Abstract
Randomized controlled pilot evaluated effect of conditional economic incentives (CEIs) on number of sex partners, condom use, and incident sexually transmitted infections (STIs) among male sex workers in Mexico City. Incentives were contingent on testing free of new curable STIs and/or clinic attendance. We assessed outcomes for n=227 participants at 6 and 12 months (during active phase with incentives), and then at 18 months (with incentives removed). We used intention-to-treat and inverse probability weighting. During active phase, CEIs increased clinic visits (10–13 percentage points) and increased condom use (10–15 percentage points) for CEI groups relative to controls. The effect on condom use was not sustained once CEIs were removed. CEIs did not have an effect on number of partners or incident STIs. Conditional incentives for male sex workers can increase clinical interaction and may reduce some HIV/STI risks such as condomless sex, while incentives are in place.
Keywords: conditional economic incentives, conditional cash transfers, HIV prevention, sexually transmitted infections prevention, male sex workers, Mexico
Introduction
Conditional economic interventions previously used for poverty alleviation and economic development (often under the name of conditional cash transfers) [1] are now being utilized as an intervention strategy specifically targeted to people highly vulnerable to HIV and other sexually transmitted infections (STIs) [2–4]. Conditional economic incentives (CEIs) involve reinforcing positive behavioral change through using cash or other material incentives in exchange for meeting specified behavioral goals or conditions. These behavioral economic interventions share common theoretical tenets with contingency management in the psychology literature [5–7]. While CEIs have demonstrated promise for various health conditions such as substance use, research on the use of CEIs in the HIV field is nascent.
There is growing evidence from a handful of studies in the HIV field that CEIs can reduce HIV/STI risks. For example, a program in Tanzania used incentives to help heterosexual adults stay free of new curable STIs and reduce prevalence of combined STIs [8]. Similarly, conditional and unconditional cash transfers reduced prevalence of HIV, herpes simplex virus type 2, unplanned pregnancy, and marriage rates among young women who stayed in high school in Malawi [3,9]. Also in Malawi, incentives motivated participants to return for their HIV test results [10]. In Lesotho, a lottery draw conditional on staying free of new curable STIs reduced HIV incidence among young adults in rural areas [11]. However, we have not yet investigated whether incentives are a feasible and acceptable intervention strategy for men who have sex with men (MSM), a key population at risk for HIV and STIs.
Prevention science around conditional economic incentives needs further development. While CEIs might offer a novel intervention strategy for high-risk MSM, we first need to build our understanding of this intervention approach in three areas. First, we need to explore whether incentives can help increase engagement in health services, which has been challenging historically. Second, we need to investigate whether incentives with different conditionalities (e.g., staying free of new curable STIs vs. just attending study visits) can have different effects. Third, we need to determine whether the interaction of the type of conditionality and the level (amount) of incentives have different effects on HIV/STI risk reduction. In this paper, we report on a study that tested the feasibility and acceptability of a conditional economic incentive intervention for male sex workers in Mexico City. We used a prospective, parallel 4-arm randomized controlled pilot trial with outcomes measured by study visits at 6 and 12 months (while incentives are in place) and at 18 months (once the incentives were removed). The latter outcome assessment was intended to explore the sustainability of effects beyond the receipt of incentives, often referred to as maintenance or habit formation in the psychology and economics literatures. We measured the preliminary efficacy of the incentives intervention on total number of sexual partners, condom use, and composite incident HIV/STIs (i.e., new cases of: Chlamydia, gonorrhea, syphilis, and HIV).
We focused on Mexico because the HIV epidemic is concentrated among MSM, of whom male sex workers are a subgroup at particularly high risk of HIV acquisition and transmission. Compared to other MSM, male sex workers (MSWs) have: higher prevalence and incidence of HIV/STIs, higher use of drugs and alcohol, higher probability of experiencing job-related violence, higher probability of homelessness, lower rates of insurance and social protection, and lower rates of linkage to the healthcare system [12]. Mexico City (population > 20 million) does not penalize sex work, and has reduced institutionalized homophobia (e.g., same-sex unions have been legal since 2006). Ideally, prevention programs should address the biological drivers of HIV infection, the social context in which sex workers engage in commercial sex, and should provide comprehensive health services [13,14]. The reality, however, is that sex workers continue to face stigma, discrimination and limited access to health services worldwide [15–17].
Behavioral economic incentives offer great promise as an intervention strategy that can reduce HIV and STI risk by motivating behavior change in a complex context that presents a constellation of challenges for MSM, especially MSWs in Mexico. Our central hypothesis is that CEIs can reduce HIV/STI risks by affecting the salience and temporality of costs and benefits related to sexual risk behaviors among sex workers; the costs of unprotected sex will occur sooner and the benefits of protection will become more important. Thus, CEIs can be a conduit for greater empowerment and self-protection against HIV/STI risks. The rationale stems from our formative research showing that MSWs are in principle willing to participate and would readily accept economic incentives to stay free of STI, if offered [18,19]; and that a successful intervention could have important implications because MSWs represent a core population that can help to maintain and increase HIV transmission in the general population.[20]
Methods
Participants and Study Design
This study, locally known as Punto Seguro (or “Secure Point”), took place from January 2012 to May 2014. Trained staff members conducted initial outreach in community sites in Mexico City where MSWs congregate to work, as identified in previous studies [18,19]; and at the clinical site: Clínica Condesa, the largest HIV treatment provider in Mexico.1 Potential participants were eligible if they were: (1) men; (2) 18 to 40 years of age; and (3) self-identified as MSWs, or if they received money in exchange for sex with other men within the past six months and had 10 or more sex partners in the past month. Transgender women were excluded because Clínica Condesa has a separate program for them. Living with HIV was not an exclusion criteria; participants could enroll in the trial regardless of HIV status.
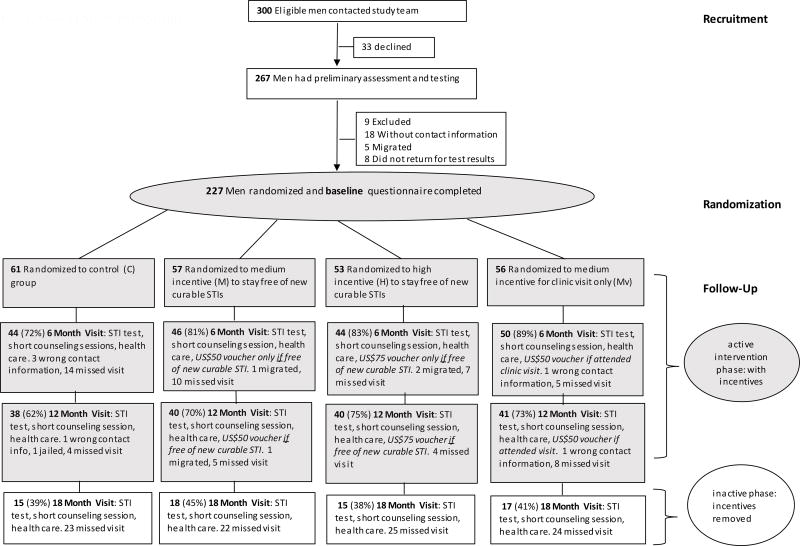
After intensive outreach using posters, flyers and palm cards, 300 men contacted the study team, of which 267 agreed to undergo HIV/STI testing and assessment for eligibility (Figure 1). Of those, 227 returned to receive their test results, completed the baseline survey, and proceeded to randomization. Participants were randomized (by drawing a labelled ping-pong ball from an opaque canvas bag), and allocated into one of four groups: control (C); medium incentive (M) to stay free of new curable STIs; high incentive (H) to stay free of new curable STIs; or medium incentive to attend study visits (Mv).
Fig. 1.
Flow of Study Participants
We based the incentive amounts on previous willingness-to-accept formative research conducted in preparation for this trial.2 [18] Participants in the M group received an incentive of 600 pesos (~USD $50 at the exchange rate at that time) each time, if they attended the study visit and were free of new curable STIs, each time, at visits 6 and 12 months after randomization. Participants in the H group received an incentive of 900 pesos (~$75) each time, if they attended the study visit and were free of new curable STIs, each time, at 6 and 12 months after randomization. Participants in the Mv group received 600 pesos (~$50) each time, for attending a study visit, regardless of their STI status, each time, at 6 and 12 months after randomization. No CEIs were provided at the 18-month assessment. For the analysis in U.S. dollars (USD), we used an exchange rate of 12 Mexican pesos per USD $1 [21]. The incentives were provided in the form of supermarket vouchers (for food and groceries) to facilitate administrative transparency and reduce consumption of harmful substances (alcohol and illicit drugs). At baseline and each follow-up visit, participants completed a computer-based survey and underwent HIV/STI testing. The National Institute of Public Health (INSP) in Cuernavaca, Mexico, and Brown University in Providence, USA provided human subjects research approval.
We used active follow-up procedures to locate participants for assessment visits that occurred at 6, 12 and18 months after randomization. The window period to complete a scheduled visit was four weeks before or after the scheduled date. That is, if participants came four weeks before or after when they had an appointment for the first follow-up (at six months after randomization) or the second follow-up (at 12 months after randomization), they received credit for the visit. In the event that a participant came to the clinic at an earlier or later date than the 4-week window period, to be tested, asking for HIV post-exposure prophylaxis (PEP) or for STI care, he received the services but it did not count as a protocol visit.
Outcome measures
The first intermediate outcome to gauge feasibility and acceptability was retention, defined as Rt=1 if participant returned to the study visit, at each time t (for t=1, 2, 3, representing the 6, 12, and 18 month visits, respectively), and Rt=0 if he did not. The primary behavioral outcome was total number of sexual partners in the last week prior to the study visit; the secondary behavioral outcome was condom use with last paying sexual partner (client); and composite incident STIs (chlamydia, gonorrhea, syphilis, and HIV) was an exploratory biological outcome (Appendix A1). We included new HIV cases in the composite incident STI variable; and because we expected high HIV prevalence at baseline, we would continue to include HIV+ individuals in the denominator for the follow-up visits as they would still be susceptible to STIs (other than HIV).
To minimize social desirability bias on sensitive behavioral questions, we used computerized surveys that incorporated audio computer-assisted self-interviewing software (ACASI). Research assistants, hired independently from the clinic, processed the survey data. Number of sex partners was asked separately for clients and non-paying partners, and then summed up to total number of sexual partners. Separate questions asked about condom use by participant and/or partner (client or non-paying partner), and then questions were collapsed to result in the analytical measure of any condom used.
If any participant was positive for a curable STI at baseline or at any of the follow-up assessments, he received appropriate free treatment at Clínica Condesa (e.g., standard penicillin treatment for syphilis). Participants in the medium and high (M and H) groups received incentives contingent on testing negative for chlamydia, gonorrhea and active syphilis; those with HIV and/or viral hepatitis were still eligible to stay free of new curable STIs (chlamydia, gonorrhea and syphilis), and they could still receive the incentives.
Statistical analysis
The target sample size of n=55 participants per arm was based on previous data for MSWs [22]. Using baseline and two follow-up measurements, and assuming five sex partners (clients and non-paying partners) per week and an effect of 30% for the intervention (i.e., a reduction from 5 to 3.5 sex partners per week), this sample size would provide 90% power to detect an effect (two sided α=0.05). We also allowed for up to 40% loss-to-follow-up of participants at month 12.
First, we tested for baseline differences across study groups by conducting balancing tests for each variable comparing (CEIs) intervention against control groups, and then each intervention group against each other.
Second, we performed an analysis of retention across study groups. We implemented a model of the retention vector, R, containing R1, R2 and R3 where we defined R1 as a retention dummy equal to 1 if the participant returned to the first follow-up (at month 6); and equal to 0 if he did not. R2, and R3 were defined analogously for the second follow-up (at month 12), and third follow-up (at month 18). In the full model regressions we included the following covariates: personal characteristics (age, schooling, marital status, age of first sex, previous STI and HIV tests, as well as housing characteristics); quintiles of distance to the clinic (in kilometers); and lagged outcomes (of number of sexual partners, condom use and incident sexually transmitted infections). We chose these covariates because they were highly predictive of the retention rates.
Third, to evaluate the effects of the intervention, we first used an intent-to-treat (ITT) approach using the observations for which we had complete data. The underlying assumption was that data were missing completely at random (MCAR) [23]. Adjusted models controlled for: age, education level, marital status, age of first sex with a man, previous HIV test at baseline and previous STI test at baseline (Appendix A1 presents the details on how the questions were asked and the variables constructed). The covariates helped to predict HIV/STI risk outcomes and made the estimates more precise.
In addition, we expected high attrition and knew that: “if the probability of attrition is correlated with experimental response, then traditional statistical techniques will lead to biased and inconsistent estimates of the experimental effect” (p. 456) [24]. Thus, we used an analytical method to address the issue of potentially differential attrition: inverse probability weighting. Under the inverse probability weighting (IPW) approach, we used inverse probability weights as outlined in 1995 by Robins and colleagues [25] and subsequent works [26–28], as well as a comparable methods presented in the econometric literature in 1999 [29] and related applications [30,31]. We implemented a model of the attrition vector, A, containing A1, A2 and A3 (the opposite of the retention outcome R defined above). We defined A1 as an attrition dummy equal to one if the observation was missing at the first follow-up (at month 6) due to attrition; and equal to zero otherwise. A2, and A3 were defined analogously for the second follow-up (at month 12), and third follow-up (at month 18). The model included time-invariant characteristics at baseline (X), covariates, and lagged variables including lagged outcome variables. We then applied weighted linear models with normalized weights to the general regression framework presented above.
We used generalized estimating equation (GEE) models for categorical outcomes and generalized linear models for continuous outcomes. For each separate outcome, we were interested in the main effects models with intervention group dummies (M, H or Mv) leaving the controls (C) as the reference group, and continuous time (follow-up visits coded as baseline=0, first follow-up at six months=1, second follow-up visit at 12 months=2, and third follow-up without incentives at 18 months=3).
Effects-by-time analyses
Furthermore, if we found an initial main effect, and to explore potential effect differences at different times, we also used fully-interacted models with the main effects of intervention groups, time (visit) dummies, and interaction terms (intervention group × time). Having the effects by each intervention group at each time allowed us also to evaluate if any of the average main effects were sustained after the incentives were removed.
Sensitivity analyses
To check on additional impacts of interest, we first conducted an analysis of the effects of any CEIs intervention (M, H or Mv) on commercial sex clients. Second, we checked for differential effects by housing status, because the literature suggests that unstable housing is often correlated with worse HIV outcomes.[32] Unstable housing was defined as living in a municipal shelter or on the street, as opposed to: own/rented apartment or home, staying at friend’s house/apartment, or renting room in hotel, motel or pension. Third, we also conducted sensitivity analyses of any statistically significant main effects by HIV status.
We conducted all analyses using Stata SE 14.2 (College Station, TX) with xtgee and glm commands for the GEE and generalized linear models using robust standard errors clustered at the individual level.
Results
Baseline Characteristics
Table 1 presents baseline characteristics of participants by intervention group. Differences across intervention groups were small in magnitude, and p-values were smaller than 0.10 for six out of 50 tests, suggesting that the randomization was successful at creating comparable groups. Column (1) for the full sample, shows that the mean age was 24.4 years, with slightly older participants in Mv and H groups (who were 25). About 13% of participants finished elementary school, 29% completed middle school, 39% finished high school, and 19% attended some tertiary-level institution. Most participants (80%) were single; the rest were married or in civil unions (18%, including common law partners of either sex). The average age of first sex with another man was 15 years, with slightly older age for the H group (who were 16 at sexual debut). At baseline, over a third (37%) of participants had ever received an STI test (other than HIV), and three-fourths (75%) had ever received an HIV test before. Baseline HIV/STI rates did not differ significantly across study conditions (not shown); and, as suspected, the prevalence of HIV and other STIs was high at baseline: Almost a third of the participants were HIV positive (+) (32.2%); about one-fifth of men had active syphilis (18.5%); while chlamydia and gonorrhea were less prevalent, with rates of 10% and 2% respectively.
Table 1.
Baseline characteristics of participants, by intervention group
| Full Sample (n=227) |
Control (C) (n=61) |
Medium Incentive for staying free of STIs $50/6mos. (M) (n=57) |
High Incentive for staying free of STIs $75/6mos. (H) (n=53) |
Medium Incentive for study visits only $50/6mos. (Mv) (n=56) |
p-value for test that: | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| M = C | H = C | Mv = C | M = Mv | H = Mv | ||||||
| Age | 24.44 (4.596) | 23.18 (4.116) | 24.36 (4.762) | 24.94 (4.259) | 25.40 (5.014) | (0.152) | (0.027) | (0.010) | (0.260) | (0.609) |
| Elementary school | 0.128 (0.335) | 0.180 (0.388) | 0.0702 (0.258) | 0.113 (0.320) | 0.143 (0.353) | (0.074) | (0.320) | (0.587) | (0.213) | (0.647) |
| Middle school | 0.291 (0.455) | 0.246 (0.434) | 0.386 (0.491) | 0.245 (0.434) | 0.286 (0.456) | (0.103) | (0.994) | (0.629) | (0.263) | (0.637) |
| High school | 0.388 (0.488) | 0.443 (0.501) | 0.368 (0.487) | 0.377 (0.489) | 0.357 (0.483) | (0.417) | (0.485) | (0.350) | (0.902) | (0.829) |
| College or higher | 0.194 (0.396) | 0.131 (0.340) | 0.175 (0.384) | 0.264 (0.445) | 0.214 (0.414) | (0.508) | (0.074) | (0.236) | (0.606) | (0.546) |
| Married/ Civil Union | 0.185 (0.389) | 0.213 (0.413) | 0.123 (0.331) | 0.189 (0.395) | 0.214 (0.414) | (0.194) | (0.748) | (0.988) | (0.197) | (0.742) |
| Single | 0.802 (0.400) | 0.770 (0.424) | 0.877 (0.331) | 0.811 (0.395) | 0.750 (0.437) | (0.132) | (0.598) | (0.797) | (0.084) | (0.445) |
| Age of first sex | 15.29 (3.374) | 14.51 (3.880) | 15.28 (3.167) | 16.06 (2.749) | 15.41 (3.426) | (0.240) | (0.017) | (0.187) | (0.834) | (0.282) |
| Had received STI test before | 0.370 (0.484) | 0.426 (0.499) | 0.368 (0.487) | 0.377 (0.489) | 0.304 (0.464) | (0.526) | (0.600) | (0.172) | (0.470) | (0.421) |
| Had received HIV test before | 0.753 (0.432) | 0.770 (0.424) | 0.807 (0.398) | 0.736 (0.445) | 0.696 (0.464) | (0.631) | (0.672) | (0.369) | (0.176) | (0.652) |
Notes: On the left side, table shows mean and standard deviations in parentheses; on the right side, table shows p-values of statistical difference tests in parentheses.
Approximate average exchange rate at time of study (2012–14): 12 Mexican Pesos per $1 (USD).
There were no statistically significant differences across any of the groups in terms of outcomes (number of partners, condom use, or any of the prevalent STIs including HIV).
Retention Rates
Table 2 analyzes retention rates; the dependent variable, Rt (return to visit at time t) is a binary indicator equal to one if the participant returned to the assessment visit at time t (for each t=1, 2, 3), and zero otherwise. Unadjusted results in Model 1 show that participants in the H and Mv groups were more likely to return. In comparison to the control group, the H group was 9.3 percentage points (pp) more likely to return to study visits; and participants in the Mv group were 11.3 pp more likely to return. Model 2 is a comprehensive model which adds distance from the participant's home to the clinic, socio-demographic variables (age, marital status and education), type of housing and sexual risk variables (age of first sex, and whether participant had received an STI test), as well as lagged dependent variables. This adjusted Model 2 was able to explain more than 28% of the variation in retention rates. Any of the intervention groups (M, H or Mv) had higher rates of returning to the clinic in comparison to controls (by 13, 12 and 10 pp respectively). Importantly, the coefficients across intervention arms were statistically the same: the p-values of the statistical difference tests across study arm coefficients were not significant. Given that the regression results showed that there were differences between study arms in the likelihood of having returned to study visit, we needed to make additional corrections to the analyses (using IPW) to make sure that any findings of program impact were not due to differential return rates.
Table 2.
Retention models Dependent variable: Rt=1 if participant returned to the study visit, Rt=0 otherwise
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
|
|
|||
| Coeff. | AME | Coeff. | AME | |
| Medium Incentive for staying free of STIs (M) | 0.319 (0.216) | 0.078 (0.052) | 0.938** (0.394) | 0.132** (0.054) |
| High Incentive for staying free of STIs (H) | 0.380* (0.221) | 0.093* (0.053) | 0.841* (0.433) | 0.120** (0.059) |
| Medium Incentive for study visits only (Mv) | 0.467** (0.219) | 0.113** (0.052) | 0.719* (0.433) | 0.104* (0.061) |
| Covariates for: distance to clinic (quintiles) | N | Y | ||
| personal characteristics | N | Y | ||
| lagged outcomes | N | Y | ||
| Pseudo R-squared | 0.006 | 0.283 | ||
| Observations | 681 | 397 | ||
| p-value (test: M = H) | 0.79 | 0.81 | ||
| p-value (test: M = Mv) | 0.51 | 0.61 | ||
| p-value (test: H = Mv) | 0.71 | 0.79 | ||
Notes:
p<0.10;
p<0.05;
p<0.01.
Table presents coefficients from logit models and average marginal effects (AME). The dependent variable is the vector R which contains R1, R2 and R3. We defined R1 as a retention dummy equal to 1 if the participant returned to the first follow-up (at month 6); and equal to 0 if he did not. R2, and R3 were defined analogously for the second follow-up (at month 12), and third follow-up (at month 18).
Reference category was the control group. Covariates: Quintiles of distance to the clinic (in kilometers); personal characteristics (age, schooling, marital status, age of first sex, previous STI and HIV tests, as well as housing characteristics); and lagged outcomes (of number of sexual partners, condom use and incident sexually transmitted infections).
Number of sexual partners in week prior to study visit
Table 3 shows the effect of CEIs on the total number of sexual partners in the week prior to the study visit. Participants had an average of four sexual partners in the week prior to the baseline study visit. Columns (1) and (2) present the intent-to-treat models, first unadjusted and then adjusted for all covariates: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline. Columns (3) and (4) present unadjusted and covariate-adjusted inverse-probability-weighed models to correct for differential retention. Under all model specifications, compared to the control group, none of the intervention arms (M, H, or Mv) were associated with changes in the total number of sexual partners in the week prior to the study visit.
Table 3.
Effect of conditional economic incentives on number of sexual partners in week prior to study visit
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Medium incentive for staying free of STIs (M) | 0.078 (0.799) | 0.308 (0.794) | −0.407 (0.877) | −0.133 (0.880) |
| High incentive for staying free of STIs (H) | −0.110 (0.848) | 0.211 (0.842) | −0.463 (0.964) | −0.115 (0.944) |
| Medium incentive for study visits only (Mv) | 0.867 (0.889) | 1.309 (0.880) | 0.614 (1.056) | 1.049 (0.993) |
| Follow-up visits¶ | 0.077 (0.155) | −0.041 (0.167) | 0.241 (0.193) | 0.150 (0.187) |
| Observations | 635 | 635 | 510 | 510 |
| Covariates | N | Y | N | Y |
| Mean of dependent variable at baseline (control) | 4.2 | 4.4 | ||
| p-value (test: M = H) | 0.80 | 0.90 | 0.94 | 0.98 |
| p-value (test: M = Mv) | 0.31 | 0.21 | 0.26 | 0.19 |
| p-value (test: H = Mv) | 0.24 | 0.18 | 0.28 | 0.21 |
Notes: Table presents effect of CEI on total number of sexual partners (in week prior to study visit) at months 6 and 12 (during the active phase) and at 18 months (once incentives were removed), with robust standard errors in parentheses ():
p<0.1;
p<0.05;
p<0.01.
ITT are intent-to-treat models and IPW are inverse probability weighted models. Regressions in columns (2) and (4) control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits were coded as: 0=baseline, 1=six-month visit, 2=twelve-month visit, and 3=eighteen-month visit
Condom use with last client
Table 4 shows the effects on condom use with the last paying sexual partner (client). At baseline, about 85% of the participants used a condom with their last client. In the unadjusted ITT model (column 1), the M group was 10.8 pp more likely to use condoms with the last client than the control group. In the adjusted ITT models in column (2), compared to controls, individuals in the M group were more likely to use condoms (by 12.8 pp), as were those in the H group (by 10.7 pp) and those in the Mv group (by 10 pp). In the adjusted IPW models in column (4), compared to controls, all intervention arms were associated with increased condom use: Individuals in the M group were more likely to use condoms (by 10 pp), as were those in the H group (by 11.3 pp) and those in the Mv group (by 10.6 pp). Statistically, the M and H coefficients were the same. Similarly, we could not reject the null hypothesis that the Mv coefficient was significantly different from those for M or H.
Table 4.
Effect of conditional economic incentives on condom use with last client
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Medium incentive for staying free of STIs (M) | 0.108** (0.048) | 0.128*** (0.049) | 0.072 (0.054) | 0.100* (0.056) |
| High incentive for staying free of STIs (H) | 0.074 (0.053) | 0.107* (0.056) | 0.070 (0.057) | 0.113* (0.060) |
| Medium incentive for study visits only (Mv) | 0.076 (0.051) | 0.100* (0.053) | 0.074 (0.059) | 0.106* (0.062) |
| Follow-up visits¶ | −0.014 (0.013) | −0.015 (0.013) | 0.017 (0.013) | 0.014 (0.014) |
| Observations | 577 | 577 | 466 | 466 |
| Covariates | N | Y | N | Y |
| Mean of dependent variable at baseline (control) | 0.85 | 0.84 | ||
| p-value (test: M = H) | 0.38 | 0.60 | 0.96 | 0.76 |
| p-value (test: M = Mv) | 0.34 | 0.42 | 0.97 | 0.88 |
| p-value (test: H = Mv) | 0.97 | 0.88 | 0.94 | 0.89 |
Notes: Table presents effect of CEI on condom use (with last client) at months 6 and 12 (during the active phase) and at 18 months (once incentives were removed), with robust standard errors in parentheses ():
p<0.1;
p<0.05;
p<0.01.
ITT are intent-to-treat models and IPW are inverse probability weighted models. Regressions in columns (2) and (4) control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits were coded as: 0=baseline, 1=six-month visit, 2=twelve-month visit, and 3=eighteen-month visit
New composite STI/HIV cases
Table 5 shows the effect of CEIs on new composite STI/HIV cases.3 In the unadjusted ITT model, the H group had higher STI/HIV rates (by 4.6 pp), but there were no other significant effects on incident STI/HIV for any of the intervention groups under any of the model specifications. Of note, however, under all specifications, the rate of new STI/HIV cases grew between 2 and 3 pp for each additional follow-up visit (i.e., every six months), indicating vividly the high need for prevention and treatment services in the MSWs population.
Table 5.
Effect of conditional economic incentives on composite incident sexually transmitted infections/HIV
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Medium incentive for staying free of STIs (M) | 0.035 (0.024) | 0.028 (0.023) | 0.028 (0.026) | 0.023 (0.024) |
| High incentive for staying free of STIs (H) | 0.046* (0.027) | 0.037 (0.028) | 0.048 (0.032) | 0.044 (0.034) |
| Medium incentive for study visits only (Mv) | 0.009 (0.020) | −0.003 (0.021) | 0.003 (0.022) | −0.006 (0.022) |
| Follow-up visits¶ | 0.024*** (0.008) | 0.024*** (0.008) | 0.030*** (0.009) | 0.032*** (0.009) |
| Observations | 635 | 635 | 510 | 510 |
| Covariates | N | Y | N | Y |
| Mean of dependent variable at baseline (control) | 0.00 | 0.00 | ||
| p-value (test: M = H) | 0.73 | 0.79 | 0.28 | 0.31 |
| p-value (test: M = Mv) | 0.31 | 0.22 | 0.79 | 0.95 |
| p-value (test: H = Mv) | 0.20 | 0.19 | 0.41 | 0.31 |
Notes: Table presents effect of CEI on new composite STI/HIV cases at months 6 and 12 (during the active phase) and at 18 months (once incentives were removed), with robust standard errors in parentheses:
p<0.1;
p<0.05;
p<0.01.
ITT are intent-to-treat models and IPW are inverse probability weighted models. Regressions in columns (2) and (4) control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits were coded as: 0=baseline, 1=six-month visit, 2=twelve-month visit, and 3=eighteen-month visit.
Composite incident STI/HIV includes new cases of: Chlamydia, gonorrhea, syphilis and HIV.
We kept the participants who were HIV+ at baseline in the analyses because they were still susceptible for other STIs at follow-up visits.
Effects-by-time
We analyzed fully interacted effects-by-time models with main effects and interactions for condom use with last client to explore differential effects at specific times (Appendix A2). Three group-by-visit interactions were significant. In the unadjusted ITT model for condom use (column 1), the interaction of M and month 18 was associated with an increase of 27 pp. In the adjusted ITT model (column 2), both the M and H groups had significant interactions at 18 months as follows: increases of 30.3 and 27.9 pp respectively. In the adjusted IPW model (column 4), however, those interactions at 18 months were not significant (suggesting that there was no maintenance or habit formation once we corrected for differential retention). The only significant interactions in the adjusted IPW model was the H group at the 12-month visit, reporting higher condom use (by 17.3 pp).
Sensitivity analyses
In addition to exploring the effects by time, we performed three other sensitivity analyses. We first checked if any of the CEI interventions (M, H, or Mv) had effect on the number of commercial sex clients in the week prior to the study visit (Appendix A3). The combined coefficient of any CEI intervention was not associated with changes in the number of clients in comparison to the control group. Nevertheless, the cohort as a whole reduced the number of clients at an average rate of 0.268 to 0.345 clients per follow-up visit (i.e., every six months).
Second, we checked if there were any differential effects on condom use for participants at higher risk because they had unstable housing (Appendix A4). The main effect of any CEI was significant, but the main effect of unstable housing was not. The interaction term of any CEI and unstable housing was not significant either. The average main effect of any CEI (M, H or Mv combined) on condom use ranged from 10 to 12 pp increase (depending on the model) when compared to the control group.
Third, we checked for differential effects of the CEI intervention on condom use by HIV status (Appendix A5). The main effect of any CEI was significant, but the main effect of HIV+ status was not. The interaction term of any CEI and HIV+ was not significant either. As before, the average main effect of any CEI (M, H or Mv combined) on condom use ranged from 10 to 13 pp increase (depending on the model) when compared to the control group.
Discussion
This study used a randomized controlled design to test different types of conditionality and their effect on number of sexual partners, condom use and composite incident HIV/STIs among male sex workers in Mexico City. A few points are worth highlighting. First, the intervention was successful in reaching a population with high HIV risk and vulnerability, and it showed feasibility in various realms. The baseline prevalence of HIV among the randomized MSWs (32%) was higher than the HIV prevalence for the general population (0.23%) by over two orders of magnitude [33], and it was greater than a nationally-representative sample of MSM (16.9%) [34] and a nationally-representative sample of MSWs (24. 1%) [35]. This pilot trial proved that it was feasible to conduct a randomized intervention in this otherwise difficult-to-reach population. Similarly, the trial showed it was feasible to recruit and retain MSWs particularly in the incentive groups. It was feasible as well in terms of the integrity in delivery of the intervention: the participants understood the randomization procedures and accepted the format.
Second, the trial showed that modest incentives (of $50 or $75 every six months) were feasible and acceptable to street-based MSWs in a large metropolitan area, such as Mexico City, to engage in regular follow-up study visits; and all incentive groups were associated with increased condom use, while the incentives were place. Also, while the incentives were in place (at 6- and 12-month visits), the retention rates were relatively high for this population (>70%) in all of the intervention groups. The results corroborate earlier qualitative and willingness-to-accept research that suggested high interest in this type of conditional-incentive-based interventions [18,19,36]. The incentive levels were based on careful formative work to find the optimal incentive level: high enough to motivate potential changes in behaviors, yet not so high as to be perceived as undue influence [18]. The incentive levels were relatively low in comparison to other incentives in Mexico [37], yet compared favorably with the minimum wage (at the time of the intervention) of about $5 per day [38]. Incentive levels were equivalent to amounts received from about 2–3 clients, who paid about $25 per sex act on average [19,36,39]. Nevertheless, once the incentives were removed, engagement in care fell dramatically at the 18-month visit, suggesting that basic support would be needed to maintain clinical care continuity. High attrition may not be an issue with interventions that occur at a single visit (e.g., male circumcision) [40]; yet among sex workers it is imperative to link them to regular care and treatment for STI/HIV, requiring continual follow-up, which may not be easily attained without material motivators. At the same time, new research questions emerge: Are other combinations of incentives and conditionalities more effective? Are incentives more cost-effective at certain timepoints of intervention (such as high risk) or certain stages in life? Can incentives as an intervention strategy be linked with other interventions in a combination approach? And if so, what is the ideal combination for this high risk population?
Third, the comparison between the different types of conditionality merits attention. One of the most policy-relevant results may be that medium incentives for clinical visits (Mv) were shown to be as efficacious as the medium (M) or the high (H) incentive to stay free of new curable STIs in terms of retention and condom use. This provides support for developing administratively and programmatically simpler programs in general [41], and informs the debate on the topic as it relates to HIV prevention as well [42,43]. If the effectiveness is the same, an HIV/STI prevention program that requires only clinical visits as a conditionality, instead of testing for STIs and negative results, would be easier to implement and likely more cost-effective.
Finally, the hypothesis of greater effect for larger amount of conditional incentives was not supported by the evidence: we were not able to reject differences in retention or condom use between participants receiving medium (M) or high (H) incentives to stay free of STIs. On the one hand, this result suggests that the formative willingness-to-accept work and the use of an optimal incentive level was moderately successful, at least for actual participation in the pilot trial. On the other hand, the expected behavior changes targeted as main outcomes (about which we also asked in the formative work) were realized only partially. This is consistent with previous literature showing potentially important differences and discrepancies between stated preference surveys, actual (implemented) behaviors and behavioral change. [44]
This paper contributes to prevention science in three ways. First, this is to the best of our knowledge, the first paper to present an experimental test of the effect of conditional economic incentives on HIV/STI risks among MSWs. This evidence is important because MSWs are a key group in terms of acquisition and transmission of STI/HIV, and previous papers have focused on general populations [2–4,8–11]. Second, and more generally, the experimental comparison between the different type of conditionality and the level of incentives contributes to the debate in the health and development fields [41,43,45]. Third, in terms of methods, the issue of attrition is common in both experimental and non-experimental methods, yet rigorous techniques to address it, such as inverse probability weighting, have not yet been fully utilized in the context of economic incentives to reduce HIV/STI risks, with only a few applications in health economics [46,47].
This study has several limitations. First, due to the nature of this pilot study, the trial had a relatively small sample size. Second, the modality of the incentives (supermarket vouchers) may be less effective than other modes, such as cash. Third, a full range of comprehensive services (psychological and mental health, dental care, human rights module, STI testing and treatment, etc.) that are offered at Clínica Condesa were available to all participants in all arms, including the control group, thus potentially attenuating the effect of the intervention groups. Regardless of the impact that these additional services might have had on trial outcomes, offering those services to all participants in all study arms was the most ethical approach and vital to ensure the balance of benefits and harms with this potentially vulnerable population. Another important limitation may be that condom use is a self-reported outcome, and thus could result in potential misclassification due to social desirability. Condom use data was collected via ACASI and participants were reminded that any incentives were tied to new STI test results (and not on self-report). Nonetheless, we acknowledge that researchers are skeptical of self-reported measures of sexual behavior. A body of literature details this issue, including studies that compare condom use with biomarkers of recent sex [48]. We recognize this limitation and try to counter it by putting in multiple measures of sexual risk behavior (including biomarker measures) as well as rigorous methods to limit social desirability bias such as ACASI. When combined with the data of the intervention on retention in care - an objective outcome – the condom use data seems promising and helps to bolster the case for further study on using incentives in this population.
Lastly, Clínica Condesa is the largest HIV treatment provider in Mexico and there may be questions raised about whether an HIV care provider can also be effective at HIV prevention. Clínica Condesa has successfully expanded its services to include STI/HIV prevention as well. The fact that its primary mission is to be an HIV clinic does not seem to have affected whether HIV-negative men would want to receive prevention services there. Although stigma associated with attending an HIV clinic may be strong (especially among HIV-uninfected patients), the need for STI treatment and other services may be stronger among underserved populations such as MSWs, and thus treating (particularly symptomatic STIs) may be more important to sustain a MSW's livelihood than the potential loss of clients perceiving a MSW to be HIV+. This same issue is becoming even more relevant as the Clinic embarks in a new pre-exposure prophylaxis (PrEP) demonstration intervention (i.e., again explicitly engaging HIV-uninfected participants at the HIV clinic). Having a separate STI clinic was essential for recruiting HIV-negative MSW, and we suspect it will benefit PrEP services in the future as well. The services for MSWs (HIV positive and negative) have grown larger as part of Punto Seguro, as a service program without incentives now (rather than as a pilot trial with incentives as evaluated in this paper). Currently, the clinic has seen n>1,100 MSWs at least once for STI testing and treatment or other services. So, the clinic seems to have become a regional reference center for providing free services in a respectful and non-discriminatory environment.
Further research should include mixed methods, including in-depth qualitative interviews to understand the effects and/or lack thereof as well as any potential pathways. It is worth noting that supply-side incentives may be a possibility as well [49], alone or in combination with the type of demand-side incentives tested so far. Also more research is needed in terms of incentives for combination prevention and newer methods such as PrEP. The extant results suggest that CEIs, through increasing clinic visits, could help MSWs to adhere to prevention services such as PrEP if HIV-negative, or to increase linkage and retention rates for those who become HIV+. Still MSWs exhibit suboptimal adherence to medications and may require other targeted interventions. In particular, HIV+ MSW do not optimally adhere to antiretroviral therapy: The estimated rate of viral suppression among HIV+ MSWs at Clínica Condesa was 61%.[20]
Conclusions
Economic incentives with different conditionality seem to have helped MSWs in Mexico City to increase rates of clinic follow-ups and condom use, while the incentives were in place. Incentives conditional on staying free of new curable STIs had similar effects in terms of retention and condom use as incentives conditional on simply attending study visits. Nevertheless, once the incentives were removed, study visit rates dropped dramatically: There was no evidence of maintenance or habit formation in any of the groups. For highly vulnerable and at-risk populations, such as MSWs, changing the level of incentives offered (as well as format) may be necessary to sustain regular clinic attendance for HIV/STI testing, treatment and control.
Given the early nature of the research on this type of intervention strategy as applied to HIV/STI prevention, it is expected that some components may work and some may not. Future studies should investigate what types of conditioning matter the most, in addition to the amounts and modalities of incentives. Similarly, more research is needed on the use of CEIs in combination with other prevention methods and prevention support. CEIs as an intervention strategy need much more exploration and continue to hold great promise for expanding our theoretical and behavioral toolbox for HIV/STI prevention.
Acknowledgments
Funding:
U.S. National Institutes of Health (R21HD065525 Economic incentives to reduce HIV/STI risk: A pilot in Mexico, PI: Galárraga O; and R24HD041020 Population Studies and Training Center at Brown University) with additional support provided by the Mexican National Center for HIV/AIDS Control and Prevention (CENSIDA: Proy-2014-0262). The funders had no role in study design; data collection, analysis or interpretation; report writing; or decision to submit article for publication. The findings, interpretations and conclusions expressed in this paper are entirely those of the authors.
We acknowledge useful comments, at different stages of development of these ideas, from Sergio Bautista-Arredondo, Stefano Bertozzi, Damien de Walque, Will Dow, Paul Gertler, Manisha Shah, Kristen Underhill, David M. Williams and Ira Wilson. Preliminary results presented at scientific conferences: International AIDS Society (IAS); HIV in the Americas; HIV Research for Prevention (R4P); Northeastern Universities Development Conference (NEUDC) at Massachusetts Institute of Technology (MIT). As well as seminars at: Brown University; Centro de Investigación y Docencia Económicas, CIDE; Clínica Condesa; Harvard Center for Population and Development Studies; McGill University; National Institute of Public Health. We gratefully acknowledge all staff members; particularly Octavio Parra, and Jehovani Tena; as well as the clinical staff including Arturo Martínez and Florentino Badial. Santa García conducted the PCR diagnosis of chlamydia and gonococcus in urine samples. The Consortium for HIV/AIDS Research (CISIDAT, A.C.) provided project management and administration support. We especially thank the participants for agreeing to become part of Punto Seguro.
Appendix
Appendix A1.
Description of main covariates and outcome variables
| Question | Answer Options | Coding for the analysis | |
|---|---|---|---|
| Socio-demographic baseline covariates | |||
|
| |||
| Age | How old are you? | Discrete variable 99. I don´t want to answer | Discrete variable |
|
| |||
| Schooling | What is the highest level of education that you have completed? | 1. Didn´t complete primary 2. Primary 3. Secondary 3. High school 4. College 5. Graduate school 99. I don´t want to answer | 1. Elementary school 2. Middle school 3. High school 4. College or higher |
|
| |||
| Marital status | What is your marital status? | 1. Single 2. Married to a woman 3. Married to a man 4. Divorced 5. Separated 6. Widower 7. Free union 8. Other 99. I don´t want to answer | 1. Married/Civil union 2. Single 3. Divorced/ Separated/widower. |
|
| |||
| Age of first sexual contact with a man | At what age did you have sex with another man (oral or anal) for the first time? | Discrete variable 99. I don´t want to answer | Discrete variable |
| Behavioral and biological outcome variables | |||
|
| |||
| Total number of sexual partners | How many persons did you have vaginal or anal or oral sex with, during the last week, with payment?¥ | Number of male sexual partners (discrete variable) Number of female sexual partners (discrete variable) 999. I don´t want to answer | Number of clients and non-paying sexual partners (discrete variable) |
|
| |||
| Condom use | If you had sex with penetration with your last client, did you use a condom?° | 1. Yes, I did 2. Yes, he did 3. Yes, we both did 4. No 99. I don´t want to answer | 0. No 1. Yes (any condom use) |
|
| |||
| New sexually transmitted infections (STIs) and HIV | Participants provided blood and urine samples, collected following the local biosafety protocols by trained staff and analyzed by lab technicians. Urine specimens were tested for gonorrhea and chlamydia (PCR Cobas-Amplicor; Roche, Basel, Switzerland); blood specimens served to measure presence of HIV, hepatitis B, hepatitis C and syphilis antibodies (Abbott HIV-1 and HIV-2, Ag/Ab Combo, anti-HBc, anti-HCV and syphilis TP quimioluminiscence immunoassay; Abbott Laboratories, North Chicago, IL, USA) running in Architect i2000 (Abbott). HIV+ samples were confirmed with HIV-1 and HIV-2 CombFirm (Orgenics, Yavne, Israel); and anti-HBcwas tested with Determine HBsAg and syphilis TP (Abbott) with tittered VDRL, the Venereal Disease Research Laboratory test. Two subgroups were defined for the markers of Syphilis: antibody positivity was regarded as a lifetime marker of past or present infection, whereas treponemic antibody positivity together with VDRL demonstrated active syphilis. | Coding: 0.No 1. Yes (any new STI/HIV) | |
the same question was asked about next-to-last client and second-to-last client; as well as non-paying partners.
similar question asked about non-paying sexual partners
Appendix A2.
Effects-by-time on condom use with last client
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Medium incentive for staying free of STIs (M) | 0.062 (0.063) | 0.074 (0.063) | 0.062 (0.063) | 0.082 (0.064) |
| High incentive for staying free of STIs (H) | 0.026 (0.070) | 0.050 (0.071) | 0.026 (0.070) | 0.053 (0.072) |
| Medium incentive for study visits only (Mv) | 0.067 (0.066) | 0.087 (0.065) | 0.067 (0.066) | 0.089 (0.066) |
| 6-month visit§ | 0.026 (0.042) | 0.019 (0.045) | −0.007 (0.050) | −0.021 (0.052) |
| 12-month visit | −0.039 (0.064) | −0.049 (0.067) | −0.029 (0.077) | −0.050 (0.077) |
| 18-month visit | −0.188 (0.129) | −0.217* (0.125) | 0.096 (0.073) | 0.031 (0.085) |
| M × 6-month visit | −0.011 (0.064) | −0.001 (0.064) | 0.009 (0.072) | 0.017 (0.072) |
| M × 12-month visit | 0.072 (0.084) | 0.089 (0.086) | 0.047 (0.099) | 0.064 (0.099) |
| M × 18-month visit | 0.271** (0.135) | 0.303** (0.131) | −0.012 (0.084) | 0.043 (0.098) |
| H × 6-month visit | −0.005 (0.069) | 0.009 (0.070) | 0.040 (0.072) | 0.059 (0.073) |
| H × 12-month visit | 0.083 (0.087) | 0.102 (0.091) | 0.148 (0.092) | 0.173* (0.093) |
| H × 18-month visit | 0.240 (0.154) | 0.279* (0.161) | 0.023 (0.089) | 0.133 (0.112) |
| Mv × 6-month visit | 0.007 (0.050) | 0.015 (0.053) | 0.024 (0.056) | 0.033 (0.059) |
| Mv × 12-month visit | 0.068 (0.086) | 0.080 (0.089) | 0.060 (0.101) | 0.080 (0.105) |
| Mv × 18-month visit | −0.086 (0.178) | −0.072 (0.176) | −0.228 (0.204) | −0.159 (0.219) |
| Covariates | N | Y | N | Y |
| Mean of dependent variable (control) | 0.85 | 0.85 | ||
| Observations | 577 | 577 | 466 | 466 |
Notes: Table presents effect-by-time (CEI groups fully interacted with time) models for condom use (with last client) at months 6 and 12 (during active phase), and at 18 months (once incentive were removed) with robust standard errors in parentheses ():
p<0.1;
p<0.05;
p<0.01.
Regressions control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits at 6, 12 and 18 months coded as dummy variables =1 if participant attended study visit, and =0 if he did not.
Appendix A3.
Effects of any incentive on number of clients in week prior to visit
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Any conditional economic incentive (CEI) | 0.355 (0.478) | 0.498 (0.527) | 0.184 (0.529) | 0.332 (0.558) |
| Follow-up visits¶ | −0.268* (0.146) | −0.326** (0.156) | −0.336** (0.156) | −0.345** (0.166) |
| Observations | 427 | 427 | 346 | 346 |
| Covariates | N | Y | N | Y |
| Mean of dependent variable at baseline (control) | 3.5 | 3.7 | ||
Notes: Table presents effect of any CEI on the number of commercial sex clients (in week prior to study visit) at months 6 and 12 (during the active phase) and at 18 months (once incentives were removed), with robust standard errors in parentheses ():
p<0.1;
p<0.05;
p<0.01.
Any conditional economic incentive (CEI) includes: medium (M) or high (H) incentive to stay free of new curable STIs or medium incentive for study visits only (Mv).
Regressions in columns (2) and (4) control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits coded as: 0=baseline, 1=six-month visit, 2=twelve-month visit, and 3=eighteen-month visit.
Appendix A4.
Effects of any incentive on condom use by unstable housing status
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Any conditional economic incentive (CEI) | 0.101** (0.048) | 0.126** (0.049) | 0.082 (0.053) | 0.115** (0.056) |
| Unstable housing | 0.092 (0.095) | 0.041 (0.094) | 0.042 (0.117) | −0.045 (0.119) |
| Interaction (any CEI × unstable housing) | −0.158 (0.109) | −0.125 (0.107) | −0.116 (0.143) | −0.063 (0.141) |
| Follow-up visits¶ | −0.013 (0.013) | −0.014 (0.014) | 0.018 (0.013) | 0.014 (0.013) |
| Observations | 577 | 577 | 466 | 466 |
| Covariates | N | Y | N | Y |
| Mean of dependent variable at baseline (control) | 0.84 | 0.83 | ||
Notes: Table presents effect of any CEI on condom use with last client interacted with unstable housing status at months 6 and 12 (during the active phase) and at 18 months (once incentives were removed), with robust standard errors in parentheses ():
p<0.1;
p<0.05;
p<0.01.
Any CEI includes medium or high incentive to stay free of new curable STIs or medium incentive for study visits only.
Regressions control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits coded as: 0=baseline, 1=six-month visit, 2=twelve-month visit, and 3=eighteen-month visit.
Appendix A5.
Effects of any incentive on condom use by HIV status
| ITT | IPW | |||
|---|---|---|---|---|
|
|
|
|||
| (1) | (2) | (3) | (4) | |
| Any conditional economic incentive (CEI) | 0.112** (0.056) | 0.134** (0.058) | 0.103 (0.065) | 0.133** (0.066) |
| HIV+ | 0.087 (0.069) | 0.070 (0.072) | 0.115 (0.087) | 0.091 (0.085) |
| Interaction (any CEI × HIV+) | −0.084 (0.076) | −0.072 (0.080) | −0.107 (0.093) | −0.096 (0.095) |
| Follow-up visits¶ | −0.015 (0.013) | −0.016 (0.014) | 0.015 (0.013) | 0.012 (0.014) |
| Observations | 577 | 577 | 466 | 466 |
| Covariates | N | Y | N | Y |
| Mean of dependent variable at baseline (control) | 0.82 | 0.81 | ||
Notes: Table presents effect of any CEI on condom use with last client interacted with HIV status at months 6 and 12 (during the active phase) and at 18 months (once incentives were removed), with robust standard errors in parentheses ():
p<0.1;
p<0.05;
p<0.01.
Any CEI includes medium or high incentive to stay free of new curable STIs or medium incentive for study visits only.
Regressions control for the covariates presented in Table 1: age, schooling, marital status, age of first sexual contact, having received a previous HIV test at baseline, having received previous STI test other than HIV at baseline.
Follow-up visits coded as: 0=baseline, 1=six-month visit, 2=twelve-month visit, and 3=eighteen-month visit.
Footnotes
Competing interests: The authors declare that they have no competing interests.
At the time of the study, Clínica Condesa had over 10,000 HIV+ patients in care, and provided over 23,000 annual HIV tests as well as STI testing and treatment to both HIV+ and HIV− populations.
The amounts of the incentives were established using an economic approach for willingness-to-accept (WTA). We used a computer experiment to measure the optimal level of incentives by interviewing 1,745 MSM including male sex workers, and found that at an offer of USD$288 a year, more than three-quarters of the men would attend prevention talks, engage in testing for STIs, and attempt to stay free of STIs. To obtain a similar level of participation among the sub-sample who engaged in sex work, the price was lower: USD$156 a year.
The composite incident STI/HIV measure included new cases of: Chlamydia, gonorrhea, syphilis, and HIV. At six months, there were 3 new cases in the control group, 6 in the M, 6 in the H, and 5 in the Mv groups; at 12 months, the new cases were 1 in the control group, 3 in M, 5 in H, and 2 in Mv. There were seven documented new HIV infections during the active trial (2 in the control group, 1 in M, 2 in H, and 2 in the Mv group).
References
- 1.Fiszbein A, Schady NR, Ferreira FHG. Conditional cash transfers : reducing present and future poverty. Washington D.C.: World Bank; 2009. [Google Scholar]
- 2.Baird S, Chirwa E, McIntosh C, Ozler B. The Short-Term Impacts of a Schooling Conditional Cash Transfer Program on the Sexual Behavior of Young Women. Health Econ. 2010 Sep;19:55–68. doi: 10.1002/hec.1569. [DOI] [PubMed] [Google Scholar]
- 3.Baird S, McIntosh C, Ozler B. Cash or Condition? Evidence from a Cash Transfer Experiment. Q J Econ. 2011 Nov;126(4):1709–1753. [Google Scholar]
- 4.Baird S, de Hoop J, Ozler B. Income Shocks and Adolescent Mental Health. J Hum Resour. 2013 Spr;48(2):370–403. [Google Scholar]
- 5.Higgins ST. Comments on contingency management and conditional cash transfers. Health Econ. 2009 Aug 7; doi: 10.1002/hec.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haug NA, Sorensen JL. Contingency management interventions for HIV-related behaviors. Current HIV/AIDS reports. 2006 Nov;3(4):154–159. doi: 10.1007/s11904-006-0010-5. [DOI] [PubMed] [Google Scholar]
- 7.Hanlon J, Barrientos A, Hulme D. Just give money to the poor : the development revolution from the global south. Sterling, VA: Kumarian Press; 2010. [Google Scholar]
- 8.de Walque D, Dow WH, Nathan R, et al. Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural Tanzania. BMJ Open. 2012;2:e000747. doi: 10.1136/bmjopen-2011-000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird SJ, Garfein RS, McIntosh CT, Ozler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012 Apr 7;379(9823):1320–1329. doi: 10.1016/S0140-6736(11)61709-1. [DOI] [PubMed] [Google Scholar]
- 10.Thornton RL. The Demand for, and Impact of, Learning HIV Status. Am Econ Rev. 2008 Dec;98(5):1829–1863. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Björkman-Nyqvist M, Corno L, de Walque D, Svensson J. Using Lotteries to Incentivize Safer Sexual Behavior: Evidence from a Randomized Controlled Trial on HIV Prevention. World Bank Policy Research Working Paper 7215. 2015 [Google Scholar]
- 12.Galárraga O, Sosa-Rubí SG. Male sex workers: HIV risk and behavioral economics. In: Shah M, Cunningham S, editors. Handbook on the Economics of Prostitution. New York: Oxford University Press; 2016. [Google Scholar]
- 13.Beyrer C, Crago AL, Bekker LG, et al. An action agenda for HIV and sex workers. Lancet. 2015 Jan 17;385(9964):287–301. doi: 10.1016/S0140-6736(14)60933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baral SD, Friedman MR, Geibel S, et al. Male sex workers: practices, contexts, and vulnerabilities for HIV acquisition and transmission. Lancet. 2015 Jan 17;385(9964):260–273. doi: 10.1016/S0140-6736(14)60801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggleton P, Parker RG. Men who sell sex : global perspectives. New York: Routledge; 2015. [Google Scholar]
- 16.Minichiello V, Scott J. Male sex work and society. New York: Harrington Park Press; 2014. [Google Scholar]
- 17.Aggleton P. Men who sell sex : international perspectives on male prostitution and HIV/AIDS. Philadelphia: Temple University Press; 1999. [Google Scholar]
- 18.Galárraga O, Sosa-Rubi SG, Infante C, Gertler PJ, Bertozzi SM. Willingness-to-accept reductions in HIV risks: conditional economic incentives in Mexico. The European journal of health economics : HEPAC : health economics in prevention and care. 2014 Jan;15(1):41–55. doi: 10.1007/s10198-012-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infante C, Sosa-Rubi SG, Cuadra SM. Sex work in Mexico: vulnerability of male, travesti, transgender and transsexual sex workers. Cult Health Sex. 2009 Feb;11(2):125–137. doi: 10.1080/13691050802431314. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro JF, Marshall BD, Escudero D, et al. Preventing HIV Transmission Among Partners of HIV-Positive Male Sex Workers in Mexico City: A Modeling Study. AIDS and behavior. 2014 Oct 12; doi: 10.1007/s10461-014-0915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tipos de Cambio y Resultados Históricos de las Subastas [Exchange Rates and Historical Series] Banco de México: Central Bank of Mexico; 2014. http://www.banxico.org.mx. [Google Scholar]
- 22.Sethi G, Holden BM, Gaffney J, Greene L, Ghani AC, Ward H. HIV, sexually transmitted infections, and risk behaviours in male sex workers in London over a 10 year period. Sexually transmitted infections. 2006 Oct;82(5):359–363. doi: 10.1136/sti.2005.019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council. The Prevention and Treatment of Missing Data in Clinical Trials. Washington (DC): 2010. [Google Scholar]
- 24.Hausman JA, Wise DA. Attrition Bias in Experimental and Panel Data - Gary Income-Maintenance Experiment. Econometrica. 1979;47(2):455–473. [Google Scholar]
- 25.Robins JM, Rotnitzky A, Zhao LP. Analysis of Semiparametric Regression-Models for Repeated Outcomes in the Presence of Missing Data. J Am Stat Assoc. 1995 Mar;90(429):106–121. [Google Scholar]
- 26.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000 Sep;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000 Sep;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Hernán MA, Robins JM. Causal Inference. Boca Raton: Chapman & Hall/CRC; 2016. (in press) [Google Scholar]
- 29.Moffitt R, Fitzgerald J, Gottschalk P. Sample Attrition in Panel Data: The Role of Selection on Observables. Annales d'Economie et de Statistique. 1999;55(56):129–152. [Google Scholar]
- 30.Fitzgerald J, Gottschalk P, Moffitt R. An analysis of the impact of sample attrition on the second generation of respondents in the Michigan Panel Study of Income Dynamics. J Hum Resour. 1998 Spr;33(2):300–344. [Google Scholar]
- 31.Fitzgerald J, Gottschalk P, Moffitt R. An analysis of sample attrition in panel data - The Michigan Panel Study of Income Dynamic. J Hum Resour. 1998 Spr;33(2):251–299. [Google Scholar]
- 32.Aidala AA, Wilson MG, Shubert V, et al. Housing Status, Medical Care, and Health Outcomes Among People Living With HIV/AIDS: A Systematic Review. Am J Public Health. 2016 Jan;106(1):e1–e23. doi: 10.2105/AJPH.2015.302905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CENSIDA. Mexico: UNGASS Report 2014. Mexico City: Centro Nacional para la prevención y el control del VIH y el sida (CENSIDA); http://www.unaids.org/sites/default/files/country/documents//MEX_narrative_report_2014.pdf;2014. [Google Scholar]
- 34.Bautista-Arredondo S, Colchero MA, Romero M, Conde-Glez CJ, Sosa-Rubi SG. Is the HIV epidemic stable among MSM in Mexico? HIV prevalence and risk behavior results from a nationally representative survey among men who have sex with men. PloS one. 2013;8(9):e72616. doi: 10.1371/journal.pone.0072616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CENSIDA. Mexico: UNGASS Report 2015. Mexico City: Centro Nacional para la prevención y el control del VIH y el sida (CENSIDA); http://www.unaids.org/sites/default/files/country/documents/MEX_narrative_report_2015.pdf;2015. [Google Scholar]
- 36.Infante C. Vulnerability and Risk for HIV Infection among Male, Transvestite, Transgender and Transsexual Sex Workers in Mexico City. Cult Health Sex. 2009 Apr;11(S1):S70–S71. doi: 10.1080/13691050802431314. [DOI] [PubMed] [Google Scholar]
- 37.Mexico City Government. Gaceta Oficial del Distrito Federal. In: GdDF, editor. No. 137 (page 199) Mexico City: 2015. http://www.cmicdf.org/docs/gaceta_GDF/2015/Julio/21-07-15-137.pdf. [Google Scholar]
- 38.SAT. Minimum daily wages in Mexico. In: Servicio de Administración Tributaria (SAT) [Mexican Internal Revenue Service], editor. Mexico City: 2015. http://www.sat.gob.mx/informacion_fiscal/tablas_indicadores/Paginas/salarios_minimos.aspx. [Google Scholar]
- 39.Galarraga O, Sosa-Rubi SG, Gonzalez A, et al. The disproportionate burden of HIV and STIs among male sex workers in Mexico City and the rationale for economic incentives to reduce risks. J Int AIDS Soc. 2014;17:19218. doi: 10.7448/IAS.17.1.19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thirumurthy H, Masters SH, Rao S, et al. Effect of providing conditional economic compensation on uptake of voluntary medical male circumcision in Kenya: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2014 Aug 20;312(7):703–711. doi: 10.1001/jama.2014.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pega F, Liu SY, Walter S, Lhachimi SK. Unconditional cash transfers for assistance in humanitarian disasters: effect on use of health services and health outcomes in low- and middle-income countries. The Cochrane database of systematic reviews. 2015;9:CD011247. doi: 10.1002/14651858.CD011247.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettifor A, MacPhail C, Nguyen N, Rosenberg M. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS and behavior. 2012 Oct;16(7):1729–1738. doi: 10.1007/s10461-012-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohler HP, Thornton R. Conditional Cash Transfers and HIV/AIDS Prevention: Unconditionally Promising? The World Bank economic review. 2012 Jun 1;26(2):165–190. doi: 10.1093/wber/lhr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.List JA, Gallet CA. What experimental protocol influence disparities between actual and hypothetical stated values? Environ Resour Econ. 2001 Nov;20(3):241–254. [Google Scholar]
- 45.Medlin C, Walque Dd World Bank. Policy research working paper 4673. Washington, D.C.: World Bank; 2008. Potential applications of conditional cash transfers for prevention of sexually transmitted infections and HIV in Sub-Saharan Africa. http://econ.worldbank.org/external/default/main?pagePK=64165259&theSitePK=469372&piPK=64165421&menuPK=64166093&entityID=000158349_20080722084441. [Google Scholar]
- 46.Cheng TC, Trivedi PK. Attrition Bias in Panel Data: A Sheep in Wolf's Clothing? A Case Study Based on the Mabel Survey. Health Econ. 2015 Sep;24(9):1101–1117. doi: 10.1002/hec.3206. [DOI] [PubMed] [Google Scholar]
- 47.Jones AM, Koolman X, Rice N. Health-related non-response in the British household panel survey and European community household panel: using inverse-probability-weighted estimators in non-linear models. J R Stat Soc a Stat. 2006;169:543–569. [Google Scholar]
- 48.Minnis AM, Steiner MJ, Gallo MF, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009 Oct 01;170(7):918–924. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Walque D, Gertler PJ, Bautista-Arredondo S, et al. Using provider performance incentives to increase HIV testing and counseling services in Rwanda. J Health Econ. 2015 Mar;40:1–9. doi: 10.1016/j.jhealeco.2014.12.001. [DOI] [PubMed] [Google Scholar]