Abstract
Multiparametric magnetic resonance imaging (mpMRI) has managed to change the paradigms on prostate cancer detection and risk classification. The most clear-cut indication of mpMRI in guidelines is the patients with a history of negative biopsy/increasing prostate-specific antigen (PSA), and presence of additional findings supporting its use in non biopsied patients and active surveillance. mpMRI complements standard clinical exam, PSA measurements, and systematic biopsy, and will miss some tumors that lack enough size or change in tissue density. Use of mpMRI is likely to increase, and further developments in the technique will be important for safe adoption of focal therapy concepts. Here we present a brief summary about mpMRI and its use in detection, risk classification and follow-up of prostate cancer.
Keywords: Focal therapy, fusion biopsy, multiparametric MRI, prostate cancer
The Need for Progress in Prostate Imaging and Diagnosis
According to 2016 cancer data, prostate cancer (PCa) was the most commonly diagnosed non-cutaneous cancer and and the second cause of death in men, and nearly 200.000 new cases were diagnosed in the United States.[1,2]
The diagnosis of PCa is determined by histopathology of the biopsy taken in case of clinical suspicion, raised prostate specific antigen (PSA) levels, and/or abnormal digital rectal examination (DRE). Many strategies for prostate biopsy have been described, and the gold standard remains to be the 10–12 core format under transrectal ultrasound (TRUS) guidance which was defined by Hodge et al.[3].
However, 12 core-TRUS biopsy has its challenges due to the limitations of prostate ultrasound resolution of early tumors, and 20–25% of frequently multifocal samples represent undersampling.[4,5] Samples made from standard TRUS biopsy with poor visualization of lesions, can cause underestimation of a high risk prostate cancer, overestimation of a clinically insignificant cancer or false risk stratification.[6,7] Therefore, repeat biopsies are often clinically indicated, with yields of 14–22% for the first repeat biopsy, and subsequent lowering of yields with >2 biopsies but rarely zero yield.[8] Therefore, many erroneous observations have been reported when comparing ultrasound biopsy findings to surgical pathology, such as: 1) missing adjacent dominant lesion in low grade disease, 2) final tumor grade accuracy in only 37% of the biopsized cases, and 3) dominant tumor localization in only 55% of the cases.[9] In some clinical situations, focal or subtotal tissue destruction is desired instead of full organ removal or radiation-a challenge in patient selection in that 72% of the cases with unilateral disease detected on TRUS biopsy have also contralateral disease.[9,10]
In aggregate, TRUS biopsy is a gold standard, but its technique and utilization creates many negative yield procedures, costs, and complications. Therefore, in the last 10 years, the technique, reported experience, and impact of magnetic resonance imaging (MRI) in prostate cancer has increased in the literature. The major advancements included both more powerful 3T magnets and multiparametric (mp) techniques.
Multiparametric Magnetic Resonance Imaging (mpMRI)
Although initial studies of MRI in prostate screening were performed at the beginning of the 1980’s with 0.35 T magnetized device,[11] clinical applications were limited to experimental work due to the low magnetic field values and poor image quality of the single sequence sections. Single sequence sections (T1W) did not clearly distinguish between the prostate and surrounding tissues, showed minimal intraprostatic tissue resolution, and further artifacts occurred from bowel motility. Renewed clinical interest occurred with increased magnet quality from 0.3T–0.5T to 1.5T and even up to 3T, with further incremental gains from endorectal coil (ERC) technique and multiphasic sequencing.[12–14]
Multiparametric MRI (mpMRI) can be briefly summarized as a method of trying to obtain an ideal three-dimensional (3D) prostate image by combining T2-weighted (T2WI), diffusion weighted (DWI), dynamic contrast enhanced (DCEI) and, if desired, MR spectroscopy (MRSI) images. Although many centers have developed their own MRI interpretation system using some of these sections, there is no standard defined combination yet.[15,16] Today, in many centers, intestinal motility-reducing drugs and endorectal coil (ERC) are used together with the 1.5T magnetized MRI device to prevent signal artifacts caused by intestinal peristaltisms [to increase signal-to-noise ratio (SNR)] which is already strongly recommended by the American College of Radiology (ACR) and the European Society of Urogynecologic Radiology (ESUR).[15,17,18] In 3T magnet devices, also the static magnetic field is stronger and the SNR is linearly higher than 1.5T and the ERC requirement is minimal, and its use is recommended.
Multiparametric MRI Sequences
T1- weighted and T2- weighted sequences
Both provide images according to the principle of calculating the water density in the tissue which are obtained in all MRIs. T1WI is especially used to identify post-biopsy hemorrhage in the prostate and seminal vesicles. It is also useful for detecting the status of lymph nodes and skeletal metastases, especially after administration of intravenous gadolinium-based contrast agent (GBCA).[18]
T2WI detects low signal intensity in the tumoral tissue. The high resolution shows a sharp demarcation line in the prostate capsule-especially where the fat content is present just outside of the anatomic pseudocapsule. For this reason, it is useful to use prostate zones, lesions within the prostate, seminal vesicle (SV) invasion, extraprostatic extension and evaluation of cancer staging. However, T2WI-sequences are not sufficient to detect transitional zone and central zone cancers.[16,18,19]
Diffusion-weighted sequences
The process is based on the proton diffusion property of water atoms, and reflects the random movement of water molecules. Thus, a functional image is provided. Presented images should include the apparent diffusion coefficient (ADC) map and high b-value images. Most or the clinically significant prostate cancers (CSPCa) appear hypointense in the ADC maps, as compared to normal tissue, due to restricted/impeded diffusion, i.e. “trapped” water molecule motion.
High b-value images protect the signal in cancer areas that have restricted/impeded diffusion compared to normal tissues.[18] Compared with ADC map high b-value images show high signal intensities, especially in CSPCa which are adjacent to or invasive the anterior fibromuscular stroma, in the subcapsular area and in the apex or base of the gland, but ADC map shows low signal intensity.[18] DWI shows better CZ and TZ tumors and cancer aggressiveness, but the resolution is poor and the image becomes impaired.[18]
Dynamic contrast-enhanced imaging
These are sequences that require GBCA. These sequences visualize tumor angiogenesis. DCEI evaluates the vascularity of tumor, before, during and after contrast agent injections.[20,21] The amount of contrast agent increases due to increase in the microvascular density in the capillary wall (wash-in), and depending on the increase in the distribution of degradation speed (wash-out).[12] Because of the detection of neovascularization in the tissue, it is particularly useful in locating recurrence after surgical treatment.[16,22] However, due to neovascularity, it may also give false positive results, especially in cases of inflammation.[23] In addition, the operation lasts longer.
MR spectroscopy imaging
MRSI is sometimes included in MP-MRI depending upon 3rd party payor rules. It may be useful for assessing the malignancy risk of a region of interest (ROI), but it is less useful in localization of maligancy, and predicting pathologic stage. The key observation is that malignant cells are more likely to be in rapid cellular turnover, and will therefore use more zinc, utilized and so reduced in intracellular spaces.[24]
This accelerates the oxidation. With increased oxidation, intracellular citrate levels are reduced. Cells with rapid turnover, such as cancer cells, have also higher level of choline. The higher the choline/citrate ratio related to the rapid turnover, the greater the aggressiveness of the cancer. MRSI calculates the choline/citrate (C/C) ratio and determines the degree of aggressiveness of the cancer dependent on the C/C ratio.[25–28] However, when 1.5T magnet devices are used, this process takes 13–20 min., and assessment is rather complex and requires additional training and expertise. For this reason, its use in routine urology practice is rather limited and it is usually used for research purposes.[29]
Prostate Imaging Reporting and Data System (PI-RADS™)
With the development of mpMRI devices, high resolution prostate images have begun to be obtained. However, no standard terminology is used in the evaluation of these images-a challenge for clinicians trying to translate non-structured narrative reports into clinical decisions. In 2012, ESUR aimed to establish a common terminology for evaluating prostate MRI. Thus they adapted the Breast Imaging Reporting and Data System (BI-RADS™) (which is used in breast MRI evaluation routinely) to the prostate imaging, and the first version of Prostate Imaging Reporting and Data System (PI-RADS™) was published.[15]
PI-RADS™ is briefly summarized as follows, the images in different sequences are obtained, the results obtained separately after first scoring are combined, giving MRI a score of 1–5 for the suspected lesion.[18] However, in this first version, some conceptual confusion and differences in the evaluation process persisted. For this reason, ESUR, ACR and AdMeTech Foundation established a Steering Committee for the development of the first version. As a result of the work, in 2015 sophisticated form of PI-RADS™v2 was announced.[18] PI-RADS™v2 divided the prostate and surrounding tissues into 39 sectors (36 sectors for prostate, 2 sectors for SV and 1 sector for external urethral sphincter) and redefined the scoring system.
The PI-RADS™v2 assessment system shown in Table 1 and its sectoral map is shown in Figure 1. With the sectoral map and new scoring that integrated standard use with recommendations of the guidelines, the prostatic lesions became more inferable with better localization in MRI reports. It has been shown that PI-RADS™v2 has higher sensitivity and specificity than the first version as confirmed with studies in the literature. PI-RADS™ 3 lesions were evaluated as negative or positive instead of scoring with the change in DCEI, and when positive, the lesion was upregulated to PI-RADS™ 4 lesion But still this lesion is mostly discussed and exposed to different approaches and continued to be called “equivocal” lesions.
Table 1.
PI-RADS ™v2 assesment categories. (The figure was adapted here with the permission of American College of Radiology[18])
| PIRADS1 | Very low (clinically significant cancer is highly unlikely to be present) |
| PIRADS2 | Low (clinically significant cancer is unlikely to be present) |
| PIRADS3 | Intermediate (the presence of clinically significant cancer is equivocal) |
| PIRADS4 | High (clinically significant cancer is likely to be present) |
| PIRADS5 | Very high (clinically significant cancer is highly likely to be present) |
Figure 1.
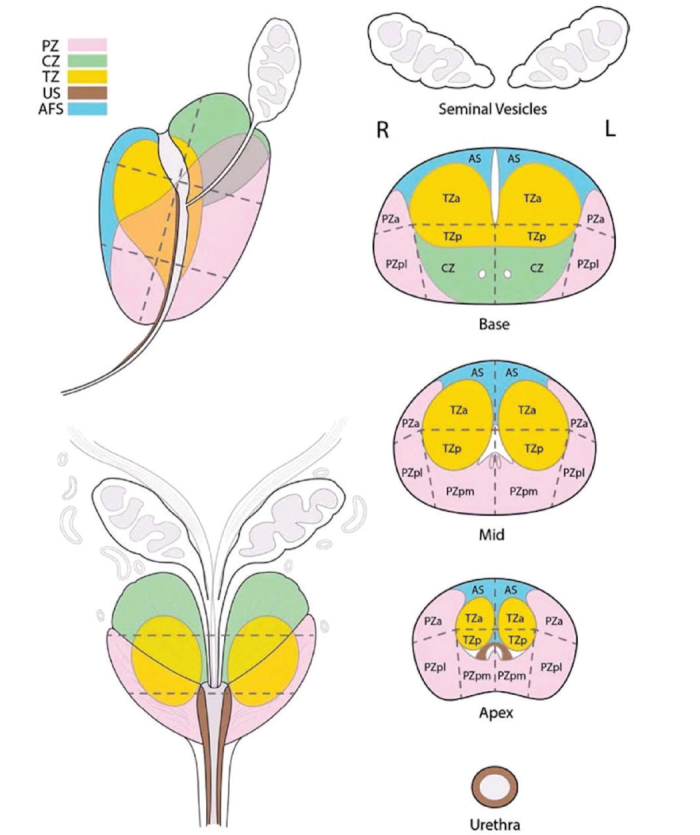
Sectoral Map of prostate, according to PI-RADS ™v2. (The figure was adapted here with the permission of American College of Radiology[18])
PZ: periferal zone; CZ: central zone; TZ: transitional zone; US: urethral stroma; AFS: anterior fascial stroma
mpMRI usage in prostate biopsy
With mpMRI imaging improved in both technique and reporting, the application to prostate biopsy was then suitable for renewed study. There are three different MRI-guided biopsy techniques.
Cognitive fusion biopsy
The easiest technique in fusion biopsies is the “cognitive fusion biopsy”, as it would not requiring any extra hardware or software. However, the practitioner must be highly experienced and able to combine mpMRI images with the real-time TRUS imaging. In some studies, it has been shown that the cancer detection rates of cognitive versus software fusion are not significantly different from each other, but both are better than the standard TRUS-guided biopsy.[30]
Image-guided targeted prostate biopsy (IGTpBx)
Pre-processed MRI images merge with real-time ultrasound (US) images using the appropriate device with the help of special software developed for this procedure. In fact, this is the process that the practitioner performs during cognitive fusion biopsy. A 3D image of the prostate is created on the monitor of the device and the location and shape of the suspicious, targeted lesion (region of interest-ROI) within the tissue is displayed. It also allows to save images. These techniques require the purchase of additional hardware and software, and at least 4 systems are commercially available. The United States Food and Drug Administration (FDA) approved four of the MRI/Ultrasound fusion biopsy devices. Their explanations are listed in Table 2.[31]
Table 2.
Explanation and comparison of MRI-ultrasound fusion biopsy devices
| Manufacturer/trade name | US image acquisition | Biopsy route | Tracking Mechanism | Year of FDA Approval | Comments |
|---|---|---|---|---|---|
| Philips/UroNav | Manual US sweep from base to apex | Transrectal | External magnetic field generator | 2005 | Prospective targeting, integrated with existing ultrasound device freehand manipulation |
| Eigen/Artemis | Manual rotation along fixed axis | Transrectal | Mechanical arm with encoders | 2008 | Prospective targeting, stabilized TRUS probe |
| Koelis/Urostation | Automatic US probe rotation | Transrectal | Real time TRUS-TRUS registration | 2010 | Retrospective targeting, real time elastic registration |
| Hitachi/HI-RVS (real time virtual sonography) | Real-time biplanar TRUS | Transrectal or transperineal | External magnetic field generator | 2010 | Prospective targeting, integrated with existing ultrasound device |
| BioJet/Jetsoft/Geoscan | Manual US sweep in sagittal | Transrectal or transperineal | Mechanical arm with encoders; uses stepper | 2012 | Prospective targeting, rigid registration |
US: ultrasound; TRUS: transrectal ultrasound
The figure was adapted from Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. Jan 2013;23(1):43–50; with the permission of Leonard S. Marks, M.D.
In-Bore biopsy
Technically the most challenging, and the most hardware-requiring method is In-Bore biopsy. The procedure is performed directly during real-time imaging performed with the MRI device. It is performed with the patient in the prone position and under absolute general anesthesia. The process time is considerably longer than other methods. The procedure is performed after confirming the position of the biopsy needle by real-time MRI, which is inserted according to previously recorded MRI images. An ERC should be used during the process. Because of the added procedure times, only the ROIs specified in the MRI are sampled, and systematic 12 core biopsies would be done another time. Complication rates of this biopsy are significantly lower than others due to limited and non-transrectal sampling. Available centers skilled in-Bore biopsy may be limited.
Use of mpMRI in biopsy naive or priorly negative biopsy patients
In patients with clinical suspicion whose initial prostate biopsies were negative, a repeat prostate biopsy has been advocated as an option, and might involve changes in the number of cores and strategies of prostate zone targeting, i.e. adding transition zone cores. However, repeat TRUS guided biopsies may still miss an apical or anterior lobe tumor.[32,33]
The technique of saturation biopsies have been reported by Barzell et al.[34] but its usefulness remains controversial. Due to increased number of cores (≥24), the percentage of insignificant cancer detection rate could increase as well as biopsy related pain, bleeding, and/or infectious complication rates.[35]
As with repeat and/or saturation biopsies, the early endpoint of studying mpMRI-based biopsies is overall and clinically significant (CSPCA-Gleason score >6) cancer detection rates. Nearly all of these studies have reported that the detection rates of MPI-based biopsies are better than the standard TRUS biopsies. Hoeks et al.[36] reported (from 438 patients who had at least one negative biopsy result) that PCa detected in 41% of 265 patients who undergone MRI guided prostate biopsies and that 87% of those PCa were CSPCa. Sonn et al.[37] reported that PCA was detected in 34% of 105 patients, whereas 72% of the detected PCa were CSPCa.
There have been a number of studies indicating that even lower insignificant but higher CSPCa cancer detection rates in patients biopsied as the first biopsy with mpMRI guided biopsy compared to standard TRUS guided biopsy alone.[38,39] After this new data has been evaluated, MRI-guided biopsies have been introduced into the guidelines in 2014. They are recommended after one prior negative biopsy by National Comprehensive Cancer Network (NCCN) American Urological Association (AUA) and European Association of Urology (EAU) guidelines.[40–42]
Use of mpMRI in biopsy positive patients
In select patients with prior positive biopsy results, active surveillance (AS) can be the initial strategy, such based on the Prostate Cancer Research International Active Surveillance (PRIAS) criteria and follow-up. PRIAS criteria are briefly summarized as, PSA ≤10 ng/mL, tumor with a Gleason score ≤3 + 3 and ≤T2a cancer, less than three cores of cancer positivity and positivity rate must be <50% in any core.[43] For patients who are not eligible for AS, surgical treatment may be performed if the patient’s performance status and the condition of the disease are appropriate for surgery. If a high risk disease is present, different treatment modalities may be applied depending on the situation. In treatment scenarios, mpMRI may be a useful method of treatment planning.
However it is recommended by ACR that, if mpMRI is required for any reason after the first biopsy, it at least six weeks should be awaited because bleeding and inflammation in the tissue cause deterioration of image quality and artefacts.[18]
mpMRI and the Index Theory
It has been reported that 80% of PCa originate from a cell clone of a single lesion. This lesion is called “index lesion”.[44] The index lesion may be presented as a single lesion in the prostate tissue, the largest volume tumor, or the highest grade tumor. Other studies have tried to link the index tumor to metastatic lesions.[45–50] If index lesion mpMRI can be clearly distinguished and the patient’s condition is also appropriate, any of the focal ablative therapy (FT) methods can be considered which are the topics of more recent research. Destruction of the index lesion with AS of the remaining prostate may be an “adjuvant therapy” model for AS an attempted compromise between early full organ definitive treatment and AS for a lesion likely to progress.
Following treatment of an index lesion, there is no consensus yet on the treatment of other lesions. However, if the lesion has not remained after the index lesion treatment with FT, the patient can be followed up. If the residual lesions are at low risk, the patient can be followed according to AS criteria. In the event of failure of FT, or any of the residual low-risk lesions becoming CSPCa in the future, a definitive treatment (radical prostatectomy or radiotherapy) may be performed according to the patient’s condition. There are also studies in which these patients can be treated again with FT. However, it should not be forgotten that a secondary intervention will always be challenging because of the sequelae of the first treatment.[51,52]
There are many FT methods of tissue destruction, and selection of one method is likely to be less critical when compared to the image guidance and patient selection. Technical platforms include cryotherapy, High Intensity Focused Ultrasound (HIFU), laser ablation, focal photodynamic therapy, irreversible electrovaporation, brachytherapy and radiofrequency (RF) ablation. The two methods that are the most studied, and whose results are more prominent than others, are cryotherapy and HIFU. There are not sufficient studies yet on other methods. All FT methods require additional devices.
In 2015 The Delphi Consensus, best practice statements were offered for FT and mpMRI, including the recommendation that FT be planned based upon an MRI-based biopsy procedure.[53]
mpMRI and Active Surveillance
Active surveillance is especially suitable for patients in the low risk group. However, frequent follow-up and recurrent biopsies result in significant increases in cost burden, in complications, anxiety in patients, distrust of care, and reluctance of patients.[44] In addition, over time, it has been understood that 33% of AS patients were exposed to definitive treatment within 2–5 years because of understaging of first diagnosis and progression of the existing disease.[54] Clinically significant PCa was detected in the final pathology in 77% of the patients who had undergone surgery.[55] An ongoing hypothesis of study is whether the use of mpMRI in follow-ups will reduce the number of annual biopsies as long as the lesion does not increase in PIRADS. In addition, using one of the MRI-based biopsy methods may prevent underdiagnosis or missing the cancer. Siddiqui et al.[56] reported in their study that mpMRI is reducing repeat biopsy rates by 68%. Abdi et al.[57] also reported that MRI fusion biopsies better detect PCa progression in patients followed by AS in their studies with 603 patients. In another study, Walton Diaz et al.[58] showed that, in 152 patients the final pathology reports and the mpMRI reports are compatible with each other. The concept is clear: use mpMRI based biopsy to find significant cancer if any (a “true positive”) and to become confident that it is not being missed despite negative biopsy results (a “true negative”).
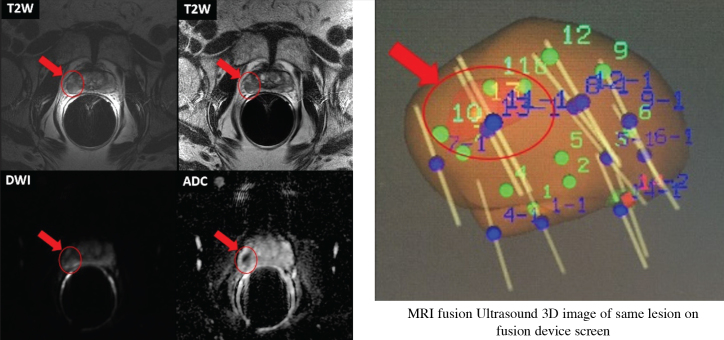
Different mpMRI sequence images and 3D images of prostatic lesion of a 69-year-old AS patient from our clinic are shown in Figure 2.
Figure 2.
The different mpMRI sequence images and 3D images of prostatic lesion of a 69-year-old active surveillance patient from our clinic. His last PSA was 4.3 ng/mL, and has Gleason grade 3+3 PCa. mpMRI was performed by 1.5T magnet device with ERC.
- Prostate volume is estimated 19 cc.
- Dominant lesion measures 1.1 × 1.0 cm and is located in the right peripheral zone at the level of the midgland at 7–9 o’clock position.
- Moderate low T2 signal, ADC signal is reduced.
- Suspicious focal enhancement is seen. Qualitative suspicion of clinically significant disease: 4. Likely.
- There is indistinctness between the lesion and the prostatic capsule on the coronal images, suspicious for early extraprostatic extension.
mpMRI after radical prostatectomy
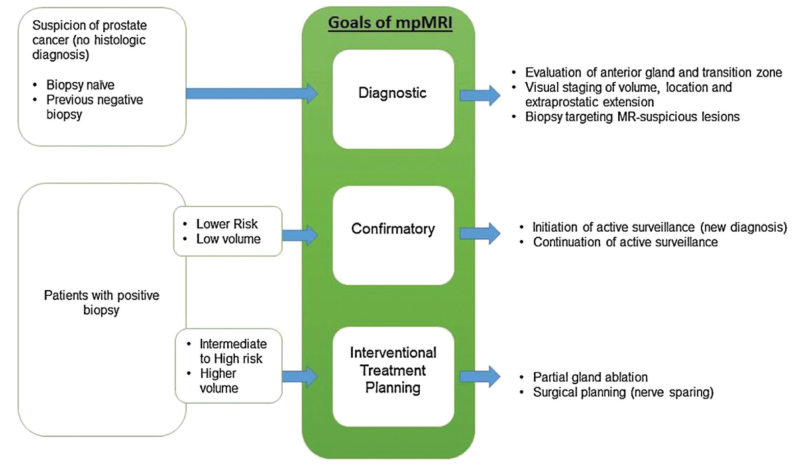
After RP, the first sign of recurrence is usually PSA elevation. Distant metastasis or local recurrence should be remembered if the PSA value begins to rise again after PSA nadir level. In the first year, distant metastasis should be considered if the elevation is rapid, whereas local recurrence should be considered if it is slowly increasing. When considering local recurrence, an mpMRI may show vascularization and contrast enhancement in the operation area.[59,60] In addition, involvement of the lymph nodes or distant metastases may be seen. Although mpMRI is claimed to be insufficient in detecting low-risk and low-volume lesions, there are also studies that show the opposite.[61] Recently, Schulman et al.[62], published a descriptive summary algorithm in a review article for the use of mpMRI in diagnosis, treatment planning and follow-up of PCa (Figure 3).
Figure 3.
Clinical stratification and relevant goals of prostate mpMRI. (The figure was adapted here with permission Thomas J. Polascik (corresponding author), and Springer (publisher)[62])
Limitations of mpMRI
Although serious progress has been made with mpMRI in the evaluation of prostate, it is still not perfect and there are some limitations that lead to controversy. mpMRI guided biopsies may miss the index lesions by 5–20%.[63–66] When fusion biopsies are combined with standard TRUS guided biopsies, the missing rates can be reduced to the minimum. Moreover, it currently lacks resolution to detect tumors with smaller volume and lower Gleason grade. It can detect lesions in the base and mid gland of prostate better, but it is not sufficiently effective in the detection of apical lesions.[64,67] In some comparative studies, 64% of multifocal cancers were found in the final pathology of RP materials, whereas mpMRI was only able to detect 21% of these foci.[64] There are also studies on missed tumors with smaller volume and shape, which is a continued concern for cases where FT is planned.[68–72]
In conclusion, mpMRI has managed to change the paradigms on prostate cancer detection and risk classification. The most clear-cut use in guidelines is for the prior negative biopsy/increasing PSA case, and additional findings are supporting use in non-biopsied patients and AS. mpMRI complements standard clinical exam, PSA, and systematic biopsy, and will miss some tumors that lack enough size or change in tissue density. mpMRI is likely to be used increasingly and further advances will be important to its safe adoption of focal therapy concepts.
Keypoints.
Avoid mpMRI with magnet strengths <1.5 T.
Endorectal coil has to be used for prostate visualization especially in older model 1.5 T magnet devices. On new 1.5 T magnet devices and 3T magnet devices, use of ERC is recommended because it increases the image quality.
PI-RADS™v2 should be used for achieving standardization on mpMRI evaluation.
If the first prostate biopsy is negative but the clinical suspicion persists, it is useful to perform one of the mpMRI-based fusion biopsies, if necessary technical equipment is available.
Although PI-RADS™ 3 lesions are better explained in PI-RADS™v2 than its first version, they are still called “equivocal”.
Multiparametric MRI use is beneficial mainly because of the better CSPCa detection rate in patients who have negative first biopsies.
Due to reduction in the frequency of annual biopsy in AS patients, mpMRI reduces the cost of treatment and complication rates. As a result, patients’ compliance with AS may increase.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept - H.C.D., J.W.D.; Design - H.C.D., J.W.D.; Supervision - J.W.D.; Data Collection and/or Processing - H.C.D., J.W.D.; Analysis and/or Interpretation - H.C.D., J.W.D.; Literature Search - H.C.D., J.W.D.; Writing Manuscript - H.C.D., J.W.D.; Critical Review - H.C.D., J.W.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. https://doi.org/10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. https://doi.org/10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70. doi: 10.1016/s0022-5347(17)38663-9. https://doi.org/10.1016/S0022-5347(17)38663-9. [DOI] [PubMed] [Google Scholar]
- 4.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. https://doi.org/10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Mian BM, Lehr DJ, Moore CK, Fisher HA, Kaufman RP, Jr, Ross JS, et al. Role of prostate biopsy schemes in accurate prediction of gleason scores. Urology. 2006;67:379–83. doi: 10.1016/j.urology.2005.08.018. https://doi.org/10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Marshall S, Taneja S. Focal therapy for prostate cancer: The current status. Prostate Int. 2015;3:35–41. doi: 10.1016/j.prnil.2015.03.007. https://doi.org/10.1016/j.prnil.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira RA, Costa RS, Muglia VF, Silva FF, Lajes JS, Dos Reis RB, et al. Gleason score and tumor laterality in radical prostatectomy and transrectal ultrasound-guided biopsy of the prostate: A comparative study. Asian J Androl. 2015;17:815–20. doi: 10.4103/1008-682X.146970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanthabalan A, Emberton M, Ahmed HU. Biopsy strategies for selecting patients for focal therapy for prostate cancer. Curr Opin Urol. 2014;24:209–17. doi: 10.1097/MOU.0000000000000046. https://doi.org/10.1097/MOU.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 9.Washington SL, Bonham M, Whitson JM, Cowan JE, Carroll PR. Transrectal ultrasonography-guided biopsy does not reliably identify dominant cancer location in men with low-risk prostate cancer. BJU Int. 2012;110:50–5. doi: 10.1111/j.1464-410X.2011.10704.x. https://doi.org/10.1111/j.1464-410X.2011.10704.x. [DOI] [PubMed] [Google Scholar]
- 10.Sinnott M, Falzarano SM, Hernandez AV, Jones JS, Klein EA, Zhou M, et al. Discrepancy in prostate cancer localization between biopsy and prostatectomy specimens in patients with unilateral positive biopsy: Implications for focal therapy. Prostate. 2012;72:1179–86. doi: 10.1002/pros.22467. https://doi.org/10.1002/pros.22467. [DOI] [PubMed] [Google Scholar]
- 11.Hricak H, Williams RD, Spring DB, Moon KL, Jr, Hedgcock MW, Watson RA, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR Am J Roentgenol. 1983;141:1101–10. doi: 10.2214/ajr.141.6.1101. https://doi.org/10.2214/ajr.141.6.1101. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson SK, Chang EK, Marks LS. Screening and detection advances in magnetic resonance image-guided prostate biopsy. Urol Clin North Am. 2014;41:315–26. doi: 10.1016/j.ucl.2014.01.007. https://doi.org/10.1016/j.ucl.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DC, Reiter RE. Multi-parametric magnetic resonance imaging as a management decision tool. Transl Androl Urol. 2017;6:472–82. doi: 10.21037/tau.2017.05.22. https://doi.org/10.21037/tau.2017.05.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyson MD, Arora SS, Scarpato KR, Barocas D. Magnetic resonance-ultrasound fusion prostate biopsy in the diagnosis of prostate cancer. Urol Oncol. 2016;34:326–32. doi: 10.1016/j.urolonc.2016.03.005. https://doi.org/10.1016/j.urolonc.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. Esur prostate mr guidelines 2012. Eur Radiol. 2012;22:746–57. doi: 10.1007/s00330-011-2377-y. https://doi.org/10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo S, Kim JK, Jeong IG. Multiparametric magnetic resonance imaging for prostate cancer: A review and update for urologists. Korean J Urol. 2015;56:487–97. doi: 10.4111/kju.2015.56.7.487. https://doi.org/10.4111/kju.2015.56.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah ZK, Elias SN, Abaza R, Zynger DL, DeRenne LA, Knopp MV, et al. Performance comparison of 1.5-t endorectal coil mri with 3.0-t nonendorectal coil mri in patients with prostate cancer. Acad Radiol. 2015;22:467–74. doi: 10.1016/j.acra.2014.11.007. https://doi.org/10.1016/j.acra.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American college of radiology. Mr prostate imaging reporting and data system version 2.0 [internet] Available from: Http://www.Acr.Org/quality-safety/resources/pirads/ [cited 2015 jun 16]. In.
- 19.Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with t2-weighted imaging in diagnosing prostate carcinoma: A systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199:103–10. doi: 10.2214/AJR.11.7634. https://doi.org/10.2214/AJR.11.7634. [DOI] [PubMed] [Google Scholar]
- 20.Collins DJ, Padhani AR. Dynamic magnetic resonance imaging of tumor perfusion. Approaches and biomedical challenges. IEEE Eng Med Biol Mag. 2004;23:65–83. doi: 10.1109/memb.2004.1360410. https://doi.org/10.1109/MEMB.2004.1360410. [DOI] [PubMed] [Google Scholar]
- 21.Hara N, Okuizumi M, Koike H, Kawaguchi M, Bilim V. Dynamic contrast-enhanced magnetic resonance imaging (dce-mri) is a useful modality for the precise detection and staging of early prostate cancer. Prostate. 2005;62:140–7. doi: 10.1002/pros.20124. https://doi.org/10.1002/pros.20124. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, et al. Overview of dynamic contrast-enhanced mri in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198:1277–88. doi: 10.2214/AJR.12.8510. https://doi.org/10.2214/AJR.12.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loffroy R, Chevallier O, Moulin M, Favelier S, Genson PY, Pottecher P, et al. Current role of multiparametric magnetic resonance imaging for prostate cancer. Quant Imaging Med Surg. 2015;5:754–64. doi: 10.3978/j.issn.2223-4292.2015.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ogura K, Maekawa S, Okubo K, Aoki Y, Okada T, Oda K, et al. Dynamic endorectal magnetic resonance imaging for local staging and detection of neurovascular bundle involvement of prostate cancer: Correlation with histopathologic results. Urology. 2001;57:721–6. doi: 10.1016/s0090-4295(00)01072-4. https://doi.org/10.1016/S0090-4295(00)01072-4. [DOI] [PubMed] [Google Scholar]
- 25.Mazaheri Y, Shukla-Dave A, Hricak H, Fine SW, Zhang J, Inurrigarro G, et al. Prostate cancer: Identification with combined diffusion-weighted mr imaging and 3d 1h mr spectroscopic imaging--correlation with pathologic findings. Radiology. 2008;246:480–8. doi: 10.1148/radiol.2462070368. https://doi.org/10.1148/radiol.2462070368. [DOI] [PubMed] [Google Scholar]
- 26.Jung JA, Coakley FV, Vigneron DB, Swanson MG, Qayyum A, Weinberg V, et al. Prostate depiction at endorectal mr spectroscopic imaging: Investigation of a standardized evaluation system. Radiology. 2004;233:701–8. doi: 10.1148/radiol.2333030672. https://doi.org/10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 27.Scheenen TW, Heijmink SW, Roell SA, Hulsbergen-Van de Kaa CA, Knipscheer BC, Witjes JA, et al. Three-dimensional proton mr spectroscopy of human prostate at 3 t without endorectal coil: Feasibility. Radiology. 2007;245:507–16. doi: 10.1148/radiol.2451061444. https://doi.org/10.1148/radiol.2451061444. [DOI] [PubMed] [Google Scholar]
- 28.Walker P, Provent P, Tizon X, Crehange G, Duchamp O, Brunotte F, et al. In-vivo metabolic characterization of healthy prostate and orthotopic prostate cancer in rats using proton magnetic resonance spectroscopy at 4.7 t. Acta Radiol. 2013;54:121–6. doi: 10.1258/ar.2012.120232. https://doi.org/10.1258/ar.2012.120232. [DOI] [PubMed] [Google Scholar]
- 29.Durmus T, Baur A, Hamm B. Multiparametric magnetic resonance imaging in the detection of prostate cancer. Rofo. 2014;186:238–46. doi: 10.1055/s-0034-1365937. https://doi.org/10.1055/s-0034-1365937. [DOI] [PubMed] [Google Scholar]
- 30.Puech P, Rouviere O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: Multiparametric mr-targeted biopsy with cognitive and transrectal us-mr fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013;268:461–9. doi: 10.1148/radiol.13121501. https://doi.org/10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 31.Marks L, Young S, Natarajan S. Mri-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43–50. doi: 10.1097/MOU.0b013e32835ad3ee. https://doi.org/10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright JL, Ellis WJ. Improved prostate cancer detection with anterior apical prostate biopsies. Urol Oncol. 2006;24:492–5. doi: 10.1016/j.urolonc.2006.03.003. https://doi.org/10.1016/j.urolonc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Moussa AS, Meshref A, Schoenfield L, Masoud A, Abdel-Rahman S, Li J, et al. Importance of additional “extreme” anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010;75:1034–9. doi: 10.1016/j.urology.2009.11.008. https://doi.org/10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Barzell W, Whitmore WF., III Transperineal template guided saturation biopsy of the prostate: Rationale, indications and technique. Urology Times. 2003;31:41–2. [Google Scholar]
- 35.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011;186:850–4. doi: 10.1016/j.juro.2011.04.069. https://doi.org/10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 36.Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: Detection of clinically significant prostate cancers. Eur Urol. 2012;62:902–9. doi: 10.1016/j.eururo.2012.01.047. https://doi.org/10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 37.Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–15. doi: 10.1016/j.eururo.2013.03.025. https://doi.org/10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of mr/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. doi: 10.1001/jama.2014.17942. https://doi.org/10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pokorny MR, de Rooij M, Duncan E, Schroder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (mr) imaging with subsequent mr-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9. doi: 10.1016/j.eururo.2014.03.002. https://doi.org/10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, vander Kwast T, et al. Eau guidelines on prostate cancer. Part 1:Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. https://doi.org/10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 41.Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: A consensus statement by aua and sar. J Urol. 2016;196:1613–8. doi: 10.1016/j.juro.2016.06.079. https://doi.org/10.1016/j.juro.2016.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.12. National comprehensive cancer network (nccn) Nccn clinical practice guidelines in oncology. Prostate cancer early detection. Version 2.2015. Dec 14, 2015. Available from: http://www.Nccn.Org/professionals/physician_gls/pdf/prostate_detection.Pdf. [DOI] [PubMed]
- 43.van den Bergh RC, Roemeling S, Roobol MJ, Roobol W, Schroder FH, Bangma CH. Prospective validation of active surveillance in prostate cancer: The prias study. Eur Urol. 2007;52:1560–3. doi: 10.1016/j.eururo.2007.05.011. https://doi.org/10.1016/j.eururo.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13:641–53. doi: 10.1038/nrurol.2016.177. https://doi.org/10.1038/nrurol.2016.177. [DOI] [PubMed] [Google Scholar]
- 45.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–9. doi: 10.1016/s0090-4295(02)01728-4. https://doi.org/10.1016/S0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–65. doi: 10.1038/nm.1944. https://doi.org/10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361:1704–6. doi: 10.1056/NEJMcibr0905562. https://doi.org/10.1056/NEJMcibr0905562. [DOI] [PubMed] [Google Scholar]
- 48.Villers A, McNeal JE, Freiha FS, Stamey TA. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992;70:2313–8. doi: 10.1002/1097-0142(19921101)70:9<2313::aid-cncr2820700917>3.0.co;2-t. https://doi.org/10.1002/1097-0142(19921101)70:9<2313::AID-CNCR2820700917>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 49.Algaba F, Montironi R. Impact of prostate cancer multifocality on its biology and treatment. J Endourol. 2010;24:799–804. doi: 10.1089/end.2009.0462. https://doi.org/10.1089/end.2009.0462. [DOI] [PubMed] [Google Scholar]
- 50.Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185:121–5. doi: 10.1016/j.juro.2010.08.082. https://doi.org/10.1016/j.juro.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 51.Postema AW, De Reijke TM, Ukimura O, Van den Bos W, Azzouzi AR, Barret E, et al. Standardization of definitions in focal therapy of prostate cancer: Report from a delphi consensus project. World J Urol. 2016;34:1373–82. doi: 10.1007/s00345-016-1782-x. https://doi.org/10.1007/s00345-016-1782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rukstalis DB, Goldknopf JL, Crowley EM, Garcia FU. Prostatecryoablation: A scientific rationale for future modifications. Urology. 2002;60(2 Suppl 1):19–25. doi: 10.1016/s0090-4295(02)01680-1. https://doi.org/10.1016/S0090-4295(02)01680-1. [DOI] [PubMed] [Google Scholar]
- 53.Muller BG, van den Bos W, Brausi M, Futterer JJ, Ghai S, Pinto PA, et al. Follow-up modalities in focal therapy for prostate cancer: Results from a delphi consensus project. World J Urol. 2015;33:1503–9. doi: 10.1007/s00345-014-1475-2. https://doi.org/10.1007/s00345-014-1475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. https://doi.org/10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 55.de la Rosette J, Ahmed H, Barentsz J, Johansen TB, Brausi M, Emberton M, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24:775–80. doi: 10.1089/end.2009.0596. https://doi.org/10.1089/end.2009.0596. [DOI] [PubMed] [Google Scholar]
- 56.Siddiqui MM, Truong H, Rais-Bahrami S, Stamatakis L, Logan J, Walton-Diaz A, et al. Clinical implications of a multiparametric magnetic resonance imaging based nomogram applied to prostate cancer active surveillance. J Urol. 2015;193:1943–9. doi: 10.1016/j.juro.2015.01.088. https://doi.org/10.1016/j.juro.2015.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdi H, Pourmalek F, Zargar H, Walshe T, Harris AC, Chang SD, et al. Multiparametric magnetic resonance imaging enhances detection of significant tumor in patients on active surveillance for prostate cancer. Urology. 2015;85:423–8. doi: 10.1016/j.urology.2014.09.060. https://doi.org/10.1016/j.urology.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 58.Walton Diaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol. 2015;33:e201–7. doi: 10.1016/j.urolonc.2015.01.023. https://doi.org/10.1016/j.urolonc.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linder BJ, Kawashima A, Woodrum DA, Tollefson MK, Karnes J, Davis BJ, et al. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can J Urol. 2014;21:7283–9. [PubMed] [Google Scholar]
- 60.Roy C, Foudi F, Charton J, Jung M, Lang H, Saussine C, et al. Comparative sensitivities of functional mri sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR Am J Roentgenol. 2013;200:W361–8. doi: 10.2214/AJR.12.9106. https://doi.org/10.2214/AJR.12.9106. [DOI] [PubMed] [Google Scholar]
- 61.Alfarone A, Panebianco V, Schillaci O, Salciccia S, Cattarino S, Mariotti G, et al. Comparative analysis of multiparametric magnetic resonance and pet-ct in the management of local recurrence after radical prostatectomy for prostate cancer. Crit Rev Oncol Hematol. 2012;84:109–21. doi: 10.1016/j.critrevonc.2012.01.006. https://doi.org/10.1016/j.critrevonc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Schulman AA, Sze C, Tsivian E, Gupta RT, Moul JW, Polascik TJ. The contemporary role of multiparametric magnetic resonance imaging in active surveillance for prostate cancer. Curr Urol Rep. 2017;18:52. doi: 10.1007/s11934-017-0699-2. https://doi.org/10.1007/s11934-017-0699-2. [DOI] [PubMed] [Google Scholar]
- 63.Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: Correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015;67:787–94. doi: 10.1016/j.eururo.2014.08.077. https://doi.org/10.1016/j.eururo.2014.08.077. [DOI] [PubMed] [Google Scholar]
- 64.Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: Correlation with whole-mount histopathology. Eur Urol. 2015;67:569–76. doi: 10.1016/j.eururo.2014.08.079. https://doi.org/10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 65.Radtke JP, Schwab C, Wolf MB, Freitag MT, Alt CD, Kesch C, et al. Multiparametric magnetic resonance imaging (mri) and mri-transrectal ultrasound fusion biopsy for index tumor detection: Correlation with radical prostatectomy specimen. Eur Urol. 2016;70:846–53. doi: 10.1016/j.eururo.2015.12.052. https://doi.org/10.1016/j.eururo.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 66.Russo F, Regge D, Armando E, Giannini V, Vignati A, Mazzetti S, et al. Detection of prostate cancer index lesions with multiparametric magnetic resonance imaging (mp-mri) using whole-mount histological sections as the reference standard. BJU Int. 2016;118:84–94. doi: 10.1111/bju.13234. https://doi.org/10.1111/bju.13234. [DOI] [PubMed] [Google Scholar]
- 67.Tan N, Margolis DJ, Lu DY, King KG, Huang J, Reiter RE, et al. Characteristics of detected and missed prostate cancer foci on 3-t multiparametric mri using an endorectal coil correlated with whole-mount thin-section histopathology. AJR Am J Roentgenol. 2015;205:W87–92. doi: 10.2214/AJR.14.13285. https://doi.org/10.2214/AJR.14.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coakley FV, Kurhanewicz J, Lu Y, Jones KD, Swanson MG, Chang SD, et al. Prostate cancer tumor volume: Measurement with endorectal mr and mr spectroscopic imaging. Radiology. 2002;223:91–7. doi: 10.1148/radiol.2231010575. https://doi.org/10.1148/radiol.2231010575. [DOI] [PubMed] [Google Scholar]
- 69.Mazaheri Y, Hricak H, Fine SW, Akin O, Shukla-Dave A, Ishill NM, et al. Prostate tumor volume measurement with combined t2-weighted imaging and diffusion-weighted mr: Correlation with pathologic tumor volume. Radiology. 2009;252:449–57. doi: 10.1148/radiol.2523081423. https://doi.org/10.1148/radiol.2523081423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turkbey B, Mani H, Aras O, Rastinehad AR, Shah V, Bernardo M, et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol. 2012;188:1157–63. doi: 10.1016/j.juro.2012.06.011. https://doi.org/10.1016/j.juro.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cornud F, Khoury G, Bouazza N, Beuvon F, Peyromaure M, Flam T, et al. Tumor target volume for focal therapy of prostate cancer-does multiparametric magnetic resonance imaging allow for a reliable estimation? J Urol. 2014;191:1272–9. doi: 10.1016/j.juro.2013.12.006. https://doi.org/10.1016/j.juro.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: Use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017;197:320–6. doi: 10.1016/j.juro.2016.07.084. https://doi.org/10.1016/j.juro.2016.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]