Abstract
The goal of this study was to identify factors contributing to hippocampal atrophy rate (HAR) in clinically-normal older adults (NC) and participants with mild cognitive impairment (MCI). Longitudinal HAR was measured on T1-weighted MRI, and the contribution of age, gender, ApoE ε4 status, intracranial volume, white matter lesions, and β-amyloid (Aβ) levels to HAR was determined using linear regression. Age-related effects of HAR were compared in Aβ positive (Aβ+) and Aβ negative (Aβ−) participants. Age and Aβ levels had independent effects on HAR in NC, while gender, ApoE ε4 status, and Aβ levels were associated with HAR in MCI. In multivariable models, Aβ levels were associated with HAR in NC; ApoE ε4 and Aβ levels were associated with HAR in MCI. In MCI, age was a stronger predictor of HAR in Aβ− versus Aβ+ participants. HAR was higher in Aβ+ participants, but the majority of HAR was due to factors other than Aβ status. Age-related effects on HAR did not differ between NC versus MCI participants with the same Aβ status. Therefore we conclude that even when accounting for other covariates, Aβ status, and not age, is a significant predictor of HAR; and that the majority of HAR is not accounted for by Aβ status in either NC or MCI.
Keywords: Alzheimer’s disease, normal aging, hippocampal atrophy rate, longitudinal study, β-amyloid
1. Introduction
Alzheimer’s disease (AD), characterized by plaques of β-amyloid (Aβ), tangles of tau/phospho tau, and neurodegeneration, begins in the entorhinal cortex and hippocampus (Cardenas et al., 2003; Du et al., 2003; Glenner and Wong, 1984; Jack et al., 2013; Jack et al., 2010; Kosik et al., 1986). AD pathology is accompanied by progressive memory dysfunction leading to a continuum of mild cognitive impairment (MCI) and finally dementia (Grundman et al., 2004; Morris et al., 2001; Petersen et al., 1999). An essential focus of current AD research is identification of biomarkers associated with, and predictive of, AD.
Hippocampal atrophy, a prominent feature of AD that correlates with cognitive decline, is accelerated in AD and associated with AD pathology (Seab et al., 1988), but also occurs across the lifespan in normal aging (Du et al., 2006; Fjell et al., 2009; Honea et al., 2011; Morra et al., 2009). Because increased hippocampal atrophy rate (HAR) is associated with both normal aging and AD, it is not clear how much HAR in clinically-normal older adults (NC) is due to presymptomatic AD pathology. Previous studies have been limited by several factors, including the lack of longitudinal hippocampal volume measurements, the exclusion of brain Aβ levels from the analyses, and focus on a singular contributing factor such as ApoE ε4 or age without the inclusion of models accounting for multiple variables simultaneously. Since studies aimed at understanding the relationship between HAR, normal aging, and AD are essential for using hippocampal atrophy as a sensitive outcome measure in evaluation of potential AD treatments, the goal of this study was to identify the main factors that contribute to HAR in a non-demented cohort of older adults.
Accumulation of fibrillar brain Aβ can be directly assessed in vivo by positron emission tomography (PET) using the radiotracers Pittsburg Compound B (PIB) or (18)Florbetapir (AV45) (Clark et al., 2011; Jack et al., 2008b; Rowe et al., 2007), or indirectly via decreased cerebrospinal fluid levels of Aβ1–42 (CSFAβ1–42) (Shaw et al., 2009). The 20–30% of older adult, cognitively-normal individuals with high levels of brain Aβ may represent a cohort that will eventually decline to MCI and/or AD (Dickerson et al., 2011; Morris et al., 2009; Rowe et al., 2010). Fibrillar Aβ deposition is associated with whole brain atrophy (Becker et al., 2011; Fagan et al., 2009), including hippocampal atrophy (Mormino et al., 2009) (Fjell et al., 2010; Schott et al., 2010; Storandt et al., 2009; Tosun et al., 2010) in older adult non-demented participants However, sensitivity in linking brain Aβ to HAR has been limited in previous studies, because of small sample sizes and limited longitudinal follow-up.
Additional factors that may be associated with HAR include apolipoprotein E (ApoE) genotype, gender, white matter lesions (WML), and intracranial volume (ICV). The ε4 allele of ApoE gene is associated with increased risk of AD and increased deposition of Aβ, and normal older adults ApoE ε4+ homozygotes have a higher rate of brain atrophy (Crivello et al., 2010) and low levels of CSFAβ1–42 (Schott et al., 2010). Evidence exists for sex differences in brain structure and cognition in normal aging and AD, with differential regional volume reductions in males and females (Cowell et al., 1994; Sowell et al., 2007). In addition to amyloidosis, WMLs and ICV are additional age-associated imaging features which are risk factors for AD that may contribute to outcome measures such as cognition (Carmichael et al., 2010; Constans et al., 1995; Farias et al., 2012; Jagust et al., 2008).
Understanding the relative contribution of multiple factors to HAR is crucial for understanding normal aging and AD disease progression, and for designing powerful AD clinical studies that rely on HAR as an outcome measure. Here we assess the association of multiple variables, especially age and Aβ status, with HAR in NC and MCI participants in ADNI.
2. Participants and Methods
Participants
The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.
The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and UCSF. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and participants have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 participants but ADNI has been followed by ADNI-GO and ADNI-2. To date these three protocols have recruited over 1500 adults, ages 55 to 90, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO. Participants originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. For up-to-date information, see www.adni-info.org.
All ADNI data was downloaded from the ADNI database (www.loni.ucla.edu/ADNI). We included ADNI participants with successful longitudinal FreeSurfer processing of MRI images (average number of images per participant=4.6), as well as either valid test result for CSFAβ1–42, PIB, or AV45 imaging (Table 1). The number of participants with multiple Aβ measurements is listed in Table 1.
Table 1.
Demographics of NC and MCI ADNI participants used in this study
| NC | MCI | |
|---|---|---|
| Number of participants | 208 | 359 |
| Age (mean ± SD) | 75.79±5.06 | 74.61±7.15 |
| Gender (% Female) | 49% | 36.8%* |
| Years of Education (mean ± SD) | 16.02±2.87 | 15.63±3.01 |
| ApoE ε4+ (% of total) | 55 (26.4) | 198 (55.2)* |
| Total PIB (% positive) | 19 (42.1) | 60 (68.3)* |
| Total AV45 (% positive) | 80 (34.0) | 94 (64.0)* |
| Total CSFAβ1–42 (% positive) | 108 (38.9) | 183 (74.3)* |
| Number of participants with multiple Aβ measurements | 57 | 79 |
| AV45 and CSFAβ1–42 | 46 | 50 |
| PIB and CSFAβ1–42 | 11 | 32 |
| AV45 and PIB | 8 | 27 |
| AV45, PIB, and CSFAβ1–42 | 4 | 15 |
p<0.01 versus NC
T1-weighted Magnetic Resonance Imaging (MRI)
Participants underwent a standardized 1.5 Tesla MRI protocol (http://www.loni.ucla.edu/ADNI/Research/Cores/index.shtml) that was previously validated across sites (Jack et al., 2008a). The ADNI MRI quality control center at the Mayo Clinic selected the MP-RAGE image with higher quality and corrected for system-specific image artifacts, as described in (Jack et al., 2008a).
FreeSurfer Longitudinal processing
Automated hippocampal volume measures were performed with FreeSurfer software package version 4.4. To reduce confounding effects of intra-participant morphological variability, each participant’s data series was processed by FreeSurfer longitudinal workflow (Fischl et al., 2002; Fischl et al., 2004), and see http://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing. Details of quality control procedures are posted online (http://www.loni.ucla.edu/twiki/pub/ADNI/ADNIPostProc/UCSFFreeSurferMethodsSummary.pdf).
PET Imaging
PIB and AV45 images were collected at multiple sites. Participants were injected with 15 mCi PIB or 10 mCi AV45, four dynamic acquisition frames were obtained 50–70 minutes post injection and coregistered to one another, averaged, interpolated to a uniform image and voxel size (160×106×96, 1.5mm3), and smoothed to a uniform resolution (8mm FWHM). See also: adni.loni.ucla.edu/about-data-samples/image-data. A mean cortical standardized uptake value ratio (SUVR) measure was derived by normalizing average retention values of 9 cortical regions (anterior cingulate, frontal, sensorimotor, lateral temporal, medial temporal, parietal and occipital cortex, occipital pole, and precuneus), to retention value of cerebellar cortex. For each participant, the SUVR was calculated from the average SUVR of scans taken at various time points from initial participant screen. For PIB, scans were performed at baseline (n=20), month 12 (n=84), month 24 (n=78), month 36 (n=40), and month 48 (n=2). For AV45 scans were performed at month 36 (n=7), month 48 (n=95), month 60 (n=73), or month 72 (n=6).
CSFAβ1–42
CSF samples were obtained by individual centers, then banked and batch-processed using a standardized protocol, under the direction of Drs. Leslie Shaw and John Trojanowski of the ADNI Biomarker Core at the University of Pennsylvania School of Medicine. Details regarding CSF collection and CSFAβ1–42 measurements are provided in the ADNI procedural manual (www.adni-info.org) and in (Shaw et al., 2009). CSFAβ1–42 was measured using multiplex xMAP Luminex platform (Luminex Corporation, Austin, TX) with Innogenetics (INNO-BIA AlzBio3, Ghent, Belgium) immunoassay kit-based research-use only reagents including monoclonal antibodies 4D7A3 (capture) and 3D6 (detector) for Aβ42. All CSFAβ1–42 samples were taken within weeks of the first hippocampal volume measure.
Statistical Analysis
Repeated measures of hippocampal volumes over 4 years were modeled using linear mixed-effects regression. To determine HAR, hippocampal volume was regressed on time since initial scan with both a random intercept and slope to estimate individual HAR, assuming a compound symmetry correlation structure. In a second step, HAR were then regressed on age, gender, APOE ε4 carrier status, WML, ICV, AV45, PIB, and CSFAβ1–42 using ordinary least squares regression. The variance of each predictor was estimated using 500 bootstrap samples to account for the initial step of estimating individual HAR. Each predictor was evaluated both separately and in a full model, adjusting for all other predictors. An interaction between Aβ status and age was also considered, using the following thresholds for Aβ positivity: 1.11 SUVR for AV45, 1.5 SUVR for PIB, and 192 pg/ml for CSFAβ1–42. Ridge regression was performed to evaluate the collective predictive ability of the Aβ measures while adjusting for high correlation between measures; the penalty term was selected via 10-fold cross validation. In the full model, a separate multivariable model was made using each of the Aβ variables due to the high correlation between the 3 measurements. The associations between predictors and HAR were assessed using Wald tests and R2. Comparisons between MCI and NC participants on baseline variables were done using the Wilcoxon-Mann-Whitney test and Fisher’s exact test. Model fits were inspected by an analysis of the residuals. All statistics were done using R (v. 2.8.1, The R Foundation for Statistical Computing).
3. Results
Factors associated with HAR in NC Participants
When tested separately in univariable models, only advanced age and increased Aβ levels were significantly associated with HAR in NC participants (Table 2). There was a trend towards a significant association between female gender and increased HAR (p=0.07). Both WM hyperintensities (p=0.05) and hypointensities (p=0.07) also showed strong trends for positive correlation with HAR. Neither ICV nor ApoE ε4 genotype was significantly associated with HAR (Table 2). The three Aβ measures were highly correlated with each other (CSFAβ1–42 versus AV45 r=−0.7835, CSFAβ1–42 versus PIB r=−0.6198, AV45 versus PIB r=0.7898) and ridge regression analysis confirmed that including multiple Aβ measures in the multivariable regression did not increase our ability to predict HAR. Therefore, we generated a multivariable model using a single Aβ measure, CSFAβ1–42. In this model, only Aβ levels were positively associated with HAR when adjusting for effects of all variables (Table 3). Thus, the effects of gender, age, and WML are likely confounded by brain Aβ levels since their associations with HAR go away in a multivariable model accounting for Aβ. Further, in separate multivariable models using either PIB or AV45 as the Aβ measure, Aβ levels were significantly associated with HAR when adjusting for effects of all variables (p=0.05 for AV45, p=0.006 for PIB), confirming that the association of Aβ and HAR holds up when accounting for other variables that may affect HAR.
Table 2.
Factors contributing to HAR in NC and MCI in univariable regression models
| Estimate | Std. Error | T value | P value | R2 | |
|---|---|---|---|---|---|
|
Age NC MCI |
−8.14 −2.92 |
3.457 3.282 |
−2.354 −0.089 |
0.02* 0.37 |
0.02619 0.00222 |
|
Gender NC MCI |
−12.42 −17.74 |
6.937 6.740 |
−1.79 −2.632 |
0.07 <0.001*** |
0.01532 0.01904 |
|
ICV NC MCI |
1.89 4.82 |
3.492 3.276 |
0.538 1.47 |
0.591 0.142 |
0.001404 0.006019 |
|
ApoE ε4+ NC MCI |
−11.42 −38.11 |
7.7884 6.281 |
−1.448 −6.067 |
0.15 <0.001*** |
0.01008 0.09347 |
|
WM Hypo NC MCI |
−6.35 −1.51 |
3.475 3.285 |
−1.826 −0.458 |
0.07 0.65 |
0.01593 0.00059 |
|
WM Hyper NC MCI |
−6.81 −2.09 |
3.472 3.284 |
−1.961 −0.636 |
0.05 0.53 |
0.01376 0.001671 |
|
AV45 NC MCI |
−49.14 −80.03 |
24.29 22.34 |
−2.023 −3.682 |
0.047** <0.001*** |
0.08497 0.1829 |
|
PIB NC MCI |
−99.34 −77.40 |
29.11 23.146 |
−3.412 −3.344 |
<0.001** 0.00145** |
0.4065 0.1616 |
|
CSF Aβ1–42 NC MCI |
0.21 0.39 |
0.08524 0.07812 |
2.460 5.00 |
0.02* <0.001*** |
0.05402 0.1214 |
p<0.05,
p<0.01,
p<0.001
Table 3.
Factors contributing to HAR in NC and MCI in a multivariable regression model.
| Estimate | Std. Error | T value | P value | |
|---|---|---|---|---|
|
Age NC MCI |
−1.113 −4.81 |
0.715 0.804 |
−1.557 −0.598 |
0.12 0.55 |
|
Gender NC MCI |
−3.03 −12.49 |
9.96 11.33 |
−0.332 −1.102 |
0.74 0.27 |
|
ICV NC MCI |
0.000 0.000 |
0.000 0.000 |
0.778 0.781 |
0.44 0.44 |
|
ApoE ε4+ NC MCI |
0.126 −29.74 |
14.33 11.14 |
0.009 −2.70 |
0.99 0.008** |
|
WM Hypo NC MCI |
0.000 0.000 |
0.000 0.007 |
−0.736 −0.018 |
0.46 0.99 |
|
CSF Aβ1–42 NC MCI |
0.220 0.256 |
0.093 0.100 |
−2.371 2.546 |
0.02* 0.01* |
Adjusted R2=0.03072 for NC, R2=0.1827 for MCI.
p<0.05,
p<0.01,
p<0.001
Factors associated with HAR in MCI Participants
In MCI participants, female gender, ApoE ε4+ status, and all three Aβ measures were significantly associated with increased HAR (Table 2) in univariable models. As with NC participants, Aβlevels measured by all 3 Aβ measures showed the strong association with HAR (Table 2). In the MCI cohort there was no significant association between HAR and age, ICV, or WML (Table 2). Using a multivariable regression model, with CSFAβ1–42 as the Aβ measure, only ApoE ε4 status and Aβ level retained a significant association with HAR (Table 3). Thus, the effect of gender on HAR is likely confounded by only ApoE ε4, and/or Aβ levels in MCI participants. We also found the significant association between Aβ and HAR in multivariable regression models using each of the other Aβ measures (p=0.03 for AV45, p=0.02 for PIB).
In summary, although multiple variable are associated with HAR in univariable models, only Aβ levels are significantly associated with HAR in both NC and MCI in a model adjusting for effects of age, gender, WMLs, and ICV in a single model. In MCI, ApoE ε4+ genotype is also significantly associated with HAR in both the univariable and multivariable models. Thus, a comparison of univariable and multivariable models reveals that the effects of such variables as age and gender on HAR can be accounted for by brain Aβ levels in NC and MCI participants, and additionally by ApoE ε4 status in MCI participants. In both NC and MCI, Aβ levels, measured by PIB, AV45, or CSF Aβ1–42, showed the strongest association with HAR. Thus, in both NC and MCI participants, Aβ levels show the most robust association with HAR even when multiple demographic and neuroimaging factors are taken into account.
The effect of age and Aβ status on HAR
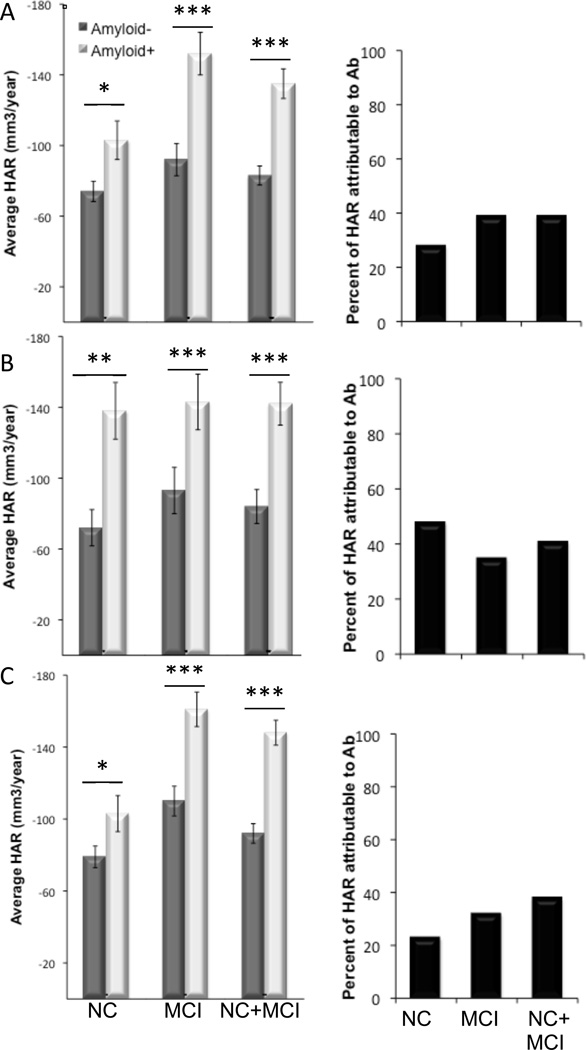
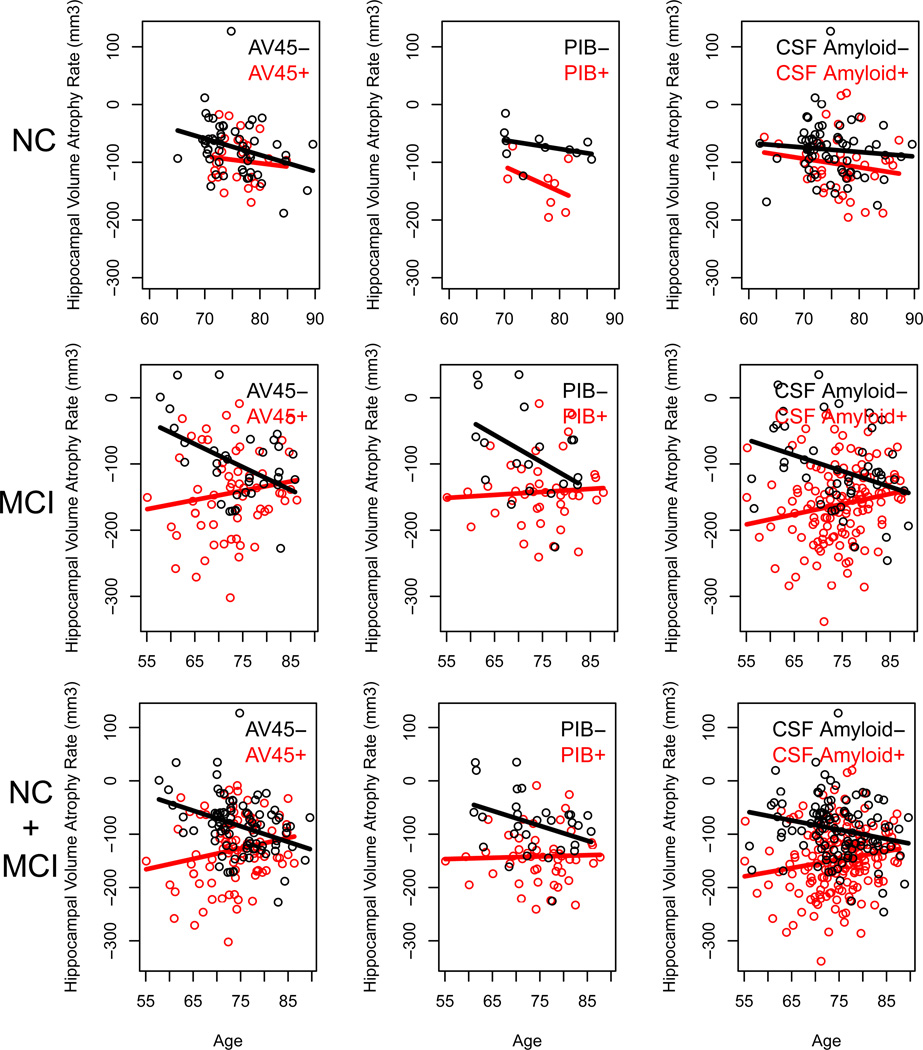
We next tested for differential age-related effect on HAR in participants classified as Aβ positive (Aβ+) or Aβ negative (Aβ−) according to previously-established thresholds (Aizenstein et al., 2008; Jack et al., 2008a; Johnson et al., 2013; Joshi et al., 2012; Mormino et al., 2012; Shaw et al., 2009; Weigand et al., 2011) We found significant HAR in both the Aβ+ and Aβ− subgroups, with higher age-adjusted HAR in the Aβ+ cohort for both NC and MCI (Figure 1). Next, we calculated the percent of HAR that was accounted for by Aβ status as the difference in HAR between the Aβ+ and Aβ− subgroups, and found that the majority of HAR was not attributable to Aβ status in NC, MCI, or a combined cohort (Figure 2). In MCI alone, or a combined cohort of NC+MCI, there was a significant interaction between age and Aβ status, with a stronger association between age and HAR in the Aβ− subgroup (p=0.007 for AV45 and CSFAβ1–42, p=0.03 for PIB) (Figure 3). In fact, for Aβ+ participants, there was a slight decrease in HAR associated with increased age (Figure 3). Thus, the association between age and HAR depends on Aβ status in MCI participants, likely because this represents a group in which disease accelerates HAR and eclipses the effect of age alone.
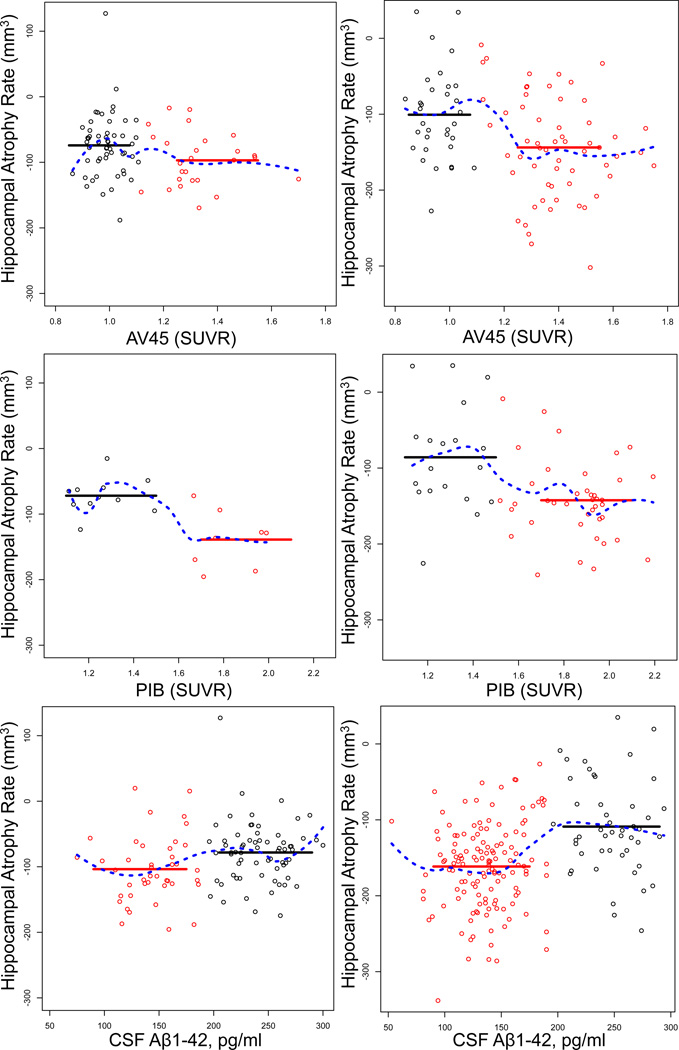
Figure 1. The effect of Aβ levels on HAR in NC and MCI.
Scatter plots showing the correlation between 3 different measures of Aβ levels (AV45, A and D; PIB, B and E; CSFAβ1–42, C and F) and HAR in NC (A–C) and MCI (D–F) participants. Regression analysis (blue dotted line) shows the best fit as a sigmoid that delineates two subpopulations of participants, Aβ+ (red squares) and Aβ− (black squares). The threshold values used are 1.11 SUVR for AV45, 1.5 SUVR for PIB, and 192 pg/ml. for CSFAβ1–42. Solid red and black lines represent the mean values for the Aβ+ (red) and Aβ− (black) subgroups.
Figure 2. HAR attributable to Aβ in NC and MCI.
Bar plots showing average HAR in Aβ+ (dark bars) and Aβ− (light bars) subgroups of NC, MCI, and the combined cohorts (NC+MCI) for AV45 (A), PIB (B) and CSFAβ1–42 (C). Black bars show the percentage of total HAR attributable to Aβ for each Aβ measure. *p<0.01, **p<0.001, ***p<0.0001.
Figure 3. The effect of age and Aβ status on HAR.
Scatter plots showing the effect of age on HAR in a cohort of NC, MCI, or NC+MCI participants classified as Aβ+ (red circles) or Aβ− (black circles) according to levels of AV45, PIB, or CSFAβ1–42. Linear regression analyses (solid black and red lines) show a significant interaction between age and Aβ status in MCI participants (p=0.007 for AV45, p=0.03 for PIB, and p=0.001 for CSFAβ1–42) as well as in the combined cohort of NC+MCI (p<0.001 for AV45, p=0.07 for PIB, and p=0.002 for CSF Aβ1–42), but not in NC participants (p=0.56 for AV45, p=0.44 for PIB, and p=0.70 for CSF Aβ1–42).
Based on our results, we conducted a power analysis of a hypothetical two-year clinical trial using HAR as an outcome measure for testing drug effect, with HA measurements performed every 6 months. We found that 1591 NC participants per arm would be required to detect a 25% slowing of Aβ−related HAR. However, detecting a 25% slowing of total HAR only in Aβ+ or Aβ− NC would require 84 or 210 participants per arm, respectively. Thus, the use of Aβ status as inclusion criterion in a NC cohort confers adequate statistical power using fewer participants.
The effect of diagnosis on age-related HAR in Aβ + and Aβ− subgroups
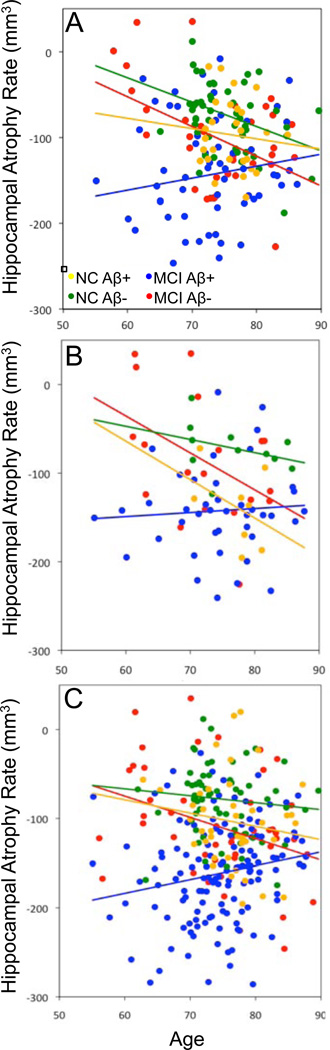
To assess the contribution of participant diagnosis to age-related HAR, we determined whether participants who share the same Aβ status differ in the effect of age on HAR by testing for a age × diagnosis interaction separately in the Aβ+ and Aβ− subgroups (Figure 4). As stated above, we found no significant association between age and HAR using the full model. Furthermore, we found no significant interaction between age and diagnosis in Aβ+ participants (p=0.44), or Aβ− participants (p=0.64) (Figure 4). To confirm whether participant diagnosis is significantly associated with overall HAR, we performed linear regression on the Aβ+ and Aβ− subgroups separately, using diagnosis, age, gender, and ApoE ε4 status as explanatory variables and HAR as the outcome variable. In the Aβ+ cohort, ApoE ε4+ status (p=0.02), MCI diagnosis (p<0.001) and female gender (p=0.05) were all associated with increased HAR, whereas in the Aβ− cohort, only age and MCI diagnosis (p<0.001) were significantly associated with increased HAR. We conclude that Aβ status, regardless of diagnosis, is the variable that determines the trajectory of age-related HAR. This finding supports the use of Aβ status as a defining criteria for assessing features of neurodegeneration in subpopulations of participants in future studies.
Figure 4. The effect of diagnosis on age-related HAR in Aβ+ and Aβ− subpopulations.
Scatter plots showing the effect of age on HAR in NC and MCI participants dichotomized into Aβ+ and Aβ− subgroups using AV45 (A), PIB (B), or CSFAβ1–42 (C) levels. Regression analyses for each subgroup are represented by solid lines.
4. Discussion
The major findings are (1) In NC participants, only Aβ levels were significantly correlated with HAR when adjusting for age, gender, Apo ε4 genotype, WMLs and ICV in a multivariable model; (2) In MCI participants, both Aβ levels and ApoE ε4+ genotype were significantly associated with HAR in the multivariable model; (3) There was significant HAR in both the Aβ+ and Aβ− NC and MCI subgroups, and Aβ+ participants had significantly higher HAR than Aβ−; (4) In MCI or the combined cohort of NC+MCI, there was a significant interaction between age and Aβ status, with a stronger association between age and HAR in the Aβ− subgroup, indicating that age had a significantly higher association with HAR in Aβ− participants than in Aβ+ participants in MCI but not NC; (5) Less than half of age-related HAR was attributable to Aβ levels in NC, MCI, or a combined cohort of NC and MCI participants.
These findings have important implications for the design of future studies examining the association between brain volume, aging and AD related biomarkers. Modeling HAR using multiple volume measurements over a 4 year time period in a linear regression model allowed us to capture volume changes over a longer time period than many other studies, while better controlling for random variations in volume measurement over time. Compared to previous cross-sectional studies that reported an association of age and hippocampal volume in older adult NC (Brickman et al., 2008; Jack et al., 1997), we find no association between HAR and age in an adjusted multivariable model. Our work confirms that of previous studies showing a correlation between Aβ levels and HAR in older adult NC (Fjell et al., 2010; Schott et al., 2010), and extends these findings using HAR measured longitudinally over a longer time span, with multiple data points per participant.
Our inclusion of multiple variables in a single, multivariable regression model reveals novel associations between the variables used and HAR. For NC participants, the difference between the univariable and multivariable models were: (1) age is only significantly associated with HAR in the univariable model; and (2) the trend-level associations of gender and WML found in the univariable models do not hold up in the multivariable model. Thus, future studies examining associations between gender, age, and WML and HAR are likely to overestimate these associations if they do not account for Aβ levels and other variables in their analyses.
For MCI but not NC participants, we found a significant association between gender and HAR in the univariable model, but not the multivariable model. However, the difference in effect size in NC (r2=0.014) versus MCI (r2−0.019) for this association is negligible, and there is a trend level (p−0.07) association between gender and HAR in NC. Therefore, it is possible that the difference in significance between the two cohorts is due to the fact that the MCI cohort is 73% larger than the NC cohort (Table 1), conferring greater statistical power to analyses done in the MCI group. Further studies using a larger NC cohort are needed to confirm this finding. The effect of gender on HAR goes away when adjusting for the other variables in the multivariable models (Table 2), suggesting it is confounded by age, Aβ, and/or ApoE ε4 genotype. We conclude that gender has no significant influence and this finding emphasizes the importance of controlling for confounding variables in future studies examining links between gender and brain atrophy.
Another important implication of our findings is for the design of clinical trials that test the efficacy of Aβ−altering therapies in NC and MCI individuals, as also discussed in (Holland et al., 2012a; Holland et al., 2012b). We found that, although age-adjusted HAR is higher in the Aβ+ group for both NC and MCI, less than half of HAR is attributable to Aβ status. Furthermore, subsetting participants into Aβ+ and Aβ− subgroups can drastically reduce the number of participants needed to achieve adequate statistical power. According to our sample size analysis, compared to a study using a general NC cohort, a study using only Aβ+ participants would require a greater than 18 fold reduction in the number of participants (84 verses 1591 participants per arm), and a study using only Aβ− participants would require a greater than 7 fold reduction in the number of participants (210 verses 1591 participants per arm) to obtain the same level of statistical power. Our study extends previous findings by measuring the effect of age on HAR separately in Aβ+ and Aβ− subgroups. It demonstrates that the majority of hippocampal atrophy is accounted for by variables other than Aβ and/or age. Since treatments directed at reducing Aβ would not be expected to slow the non-Aβ−related HAR, our results can inform future clinical trials on expected levels of HAR reduction NC and MCI participants.
Although this study provides important new information about the factors contributing to HAR, it has some limitations. First, the ADNI cohort may not accurately reflect the population in terms of HAR in normal controls (Whitwell et al., 2012). ADNI exclusion criteria limit the range of values available for some variables we studied here. For example, ADNI does not include participants with cerebrovascular disease, which biases the WML values to a narrow range of values. This type of selection bias could lead us to underestimate the significance of the association of some variables with HAR. Thus, while these findings are highly relevant to the AD field and those conducting clinical research on an AD-focused aging population, it limits our ability to generalize to a population of older adults. Furthermore, the smaller cohort size of NC versus MCI participants used in our study increases the risk that we will overestimate the contributions of specific variables to HAR in MCI, or underestimate the same associations in NC.
Another limitation is the timing of the brain Aβ measurement in relation to the structural MRIs used to calculate HAR. The Aβ measure is a single snapshot taken at variable intervals with respect to hippocampal volume measurements. In our study, all CSFAβ1–42 samples were taken before the initial volume measurement, all AV45 scans were done after the final volume measurement, and PIB measurements were taken at variable intervals within the same timeframe as the volume measurements. However, the different Aβ measurements were still highly correlated with each other in terms of their association with HAR, and showed a high level of consistency in terms of the percent of HAR attributable to Aβ status. Finally, because we calculated HAR using a linear model, we may have overestimated contributions of Aβ and age to the extent that accelerated and/or nonlinear HAR were present in the data (Schuff et al., 2012).
In conclusion, this study shows that the presence of brain Aβ is a major predictor of HAR in older adults, but that most HAR in older adult NC and MCI participants is not associated with the presence of Aβ and is likely to be a consequence of aging and other unknown factors. The findings emphasize the importance of including longitudinal volume measurements in studies of brain atrophy, and of simultaneously accounting for the contributions of multiple variables to brain atrophy. Our results demonstrate the importance of accounting for brain Aβ accumulation when designing clinical studies of AD drugs, and therefore have important implications for the design and statistical powering of future clinical trials testing the efficacy of anti-Aβ therapeutics for AD.
Highlights.
We examine effects of multiple variables on longitudinal HAR in NC and MCI.
Multivariable linear regression analysis revealed novel associations with HAR.
In MCI there was a significant interaction between age and Aβ status.
Less than half of age-related HAR was attributable to Aβ levels in NC and MCI.
Our findings can inform the design of clinical trials of anti-Aβ therapies for AD.
Acknowledgments
Source of Funding: Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904). ADNI is funded by the NIA, the NIBIB, and through generous contributions from Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, Glaxo-SmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace Inc., Merck and Co. Inc., Novartis AG, Pfizer Inc., F. Hoffman-La Roche, Schering-Plough, Synarc Inc., as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Disclosure Statement: To fully address any financial relationships of the coauthors that may lead to a perceived conflict of interest, we would like to make the following disclosures. Dr. Shaw reports grants from NIA/NIH during the conduct of the study. Dr. Shaw previously was consultant for Innogenetics and collaborates on quality assessment activities as part of the Alzheimer's Disease Neuroimaging Initiative. Dr. Aisen has been a consultant for NeuroPhage; Elan Corporation, Wyeth, Eisai Inc., Schering-Plough Corp., Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co., Roche, Amgen, Genentech, Inc., Abbott, Pfizer Inc, Novartis, Bayer, Astellas, Dainippon, Biomarin, Solvay, Otsuka, Daiichi, AstraZeneca, Janssen, Medivation, Inc., Ichor, Toyama, Lundbeck, Biogen Idec, iPerian, Probiodrug, Somaxon, Biotie, Anavex and Kyowa Hakko Kirin Pharma. In addition, Dr. Aisen has grants or pending grants from Lilly, Baxter, NIA, and FNIH. Dr. Weiner has been on scientific advisory boards for Pfizer and BOLT Inter-national; has been a consultant for Pfizer Inc., Janssen, KLJ Associates, Easton Associates, Harvard University, inThought, INC Research, Inc., University of California, Los Angeles, Alzheimer’s Drug Discovery Foundation and Sanofi-Aventis Groupe; has received funding for travel from Pfizer, AD PD meeting, Paul Sabatier University, Novartis, Tohoku University, MCI Group, France, Travel eDreams, Inc., Neuroscience School of Advanced Studies (NSAS), Danone Trading, BV, CTAD ANT Congres; serves as an associate editor of Alzheimer’s & Dementia; has received honoraria from Pfizer, Tohoku University, and Danone Trading, BV; has research support from Merck, Avid, DOD and VA; and has stock options in Synarc and Elan.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, Chui HC, Schuff N, Weiner MW. Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol Aging. 2003;24:537–544. doi: 10.1016/s0197-4580(02)00130-6. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr, Weiner M, DeCarli C. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman EP, Zehntner SP, Skovronsky DM. Use of florbetapir-PET for imaging beta-amyloid pathology. Jama. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans JM, Meyerhoff DJ, Gerson J, MacKay S, Norman D, Fein G, Weiner MW. H-1 MR spectroscopic imaging of white matter signal hyperintensities: Alzheimer disease and ischemic vascular dementia. Radiology. 1995;197:517–523. doi: 10.1148/radiology.197.2.7480705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivello F, Lemaitre H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage. 2010;53:1064–1069. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed BR, Kramer JH, Mungas D, Yaffe K, Chui HC, Weiner MW. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed B, Carmichael O, Beckett L, Harvey D, Olichney J, Simmons A, Decarli C. Maximal brain size remains an important predictor of cognition in old age, independent of current brain pathology. Neurobiol Aging. 2012;33:1758–1768. doi: 10.1016/j.neurobiolaging.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Blennow K, Brewer JB, Dale AM. Brain atrophy in healthy aging is related to CSF levels of Abeta1–42. Cereb Cortex. 2010;20:2069–2079. doi: 10.1093/cercor/bhp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Holland D, Desikan RS, Dale AM, McEvoy LK. Rates of decline in Alzheimer disease decrease with age. PLoS One. 2012a;7:e42325. doi: 10.1371/journal.pone.0042325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, McEvoy LK, Desikan RS, Dale AM. Enrichment and stratification for predementia Alzheimer disease clinical trials. PLoS One. 2012b;7:e47739. doi: 10.1371/journal.pone.0047739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni ED, Burns JM. Progressive regional atrophy in normal adults with a maternal history of Alzheimer disease. Neurology. 2011;76:822–829. doi: 10.1212/WNL.0b013e31820e7b74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, Ward LWJC, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of magnetic resonance imaging : JMRI. 2008a;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008b;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, Ellis WG, Zarow C, Mungas D, Reed BR, Kramer JH, Schuff N, DeCarli C, Chui HC. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Sperling RA, Gidicsin CM, Carmasin JS, Maye JE, Coleman RE, Reiman EM, Sabbagh MN, Sadowsky CH, Fleisher AS, Murali Doraiswamy P, Carpenter AP, Clark CM, Joshi AD, Lu M, Grundman M, Mintun MA, Pontecorvo MJ, Skovronsky DM A.A.s. group. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9:S72–S83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM F.S.I. Florbetapir. Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer's disease and cognitively normal subjects. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, Jagust WJ. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. NeuroImage. 2012;59:1152–1160. doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Toga AW, Jack CR, Jr, Schuff N, Weiner MW, Thompson PM. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45:S3–S15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O'Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O'Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1–42. Ann Neurol. 2010;68:825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- Schuff N, Tosun D, Insel PS, Chiang GC, Truran D, Aisen PS, Jack CR, Jr, Weiner MW. Nonlinear time course of brain volume loss in cognitively normal and impaired elders. Neurobiol Aging. 2012;33:845–855. doi: 10.1016/j.neurobiolaging.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seab JP, Jagust WJ, Wong ST, Roos MS, Reed BR, Budinger TF. Quantitative NMR measurements of hippocampal atrophy in Alzheimer's disease. Magn Reson Med. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Annals of neurology. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol Aging. 2010;31:1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand SD, Vemuri P, Wiste HJ, Senjem ML, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS, Jack CR, Jr I. Alzheimer's Disease Neuroimaging. Transforming cerebrospinal fluid Abeta42 measures into calculated Pittsburgh Compound B units of brain Abeta amyloid. Alzheimers Dement. 2011;7:133–141. doi: 10.1016/j.jalz.2010.08.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Roberts RO, Boeve BF, Petersen RC, Jack CR., Jr Comparison of imaging biomarkers in the Alzheimer Disease Neuroimaging Initiative and the Mayo Clinic Study of Aging. Arch Neurol. 2012;69:614–622. doi: 10.1001/archneurol.2011.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]