Abstract
A vesicocutaneous fistula (VCF) is a tract that is formed abnormally between the bladder and the external surface of the body. VCF results in a great deal of inconvenience, discomfort, and physical disability for the affected patient. This condition can be caused by extensive trauma with pelvic bone fracture, radical pelvic surgery, irradiation of pelvic malignancies, hip arthroplasty, a large bladder calculus, and various other pathologies. The management of VCF should be approached on a case-by-case basis because of the complexity of the disease. In this report, we present a case of VCF that was managed by using vacuum-assisted closure therapy. A 72-year-old female was diagnosed with VCF as a late complication after radiotherapy for cervical cancer. After radiotherapy, she had lower urinary tract symptoms and was diagnosed as a neurogenic bladder. She started to perform clean intermittent catheterization (CIC). She was subsequently diagnosed as chronic kidney disease stage 5 due to hypertensive nephrosclerosis, and started to receive hemodialysis. Recently, she avoided CIC because of decreased urine output. Despite urinary diversion and surgical debridement, the surgical wound had not healed after several days. After vacuum assisted closure therapy, the surgical wound healed and filled with granulation tissue. This case shows that vacuum-assisted closure therapy is efficient for complicated wound healing of a VCF after radiotherapy.
Keywords: Complication, cutaneous, fistula, radiation, urinary bladder, cervical, neoplasm
Introduction
Vesicocutaneous fistula (VCF) is a tract that is formed abnormally between the bladder and the external surface of the body, and results in urine leakage from the bladder on to the body surface. VCF results in a great deal of inconvenience, discomfort, and physical disability for the affected patient. This condition can be caused by extensive trauma with pelvic bone fracture, radical pelvic surgery, irradiation of pelvic malignancies, hip arthroplasty, a large bladder calculus, and various other pathologies.[1–3] VCF should be managed based on medical condition of each paitents because of the complexity of the disease.
We report a case of VCF that developed as a late complication of radiotherapy for cervical cancer. This case is notable for management using vacuum-assisted closure therapy (VAC).
Case presentation
A 72-year-old female presented to our emergency department with complaints of suppurative discharge in the suprapubic area, lower abdominal pain, and lower urinary tract symptoms with intermittent dysuria, frequency, and gross hematuria persisting for a month. Her medical history was significant for total hysterectomy and adjuvant radiotherapy of the pelvic area for cervical cancer 13 years ago, and right side laparoscopic nephrectomy for the management of a non-functioning kidney 11 years ago. After radiotherapy, she had lower urinary tract symptoms and was diagnosed with a neurogenic bladder as a complication of radiation therapy at other medical center 12 years ago. Although she started to perform clean intermittent catheterization (CIC) and receive medical treatment due to incomplete voiding and large amount of post-voided residual urine, her lower urinary tract symptoms had not improved for a long period of time. She was subsequently diagnosed with chronic kidney disease stage 5 due to hypertensive nephrosclerosis, and started to receive hemodialysis. Recently, she avoided CIC because of decreased urine output and aversion to CIC.
On presentation to our medical center, lower abdominal pain and tenderness, skin redness, and a purulent discharge in the suprapubic area were observed on physical examination. Non-enhanced computed tomography (CT) showed a prominent inflammatory lesion between the anterior perivesical area and suprapubic area (Figure 1a). We performed cystography which demonstrated urine leakage from the anterior wall of the bladder, and vesicoureteral reflux was not found (Figure 1b). According to her medical history and image findings, we diagnosed the VCF which was caused by chronic inflammation based on radiation injury. We decided on surgical correction of VCF. Under general anesthesia, the patient was placed on the operating table in supine position. After a midline infraumbilical incision was made, we identified purulent discharge and urine spurting from the incision line, and anterior wall of the bladder was communicating with an opening of suprapubic skin (Figure 1c). Extensive surgical debridement was performed until healthy tissue was reached. However, primary closure of bladder wall was impossible due to chronic inflammatory change of surrounding tissue and tissue adhesion caused by previous radiotherapy targeted to the pelvic cavity. After massive saline irrigation, Potadine® (polyvinylpyrrolidone iodine) wet dressing was applied to the open wound. Finally, she was diagnosed with extraperitoneal rupture of the anterior bladder wall and VCF. During postoperative care, Potadine wet to dry dressings were applied three times a day to the open wound site. While dressing was changed frequently, the wound was constantly oozing due leakage of urine. Because of improper wound healing, we decided to perform urinary diversion using a percutaneous nephrostomy (PCN). Despite urinary diversion and surgical debridement, the wound did not heal over the next several days. Therefore, we decided to try VAC therapy for the open wound. Under spinal anesthesia, the VAC GranuFoam (Kinetic Concepts, Inc. USA) was applied to the suprapubic area after surgical debridement. Suction pressure of 75–125 mmHg was applied to the wounds continuously. Additional wound debridement and VAC were performed once a week. After application of VAC, the wound was healed and filled with granulation tissue (Figure 2). After the wounds were clinically healed, we consulted the department of plastic surgery for a bilateral advancement flap. The flap took well and the patient attained disease-free status (Figure 3). After wound healing, we discussed with her about further evaluations including pressure flow study or voiding cystourethrogram. However, she did not want further evaluations because of much discomfort and fear. Therefore, we recommended continuous CIC and medication including anticholinergics. Follow-up CT showed no evidence of inflammatory change or fistula; the patient still continues to perform CIC and is taking anticholinergics (Figure 4).
Figure 1. a–c.
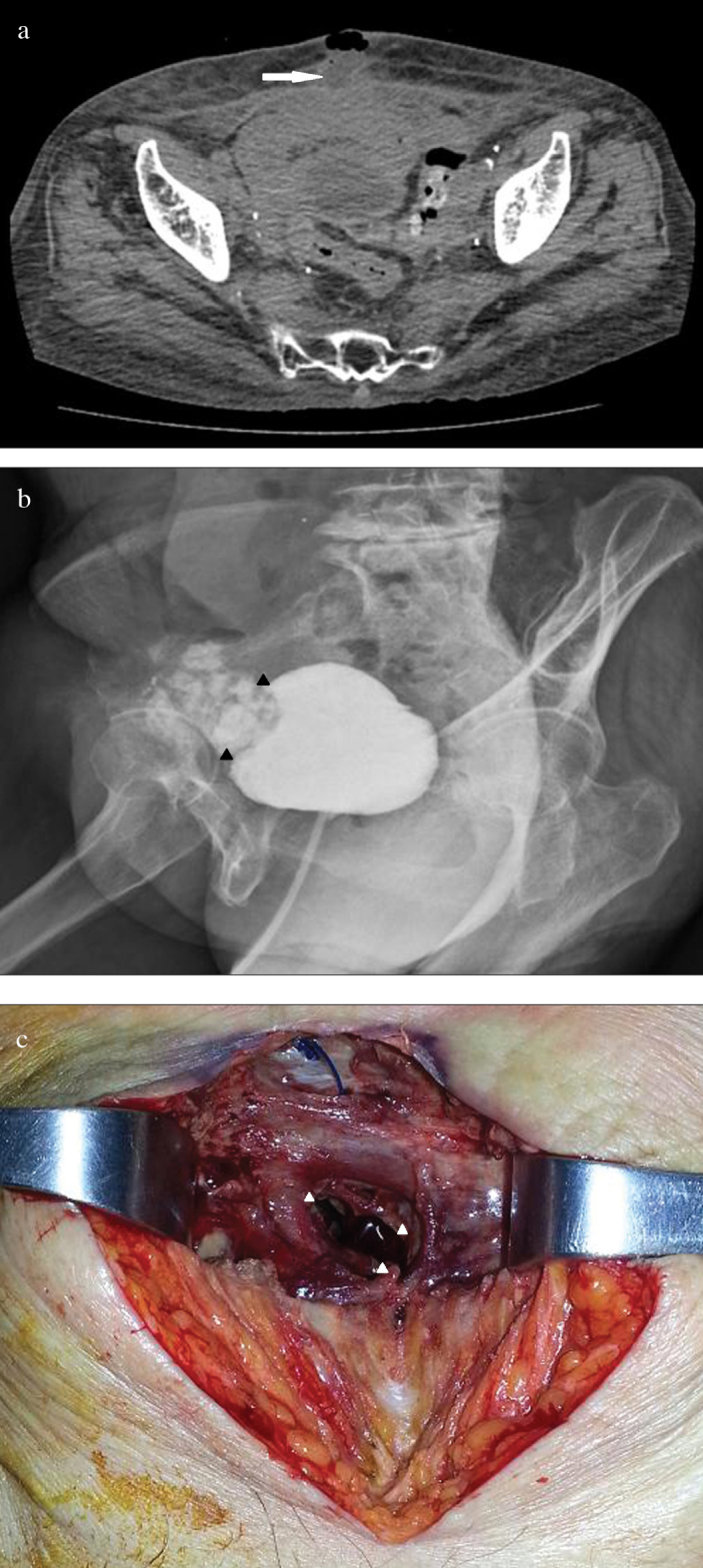
(a) Computed axial tomography of the abdomen showing the tract between the anterior wall of the bladder and suprapubic area. (b) Cystogram showing leakage at the anterior bladder wall. (c) Gross appearance of suprapubic area at surgery. An opening of suprapubic skin connected to the anterior wall of the bladder
Figure 2.
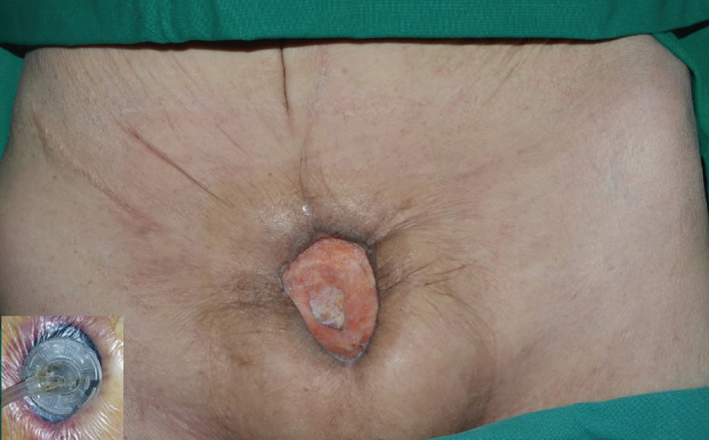
VAC applied to open wound after surgical debridement. Gross appearance of the wound, which was healed and filled in with granulation
VAC: vacuum-assisted closure therapy
Figure 3.
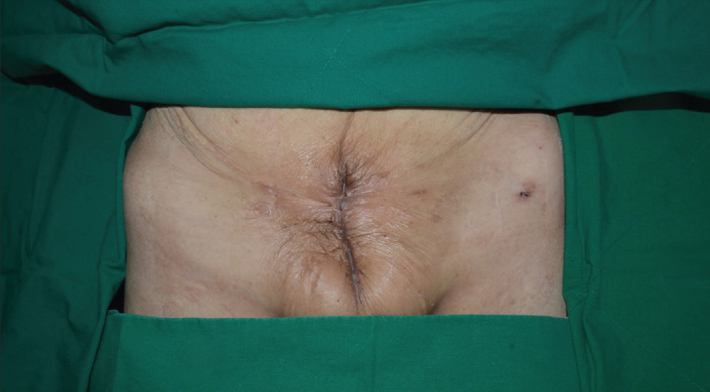
Gross appearance of the wound before discharge
Figure 4. a, b.
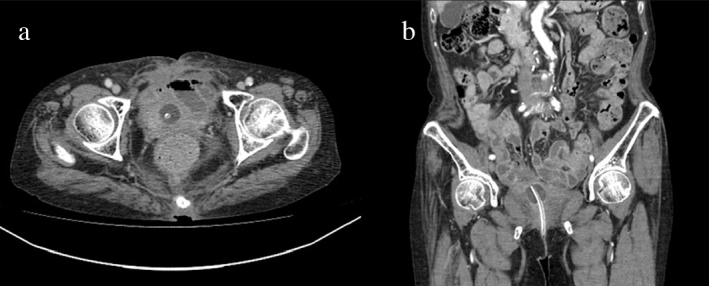
Follow up computed tomography at 1 month after external wound healing
Discussion
Vesicocutaneous fistula results in urine leakage from the bladder to the body surface. Most cases of VCF are caused by iatrogenic injury, extensive trauma, irradiation of malignancies, and various other etiologies. VCF can be remedied through proper diagnosis, supervision, inspection, and careful surgery. Although some types of VCF will heal with conservative management, surgery often assumes a role in definitive repair.
Delayed VCF formation following radiotherapy for cervical cancer was first reported in 1982.[1] Cervical cancer is a common malignancy of the female genital tract, and radiotherapy, chemotherapy, and surgical treatment are the modes of management.[4] The role of adjuvant radiotherapy directed at tumor sites is well established and forms part of the initial management plan. Despite the benefits, late-term toxicities are attributed to progressive microvascular injury with fibrosis causing ulceration, perforation, and fistulation.[5] Pieters et al.[6] reported that radiotherapy may harm the nerve, and it can result in neurogenic bladder. Additional adverse effects of previous radiotherapy are induration or late dermal necrosis.[7] In this case, we assumed that the patient had suffered from neurogenic bladder as a late complication of post-cervical cancer radiotherapy based on her medical history, and VCF was subsequently caused by chronic inflammation due to radiation injury.
If a patient with lower urinary tract symptoms, skin lesions, and/or discharge in the suprapubic area is suspected for VCF, a cystogram and cystoscopy are useful in making a diagnosis. In this case, urine leakage was observed at the initiation of cystogram. Although we could not measure the definite bladder volume, we guess her bladder volume had been less than 100 mL based on cystogram. As explained in the previous revision, only subjective evaluation is possible in this case. So, authors think it is better to leave this sentence intact. Other imaging studies such as computed tomography and magnetic resonance imaging can be conducted if the fistulous tract is a complicated lesion or suspicious for malignancy.
The factors to consider in deciding the surgical treatment method are as follows: the anatomical position of the fistula, the primary causes of the VCF, the activity level of the patient, and the presence of tumor. In the setting of radiation-induced VCF, a urinary diversion procedure such as PCN is the mainstay of management.[8] Tissue healing in a previously irradiated field will be severely compromised and surgery is likely to result in further morbidity.[1] In this case, we were able to suppress the persistent urinary leakage of VCF through urinary diversion using PCN. Although the urinary diversion was performed in this case, an additional procedure was required to promote granulation of the VCF. Therefore, we decided to apply negative pressure dressings using VAC. Negative pressure dressings were first described by Fleischmann et al. in 1993.[9] VAC is reportedly able to reduce wound volume where there has been tissue loss, and can promote granulation tissue formation and reduce wound surface area with edge contraction.[10] Moreover, it has been used to prepare wound beds for grafting or flap closure.[11] Recently, VAC is used for the management of complicated wound in urological field. Katsuragi et al.[12] reported a case with a huge VCF that was successfully treated with an ileal conduit, VAC and a rectus femoris musculocutaneous flap. They showed that the complicated wound was considerably reduced in size after application of VAC. In addition, Freeman et al.[13] reported a rare case of VCF in a patient with a history of radiation therapy and recent abdominal surgery. Similar to our case, they successfully treated VCF using urinary diversion via a nephrostomy tube and VAC. Recently, VAC also proved a beneficial outcome in an adolescent patient.[14]
In this case, further evaluations including urodynamic study or voiding cystourethrogram were important for the proper management. Unfortunately, we did not acquire the urodynamic results she had undergone at another medical center. In addition, we could not perform an urodynamic study after completing treatment of VCF because she did not want further evaluations. After wound healing, she just underwent CIC and anticholinergic medication.
Vesicocutaneous fistula is a potential complication in an unmanaged neurogenic bladder patient previously treated with radiotherapy. Tissue exposed to radiotherapy can be stiff and adhesive. In this case, primary bladder closure was not possible due to failure of approximation of wound edges. To facilitate wound healing, urinary flow was diverted and negative pressure dressings using VAC were applied. For decades, radiotherapy has been an accepted adjuvant therapy for cervical, prostate, and rectal cancers. Thus, the physician should be aware of VCF following radiotherapy performed for several types of cancer. In addition, urinary diversion and VAC are worth considering when wound healing is expected to be difficult.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – J.N.L.; Design – S.W.K., J.N.L.; Supervision – J.N.L., E.S.Y.; Resources – S.W.K., J.N.L.; Materials – S.W.K., H.T.K.; Data Collection and/or Processing – S.W.K., J.N.L.; Analysis and/or Interpretation – S.W.K., J.N.L.; Literature Search – S.W.K., H.T.K.; Writing Manuscript – S.W.K., J.N.L., E.S.Y.; Critical Review – J.N.L., H.T.K., E.S.Y.; Other – H.T.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lau KO, Cheng C. A case report--delayed vesicocutaneous fistula after radiation therapy for advanced vulvar cancer. Ann Acad Med Singapore. 1998;27:705–6. [PubMed] [Google Scholar]
- 2.Toufique H, Merani AJ. Vesicocutaneous fistula. J Pak Med Assoc. 2011;61:918–9. [PubMed] [Google Scholar]
- 3.Kotkin L, Koch MO. Morbidity associated with nonoperative management of extraperitoneal bladder injuries. J Trauma. 1995;38:895–8. doi: 10.1097/00005373-199506000-00012. https://doi.org/10.1097/00005373-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Turina M, Mulhall AM, Mahid SS, Yashar C, Galandiuk S. Frequency and surgical management of chronic complications related to pelvic radiation. Arch Surg. 2008;143:46–52. doi: 10.1001/archsurg.2007.7. https://doi.org/10.1001/archsurg.2007.7. [DOI] [PubMed] [Google Scholar]
- 5.Hennessey DB, Bolton E, Thomas AZ, Lynch TH. Vesicocutaneous fistula following adjuvant radiotherapy for prostate cancer. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-008986. pii: bcr2013008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pieters RS, Niemierko A, Fullerton BC, Munzenrider JE. Cauda equina tolerance to high-dose fractionated irradiation. Int J Radiat Oncol Biol Phys. 2006;64:251–7. doi: 10.1016/j.ijrobp.2005.04.019. https://doi.org/10.1016/j.ijrobp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3–11. doi: 10.2214/ajr.177.1.1770003. https://doi.org/10.2214/ajr.177.1.1770003. [DOI] [PubMed] [Google Scholar]
- 8.Lentz SS, Homesley HD. Radiation-induced vesicosacral fistula: treatment with continent urinary diversion. Gynecol Oncol. 1995;58:278–80. doi: 10.1006/gyno.1995.1227. https://doi.org/10.1006/gyno.1995.1227. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg. 1993;96:488–92. [PubMed] [Google Scholar]
- 10.Gupta S. The impact of evolving V.A.C (R) Therapy technology on outcomes in wound care. Prologue. Int Wound J. 2012;9(Suppl 1):iii–vii. doi: 10.1111/j.1742-481X.2012.00978.x. https://doi.org/10.1111/j.1742-481X.2012.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917–22. doi: 10.1097/01.prs.0000133168.57199.e1. https://doi.org/10.1097/01.PRS.0000133168.57199.E1. [DOI] [PubMed] [Google Scholar]
- 12.Katsuragi Y, Ueda K, Kajikawa A, Tateshita T, Okochi H. Repair of a huge vesicocutaneous fistula with the rectus femoris musculocutaneous flap and VAC. J Wound Care. 2010;19:157–9. doi: 10.12968/jowc.2010.19.4.157. https://doi.org/10.12968/jowc.2010.19.4.157. [DOI] [PubMed] [Google Scholar]
- 13.Freeman JJ, Storto DLP, Berry-Caban CS. Repair of a vesicocutaneous fistula using negative-pressure wound therapy and urinary diversion via a nephrostomy tube. J Wound Ostomy Cont. 2013;40:536–8. doi: 10.1097/WON.0b013e3182a46549. https://doi.org/10.1097/WON.0b013e3182a46549. [DOI] [PubMed] [Google Scholar]
- 14.Elizondo RA, Au JK, Gargollo PC, Tu DT. Vacuum-assisted closure of a vesicocutaneous fistula in a pediatric patient after bladder cystoplasty. Urology. 2016;95:190–1. doi: 10.1016/j.urology.2016.04.001. https://doi.org/10.1016/j.urology.2016.04.001. [DOI] [PubMed] [Google Scholar]


