y, Fujie H, et al. Serum lipid profile of patients with genotype 1b hepatitis C viral infection in Japan. Hepatol Res. 2003;25:371–376. doi: 10.1016/S1386-6346(02)00309-1. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi N, Izumi N, Kumada H, et al. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219–227. doi: 10.1016/j.jhep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi N, Okanoue T, Tsubouchi H, Toyota J, Chayama K, Kumada H. Efficacy and safety of telaprevir, a new protease inhibitor, for difficult-to-treat patients with genotype 1 chronic hepatitis C. J Viral Hepat. 2012;19:e134–e142. doi: 10.1111/j.1365-2893.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 20.Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corey KE, Kane E, Munroe C, et al. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030–1037. doi: 10.1002/hep.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade RJ, García-Escaño MD, Valdivielso P, Alcántara R, Sánchez-Chaparro MA, González-Santos P. Effects of interferon-beta on plasma lipid and lipoprotein composition and post-heparin lipase activities in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2000;14:929–935. doi: 10.1046/j.1365-2036.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara E, Yamashita S, Kihara S, et al. Interferon alpha induces disorder of lipid metabolism by lowering postheparin lipases and cholesteryl ester transfer protein activities in patients with chronic hepatitis C. Hepatology. 1997;25:1502–1506. doi: 10.1002/hep.510250632. [DOI] [PubMed] [Google Scholar]
- 25.Schectman G, Kaul S, Mueller RA, Borden EC, Kissebah AH. The effect of interferon on the metabolism of LDLs. Arterioscler Thromb. 1992;12:1053–1062. doi: 10.1161/01.ATV.12.9.1053. [DOI] [PubMed] [Google Scholar]
- 26.Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–2091. doi: 10.1002/hep.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meissner EG, Lee YJ, Osinusi A, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790–801. doi: 10.1002/hep.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto S, Yatsuhashi H, Abiru S, et al. Rapid increase in serum low-density lipoprotein cholesterol concentration during hepatitis C interferon-free treatment. PLoS One. 2016;11:e0163644. doi: 10.1371/journal.pone.0163644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younossi ZM, Elsheikh E, Stepanova M, et al. Ledipasvir/sofosbuvir treatment of hepatitis C virus is associated with reduction in serum apolipoprotein levels. J Viral Hepat. 2015;22:977–982. doi: 10.1111/jvh.12448. [DOI] [PubMed] [Google Scholar]
- 30.Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41(Suppl 2):97–101. doi: 10.2337/diab.41.2.S97. [DOI] [PubMed] [Google Scholar]
- 31.Jung HJ, Kim YS, Kim SG, et al. The impact of pegylated interferon and ribavirin combination treatment on lipid metabolism and insulin resistance in chronic hepatitis C patients. Clin Mol Hepatol. 2014;20:38–46. doi: 10.3350/cmh.2014.20.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TH, Matta B, King BD, Hodges MR, Tillmann HL, Patel K. MicroRNA-122 associates with serum apolipoprotein B but not liver fibrosis markers in CHC genotype 1 infection. J Med Virol. 2015;87:1722–1726. doi: 10.1002/jmv.24230. [DOI] [PubMed] [Google Scholar]
- 33.Bassendine MF, Sheridan DA, Felmlee DJ, Bridge SH, Toms GL, Neely RD. HCV and the hepatic lipid pathway as a potential treatment target. J Hepatol. 2011;55:1428–1440. doi: 10.1016/j.jhep.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 35.Lange CM, von Wagner M, Bojunga J, et al. Serum lipids in European chronic HCV genotype 1 patients during and after treatment with pegylated interferon-alpha-2a and ribavirin. Eur J Gastroenterol Hepatol. 2010;22:1303–1307. doi: 10.1097/MEG.0b013e32833de92c. [DOI] [PubMed] [Google Scholar]
- 36.Berg T, Andreone P, Pol S, et al. Low-density lipoprotein and other predictors of response with telaprevir-based therapy in treatment-experienced HCV genotype 1 patients: REALIZE study. Liver Int. 2015;35:448–454. doi: 10.1111/liv.12703. [DOI] [PubMed] [Google Scholar]
- 37.Gopal K, Johnson TC, Gopal S, et al. Correlation between beta-lipoprotein levels and outcome of hepatitis C treatment. Hepatology. 2006;44:335–340. doi: 10.1002/hep.21261. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan DA, Price DA, Schmid ML, et al. Apolipoprotein B-associated cholesterol is a determinant of treatment outcome in patients with chronic hepatitis C virus infection receiving anti-viral agents interferon-alpha and ribavirin. Aliment Pharmacol Ther. 2009;29:1282–1290. doi: 10.1111/j.1365-2036.2009.04012.x. [DOI] [PubMed] [Google Scholar]
- 39.Harrison SA, Rossaro L, Hu KQ, et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology. 2010;52:864–874. doi: 10.1002/hep.23787. [DOI] [PubMed] [Google Scholar]
- 40.Li JH, Lao XQ, Tillmann HL, et al. Interferon-lambda genotype and low serum low-density lipoprotein cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904–1911. doi: 10.1002/hep.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meissner EG, Bon D, Prokunina-Olsson L, et al. IFNL4-deltaG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J Infect Dis. 2014;209:1700–1704. doi: 10.1093/infdis/jit827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeuzem S, Soriano V, Asselah T, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630–639. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]