Abstract
Objective
Understanding genetic polymorphisms might facilitate the analysis of differences between individuals in their susceptibility to developing cancers as a result of environmental carcinogens. Skin, lung, colon and bladder cancers emerge from biological defects in GSTM1, GSTT1 and GSTP1 gene expressions. In this study, we aimed to investigate whether there was an association between CYP1A1 and GSTP1 gene polymorphisms and bladder cancer in a Turkish population.
Material and methods
Blood samples were collected from 120 individuals (60 patients with bladder cancer and 60 healthy individuals), and their DNAs were isolated. A polymerase chain reaction-restriction fragment length polymorphism (PCR - RFLP) method was used to detect the frequencies of CYP1A1 NM_000499.3: c.*1189T > C and GSTP1 NM_000852.3: c.313A > G polymorphisms in bladder cancer patients.
Results
The frequency of the CYP1A1: c.*1189 TC genotype and C allele were significantly different between bladder cancer patients and healthy individuals (p=0.001 and p=0.005, respectively). However, there was no significant difference for the GSTP1: c.313 AG genotype or G allele between both study groups (p=0.699 and p=0.360, respectively).
Conclusion
A polymorphic site of the CYP1A1 gene might be involved in the development of bladder cancer. However, the investigated GSTP1 polymorphic site did not represent an important risk factor for the development of bladder cancer in a Turkish population.
Keywords: Bladder cancer, cytochrome, gene, glutathione, polymorphism
Introduction
Bladder cancer is the 11th most commonly diagnosed cancer in the world, and the age-standardized incidence rate (per 100,000 person-years) is 8.9 for men and 2.2 for women.[1] The mutual interaction of various genetic and carcinogenic factors is thought to be an important determinant. Genetic polymorphisms causing changes in enzyme activities leading to biotransformation were reported to have a major role in the development and progression of cancer.[2] Endogenous and exogenous xenobiotics are activated and deactivated in two metabolic steps by phase I and phase II enzymes. Human P450 enzymes are oxidative enzymes with a role in the activation and deactivation of anticancer agents and the activation of various precarcinogen-metabolic conversions.
Smoking is the most important factor assumed to cause bladder cancer. The relationship between bladder cancer and smoking has been revealed by many epidemiological studies.[3,4] Smoking is the main risk factor for bladder cancer for both men and women and it is responsible for 1/3 of bladder cancers in men and for 1/4 in women. Cigarettes have over 60 carcinogen compounds, including polycyclic aromatic hydrocarbons (PAH), such as benzopyrene, and aromatic amines, such as 2 - naphthylamine and 4 - aminodiphenyl.[5] The emergence of hazardous effects of these metabolic compounds depends on metabolic activation.[6] In particular, the carcinogenic effects of PAHs emerge from the stimulation of the metabolic activation that occurs as a result of the epoxidation and hydrolysis of these agents.[5] The metabolic activation of PAHs and aromatic amines is followed by the binding of their metabolites to the DNA structure, which elicits the main tumorigenic effects. Enzymes, generally within the cytochrome P450 (CYP450) family, initiate the metabolic effects of PAHs. In this first step, xenobiotics are transformed into hydrophilic and disposable derivations. In particular, CYP450 enzymes work as catalysts in the oxidation of many endogenous and exogenous compounds. Although most of these metabolites transform into detoxified forms, some gain electrophilic characteristics and interact with DNA.[7] The human CYP1A1 gene participates in the activation of tobacco and pro-carcinogens, including PAH and aromatic hydrocarbons, and it was reported as a potential genetic biomarker for some cancer types.[8] More than 11 alleles (gene pairs) that code amino acid changes have been identified for the CYP1A1 gene.[9] The effects of CYP1A1 gene polymorphisms on the risk of bladder cancer remain controversial in different populations. A meta-analysis revealed that the c.*1189C > T polymorphism is not associated with bladder cancer risk in Chinese, Turkish, and French populations.[10] However, several studies have indicated that the CYP1A1 gene might be strongly correlated with an increased risk of bladder cancer in populations of China and North India.[11,12]
Phase II enzymes (epoxide hydrolase, glutathione S-transferase, sulfotransferase, glucuronosyltransferase) conjugate with glutathione and glucuronides to produce excretable hydrophilic products, which results in the detoxification of primary metabolites. In total, the balance between these activation and deactivation systems determines the biologically active toxic dose and reveals the disease risk.[13] Deletions occurring in either the GSTM1 or GSTT1 genes cause the total loss of enzyme activity. Any amino acid change in the GSTP1 gene causes a change in the activation of this enzyme.[14] Skin, lung, colon and bladder cancers emerge from the biological outcomes of the defects occurring in GSTM1, GSTT1 and GSTP1 gene expressions.[15,16]
Some individuals have increased cancer risk depending upon DNA damage. Understanding genetic polymorphisms might facilitate the analysis of differences between individuals in terms of susceptibility to developing cancers as a result of environmental carcinogens. Obtaining information about gene pair variants in the metabolism of xenobiotics, DNA damage and events causing mutations may help in the diagnosis and treatment of bladder cancer. Many studies have shown a relationship between biometabolism and bladder cancer.[14,17] This case-control study examined genetic polymorphisms in the CYP1A1 and GSTP1 genes and their relationship with bladder cancer.
Material and methods
Patients
The study included 60 patients with bladder cancer and 60 healthy individuals. Patients with bladder cancer were diagnosed as having transitional cell carcinoma by histopathological examination. Blood samples were collected from 60 patients after bladder cancer diagnosis. Also blood samples from 60 healthy humans were taken and analyzed as the control group. All patients in the study group were informed about the study after taking their detailed medical history. All patients gave written informed consent before being recruited. The study was approved by the ethics committee of the Medical Faculty of Harran University (Şanlıurfa, Turkey). Exclusion criterion was receival of any form of radiotherapy or chemotherapy for the treatment of cancer.
DNA extraction
In this study, blood samples were collected in both groups and DNA was extracted according to the salting out technique from the bloods with EDTA. In the PCR, 30 ng DNA was used for each patient.[18]
Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) Technique
The CYP1A1 (NM_000499.3; GI: 1543):c.*1189T > C (rs4646903, in 3′-untranslated region (3′-UTR))and GSTP1 (NM_000852.3; GI: 2950):c.313A > G (rs1695, in exon 5) polymorphic sites were investigated by the PCR - RFLP technique. Four primers (forward and reverse) were used to determine the genotype and allele status of both genes (Table 1). With the selected primers, the DNA was amplified in a 10 - μl reaction volume containing 1xPCR buffer, 2 mM MgCl2, 0.2 μM primers (BioBasic Inc, Ontario, Canada), 200 μM each deoxynucleotide triphosphate (dNTPs, Fermentas, St. Leon-Rot, Germany), 30 ng of genomic DNA, and 0.5 U of Taq DNA polymerase (Fermentas). For theCYP1A1: c.*1189T > C polymorphic site, the PCR program was applied at 94°C for 3 min for the initial denaturation, then 35 cycles with 94°C for 45 sec, 59°C for 45 sec, and 72°C for 45 sec, and finally 72°C for 5 min for the final extension. For the GSTP1: c.313A > G polymorphism, the PCR program was the same but with an annealing temperature of 60°C.
Table 1.
Primer sequences selected on CYP1A1 ve GSTP1 genes
| Primers | Primer sequences | PCR products (bp) |
|---|---|---|
| CYP1A1: c.*1189T > C | 5′-CAGTGAAGAGGTGTAGCCGC-3′ 5′-TAGGAGTCTTGTCTCATGCC-3′ |
342 |
| GSTP1: c.313A > G | 5′-ACCCCAGGGCTCTATGGGAA-3′ 5′-TGAGGGCACAAGAAGCCCCT-3′ |
176 |
Ten microliters of PCR product in a 30-μL volume for CYP1A1: c.*1189T > C and GSTP1: c.313A > G were separately digested with 1.5 Units of MspI (HpaII) and Alw26I (BsmAI) (Fermentas, St. Leon-Rot, Germany), respectively, at 37°C for 2 hours.
The digested PCR products were separated on a 2% agarose gel prepared with 10 mM lithium borate and analyzed using the Alpha Imager System (AlphaInnotech, San Leandro, California, USA). The digested CYP1A1: c.*1189 T allele yielded two fragments of 209 and 133 bp, and the C allele yielded a 342 bp fragment (Figure 1).
Figure 1.
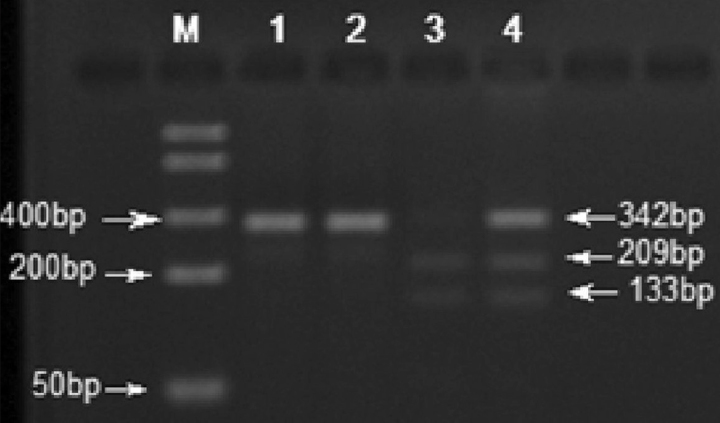
The restriction profile of CYP1A1: c.*1189T > C (MspI, HpaIIC > T). DNA Ladder (50–1500bp, Bio Basic Inc., Canada); lane 1 : undigested PCR product; lane 2: CC genotype (homozygous, polymorphic) ; lane 3 : TT genotype (homozygous, wild type); and lane 4 : TC genotype (heterozygous)
The GSTP1: c.313 A allele yielded a fragment of 176 bp, and the G allele yielded three fragments of 176, 93, and 83 bp (Figure 2).
Figure 2.
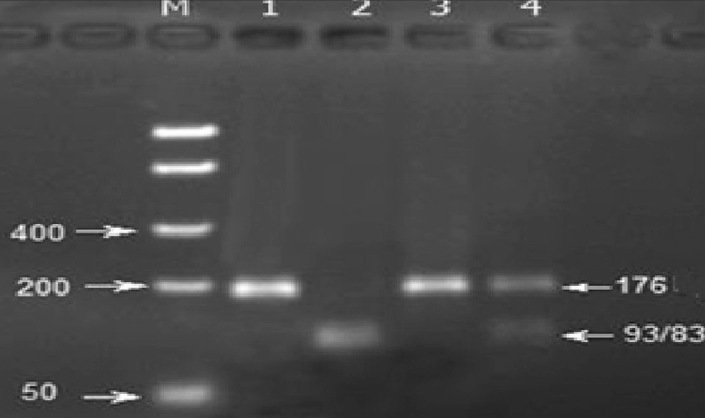
The restriction profile of the GSTP1: c.313A > G (Alw26I, BsmAI A>G). DNA Ladder (50–1500bp, Bio Basic Inc., Canada); lane 1: undigested PCR product; lane 2: AG genotype (heterozygous); lane 3: AA genotype (homozygous, wild type); and lane 4: GG genotype (homozygous, polymorphic) (the 93-and 83-bp fragments could not be separated from each other on the gel)
Statistical analysis
Student’s t - test and chi-square tests were used to determine differences in the means of the demographic and clinical profiles. The genotype and allele frequencies of CYP1A1: c.*1189T > C and GSTP1: c.313A > G were tested for Hardy-Weinberg Equilibrium using the chi-square test. The genotype and allele frequencies of these polymorphisms were analyzed with Fisher’s exact test using the Statistical Package for the Social Sciences 11.0 (SPSS In.; Chicago, IL, USA). Statistical significance was defined as p< 0.05.
Results
With regard to the patient characteristics, the number of patients; patient gender, age, and body mass index; and the number of patients with diabetes and hypertension were similar between the two groups. Only the number of smoking patients was significantly different (p<0.05). The baseline characteristics of the patients and controls are presented in Table 2.
Table 2.
Distribution of clinical and demographical parameters in patients with bladder cancer and healthy controls
| Patients with bladder cancer | Healthy control group | p | |
|---|---|---|---|
| Individuals (n) | 60 | 60 | |
| Gender (M/F) | 52/8 | 51/9 | 1.000 |
| Age (years) | 64.3±12.7 | 63.6±12.7 | 0.775 |
| BMI (kg/m2) | 25.8±3.9 | 26.7±4.6 | 0.260 |
| Diabetics | 6 | 16 | 0.32 |
| Smoking | 53 | 39 | 0.035 |
| Hypertension | 21 | 27 | 0.352 |
Average ± standard deviation, M: male; F: female
The distribution of the genotypes for the CYP1A1: c.*1189T > C and GSTP1: c.313A > G polymorphic sites were consistent with Hardy-Weinberg equilibrium in the bladder cancer and control groups (p>0.05). For CYP1A1: c.*1189, the TC heterozygous genotype and C allele frequencies in bladder cancer individuals were higher than those in healthy individuals (48.3% and 25.8% vs. 20.0% and 11.4% respectively, p<0.05). This difference was found to be significant. Furthermore, there was no significant association between bladder cancer patients and healthy controls for the GSTP1: c.313 GG genotype (5.0% vs. 8.3%) or G allele (20.8% vs. 25.8%) frequencies (Table 3).
Table 3.
SNP polymorphisms in patients with bladder cancer and control group
| SNPgenotype/allele | Bladder cancer patients (n=60) | Healthy control (n=60) | X2 | OR (95% CI) | p |
|---|---|---|---|---|---|
| CYP1A1: c.*1189T > C | |||||
| TT | 30 (50.0%) | 47 (78.3%) | 10.474 | Reference | Reference |
| TC | 29 (48.3%) | 12 (20.0%) | 10.707 | 3.742 (1.664 – 8.414 | 0.001 |
| CC | 1 (1.7%) | 1 (1.7%) | 0.000 | 1.000 (0.061–16.366) | 1.000 |
| T | 89 (74.2%) | 106 (88.3%) | 7.904 | Reference | Reference |
| C | 31 (25.8%) | 14 (11.4%) | 7.904 | 2.637 (1.321 – 5.264) | 0.005 |
|
| |||||
| GSTP1: c.313A > G | |||||
| AA | 38 (63.3%) | 34 (56.7%) | 0.556 | Reference | Reference |
| AG | 19 (31.7%) | 21 (35.0%) | 0.150 | 0.861 (0.403 – 1.840) | 0.699 |
| GG | 3 (5.0%) | 5 (8.3%) | 0.536 | 0.579 (0.132 – 2.540) | 0.464 |
| A | 95 (79.2%) | 89 (74.2%) | 0.839 | Reference | Reference |
| G | 25 (20.8%) | 31 (25.8%) | 0.839 | 0.756 (0.414 – 1.378) | 0.360 |
X2: Chi-square; OR: odds ratio; CI: confidence interval; SNP: single nucleotide polymorphism
Discussion
Genetic differences between detoxification systems in humans affect the extent to which environmental factors are involved in bladder cancer. Our study primarily examined the frequency of three genotypes of the CYP1A1 and GSTP1 genes in bladder cancer patient and control groups.
The relationship between occupational exposure to smoking and aryl amines has been well defined in terms of bladder cancer etiology.[19] Individual differences in the occurrence of cancer were defined by polymorphic variables in the bioactivation and detoxification of carcinogens.[20] For example, cigarette smoke contains many carcinogenic compounds, including PAH and aryl amines, and these are metabolized by the CYP450 and GST enzyme families. With regards to PAH, it is not known whether the changes in the catalytic activities enabling the oxidation of xenobiotics are caused by amino acid changes in the CYP450 enzymes.[9] Some studies have shown that CYP1A1 gene polymorphisms convey bladder cancer risk in some Asian populations.[11,12] Our study have demonstrated that the c.*1189 TC (heterozygote) genotype of the CYP1A1 gene was significantly higher in patients (48.3%) than in the controls (20%). Additionally, the c.*1189 C allele rates of the CYP1A1 gene were found to be significantly higher in patients (25.8%) than in the controls (11.4%). However, a meta-analysis has indicated that the CYP1A1 gene c.*1189C > T polymorphism is not associated with bladder cancer risk in Chinese, Turkish, or French populations.[10] The effects of CYP1A1 gene polymorphisms on the risk of bladder cancer remain controversial in different populations.
However, no relationship was observed between the genotype and allele rates of the c.313A > G polymorphism of the GSTP1 gene and bladder cancer in the present study.
Although a relationship between the GSTP1 Ile Val genotype and bladder cancer was observed in a previous study performed in an Iranian population, this relationship was not observed in German or Japanese populations.[21] Epidemiological studies have revealed differences in the distribution frequency of alleles and polymorphic alleles of different ethnic groups.[22] There are few studies examining the relationship between bladder cancer and gene polymorphisms in Turkey. Altaylı et al.[23] investigated the relationship between bladder cancer and CYP1A2, CYP2D6, GSTM1, GSTP1 and GSTT1 gene polymorphisms. Although no relationship was found between CYP1A2, CYP2D6, GSTM1, or GSTP1 gene polymorphisms and bladder cancer in this study, a statistical correlation was found with the GSTT1 gene and bladder cancer (OR=3.94, 95% CI=1.70–9.38, p<0.05). In addition, a significant relationship was identified between smoking and bladder cancer. In our patient group, even though the relationship between the CYP1A1 gene and bladder cancer was determined for the “TC” genotype, the “CC” genotype was not found to be significantly different as it was found in only one case. No statistically significant relationship was found for any variation in the GSTP1 gene (OR=0.86; 95% CI=0.40–1.84). Although our findings are in agreement with those of study performed by Grando et al.[24] in Brazil, they differ from those reported by Altaylı et al.[23]
There is no consensus in the literature on the extent to which variant gene forms are effective in the activation and detoxification of carcinogens or on the sources of differences within populations. Although Agundez et al.[25] reported a relationship between some cytochrome P450 enzyme polymorphisms and various cancer types, they did not clearly state the relationship with bladder cancer. In a case-control study, Brockmöller et al.[26] investigated some relevant gene polymorphisms in terms of their effects on drug metabolism and did not find any relationship between CYP1A1 gene polymorphisms and bladder cancer. Katoh et al.[27] found no relationship between CYP1A1 gene polymorphisms and bladder cancer in a Japanese sample. Johns and Houlston[28] suggested that the CYP1A1 polymorphism played a secondary role rather than creating a predisposition for cancer. Contrary to these findings, some studies have shown that the presence of aCYP1A1 allele in combination with GSTM1 null alleles is associated with an increase in cancer incidence.[28–30] In a study a relationship between aGSTM1 polymorphism, which causes gene mutations over TP53, and bladder cancer was indicated.[31] However, we did not investigate this gene polymorphism in our study.
Single nucleotide polymorphisms are important as they stress that drug metabolism is not activated on its own, even though many studies have found no significant relationship between these polymorphisms and bladder cancer. This result indicates that carcinogenesis requires the interaction of more than one gene for genetic predisposition in bladder cancer. Naccarati et al.[32] reported that different relationships with genetic polymorphisms caused differing individual sensitivities to genotoxicity and carcinogens. The present study investigated a potential relationship between different genotype variants (CYP1A1 and GSTP1) and bladder cancer.
Some recent studies have shown that CYP1A1 protein levels increase in exfoliative urothelial cells in smokers.[33] It was reported that CYP1A1-mediated materials are deposited in bladder tissues in smokers. Our study found a positive relationship between bladder cancer and the CYP1A1 C allele, and 28 patients (47%) in this group had a history of smoking. It is well known that bladder cancer is not only related to smoking but also it may be related to occupational exposure to carcinogens. In our study, no difference was found between the two gene groups according to gender in terms of the allele distribution (p>0.05).
Although some studies from other countries reported a relationship between GSTP1 polymorphisms and bladder cancer, a Turkish study by Altaylı et al.[23] found no relationship. The results of our study support this finding. Contrary to our findings, Cao et al.[30] demonstrated a relationship between GSTP1 II and IV gene variants and bladder cancer. Similar to the results of our study, some studies conducted in Turkey did not find any relationship between the GSTP1 gene polymorphism and laryngeal squamous cancers, stomach cancer or colon cancer.[34,35] According to the comparable results of these studies, we can infer that, in Turkish society, there is no relationship between GSTP1 gene polymorphisms and cancers.
Epidemiological studies show varying polymorphic allele distributions among different ethnic groups.[22] However, people in different origin commonly live in our city. Genetic variations associated with ethnicity may result in a non-homogenous distribution in the study and control groups and therefore different results may be obtained. A future study based on ethnic origins may address this issue.
One of the main deficiencies of our study is the small number of cases in our patient group. The “TC” (heterozygote) CYP1A1 genotype was more frequently seen in patients (48.3%) than in healthy controls (20%). As the “CC” (homozygote) CYP1A1 genotype was found only in one patient, it was not included in our study. A larger study cohort may help to reveal any potential relationship between the frequency of the “CC” homozygote CYP1A1 genotype and bladder cancer.
In conclusion, the results of our study suggest that the bladder cancer risk is high in individuals with the “TC” allele of the CYP1A1 gene, and predisposition to bladder cancer can be pre-determined in these individuals. TheGSTP1 c.313A > G gene polymorphic site did not represent an important risk factor for the development of bladder cancer in a Turkish population. However, larger case series studies are required to generate further evidence for this assumption.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Harran University School of Medicine (Project No: 1044).
Informed Consent: Written informed consent was obtained from all patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.S., A.A.; Design - A.A., E.Y.; Supervision - E.Y., M.S.; Resources - K.G.; Materials - A.A., F.D.; Data Collection and/or Processing - F.D., M.M.U.; Analysis and/or Interpretation - A.A., F.D., M.S.; Literature Search - A.A., İ.K., D.A.; Writing Manuscript - A.A., M.S.; Critical Review - E.Y., M.S., A.A.; Other - K.G., İ.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Babjuk M, Böhle A, Burger M, Compérat E, Kaasinen E, Palou J, et al. Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1and CIS) 2015. Available from: www.uroweb.org.
- 2.Franekova M, Halasova E, Bukovska E, Luptak J, Dobrota D. Gene polymorphisms in bladder cancer. Urol Oncol. 2008;26:1–8. doi: 10.1016/j.urolonc.2006.10.011. https://doi.org/10.1016/j.urolonc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Johansson SL, Cohen SM. Epidemiology and etiology of bladder cancer. Semin Surg Oncol. 1997;13:291–8. doi: 10.1002/(sici)1098-2388(199709/10)13:5<291::aid-ssu2>3.0.co;2-8. https://doi.org/10.1002/(SICI)1098-2388(199709/10)13:5<291::AID-SSU2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630–9. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. https://doi.org/10.1002/1097-0142(20000801)89:3<630::AID-CNCR19>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Luch A. Nature and nurture - lessons from chemical carcinogenesis. Nat Rev Cancer. 2005;5:113–25. doi: 10.1038/nrc1546. https://doi.org/10.1038/nrc1546. [DOI] [PubMed] [Google Scholar]
- 6.Yu MC, Skipper PL, Tannenbaum SR, Chan KK, Ross RK. Arylamine exposures and bladder cancer risk. Mutat Res. 2002;506–507:21–8. doi: 10.1016/s0027-5107(02)00148-3. https://doi.org/10.1016/S0027-5107(02)00148-3. [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. https://doi.org/10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Lu AYH. CYP1A induction and human risk assessment: anevolving tale of in vitro and in vivo studies. Drug Metab DisposBiol Fate Chem. 2007;35:1009–16. doi: 10.1124/dmd.107.015826. https://doi.org/10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- 9.Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–76. doi: 10.2133/dmpk.21.257. https://doi.org/10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Zhang XL, Xie L, Li TJ, He Y, Peng QL, et al. Lack of association between CYP1A1 polymorphisms and risk of bladder cancer: ameta-analysis. Asian Pac J Cancer Prev. 2014;15:4071–7. doi: 10.7314/apjcp.2014.15.9.4071. https://doi.org/10.7314/APJCP.2014.15.9.4071. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Kong CZ, Zhang Z, Yang CM, Li J. Relationships between CYP1A1 genetic polymorphisms and bladder cancer risk: ameta-analysis. DNA Cell Biol. 2014;33:171–81. doi: 10.1089/dna.2013.2298. https://doi.org/10.1089/dna.2013.2273. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava DS, Mandhani A, Mittal RD. Genetic polymorphisms of cytochrome P450 CYP1A1 (*2A) and microsomal epoxide hydrolase gene, interactions with tobacco-users, and susceptibility to bladder cancer: a study from North India. Arch Toxicol. 2008;82:633–9. doi: 10.1007/s00204-007-0276-4. https://doi.org/10.1007/s00204-007-0276-4. [DOI] [PubMed] [Google Scholar]
- 13.Thier R, Brüning T, Roos PH, Rihs HP, Golka K, Ko Y, et al. Markers of genetic susceptibility in human environmental hygiene and toxicology: the role of selected CYP, NAT and GST genes. Int J Hyg Environ Health. 2003;206:149–71. doi: 10.1078/1438-4639-00209. https://doi.org/10.1078/1438-4639-00209. [DOI] [PubMed] [Google Scholar]
- 14.Harries LW, Stubbins MJ, Forman D, Howard GC, Wolf CR. Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis. 1997;18:641–4. doi: 10.1093/carcin/18.4.641. https://doi.org/10.1093/carcin/18.4.641. [DOI] [PubMed] [Google Scholar]
- 15.Fryer AA, Ramsay HM, Lovatt TJ, Jones PW, Hawley CM, Nicol DL, et al. Polymorphisms in glutathione S-transferases and non-melanoma skin cancer risk in Australian renal transplant recipients. Carcinogenesis. 2005;26:185–91. doi: 10.1093/carcin/bgh291. https://doi.org/10.1093/carcin/bgh291. [DOI] [PubMed] [Google Scholar]
- 16.Gonlugur U, Pinarbasi H, Gonlugur TE, Silig Y. The association between polymorphisms in glutathione S-transferase (GSTM1 andGSTT1) and lung cancer outcome. Cancer Invest. 2006;24:497–501. doi: 10.1080/07357900600814813. https://doi.org/10.1080/07357900600814813. [DOI] [PubMed] [Google Scholar]
- 17.Saad AA, O’Connor PJ, Mostafa MH, Metwalli NE, Cooper DP, Povey AC, et al. Glutathione S-transferase M1, T1 and P1 polymorphisms and bladder cancer risk in Egyptians. Int J Biol Markers. 2005;20:69–72. doi: 10.5301/jbm.2008.829. https://doi.org/10.5301/JBM.2008.829. [DOI] [PubMed] [Google Scholar]
- 18.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. https://doi.org/10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Rahman SZ, el-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1genes in population studies. Cancer Lett. 1996;107:229–33. doi: 10.1016/0304-3835(96)04832-x. https://doi.org/10.1016/0304-3835(96)04832-X. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava DSL, Mishra DK, Mandhani A, Mittal B, Kumar A, Mittal RD. Association of genetic polymorphism of glutathione S-transferase M1, T1, P1 and susceptibility to bladder cancer. Eur Urol. 2005;48:339–44. doi: 10.1016/j.eururo.2005.02.007. https://doi.org/10.1016/j.eururo.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Association of genetic polymorphism of glutathione S transferase(GSTM1, GSTT1, GSTP1) with bladder cancer susceptibility. Urol Oncol. 2013;31:1193–203. doi: 10.1016/j.urolonc.2011.11.027. https://doi.org/10.1016/j.urolonc.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35:361–90. doi: 10.2165/00003088-199835050-00003. https://doi.org/10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Altayli E, Gunes S, Yilmaz AF, Goktas S, Bek Y. CYP1A2, CYP2D6, GSTM1, GSTP1, and GSTT1 gene polymorphisms in patients with bladder cancer in a Turkish population. Int Urol Nephrol. 2009;41:259–66. doi: 10.1007/s11255-008-9444-6. https://doi.org/10.1007/s11255-008-9444-6. [DOI] [PubMed] [Google Scholar]
- 24.Grando JPS, Kuasne H, Losi-Guembarovski R, Sant’ana Rodrigues I, Matsuda HM, et al. Association between polymorphisms in the biometabolism genes CYP1A1, GSTM1, GSTT1 and GSTP1 in bladder cancer. Clin Exp Med. 2009;9:21–8. doi: 10.1007/s10238-008-0015-z. https://doi.org/10.1007/s10238-008-0015-z. [DOI] [PubMed] [Google Scholar]
- 25.Agundez JAG. Cytochrome P450 gene polymorphism and cancer. Curr Drug Metab. 2004;5:211–24. doi: 10.2174/1389200043335621. https://doi.org/10.2174/1389200043335621. [DOI] [PubMed] [Google Scholar]
- 26.Brockmöller J, Cascorbi I, Kerb R, Roots I. Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res. 1996;56:3915–25. [PubMed] [Google Scholar]
- 27.Katoh T, Inatomi H, Nagaoka A, Sugita A. Cytochrome P4501A1gene polymorphism and homozygous deletion of the glutathione S-transferase M1 gene in urothelial cancer patients. Carcinogenesis. 1995;16:655–7. doi: 10.1093/carcin/16.3.655. https://doi.org/10.1093/carcin/16.3.655. [DOI] [PubMed] [Google Scholar]
- 28.Johns LE, Houlston RS. Glutathione S-transferase mu1 (GSTM1)status and bladder cancer risk: a meta-analysis. Mutagenesis. 2000;15:399–404. doi: 10.1093/mutage/15.5.399. https://doi.org/10.1093/mutage/15.5.399. [DOI] [PubMed] [Google Scholar]
- 29.Nair U, Bartsch H. Metabolic polymorphisms as susceptibility markers for lung and oral cavity cancer. Iarc Sci Publ. 2001;154:271–90. [PubMed] [Google Scholar]
- 30.Qui-ones L, Lucas D, Godoy J, Cáceres D, Berthou F, Varela N, et al. CYP1A1, CYP2E1 and GSTM1 genetic polymorphisms. The effect of single and combined genotypes on lung cancer susceptibility in Chilean people. Cancer Lett. 2001;174:35–44. doi: 10.1016/s0304-3835(01)00686-3. https://doi.org/10.1016/S0304-3835(01)00686-3. [DOI] [PubMed] [Google Scholar]
- 31.Salagovic J, Kalina I, Stubna J, Habalová V, Hrivnák M, Valanský L, et al. Genetic polymorphism of glutathione S-transferases M1and T1 as a risk factor in lung and bladder cancers. Neoplasma. 1998;45:312–7. [PubMed] [Google Scholar]
- 32.Naccarati A, Soucek P, Stetina R, Haufroid V, Kumar R, Vodickova L, et al. Genetic polymorphisms and possible gene-gene interactions in metabolic and DNA repair genes: effects on DNA damage. Mutat Res. 2006;593:22–31. doi: 10.1016/j.mrfmmm.2005.06.016. https://doi.org/10.1016/j.mrfmmm.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Cao W, Cai L, Rao JY, Pantuck A, Lu ML, Dalbagni G, et al. Tobacco smoking, GSTP1 polymorphism, and bladder carcinoma. Cancer. 2005;104:2400–8. doi: 10.1002/cncr.21446. https://doi.org/10.1002/cncr.21446. [DOI] [PubMed] [Google Scholar]
- 34.Ateş NA, Unal M, Tamer L, Derici E, Karakaş S, Ercan B, et al. Glutathione S-transferase gene polymorphisms in presbycusis. Otol Neurotol. 2005;26:392–7. doi: 10.1097/01.mao.0000169774.23668.f1. https://doi.org/10.1097/01.mao.0000169774.23668.f1. [DOI] [PubMed] [Google Scholar]
- 35.Unal M, Tamer L, Ateş NA, Akbaş Y, Pata YS, Vayisoğlu Y, et al. Glutathione S-transferase M1, T1, and P1 gene polymorphism in laryngeal squamous cell carcinoma. Am J Otolaryngol. 2004;25:318–22. doi: 10.1016/j.amjoto.2004.04.003. https://doi.org/10.1016/j.amjoto.2004.04.003. [DOI] [PubMed] [Google Scholar]


