Abstract
Determining intrabolus pressure (IBP) at the upper esophageal sphincter (UES) and in the esophagus has given compelling evidence that IBP can be a predictor for swallowing dysfunction. Studies have looked most superiorly at the low hypopharynx region but there has been no inquiry into what IBP measures throughout the entire pharynx can tell us. We present a study to describe the pressures within and surrounding the moving bolus throughout the pharynx and into the UES. Simultaneous HRM and videofluoroscopy were performed in 10 healthy subjects swallowing ten 10 mL thin-liquid barium boluses. Three events surrounding bolus movement were tracked via videofluoroscopy, two additional events were found using manometric measures. As the bolus passes through the pharynx, low pressure is created at and below the head of the bolus. A modest pressure increase is seen as the bolus passes through the pharynx and finally, high pressure is observed at the bolus tail, followed by an even larger pressure generation of a clearance event. HRM allows for greater resolution in data collection in the pharynx and in this study, aided in identifying semi unique characteristics around the hypopharynx and the UES which are consistent with the complex anatomy of the regions and the transition of the UES from active closure to relaxed opening. In the future, additional studies designed to look at aged and diseased populations may lead to better understanding of disease etiology, and treatment options.
Keywords: High-resolution manometry, Videofluoroscopy, Intrabolus Pressure, Deglutition, Deglutition Disorders
Introduction
Swallowing is a pressure-driven event requiring intricate coordination of muscle contraction for the successful movement of a bolus from the oral cavity to the esophagus. The bolus is subjected to complex forces during its passage, including gravity, contact pressure from pharyngeal structures, pressure changes due to oropharyngeal cavity shape changes, and intrabolus shear forces (1, 2). Inability to produce adequate forces in a coordinated matter can lead to inefficient or unsafe swallowing. Evaluating the pressures in and around the moving bolus has the potential to predict bolus movement efficiency (3, 4). Intrabolus pressure (IBP) has thus far been used to look at short windows in bolus transport through a closed system (4-6), and shows great potential for further use in the pharynx.
Abnormal IBP patterns can guide treatment options, predict risk, and can be used to document therapeutic change. For example, Ali and colleagues (4) used hypopharyngeal IBP to predict the successful outcome of cricopharyngeal surgery in the setting of pharyngeal dysphagia. More recently, Colizzo and colleagues (5) used IBP measured in the esophagus to distinguish between the fibrostenotic and inflammatory phenotypes of eosinophilic esophagitis. The importance of IBP during deglutition, both in the pharynx and esophagus, has been established and has shown great clinical promise.
Previously, IBP has been reported as a single point in space and time that lies somewhere between the bolus head and tail (4, 7-12), sometimes as the averaged pressure between the bolus head and tail (6). The few explorations of pharyngeal IBP using high-resolution manometry (HRM) have focused on one time point and one physiological sensor region (6, 13). Most studies that examined pharyngeal pressures using HRM focused solely on the upper esophageal sphincter (UES) and a small hypopharynx region rostral to the UES (4, 6). While determining IBP at the UES has given compelling evidence that IBP can be a predictor for swallowing dysfunction, there has been no inquiry into what IBP measures throughout the entire pharynx can tell us. It seems to reason that picking a single point in time and space when defining IBP would leave an abundance of important data unexamined, potentially omitting data that could help to define patterns and predict unsafe swallows. Although abnormal IBP measured at the center of a bolus would likely reflect abnormalities throughout the bolus, it is important to recognize that the bolus is a moving object and thus should have pressure gradients within the bolus.
Understanding these gradient patterns should provide useful information about normal swallow and a deeper understanding of dysfunctional swallow, thus further investigation into multiple parameters of IBP is warranted.
Most studies reporting IBP have used conventional manometry. Methods for quantifying the changing pressures of the pharyngeal swallow traditionally involved the use of three to five unidirectional sensors typically positioned around the UES (2, 14-16). Such studies provided important information regarding the underlying forces of bolus propulsion, but the limited capacity and number of sensors do not precisely capture the complex pressure events of the entire pharynx. High-resolution manometry (HRM) addresses these problems and has been used successfully to evaluate pharyngeal swallowing (17-20). HRM measures pressure in the pharynx and esophagus with a large number of closely-spaced sensors. The large number and circumferential nature of HRM sensors has allowed for more accurate exploration into pressure events in the asymmetrical pharynx (17, 21, 22). With the increased number and decreased spacing of sensors, HRM has the potential to measure IBP along the entirety of the pharynx and into the esophagus.
In the present study, we used simultaneous HRM and videofluoroscopy in a pilot project to explore pharyngeal IBP as a series of pressure events occurring throughout time and space in order to define a system of measurements for pharyngeal IBP during 10 mL liquid swallows. As the bolus is being actively pushed and pulled during its transit through the pharynx, it reasons that all the spatial regions in a pharyngeal swallow could be important based on the predictive nature of IBP in the UES and esophagus (4-6). We hypothesized there would be differences in pressure profiles based on both the pharyngeal location and the intra-bolus event location (bolus head, tail, or center). We hypothesized that mid-IBP would decrease along its course through the pharynx, as the bolus moved along a high to low-pressure gradient and there should be a measureable pressure gradient within the bolus with the highest pressure at the bolus tail and the lowest pressure at the head or leading the bolus.
Materials and Methods
Data Collection
Five male and five female subjects (ages 21-52, mean age 35.1) participated in this study with the approval of the Institutional Review Board of the University of Wisconsin-Madison. Subjects were excluded if they had a history of swallowing, gastrointestinal, or any neurological conditions.
Topical viscous lidocaine hydrochloride (2%) was applied to the nasal passages with a cotton swab, and the manometric catheter was lubricated with viscous lidocaine hydrochloride (2%) to ease the passage of the catheter through the nasal cavity and the pharynx. Each subject swallowed ten boluses of 10 mL thin liquid barium (40% w/v) (Varibar, Bracco Diagnostics, Monroe Township, NJ) with the head in the neutral position. Each bolus was delivered to the oral cavity via syringe. Five randomly selected swallows were analyzed for each participant. The random swallows were selected using a random number generator. A total of 43 swallows were used after excluding trials with multiple swallows and trials in which clear sensor identification was not achieved.
Equipment
High-Resolution Manometry: A solid-state high-resolution manometer was used for all data collection (ManoScan360, Given Imaging, Atlanta, GA). The catheter has an outer diameter of 2.75 mm and 36 circumferential pressure sensors spaced 1 cm apart. Each sensor spans 0.4 mm and receives input from 12 circumferential sectors. These inputs are averaged and the mean pressure is recorded as the pressure detected by that individual sensor. The system is calibrated to record pressures between −20–600 mmHg. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Given Imaging). The catheter was calibrated before each participant according to manufacturer specifications.
Videofluoroscopy: Continuous videofluoroscopy was captured in the lateral plane (OEC 9900, General Electric, Fairfield, CT). The video was digitized and recorded at 30 frames per second on a DVD+RW for offline analysis (DVO-1000MD, Sony, Park Ridge, NJ). The videofluoroscopic frame was adjusted to include the incisors, cervical vertebrae, nasal border of the soft palate, and the cervical esophagus.
Data Analysis
Manometric and videofluoroscopic data were aligned temporally with a time stamp embedded into the videofluoroscopy signal (UTG-50, Horita, Mission Viejo, CA) and recorded by the manometric system (ManoScan 2.1, Given Imaging). A specialized Matlab program was used to time-align the fluoroscopic and manometric data (The MathWorks, Inc., Natick, MA).
Videofluoroscopic video frames were imported into ImageJ (National Institutes of Health, Bethesda, MD) and rotated such that the y-axis was made parallel to a line connecting the anterior-inferior corners of the C2 and C4 vertebrae. The following coordinates were labeled on each video frame: 1) anterior-superior corner of each visible sensor; and 2) anterior tubercle of C1 vertebra. The anterior tubercle of C1 represented a stable reference point and was set as the origin of the x- and y-axes. Each visible sensor was also labeled on each video frame. The most anterior-superior and anterior-inferior corners of each sensor in view was marked and used when lining up the passing bolus and determining the pharyngeal regions of interest (23).
Regions of interest were defined manometrically using methods similar to that described previously (23-25). Sensors included in these regions of interest differed between subjects, based on individual characteristics, such as height, and catheter placement, but each was defined precisely using the manometric details that follow. Briefly, the velopharynx (VP) is a region swallowing-related pressure characterized as the most-superior two or three sensors registering swallowing-related pressure. Pressure in this region will rise from baseline inactivity before any other pressure rise in the pharynx and may display a bimodal pressure wave pattern (Figure 1). VP pressure events have been reported (18-20, 24) and are essential to define other regions of interest, but one that this study does not directly address.
Fig. 1.
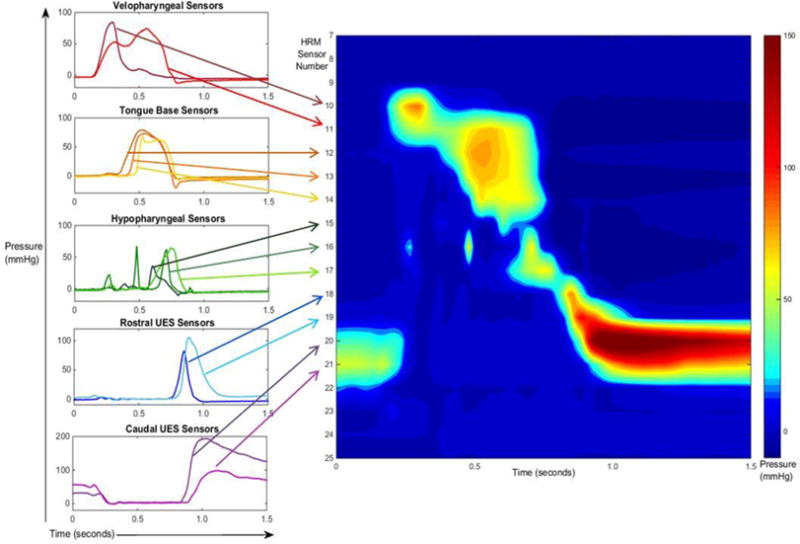
High-resolution manometry (HRM) pressure wave and spatiotemporal plot alignment of sensor regions of a 10 mL swallow. The left side of the figure outlines the pressure wave shapes that helped us determine the regions of interest, and shows where the corresponding sensors line up on the HRM spatiotemporal plot (on the right). The pressure wave and spatiotemporal plots are time aligned along the x-axis.
Previous reports have combined pressures from the tongue base and hypopharynx in a single region (18, 19, 24). For the purposes of this study, we have separated these pressures into two regions, based on the shapes of the pressure waves. The tongue base (TB) region spans two or three sensors directly inferior to the VP region and represents the region and pressures of the tongue base and posterior pharyngeal wall. These sensors have a unimodal pressure wave pattern which is generated after the start of the velopharyngeal pressure (Figure 1). The hypopharynx (HP) is the region of swallowing-related pressure between the tongue base and UES regions. The HP region is represented by one or two sensors whose pressure generation wave patterns often are comprised of multiple peaks. The multiple peaks are likely an artifact of the circumferentially averaged pressure in a region of moving structures in the anterior domain, such as the larynx, with the contracting middle and inferior pharyngeal constrictors. Anatomically, this zone is adjacent to the superior cricoid and arytenoids anteriorly, and to the interior and lower middle constrictors posteriorly. The pressures in this region occur at approximately the same time as pressures in the tongue base region, but the overall pressure pattern contrasts starkly with the unimodal pressure waves produced in the TB region. The HP swallowing-related pressure begins after the start of the TB swallowing-related pressure and before pressure in the rostral upper esophageal sphincter (UES) region (Figure 1).
The upper esophageal sphincter region is split into two distinct regions of interest based on two distinct pressure patterns outlined by Jones et al (23). The rostral upper esophageal sphincter (rUES) region of interest displays a pressure wave that has a sharp, brief departure from baseline with a fast rate of pressure release (23) (see Figure 1). These 1-3 sensors are directly caudal to the HP sensors and directly rostral to the caudal upper esophageal sphincter sensors. They can be differentiated from the HP sensors, by looking at the difference in timing of the peaks. The caudal upper esophageal sphincter (cUES) region of interest displays the same pattern as the caudal UES pressure wave pattern reiterated by Jones and colleagues (23), but identified previously by many (26-28). The cUES sensors display a pressure pattern with elevated pressure at baseline, fall to a nadir pressure during UES opening followed by a pressure burst, and return to baseline pressure (Figure 1). These 2-3 sensors are directly caudal to the rUES sensors.
Five pressure events were chosen to describe multiple time points relating to bolus movement through the pharynx. Four of those points are depicted in Figure 2. The leading bolus point was identified as the coordinate of the center of the caudal-most edge of bolus head on the videofluoroscopic image. The leading bolus point was identified on each frame of video until the bolus head leaves the visible frame. This point was compared against the coordinates of each HRM sensor to find the corresponding leading bolus pressure (2). The trailing bolus point was defined as the coordinate of the tail of the majority of the bolus body and identified as the center of the rostral-most edge of the bolus on the videofluoroscopic image (Figure 2). The trailing bolus point was identified on each frame on which it was visible. This point was compared against the coordinates of each HRM sensor to find the corresponding pressure within the HRM data and is then referred to as the trailing bolus pressure (2). The clearance point is the measure of the very end of the elongated bolus tail. The trailing bolus point is a measure of the rostral-edge of the majority of the bolus body, but the clearance point is a measure of the last part of the bolus that falls behind the majority of the bolus body (Figure 2). The clearance point was identified as the coordinate of the center of the rostral-most edge of the bolus that falls behind the majority of the bolus body, much like the trailing bolus point measurement but more rostral than the bolus tail (Figure 2). As the tail of the bolus elongates during the pharyngeal swallow, the coordinate measured for the clearance point shifts out to the end of the visible bolus trajectory. This point might be considered residue if it stops making forward progress. This point was then compared against the coordinates of each HRM sensor to find the corresponding pressure within the HRM data and is then referred to as the clearance pressure. The leading pressure is measured as the pressure from one HRM sensor caudal to where the leading bolus pressure is measured which depicts what is taking place in preparation for the bolus to move through the cavity. Leading pressure thus represents pressure directly ahead of the bolus. The mid-bolus pressure is the pressure representing the bolus between the leading bolus pressure and trailing bolus pressure points. The mid-bolus measurement was made using the trailing bolus pressure and the leading bolus pressure measurement times for each sensor and region of interest. The trailing bolus pressure and leading bolus pressure measurements were time aligned with the HRM data and superimposed upon a waveform for the corresponding sensor using a specialized Matlab program (see Figure 3, 4). The point measurement is the pressure at the time point exactly halfway between the trailing and leading bolus pressure time points (see Figure 4). For a clearer picture and enhanced understanding, Figure 5 shows the five described pressure events superimposed on the corresponding HRM spatiotemporal plot from a single swallow. As the head of the bolus is moving at a rate faster than the tail—the bolus head moves through the pharynx in approximately 15msec—it is captured in only four frames of videofluoroscopy before entering the esophagus, whereas the bolus tail is identified during the entire duration of the swallow and can be located on 15 or more video frames.
Fig. 2.
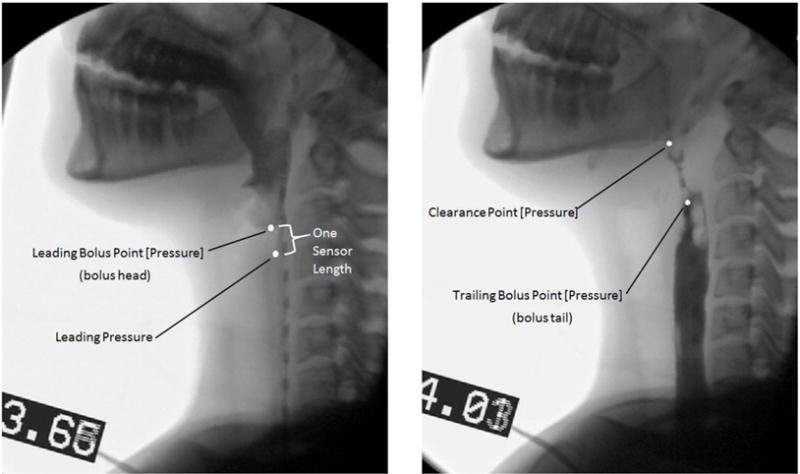
Visual Description of the Bolus Events on fluoroscopy stills. The Leading Bolus Point, Trailing Bolus Point and Clearance Point were each found using videofluoroscopy, and the coordinates were then used to find the corresponding pressure values. Leading Pressure was not found using videofluoroscopic techniques like the other points outlined on the stills, it is represented here as a way to better visualize the event that was measured manometrically.
Fig. 3.
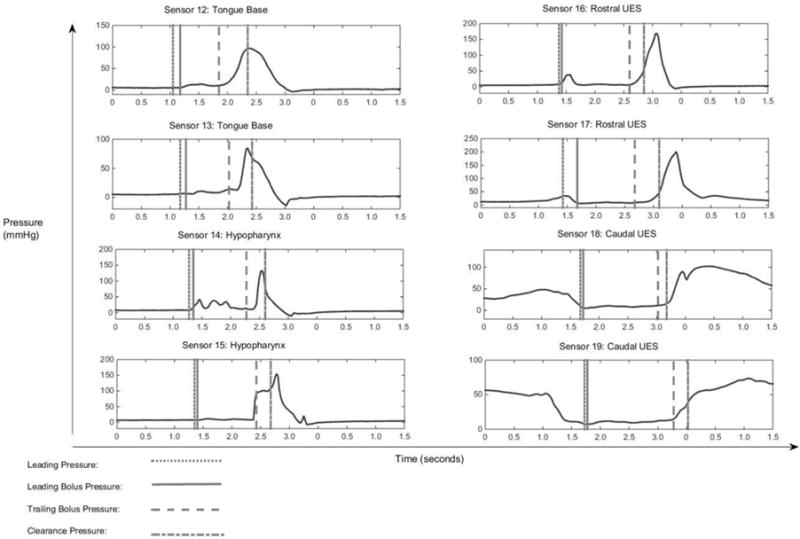
High-resolution manometry (HRM) sensor readings from a single 10mL swallow. Each measured pressure event is temporally displayed on each corresponding sensor of the measurement.
Fig. 4.
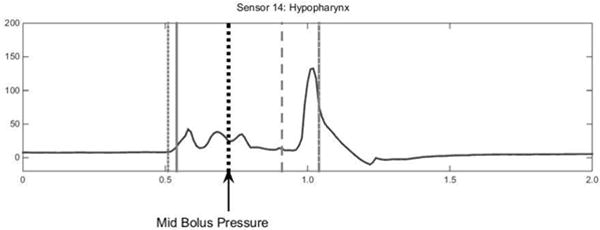
Mid Bolus measurement was made at each sensor between the Leading and Trailing Bolus Pressure events’ timing (see Figure 3 for description of bolus events).
Fig. 5.
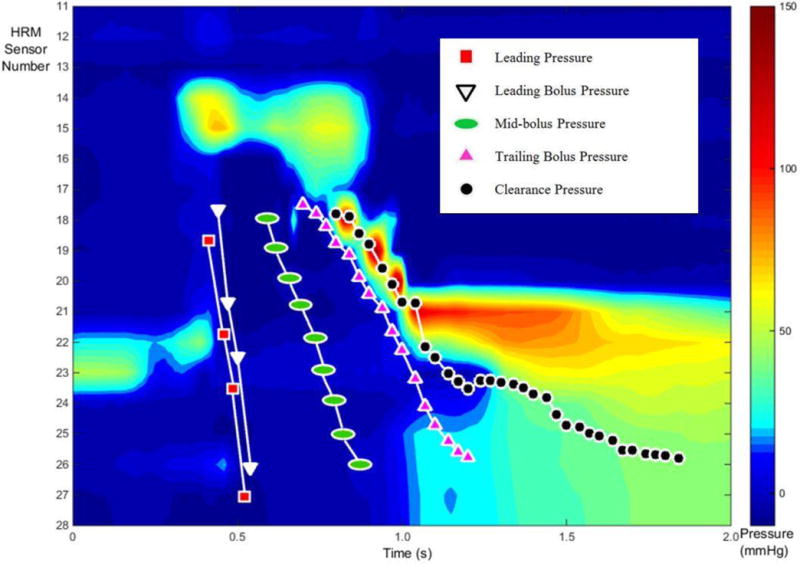
An alternate display of the five described pressure events superimposed on the corresponding HRM spatiotemporal plot from a single swallow. Each marker indicates the timing and positioning of the event as caught on videofluoroscopy, and then aligned with HRM data. The number of markers for each event varies due to the rate of movement on videofluoroscopy. For example, the bolus head moves much more rapidly than the tail and therefore has less event specific markers.
Statistical Analysis
For each individual the pressure data within each pharyngeal region was averaged yielding a single regional average for ease of analysis and understanding. A 4 × 5 repeated measures analysis of variance (ANOVA) was performed on average swallowing pressures of subjects (four sensor regions of interest, five pressure events). Pairwise comparisons were made using Fisher’s protected least significant difference (LSD) tests. An α criterion of 0.05 was selected to represent significance. Twenty percent of videofluoroscopy data were reanalyzed by a separate team member in the lab and intraclass correlation coefficients (ICC) were calculated as a measure of agreement between the two raters (ICC=0.9712).
Results
An overview of all the pressure profiles, divided out by event and then pharyngeal position are presented in Figure 6. Statistically significant differences are presented in Figure 6 and outlined in Table 1. A complete table of pressure event measurements is provided in Table 2.
Fig. 6.
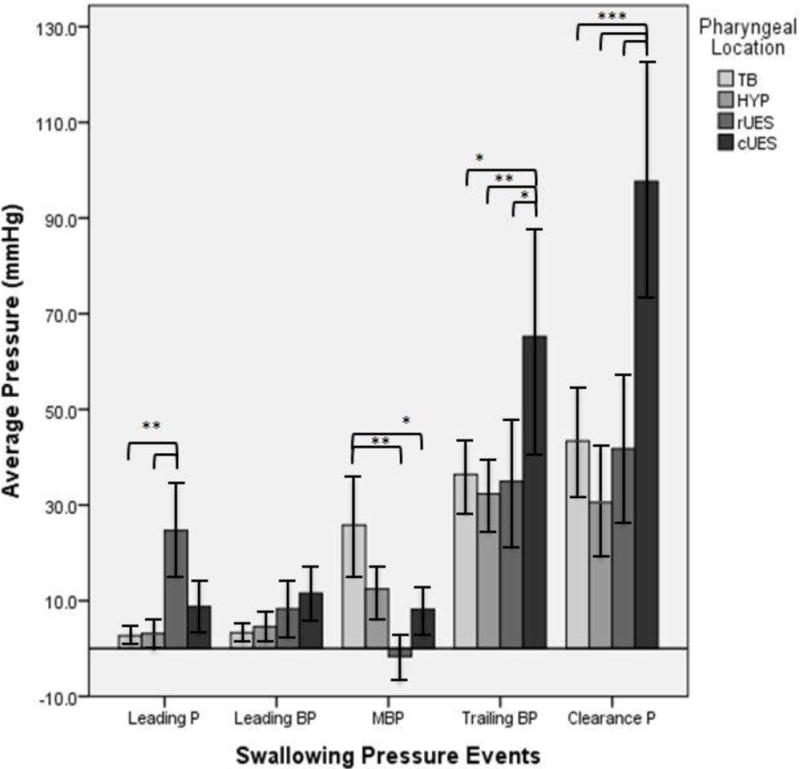
An overview of all the pressure profiles, divided out by swallowing pressure event then pharyngeal location. P = Pressure, BP = Bolus Pressure, MBP = Mid Bolus Pressure, ‘rUES’ rostral upper esophageal sphincter, and ‘cUES’ caudal upper esophageal sphincter.
Within individual pressure events, significant pressure differences were observed across regions. For the leading pressure event, average pressure at the rUES was significantly greater than at the tongue base and hypopharynx. For the mid-bolus pressure event, average pressure at the tongue base is significantly greater than at the rUES and the cUES. For the trailing bolus pressure event, average pressure at the cUES is significantly greater than at the tongue base, hypopharynx, and rUES. For the clearance pressure event, average pressure at the cUES is significantly greater than at the tongue base, hypopharynx, and rUES. Error bars indicated standard error of the mean. *P < 0.05. **P < 0.01. ***P <0 .001.
Table 1.
An overview of all the pressure profiles, divided by pharyngeal location then swallowing pressure event. Within individual pharyngeal regions, significant pressure differences were observed between swallowing pressure events.
| Tongue Base Region | Leading Pressure | Leading Bolus Pressure | Mid-Bolus Pressure | Trailing Bolus Pressure |
|---|---|---|---|---|
| Leading Bolus Pressure | t= −0.03, p=0.973 | – | – | – |
| Mid-Bolus Pressure | t= −2.18, p=0.036* | t= −2.15, p=0.039* | – | – |
| Trailing Bolus Pressure | t= −3.1, p=0.004* | t= −3.07, p=0.004* | t=0.93, p=0.361 | – |
| Clearance Pressure | t= −3.72, p<0.001* | t= −3.69, p<0.001* | t=1.54, p=0.132 | t= −0.62, p=0.541 |
| Hypopharynx Region | Leading Pressure | Leading Bolus Pressure | Mid-Bolus Pressure | Trailing Bolus Pressure |
|---|---|---|---|---|
| Leading Bolus Pressure | t= −0.21, p=0.835 | – | – | – |
| Mid-Bolus Pressure | t= −1.11, p=0.274 | t= −0.9, p=0.374 | – | – |
| Trailing Bolus Pressure | t= −3.78, p<0.001* | t= −3.57, p=0.001* | t=2.67, p=0.011* | – |
| Clearance Pressure | t= −3.35, p=0.002* | t= −3.14, p=0.003* | t=2.24, p=0.032* | t=0.44, p=0.664 |
| Rostral UES Region | Leading Pressure | Leading Bolus Pressure | Mid-Bolus Pressure | Trailing Bolus Pressure |
|---|---|---|---|---|
| Leading Bolus Pressure | t=1.36, p=0.184 | – | – | – |
| Mid-Bolus Pressure | t=2.13, p=0.040* | t=0.77, p=0.446 | – | – |
| Trailing Bolus Pressure | t= −1.19, p=0.243 | t= −2.54, p=0.016* | t=3.31, p=0.002* | – |
| Clearance Pressure | t= −1.34, p=0.19 | t= −2.69, p=0.011* | t=3.46, p=0.001* | t= −0.15, p=0.881 |
| Caudal UES Region | Leading Pressure | Leading Bolus Pressure | Mid-Bolus Pressure | Trailing Bolus Pressure |
|---|---|---|---|---|
| Leading Bolus Pressure | t= −0.13, p=0.901 | – | – | – |
| Mid-Bolus Pressure | t=0.12, p=0.9028 | t=0.25, p=0.805 | – | – |
| Trailing Bolus Pressure | t= −3.52, p=0.001* | t= −3.40, p=0.002* | t=3.65, p<0.001* | – |
| Clearance Pressure | t= −5.39, p<0.0001* | t= −5.27, p<0.0001* | t=5.51, p<0.0001* | t= −1.87, p=0.07 |
highlights a statistically significant difference (p<0.05).
Table 2.
Summary data. Values are presented as mean ± standard error of the mean. All values are pressures (mmHg).
| Bolus Movement Event | Leading Pressure | Leading Bolus Pressure | Mid-Bolus Pressure | Trailing Bolus Pressure | Clearance Pressure |
|---|---|---|---|---|---|
| Region | |||||
| Tongue Base | 2.68 ± 0.76 | 3.30 ± 1.05 | 25.82 ± 10.13 | 36.42 ± 8.31 | 43.40 ± 11.07 |
| Hypopharynx | 3.14 ± 1.36 | 4.60 ± 1.54 | 12.44 ± 5.33 | 32.32 ± 8.82 | 30.55 ± 10.93 |
| Rostral UES (rUES) | 24.70 ± 9.47 | 8.31 ± 5.80 | −1.64 ± 3.69 | 34.93 ± 13.35 | 41.78 ± 14.82 |
| Caudal UES (cUES) | 8.76 ± 5.45 | 11.58 ± 5.18 | 8.24 ± 4.50 | 65.22 ± 21.31 | 97.65 ± 22.75 |
Within pharyngeal regions, mean pressure changed significantly across the defined swallowing pressure events (Table 1). At the tongue base, the leading pressure and the leading bolus pressure were both significantly less than the pressures generated at the mid-bolus pressure point, the trailing bolus pressure, and the clearance pressure. At the level of the hypopharynx, the leading pressure, leading bolus pressure and the mid-bolus pressure were all significantly less than the trailing bolus pressure and the clearance pressure. At the level of the rostral UES, leading bolus pressure was significantly less than trailing bolus pressure and clearance pressure. Additionally, mid-bolus pressure reached a minimum in this region and was significantly less than leading pressure, trailing bolus pressure and clearance pressure. This dip in mid-bolus pressure to a subatmospheric average pressure is unique to this area. At the level of the caudal UES, the leading pressure, leading bolus pressure, and the mid-bolus pressure were all significantly less than the trailing bolus pressure and the clearance pressure.
Within individual swallowing pressure events, mean pressure changed significantly across pharyngeal location (Figure 6). Significantly higher average pressures were generated in the cUES region for the trailing bolus pressure and clearance pressure events relative to TB, HP, and rUES regions. An elevated rUES pressure is also seen for the leading pressure event, relative to the tongue base and hypopharynx. No significant pressure difference was seen across regions for the leading bolus pressure event.
Discussion
The findings of this study highlight the importance of pharyngeal manometry and videofluoroscopy to understanding the pharynx as a complex system. At every pharyngeal region of interest, significantly lower pressures are seen at the leading pressure and leading bolus pressure events. This underscores the importance of generating a low pressure environment in preparation for bolus flow through each region. The highest pressures are recorded in the trailing bolus pressures and the clearance pressures. The IBP recorded at the mid-bolus point revealed a unique pattern with pressures in the tongue base region being the highest, lower at the hypopharynx, but still greater than leading bolus pressure, and then becoming lower than leading pressures in rostral and caudal UES.
The results of this study support the long-standing idea, following fluid dynamics, that we can measure to some degree the propagation of the bolus from high to low pressure areas, though we acknowledge we did not record pressure in the oral cavity where the bolus initiated its movement. The movement from high to low pressure can be recognized discretely in each pharyngeal region of interest. When the bolus enters into each defined region, low pressures are observed in both the leading pressure and leading bolus pressure events (Table 2). As the bolus exits that same pharyngeal region of interest, high pressures are seen via the trailing bolus pressure and clearance pressure events (Table 2). The leading pressure, one sensor caudal to the bolus head (leading bolus pressure), gives us an idea of how the pharynx prepares for the incoming bolus. By recording the pressures in the UES as the bolus moves toward it and then through to the esophagus, we are provided insight into the complex activity which occurs at the UES during bolus transit. Work completed by Omari et al. addressing the mechanical states of the UES, coupled UES electromyography, and high resolution impedance manometry and illuminated the rapid transition from an active contracted state to quiescent distensible state (29). The greater leading pressure at the rUES was likely due to residual pressure present as this transition occurs. This is supported by the fact that the leading pressure at the UES was less than previously-reported average resting UES pressures—55.7mmHg (30) and 46.5mmHg (31). In the present study, the average pressure measured at the leading pressure event at the rUES was 23.5 mmHg, which may indicate a lowering in the high static pressure of the UES in preparation for the incoming bolus. The even lower leading pressure measure of 9.9 mmHg at the cUES is conceivably due to the nature of the ‘opening’ rUES segment just rostral to the region and also supports the preparatory drop in pressure idea presented above. The final active opening of the UES is a rapid process which includes distraction through elevation and anterior movement of the hyolaryngeal complex, which reaches its peak as the bolus transits the UES and is most likely accounting for the subatmospheric pressure measured at the rUES when the mid-IBP is recorded at that location (Figure 6, Table 2).
Previous work has shown that due to its rostral rise with the swallow, UES manometric recordings from this area transition as the swallow occurs (23). Prior to the swallow, sensor(s) reside above the UES at the level of the post cricoid area and then are brought in proximity to the UES as the pharynx contracts and UES rises relative to the pressure sensors. These complex events lead to an a elevated leading pressure, which quickly dissipates as the bolus head arrives and continues to decrease until the mid-bolus pressure low point at the time of maximum UES opening recorded by these sensors (Figure 6, Table 2).
As the bolus tail moves through the pharynx, elevated pressures start to appear (i.e. trailing bolus pressure) and then even larger clearance pressures (CP) follow, representing the pharyngeal stripping wave (2, 32). In all regions, the clearance pressures exceed the mid-bolus pressure and bolus head pressure (leading bolus pressure) by a significant amount (Table 1).The largest average clearance pressure was found at the cUES and the smallest average clearance pressure was found at the hypopharynx. In fact, the average clearance pressure measure found in the hypopharynx was the only average clearance pressure measure found to be lower than its trailing bolus pressure counterpart in the same pharyngeal region (Table 2). These hypopharyngeal pressure events were only considered significantly lower when compared to corresponding cUES pressure events, but the pattern is interesting nonetheless. It could be that clearance pressure generation in the hypopharynx is lower because pressure wave generation in the area is not a priority; rather keeping the bolus out of the airway may be more important. With glottal closure falling in the hypopharyngeal sensor region, it may seem reasonable that more muscle coordination is going toward airway protection rather than direct bolus propagation. Lowest normal clearance forces are consistent with presence of glottis structures which do not contribute to averaged clearance pressures in this region.
A possible explanation for largest trailing bolus pressures and clearance pressures occurring in the cUES region may be due to better catheter contact at that point. This is the only location along bolus flow were true sphincteric muscle activity occurs. As the bolus moves to the esophagus, it is likely that the cUES region has the highest pressure generation to prevent retrograde bolus flow post swallow. Pouderoux and Kahrilas described a strong UES force at the time of bolus clearance—the grabbing effect—that was attributed to the combination of UES contraction and laryngeal descent (33). The high resolution data from this study supports that the function of the grabbing effect is to prevent regurgitation with an elevated from baseline clearance pressure seen in the cUES.
Our findings support previous evaluations of IBP in the pharynx and UES. Pal and colleagues used HRM to study forces acting on the bolus during UES trans-sphincteric flow (6). They presented IBP, calculated at a single point, of a 10mL high-density barium suspension at regions approximating our definitions of rostral UES (rUES) and caudal UES (cUES). They reported pressures that were in the same range of the present study for the rUES, but presented pressures that were slightly lower than the range of the present study at the cUES. Ali, et al. reported a hypopharyngeal IBP, which was measured at the sensor immediately proximal to the UES at the pressure time point midway between the bolus head arrival and bolus tail departure at that point (4). This region likely approximates our definition of the hypopharynx, though it may only represent the lower sensors. They reported pressures that were in the same range as the present study for thy hypopharynx. Ghosh and colleagues performed a study using solid-state HRM, focusing solely on the UES (13). Using multiple algorithms and derived graphs, a median IBP was found for what can best be described as the UES during its relaxation phase for a 10mL water swallow at a region approximating our definition of caudal UES (cUES). They reported pressures that were in the same ranges of the present study.
We had hypothesized that we would see decreasing mid-IBP measurements as the bolus moved through the pharynx, demonstrating an elongating bolus with less shear forces and therefore less IBP due to the decreased amount of bolus substance. The bolus elongation begins with the very low leading pressures at the tongue base and hypopharynx and then support by the gradient pressures as the UES opens and the upper pharynx contracts on the bolus tail. The cUES is the zone of lowest capacity and the area which represents the muscular UES the best during most of the swallow. It retains positive pressure during all phases of bolus transport, which are relatively static, and reveals the highest bolus tail clearance pressures consistent with active UES muscular contraction. Bolus movement through this zone requires the elongation of the bolus and the pressure gradients created by the clearance pressures acting on the bolus tail as the mid-bolus transits this zone.
A few limitations in these studies should be noted. First, analysis was performed on a modest-sized sample of healthy participants. This is an important first step in understanding pharyngeal IBP, but in the future, it would be important to examine how the patterns observed in this study change in a larger group of healthy swallowers, in a state of dysphagia, and in an aging population. Second, analysis of videofluoroscopy was limited to 30 frames per second. Current technology does not permit a faster rate of acquisition, which may have resulted in a clearer picture of bolus flow dynamics. Additionally, the current HRM system circumferentially averages the pressures of each sensor to give one discrete pressure per sensor per time point. This gives an incomplete picture of directional pressure measurement and prevents us from making further judgements on which specific muscles are responsible for certain pressure readings with electromyography. Finally, the mid-bolus pressure measure only looks at one single point that lies directly between the leading bolus pressure and trailing bolus pressure. The point measure follows traditional methodology, but fails to capture more than a single time point. For comparison sake, using this traditional methodology is important. In the future, a method such as mean or integral should be applied to measuring the mid-bolus pressure as to evaluate all the time points, and therefore all the corresponding pressures.
Conclusion
Traditionally, a single point measurement has been most often used to represent an IBP measurement. This study was aimed at describing a more complete paradigm for IBP measurement, recognizing that the measurement is more than a single point in bolus movement, but is a system of pressures and events that changes throughout the swallow. We used five events to make a more thorough evaluation of the bolus during its movement through the pharynx: 1) Leading Pressure, a view of what pressure lies ahead of the oncoming bolus; 2) Leading Bolus Pressure, a view of the pressures at the head of the bolus; 3) Mid Bolus Pressure, a view of true IBP at the midpoint between the leading and trailing bolus pressure 4) Trailing Bolus Pressure, a view of the pressures at the tail of the bolus 5) Clearance Pressure, a view of the pressures that follow the bolus tail and clear the bolus through the pharynx. The present study’s results confirmed the patterns of bolus moving from an area of high to low pressure, followed by a strong, and driving clearance pressure. Largest average clearance pressure following the tail of the bolus was seen in the cUES, a likely consequence of better catheter contact and the prevention of retrograde bolus flow. The smallest average clearance pressure was seen in the hypopharynx region. Though this only reached our definition of statistical significance when compared to cUES clearance pressure, this finding could indicate that precise coordination, rather than pressure generation, may be the ultimate goal of the muscles in the region.
Acknowledgments
The authors would like to acknowledge University of Wisconsin Department of Surgery biostatisticians Glen Leverson, Ph.D., and Ying Shan, M.S., for their assistance with statistical analysis. The authors would also like to acknowledge Levi Brown, B.A., and William Bleifuss, B.S., for their assistance with data analysis.
Funding: This research was supported by National Institutes of Health grant number 1R21DC011130-01A1. CAJ was also supported by T32GM007507.
Footnotes
Parts of this manuscript were presented at the Dysphagia Research Society Meeting on March 6, 2014 (Nashville, TN, USA).
Conflict of Interest: Authors have no conflict of interest to declare.
References
- 1.Massey BT. Physiology of oral cavity, pharynx and upper esophageal sphincter. GI Motility online. 2006 [Google Scholar]
- 2.McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98:71–78. doi: 10.1288/00005537-198801000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Mcconnel FMHD, Jackson K, O’Connorn A. Analysis of intrabolus forces in patients with zenker’s diverticulum. The Laryngoscope. 1994;104:571–581. doi: 10.1002/lary.5541040512. [DOI] [PubMed] [Google Scholar]
- 4.Ali GN, Wallace KL, Laundl TM, Hunt DR, deCarle DJ, Cook IJ. Predictors of outcome following cricopharyngeal disruption for pharyngeal dysphagia. Dysphagia. 1997;12:133–139. doi: 10.1007/PL00009527. [DOI] [PubMed] [Google Scholar]
- 5.Colizzo JM, Clayton SB, Richter JE. Intrabolus pressure on high-resolution manometry distinguishes fibrostenotic and inflammatory phenotypes of eosinophilic esophagitis. Diseases of the Esophagus. 2015 doi: 10.1111/dote.12360. [DOI] [PubMed] [Google Scholar]
- 6.Pal A, Williams RB, Cook IJ, Brasseur JG. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2003;285:G1037–1048. doi: 10.1152/ajpgi.00030.2003. [DOI] [PubMed] [Google Scholar]
- 7.Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Normal values for esophageal high-resolution manometry. Neurogastroenterology and Motility. 2013;25:762–e579. doi: 10.1111/nmo.12167. [DOI] [PubMed] [Google Scholar]
- 8.Chen CL, Yi CH, Liu TT, Hsu CS, Omari TI. Characterization of esophageal pressure-flow abnormalities in patients with non-obstructive dysphagia and normal manometry findings. Journal of Gastroenterology and Hepatology. 2013;28:946–953. doi: 10.1111/jgh.12176. [DOI] [PubMed] [Google Scholar]
- 9.Loots C, van Herwaarden MY, Benninga MA, VanderZee DC, van Wijk MP, Omari TI. Gastroesophageal reflux, esophageal function, gastric emptying, and the relationship to dysphagia before and after antireflux surgery in children. Journal of Pediatrics. 2013;162:566–573.e562. doi: 10.1016/j.jpeds.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Dire C, Shi G, Manka M, Kahrilas PJ. Manometric characteristics of the upper esophageal sphincter recorded with a microsleeve. American Journal of Gastroenterology. 2001;96:1383–1389. doi: 10.1111/j.1572-0241.2001.03793.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams RB, Wallace KL, Ali GN, Cook IJ. Biomechanics of failed deglutitive upper esophageal sphincter relaxation in neurogenic dysphagia. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2002;283:G16–26. doi: 10.1152/ajpgi.00189.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ren J, Massey BT, Dodds WJ, Kern MK, Brasseur JG, Shaker R, Harrington SS, Hogan WJ, Arndorfer RC. Determinants of intrabolus pressure during esophageal peristaltic bolus transport. American Journal of Physiology. 1993;264:G407–413. doi: 10.1152/ajpgi.1993.264.3.G407. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2006;291:G525–531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phoniatricia et Logopaedica. 2002;54:171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 15.Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Archives of Physical Medicine and Rehabilitation. 1989;70:767–771. [PubMed] [Google Scholar]
- 16.Hind JA, Nicosia MA, Roecker EB, Carnes ML, Robbins J. Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Archives of Physical Medicine and Rehabilitation. 2001;82:1661–1665. doi: 10.1053/apmr.2001.28006. [DOI] [PubMed] [Google Scholar]
- 17.Takasaki K, Umeki H, Enatsu K, Tanaka F, Sakihama N, Kumagami H, Takahashi H. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008;118:1729–1732. doi: 10.1097/MLG.0b013e31817dfd02. [DOI] [PubMed] [Google Scholar]
- 18.McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. The Annals of Otology, Rhinology, and Laryngology. 2010;119:369–376. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umeki H, Takasaki K, Enatsu K, Tanaka F, Kumagami H, Takahashi H. Effects of a tongue-holding maneuver during swallowing evaluated by high-resolution manometry. Otolaryngology: Head Neck Surgery. 2009;141:119–122. doi: 10.1016/j.otohns.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. Laryngoscope. 2010;120:2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 22.Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia. 2014;29:2–16. doi: 10.1007/s00455-013-9494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones CA, Ciucci MR, Hammer MJ, McCulloch TM. A multisensor approach to improve manometric analysis of the upper esophageal sphincter. Laryngoscope. 2016;126:657–664. doi: 10.1002/lary.25506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng Z, Hoffman MR, Jones CA, McCulloch TM, Jiang JJ. Three-dimensional analysis of pharyngeal high-€ •resolution manometry data. The Laryngoscope. 2013;123:1746–1753. doi: 10.1002/lary.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JS, Park D, Kang JY. Application and Interpretation of High-resolution Manometry for Pharyngeal Dysphagia. J Neurogastroenterol Motil. 2015:283–287. doi: 10.5056/15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isberg A, Nilsson ME, Schiratzki H. The upper esophageal sphincter during normal deglutition. A simultaneous cineradiographic and manometric investigation. Acta Radiologica: Diagnosis (Stockholm) 1985;26:563–568. doi: 10.1177/028418518502600511. [DOI] [PubMed] [Google Scholar]
- 27.Sokol EM, Heitmann P, Wolf BS, Cohen BR. Simultaneous cineradiographic and manometric study of the pharynx, hypopharynx, and cervical esophagus. Gastroenterology. 1966;51:960–974. [PubMed] [Google Scholar]
- 28.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 29.Omari TI, Jones CAMC-S, Hammer MJ, Cock C, Dinning PG, Wiklendt L, Costa MC, McCulloch TM. Predicting the Activation States of the Muscles Governing Upper Esophageal Sphincter Relaxation and Opening. Am J Physiol Gastrointest Liver Physiol. 2016 doi: 10.1152/ajpgi.00388.2015. ajpgi.00388.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwiatek MA, Mirza F, Kahrilas PJ, Pandolfino JE. Hyperdynamic Upper Esophageal Sphincter Pressure: A Manometric Observation in Patients Reporting Globus Sensation. The American Journal of Gastroenterology. 2009;104:289–298. doi: 10.1038/ajg.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook IJ, Dent J, Shannon S, Collins SM. Measurement of upper esophageal sphincter pressure. Effect of acute emotional stress. Gastroenterology. 1987;93:526–532. doi: 10.1016/0016-5085(87)90915-2. [DOI] [PubMed] [Google Scholar]
- 32.Mason RJ, Bremner CG, DeMeester TR, Crookes PF, Peters JH, Hagen JA, DeMeester SR. Pharyngeal swallowing disorders: selection for and outcome after myotomy. Ann Surg. 1998;228:598–608. doi: 10.1097/00000658-199810000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouderoux P, Kahrilas PJ. Function of upper esophageal sphincter during swallowing: the grabbing effect. Am J Physiol. 1997;272:G1057–1063. doi: 10.1152/ajpgi.1997.272.5.G1057. [DOI] [PubMed] [Google Scholar]


