Abstract
In the last few years, increased emphasis has been devoted to understanding the contribution of mitochondria-associated endoplasmic reticulum (ER) membranes (MAM) to human pathology in general, and neurodegenerative diseases in particular. A major reason for this is the central role that this subdomain of the ER plays in metabolic regulation and in mitochondrial biology. As such, aberrant MAM function may help explain the seemingly unrelated metabolic abnormalities often seen in neurodegeneration. In the specific case of Alzheimer disease (AD), besides perturbations in calcium and lipid homeostasis, there are numerous documented alterations in mitochondrial behavior and function, including reduced respiratory chain activity and oxidative phosphorylation, increased free radical production, and altered organellar morphology, dynamics, and positioning (especially perinuclear mitochondria). However, whether these alterations are primary events causative of the disease, or are secondary downstream events that are the result of some other, more fundamental problem, is still unclear. In support of the former possibility, we recently reported that C99, the C-terminal processing product of the amyloid precursor protein (APP) derived from its cleavage by β-secretase, is present in MAM, that its level is increased in AD, and that this increase reduces mitochondrial respiration, likely via a C99-induced alteration in cellular sphingolipid homeostasis. Thus, the metabolic disturbances seen in AD likely arise from increased ER-mitochondrial communication that is driven by an increase in the levels of C99 at the MAM.
Facts
Mitochondrial bioenergetic function is decreased in AD, but the reason for this decline is unknown.
A “mitochondrial cascade hypothesis” has been put forward to explain AD pathogenesis.
ER-mitochondrial communication and MAM function are increased significantly in AD.
C99 is present in MAM, and accumulates above normal levels in AD cells and animal models.
Increased C99-mediated MAM activity induces bioenergetic dysfunction in AD cells.
Open questions
How does C99 modulate MAM function in general and bioenergetic output in particular?
What is the mechanism of mitochondrial dysfunction due to alterations in MAM behavior?
How do these alterations occur in sporadic AD, in which APP processing is presumably normal?
Introduction
Alzheimer disease (AD) is the most common adult neurodegenerative disorder1. Pathologically, it is characterized by progressive neuronal loss in the hippocampus and cortex, with the accumulation in the brain of extracellular neuritic plaques and intracellular neurofibrillary tangles. Prominent among the proteins deposited in the plaques is β-amyloid (Aβ), which is produced by cleavage of the amyloid precursor protein (APP) by presenilin-1 (PS1) and/or presenilin-2 (PS2), both of which are active components of the γ-secretase complex2. Notably, dominantly inherited mutations both in the presenilins and in APP are currently the only known causes of the familial form of AD (FAD), which has led to the most widely accepted hypothesis to explain the pathogenesis of AD, namely, the “amyloid cascade,” which proposes that deposition of Aβ in the brain is the precipitating pathological event in AD3. However, while the amyloid cascade hypothesis helps explain the development of the plaques and perhaps also the tangles, it sheds little light on the impact of other aspects of the disease, some of which occur years before the appearance of those plaques and tangles4–6. Those other aspects include altered metabolism of phospholipids and fatty acids7,8, increased levels of circulating cholesterol9, the deposition of lipid droplets within cells10–12, alterations in glucose levels13, aberrant calcium homeostasis14, increased ER stress15, and mitochondrial dysfunction16,17, the focus of our discussion here.
Mitochondrial alterations in AD
In the last few decades, many reports have demonstrated the impairment of mitochondrial function in AD. Moreover, a number of lines of biochemical and cell biological evidence have been marshaled in support of a “mitochondrial cascade hypothesis” for the pathogenesis of AD, which proposes that mitochondrial alterations initiate the cascade of pathologies characteristic of the disease18–25. However, while this possibility is intriguing, it is currently unclear whether the impairment of mitochondrial function in AD26–33 is the cause, the consequence, or merely a “bystander effect” of the biochemical and morphological changes seen in AD34,35. While mitochondria are clearly altered in AD, we believe that the mitochondrial cascade hypothesis has a number of flaws, discussed in greater detail below, that have led us to the conclusion that mitochondrial dysfunction is an early disturbance in the pathogenesis of AD but is not the driver of the pathogenesis.
Mitochondrial biochemical and dynamic alterations
As alluded to above, mitochondrial bioenergetic function is reduced in AD. Specifically, AD patients and animal models of AD exhibit reduced respiratory chain activity and lower ATP production26,28,31,36–39. Additionally, many reports have described a significant decrease in enzymes of the mitochondrial tricarboxylic acid cycle in AD patients40–42. Moreover, the levels of free radicals and reactive oxygen species, which are produced mainly by mitochondria, are elevated in AD cells38,43–46.
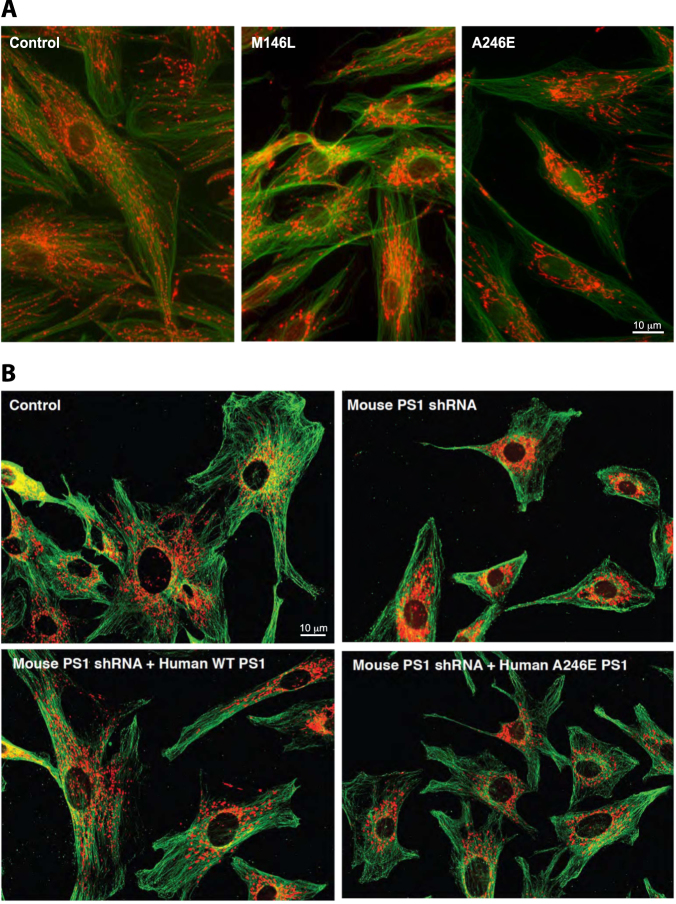
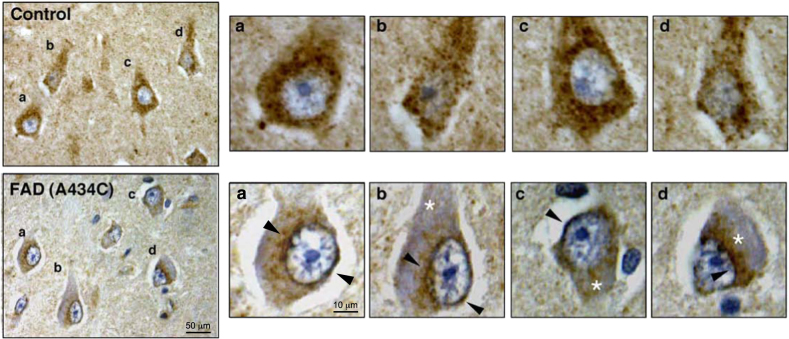
Besides the effects on bioenergetics, there are also significant changes in mitochondrial dynamics and localization in AD cells, namely, dysfunctional mitochondrial axonal transport19,47–49, deregulated organellar dynamics (e.g., mitochondrial fission and fusion)17,50–53, and a more perinuclear distribution of the organelles17,50,54,55. On this latter point, we too have observed perinuclear mitochondria in AD patient cells (Fig. 1a) and were also able to reproduce the perinuclear phenotype in mouse embryonic fibroblasts (MEFs) in which endogenous PS1 mRNA was knocked down (Fig. 1b). Equally important, we observed a reversal of the perinuclear phenotype upon overexpression of the wild-type allele of human PS1, but not a pathogenic AD-mutant allele (A246E) (Fig. 1b). Consistent with this, we obtained a similar result in the most clinically relevant tissue, namely human brain. Specifically, we used immunohistochemistry to detect mitochondria in the hippocampal CA1 region of an autoptic brain from an AD patient with a pathogenic PS1 mutation (A434C)56. Mitochondria were uniformly distributed in the cytosol of control hippocampus, as expected. In contrast, we detected a “ring” of mitochondria around the nucleus of the patient neurons, and depletion of the organelles in the distal region of hippocampal cell bodies (Fig. 2). Thus, we believe that the observation of perinuclear mitochondria in patient cells in vitro likely reflects what is occurring clinically in vivo. Moreover, it appears that γ-secretase activity and/or APP processing play a role in the altered distribution of mitochondria in at least some forms of AD17,50, but the relationship, if any, of mitochondrial maldistribution to altered APP processing has been obscure; this issue is addressed below.
Fig. 1.
a Representative mitochondrial morphology in AD-mutant cells. Human fibroblasts were stained with MitoTracker (red) and anti-tubulin (green). Note the relatively dispersed distribution of the mitochondria in the control, whereas they are more perinuclear in the FAD-PS1M146L and FAD-PS1A246E cells. b MEFs in which PS1 was knocked down (by small hairpin RNA). Cells were stained as in a. Note relatively dispersed distribution of the mitochondria in the control, whereas they are more perinuclear in the PS1-knockdown cells. This phenotype could be rescued by overexpression of WT human PS1, but not by expression of a human pathogenic PS1 mutation (A246E)
Fig. 2. Immunohistochemistry to detect mitochondria (FeS subunit of complex III of the mitochondrial respiratory chain) in the hippocampus (CA1 region) from a FAD patient with a PS1 mutation (A434C).
(Upper panels) Control subject (left), with four indicated neurons (a–d) magnified at right. Note relatively uniform stain (brown), indicating a homogeneous distribution of mitochondria in the cell body. (Lower panels) FAD-PS1A434C patient. Notation as in upper panel. Note the perinuclear distribution of immunostain (brown rings; arrowheads), with a relative paucity of immunostain in the distal regions of the cell body (asterisks)
Taken together, it is clear that there are numerous functional alterations in mitochondrial behavior in both familial and sporadic AD. However, even though many of these mitochondrial phenotypes are evident before the appearance of plaques57–59, we believe that mitochondrial dysfunction likely will not be found to be the underlying cause of the disease, for a number of reasons.
First and foremost, patients with authentic mitochondrial diseases (by which we mean diseases where bioenergetic deficits are the initiating cause of the pathology), whether due to mutations in the mitochondrial or nuclear genomes, do not evince the symptomatology of AD, even in those patients who live into their fourth and fifth decades. Generally speaking, mitochondrial diseases are characterized by numerous defects (e.g., encephalopathy, myopathy, endocrinopathy, retinopathy, and gastrointestinal and kidney disorders) that are simply not seen with any frequency in AD.
Second, mitochondrial deficiency is a common consequence of other insults, such as tissue injury. In fact, the altered mitochondrial function and dynamics seen in AD are also seen in a number of other neurodegenerative disorders that are distinct from AD. For example, perinuclear mitochondria are seen in Huntington disease60,61 and in amyotrophic lateral sclerosis62–65, and was seen in a patient with a mutation in the mitochondrial fission protein DRP166.
Finally, perinuclear mitochondria can be induced by overexpression of the mitochondrial fission protein FIS167 and also, notably, by tau68. Thus, these findings imply minimally that mitochondrial dysfunction and altered mitodynamics, while occurring early, are probably downstream consequences of other specific primary events in AD progression, and are not a fundamental cause of pathogenesis.
Mitochondria and genetics
Genetically, alterations in mitochondrial DNA (mtDNA) have been found in AD cells and tissues69. These include both qualitative changes, such as the association of specific mtDNA haplogroups with the risk of developing AD70–73 and the presence of mtDNA deletions74,75 and point mutations76–78 in patient cells and tissues, and quantitative changes, such as reduced mtDNA levels in AD cerebrospinal fluid79. At the gene expression level, one study showed that the transcription of a number of nuclear-encoded oxidative phosphorylation (OxPhos) subunits was reduced in AD blood, whereas transcription of most mtDNA-encoded subunits was elevated in AD blood80.
Nevertheless, from the genetic point of view, in spite of the implication that mtDNA mutations, and especially point mutations, should result in maternally inherited AD, only a tiny number of mtDNA variants have been ascribed specifically to the development of the disease76, and even this is controversial81–83; in fact, there is little evidence for maternal inheritance in AD at all84,85.
Finally, genetic association studies have identified numerous nuclear loci associated with increased risk for developing AD86. Along with the three FAD-linked genes (APP, PSEN1, and PSEN2), the most commonly accepted sporadic AD (SAD) risk loci (i.e. linkage to mutations either near or within known genes) are ABCA7, APOE, BIN1, CASS4, CD2AP, CD33, CELF1, CLU, CR1, EPHA1, FERMT2, INPP5D, MEF2C, MS4A, NME8, PICALM, PLD3, PTK2B, SLC24A4, SORL1, TREM2, and ZCWPW1. Notably, none of the proteins encoded by these genes are targeted to mitochondria. Of course, this lack of correlation does not prove that mitochondria are not involved in AD pathogenesis, but by the same token it provides little genetic support to the mitochondrial cascade hypothesis.
Mitochondria and γ-secretase
In support of the mitochondrial cascade hypothesis, there is an intriguing connection between mitochondria and the γ-secretase complex. First, APP and/or Aβ44,46,87–94, as well as components of the γ-secretase complex95–98, have been reported to be at or in mitochondria, presumably implicating the organelle directly in AD. Second, there are mitochondria-mediated alterations in APP processing in AD cells and tissues39. Third, PS1 enhances the expression of PGC-1α, the master regulator of mitochondrial biogenesis, and this effect is reduced in PS1-mutated cells99. Finally, incubation of cultured cells and/or isolated mitochondria with Aβ has been shown to have deleterious effects on mitochondrial functions, including effects on respiration100–104, protein import105, organellar transport19,47–49, organellar localization106, and organellar dynamics (e.g., mitochondrial fission and fusion)17,50–53. These and other reported data reveal an intriguing association between mitochondrial regulation and γ-secretase activity, but whether this link is direct or is mediated by some other, more indirect, mechanism, is not clear.
Regarding the localization studies, it is true that components of the γ-secretase complex can be found on mitochondria, but their relative concentration is very low compared to that in other membranes, such as ER. Moreover, data from our laboratory107 and others108 showed that presenilins are not imported into mitochondria, implying that while presenilins and γ-secretase exert direct effects on mitochondria, they do not behave as canonical mitochondrial proteins. Another argument in opposition to APP or γ-secretase activity being localized to mitochondria is that this protein complex is only activated in lipid raft domains109–112, which do not exist on mitochondrial membranes113.
Regarding the toxic effects of Aβ on mitochondria, previous studies showing inhibitory effects of Aβ treatment on mitochondrial function could have been due to the use of unphysiological concentrations of Aβ101. Indeed, inhibitory effects on mitochondria were observed with Aβ concentrations that were 10–100 times higher than those found in the entorhinal cortex or cerebrospinal fluid from AD patients114.
We therefore think that the pathogenesis of AD cannot be explained as resulting from those alterations in mitochondrial function that are similar to those seen in authentic mitochondrial disorders. Nevertheless, mitochondrial dysfunction is an undeniable early symptom of the disease that needs to be investigated and understood if we are to understand better the course of AD pathogenesis. How might this conundrum be reconciled?
Mitochondria-associated ER membranes, APP processing, and bioenergetics in AD
Some years ago we hypothesized that the phenotypes seen in AD, including the mitochondrial disturbances, were the downstream consequences of some primary insult in AD arising prior to plaque and tangle formation, and triggered by mutations in PS1, PS2, and APP in the case of FAD, and by unknown causes in the case of SAD115,116.
With that in mind, we first tried to clarify the subcellular localization of presenilins and γ-secretase activity and its spatial relationship to mitochondria. Intriguingly, our group found107, and others confirmed117–119, that presenilins and γ-secretase activity, while localized at the ER, as described previously120, are enriched in mitochondria-associated ER membranes or MAM. MAM is a specialized subdomain of the ER that, as opposed to the rest of the ER, has the features of a lipid raft and is rich in cholesterol and sphingomyelin121,122. MAM is critical for processes that occur at the interface between mitochondria and ER, including phospholipid biosynthesis, cholesterol esterification, calcium transport, and communication between the two organelles123.
After this initial finding, our group (and subsequently others124–126) became interested in the potential role that MAM might play in the pathogenesis of AD115,116,127,128. We therefore measured MAM activity and ER-mitochondrial connectivity in AD cell models and in cells from AD patients, and found both to be increased significantly compared to controls122.
This connection between MAM and AD, at least on theoretical grounds, is appealing, because besides its close apposition to mitochondria, many of MAM’s known functions are among the functions that are perturbed in AD beyond the accumulation of amyloid plaques and tangles129,130. These include the regulation of phospholipid, cholesterol, and calcium homeostasis123, increased ER stress131–133, and perturbed calcium homeostasis, driven, in part, by interactions between p53 and the sarco-ER calcium pump at the MAM132. In addition, at least one known MAM-localized enzyme, acyl-CoA:cholesterol acytransferase (ACAT1; gene SOAT1)134 appears to be required for the production of Aβ135,136. Moreover, altered mitochondrial function might somehow be connected to APP processing137, at least in the familial form of the disease where APP processing is clearly perturbed2. Finally, given the physical proximity of ER to mitochondria123,134,138, it is possible that the mitochondrial disturbances that are found in AD might also be due to perturbed MAM morphology and behavior133,139–141. For example, given the fact that the subcellular localization of the majority of ER is perinuclear, the increased apposition between ER and mitochondria in AD122 could help explain why mitochondria accumulate in the perinuclear region in the disease. In order to address whether perturbations in MAM are responsible for mitochondrial dysfunction in AD, we focused on the relationships among APP processing, MAM behavior, and mitochondrial regulation.
In the non-amyloidogenic pathway, full-length APP (~700 amino acids (aa) in length) is first cleaved by α-secretase at the plasma membrane to produce a long soluble N-terminal fragment (sAPPα) and a short membrane-bound 83-aa C-terminal fragment, called C83; C83 is cleaved by the γ-secretase complex to produce two peptides, P3 and the APP intracellular domain (AICD)142. In the alternative amyloidogenic pathway, full-length APP is first cleaved by β-secretase (BACE1) within endosomes to produce a slightly shorter soluble N-terminal fragment (sAPPβ) and a slightly longer 99-aa membrane-bound C-terminal fragment, called C99. C99 is then delivered to the ER, via a currently unknown mechanism, to be cleaved by the γ-secretase complex, producing two peptides, Aβ and AICD. In unaffected individuals, C99 is cleaved rapidly to Aβ40, which is ~40 aa in length. In AD, C99 is cleaved to Aβ42, which is ~42 aa in length, and there is an increase in the ratio of Aβ42:Aβ40. Since Aβ has been found to be produced in MAM107,118, it was logical to assume that the substrate for MAM-localized γ-secretase, namely C83 and/or C99, must be present in this compartment. In agreement with this supposition, we found that C99 (but not C83) was present not only in endosomes, as expected, but in MAM as well, both in cells and in tissues143, where it undergoes cleavage by γ-secretase to generate Aβ107,117,118.
Our data further showed that in cellular models of AD, in cells and tissues from AD animal models, and in cells from FAD and SAD patients, there were significant increases in C99 in MAMs that correlated with alterations in MAM structure and function143. Specifically, we found that the accumulation of C99 at MAM resulted in the upregulation of sphingomyelin hydrolysis by sphingomyelinases (SMases) within this ER subdomain, but the identity of the specific SMases that are upregulated (there are at least five) is currently unknown. We were also able to replicate the increase in SMase activity at MAM domains in SH-SY5Y cells by inhibiting γ-secretase activity (thereby promoting the accumulation of C99). Supporting this result, the inhibition of BACE1 activity (which reduces C99 formation) resulted in an attenuation of SMase activity143.
This increase in SMase activity resulted not only in reductions in the content of sphingomyelin but also in a notable elevation of the sphingomyelin hydrolysis product, ceramide143. This finding was noteworthy because ceramide is not only a pro-apoptotic molecule144 but is also an inhibitor of mitochondrial respiration145–149. Indeed, we found that in our presenilin-mutant and AD patient cells, ceramide levels were elevated and respiratory chain function was decreased, as was respiratory supercomplex formation and function143. Importantly, manipulation of the ceramide pathway (both pharmacologically and genetically) to reduce ceramide levels in these cells reversed the bioenergetic defects143. In addition, reduction of C99 levels, either via inhibition of BACE1 activity (again, both pharmacologically and genetically) or via ablation of the APP gene, also reversed the bioenergetic deficits, concomitant with a renormalization of the sphingolipid profiles143. Importantly, these phenotypes could not be replicated by the addition of physiological concentration ratios of Aβ42:Aβ40, physiological concentrations of Aβ42 oligomers, or by the overexpression of AICD. We therefore believe that the bioenergetic defects in AD are likely to be the consequence of upregulated sphingolipid turnover and increased ceramide content triggered by the accumulation of C99 at the MAM. This elevation in ceramide levels alters mitochondrial membrane properties, hindering the assembly and activity of respiratory supercomplexes, resulting or exacerbating, at least in part, in bioenergetic deficiencies. Importantly, these findings implicating C99 are consistent with the findings of others, who showed that altered APP processing, especially via MAM-localized PS2126,150, decreased bioenergetics. Interestingly, deletion of a portion of the C99 transmembrane region altered mitochondrial morphology and function in HeLa cells, including decreased ATP levels and decreased membrane potential151.
These findings are particularly important because the sphingolipid and mitochondrial phenotypes that we found in PS-mutant cells and in cells from FAD patients were also observed in cells from SAD patients143, in which the PSEN1, PSEN2, and APP genes are normal. This latter result implies that from a mitochondrial point of view, both the familial and sporadic forms of the disease have a common pathogenetic origin. In this regard, we note that the most important genetic risk factor for developing SAD is a variant of apolipoprotein E (ApoE), a protein required to ferry cholesterol within lipoprotein particles: the ε4 allele (ApoE4) confers a significantly higher risk of developing AD than does the ε3 allele (ApoE3)152. It is noteworthy, therefore, that ER-mitochondrial communication and MAM function were increased significantly in fibroblasts and neurons treated with ApoE4-containing astrocyte-conditioned media as compared to those treated with ApoE3-containing astrocyte-conditioned media153. Moreover, in spite of no obvious qualitative defect in the APP or presenilin genes in SAD, C99 is nevertheless elevated in these patients154–156.
In summary, previous data and our own results point to a direct connection between APP processing and OxPhos deficiency via C99, both in FAD and SAD. Moreover, these data imply that at least from the mitochondrial point of view, it is C99, and not Aβ (nor any other APP processing product (e.g., sAPPα, C83, or P3)137), that is the key APP-processing intermediate that is required for pathogenicity.
Concluding remarks—the “MAM hypothesis”
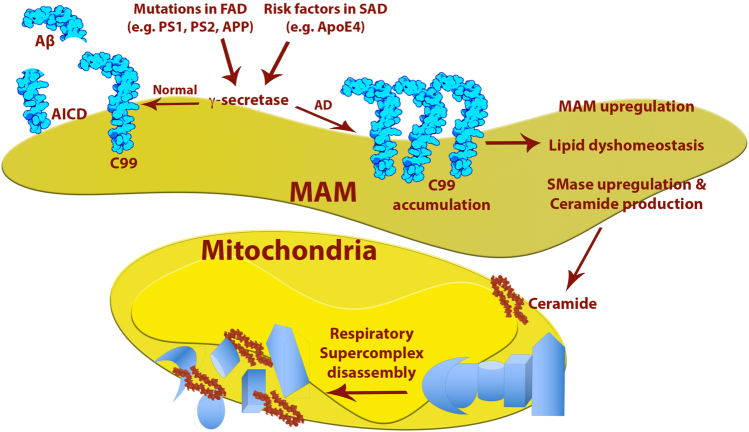
We believe that while mitochondrial dysfunction is an early and significant defect in AD, it is not a primary insult in the pathogenesis of the disease, but rather is a consequence of MAM dysfunction that is driven by an increased presence of C99 at MAM.
These results also imply that from the genetic standpoint, dominant mutations in PS1 likely result in haploinsufficiency157–159 or behave in a dominant-negative manner160 rather than resulting in a gain of function161, with the reduced PS1 activity as the likely cause of the increased levels of C99. In turn, increased MAM-localized C99 promotes the various features of the disease, including the calcium and lipid dyshomeostasis, the mitochondrial perturbations, and ultimately the plaque and tangle formation. In agreement with this view, the accumulation of C99 in mitochondrial fractions, and mitochondrial respiratory chain deficiency, has been detected in brains from animal models of AD162, and was reversed following deletion of BACE1 (thereby preventing the formation of C99)162. Finally, the idea that mitochondrial dysfunction is the result of the accumulation of C99 in the MAM rather than being a consequence of higher levels of Aβ42 helps explain previous results showing that mitochondrial alterations occur early in the pathogenesis of the disease58, well before any pathophysiological hallmark of AD becomes apparent57. Nevertheless, we believe that even though mitochondrial dysfunction is an early event upstream of plaque and tangle formation, we do not consider the organelle to be a reasonable target for therapeutic intervention, as the mitochondrial perturbations observed in AD are themselves consequences of an even earlier precipitating process, namely elevated C99 and altered lipid homeostasis (Fig. 3). Thus, it is possible that increased ER-mitochondrial connectivity and upregulated MAM behavior underlie the metabolic disturbances (and probably the other phenotypes) seen in AD122,143,163 (the “MAM hypothesis”115,116,127,128). We are currently actively engaged in deducing the mechanism(s) underlying these changes.
Fig. 3. Model of the “MAM hypothesis” for the pathogenesis of AD, with emphasis on the mitochondrial alterations.
See text for details
Finally, alterations in ER-mitochondrial communication and in MAM behavior may not be confined to AD. Other neurodegenerative disorders, such as Parkinson disease and amyotrophic lateral sclerosis, also evince altered mitochondrial function and disturbances in calcium and lipid homeostasis164. Notably, alterations in MAM behavior have also been found in both of these disorders, and are especially prominent in the familial form of these diseases, where a connection between the culprit gene and altered MAM behavior can be drawn165,166. Thus, altered ER-mitochondrial communication has the potential to play a critical, and hitherto unappreciated, role in the pathogenesis of many of the most common and devastating diseases of advanced age129.
Acknowledgements
This work was supported by grants from the U.S. Department of Defense (W911F-15-1-0169) and the J. Willard and Alice S. Marriott Foundation (to E.A.S.), the U.S. National Institutes of Health (K01AG045335 and R01AG056387 to E.A.-G.; 1R35 GM122589 and 3R01GM45735 to L.P.), Project ALS (to E.A.-G.), and IP Group (to E.A.-G. and E.A.S.).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Eduardo Bonilla is deceased.
Edited by P. Pinton.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 3.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 4.Nunomura A, et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 5.Manczak M, Sheiko T, Craigen WJ, Reddy PH. Reduced VDAC1 protects against Alzheimer’s disease, mitochondria, and synaptic deficiencies. J. Alzheimers Dis. 2013;37:679–690. doi: 10.3233/JAD-130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol. Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 2001;26:771–782. doi: 10.1023/A:1011603916962. [DOI] [PubMed] [Google Scholar]
- 8.Fraser T, Tayler H, Love S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem. Res. 2010;35:503–513. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- 9.Stefani M, Liguri G. Cholesterol in Alzheimer’s disease: unresolved questions. Curr. Alzheimer Res. 2009;6:15–29. doi: 10.2174/156720509787313899. [DOI] [PubMed] [Google Scholar]
- 10.Gómez-Ramos P, Asunción Morán M. Ultrastructural localization of intraneuronal Aβ-peptide in Alzheimer disease brains. J. Alzheimers Dis. 2007;11:53–59. doi: 10.3233/JAD-2007-11109. [DOI] [PubMed] [Google Scholar]
- 11.Pani A, et al. Altered cholesterol ester cycle in skin fibroblasts from patients with Alzheimer’s disease. J. Alzheimers Dis. 2009;18:829–841. doi: 10.3233/JAD-2009-1193. [DOI] [PubMed] [Google Scholar]
- 12.Pani A, et al. Accumulation of neutral lipids in peripheral blood mononuclear cells as a distinctive trait of Alzheimer patients and asymptomatic subjects at risk of disease. BMC Med. 2009;7:66–77. doi: 10.1186/1741-7015-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 14.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 16.Gillardon F, et al. Proteomic and functional alterations in brain mitochondria from Tg2576 mice occur before amyloid plaque deposition. Proteomics. 2007;7:605–816. doi: 10.1002/pmic.200600728. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, et al. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009;109:153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Stern D, Yan SD. Mitochondrial dysfunction and Alzheimer’s disease. Curr. Alzheimer Res. 2006;3:515–520. doi: 10.2174/156720506779025215. [DOI] [PubMed] [Google Scholar]
- 19.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spuch C, Ortolano S, Navarro C. New insights in the amyloid-beta interaction with mitochondria. J. Aging Res. 2012;2012:324968. doi: 10.1155/2012/324968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coskun P, et al. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim. Biophys. Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demetrius LA, Driver JA. Preventing Alzheimer’s disease by means of natural selection. J. R. Soc. Interface. 2015;12:20140919. doi: 10.1098/rsif.2014.0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sims NR, Finegan JM, Blass JP. Altered metabolic properties of cultured skin fibroblasts in Alzheimer’s disease. Ann. Neurol. 1987;21:451–457. doi: 10.1002/ana.410210507. [DOI] [PubMed] [Google Scholar]
- 27.Stokin GB, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 28.Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7:297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Santos RX, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. J. Alzheimers Dis. 2010;20(Suppl. 2):S401–S412. doi: 10.3233/JAD-2010-100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su B, et al. Abnormal mitochondrial dynamics—a novel therapeutic target for Alzheimer’s disease? Mol. Neurobiol. 2010;41:87–96. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young-Collier KJ, McArdle M, Bennett JP. The dying of the light: mitochondrial failure in Alzheimer’s disease. J. Alzheimers Dis. 2012;28:771–781. doi: 10.3233/JAD-2011-111487. [DOI] [PubMed] [Google Scholar]
- 32.DuBoff B, Feany M, Gotz J. Why size matters—balancing mitochondrial dynamics in Alzheimer’s disease. Trends Neurosci. 2013;36:325–335. doi: 10.1016/j.tins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Cai Q, Tammineni P. Alterations in mitochondrial quality control in Alzheimer’s disease. Front. Cell. Neurosci. 2016;10:24. doi: 10.3389/fncel.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancuso M, Siciliano G, Filosto M, Murri L. Mitochondrial dysfunction and Alzheimer’s disease: new developments. J. Alzheimers Dis. 2006;9:111–117. doi: 10.3233/JAD-2006-9203. [DOI] [PubMed] [Google Scholar]
- 35.Cabezas-Opazo FA, et al. Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2015;2015:509654. doi: 10.1155/2015/509654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redjems-Bennani N, et al. Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology. 1998;44:300–304. doi: 10.1159/000022031. [DOI] [PubMed] [Google Scholar]
- 37.Bosetti F, et al. Cytochrome c oxidase and mitochondrial F1Fo-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol. Aging. 2002;23:371–376. doi: 10.1016/S0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 38.Ohta S, Ohsawa I. Dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer’s disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. J. Alzheimers Dis. 2006;9:155–166. doi: 10.3233/JAD-2006-9208. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins HM, Swerdlow RH. Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2017;133:71–79. doi: 10.1016/j.brainresbull.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson GE, Huang HM. Mitochondrial enzymes and endoplasmic reticulum calcium stores as targets of oxidative stress in neurodegenerative diseases. J. Bioenerg. Biomembr. 2004;36:335–340. doi: 10.1023/B:JOBB.0000041764.45552.f3. [DOI] [PubMed] [Google Scholar]
- 41.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann. Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 42.Gibson GE, et al. Deficits in the mitochondrial enzyme α-ketoglutarate dehydrogenase lead to Alzheimer’s disease-like calcium dysregulation. Neurobiol. Aging. 2012;33:1121.e1113–1124. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirai K, et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manczak M, et al. Mitochondria are a direct site of Aβ accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 45.Nunomura A, et al. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 46.Du H, et al. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl Acad. Sci. USA. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Perry G, Smith MA, Zhu X. Amyloid-β-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener. Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim. Biophys. Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riemer J, Kins S. Axonal transport and mitochondrial dysfunction in Alzheimer’s disease. Neurodegener. Dis. 2013;12:111–124. doi: 10.1159/000342020. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, et al. Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl Acad. Sci. USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura T, Cieplak P, Cho DH, Godzik A, Lipton SA. S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion. 2010;10:573–578. doi: 10.1016/j.mito.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han XJ, et al. Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol. Med. Rep. 2017;16:4521–4528. doi: 10.3892/mmr.2017.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am. J. Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonda DJ, Wang X, Perry G, Smith MA, Zhu X. Mitochondrial dynamics in Alzheimer’s disease: opportunities for future treatment strategies. Drugs Aging. 2010;27:181–192. doi: 10.2165/11532140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devi G, et al. Novel presenilin 1 mutations associated with early onset of dementia in a family with both early-onset and late-onset Alzheimer disease. Arch. Neurol. 2000;57:1454–1457. doi: 10.1001/archneur.57.10.1454. [DOI] [PubMed] [Google Scholar]
- 57.Yao J, et al. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balietti M, et al. Early selective vulnerability of synapses and synaptic mitochondria in the hippocampal CA1 region of the Tg2576 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013;34:887–896. doi: 10.3233/JAD-121711. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalil B, et al. PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease. Cell Death Dis. 2015;6:e1617. doi: 10.1038/cddis.2014.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo X, et al. VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington’s disease. Nat. Commun. 2016;7:12646. doi: 10.1038/ncomms12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shan X, Chiang PM, Price DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc. Natl Acad. Sci. USA. 2010;107:16325–16330. doi: 10.1073/pnas.1003459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu YF, et al. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W, et al. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 2013;22:4706–4719. doi: 10.1093/hmg/ddt319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magrane J, Cortez C, Gan WB, Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum. Mol. Genet. 2014;23:1413–1424. doi: 10.1093/hmg/ddt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waterham HR, et al. A lethal defect of mitochondrial and peroxisomal fission. N. Engl. J. Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 67.Frieden M, et al. Ca2+ homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J. Biol. Chem. 2004;279:22704–22714. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- 68.Li XC, et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 2016;6:24756. doi: 10.1038/srep24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mancuso M, Calsolaro V, Orsucci D, Siciliano G, Murri L. Is there a primary role of the mitochondrial genome in Alzheimer’s disease? J. Bioenerg. Biomembr. 2009;41:411–416. doi: 10.1007/s10863-009-9239-1. [DOI] [PubMed] [Google Scholar]
- 70.Lakatos A, et al. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol. Aging. 2010;31:1355–1363. doi: 10.1016/j.neurobiolaging.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santoro A, et al. Evidence for sub-haplogroup H5 of mitochondrial DNA as a risk factor for late onset Alzheimer’s disease. PLoS ONE. 2010;5:e12037. doi: 10.1371/journal.pone.0012037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maruszak A, et al. The impact of mitochondrial and nuclear DNA variants on late-onset Alzheimer’s disease risk. J. Alzheimers Dis. 2011;27:197–210. doi: 10.3233/JAD-2011-110710. [DOI] [PubMed] [Google Scholar]
- 73.Ridge PG, et al. Mitochondrial haplotypes associated with biomarkers for Alzheimer’s disease. PLoS ONE. 2013;8:e74158. doi: 10.1371/journal.pone.0074158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corral-Debrinski M, et al. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23:471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- 75.Krishnan KJ, Ratnaike TE, De Gruyter HL, Jaros E, Turnbull DM. Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer’s disease. Neurobiol. Aging. 2012;33:2210–2214. doi: 10.1016/j.neurobiolaging.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Brown MD, et al. Mitochondrial DNA sequence analysis of four Alzheimer’s and Parkinson’s disease patients. Am. J. Med. Genet. 1996;61:283–289. doi: 10.1002/(SICI)1096-8628(19960122)61:3<283::AID-AJMG15>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 77.Hutchin T, Cortopassi G. A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc. Natl Acad. Sci. USA. 1995;92:6892–6895. doi: 10.1073/pnas.92.15.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc. Natl Acad. Sci. USA. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Podlesniy P, et al. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann. Neurol. 2013;74:655–668. doi: 10.1002/ana.23955. [DOI] [PubMed] [Google Scholar]
- 80.Lunnon K, et al. Mitochondrial genes are altered in blood early in Alzheimer’s disease. Neurobiol. Aging. 2017;53:36–47. doi: 10.1016/j.neurobiolaging.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 81.Zsurka G, et al. No mitochondrial haplotype was found to increase risk for Alzheimer’s disease. Biol. Psychiatry. 1998;44:371–373. doi: 10.1016/S0006-3223(97)00461-7. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez Santiago B, Casademont J, Nunes V. Is there a relation between Alzheimer’s disease and defects of mitochondrial DNA? Rev. Neurol. 2001;33:301–305. [PubMed] [Google Scholar]
- 83.Hudson G, et al. No consistent evidence for association between mtDNA variants and Alzheimer disease. Neurology. 2012;78:1038–1042. doi: 10.1212/WNL.0b013e31824e8f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Payami H, Hoffbuhr K. Lack of evidence for maternal effect in familial Alzheimer’s disease. Genet. Epidemiol. 1993;10:461–464. doi: 10.1002/gepi.1370100622. [DOI] [PubMed] [Google Scholar]
- 85.Ehrenkrantz D, et al. Genetic epidemiological study of maternal and paternal transmission of Alzheimer’s disease. Am. J. Med. Genet. 1999;88:378–382. doi: 10.1002/(SICI)1096-8628(19990820)88:4<378::AID-AJMG15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 86.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ankarcrona M, Hultenby K. Presenilin-1 is located in rat mitochondria. Biochem. Biophys. Res. Commun. 2002;295:766–770. doi: 10.1016/S0006-291X(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 88.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lustbader JW, et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 90.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hansson Petersen CA, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl Acad. Sci. USA. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pagani L, Eckert A. Amyloid-beta interaction with mitochondria. Int J. Alzheimers Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walls KC, et al. Swedish Alzheimer mutation induces mitochondrial dysfunction mediated by HSP60 mislocalization of amyloid precursor protein (APP) and beta-amyloid. J. Biol. Chem. 2012;287:30317–30327. doi: 10.1074/jbc.M112.365890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie H, et al. Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model. J. Neurosci. 2013;33:17042–17051. doi: 10.1523/JNEUROSCI.1836-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansson CA, et al. Nicastrin, presenilin, APH-1, and PEN-2 form active γ-secretase complexes in mitochondria. J. Biol. Chem. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- 96.Behbahani H, et al. Association of Omi/HtrA2 with γ-secretase in mitochondria. Neurochem. Int. 2010;57:668–675. doi: 10.1016/j.neuint.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 97.Pavlov PF, et al. Mitochondrial γ-secretase participates in the metabolism of mitochondria-associated amyloid precursor protein. FASEB J. 2011;25:78–88. doi: 10.1096/fj.10-157230. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi H, et al. HIG1, a novel regulator of mitochondrial γ-secretase, maintains normal mitochondrial function. FASEB J. 2012;26:2306–2317. doi: 10.1096/fj.11-196063. [DOI] [PubMed] [Google Scholar]
- 99.Robinson A, et al. Upregulation of PGC-1α expression by Alzheimer’s disease-associated pathway: presenilin 1/amyloid precursor protein (APP)/intracellular domain of APP. Aging Cell. 2014;13:263–272. doi: 10.1111/acel.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peterson C, Goldman JE. Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc. Natl Acad. Sci. USA. 1986;83:2758–2762. doi: 10.1073/pnas.83.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. β-Amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 102.Keil U, et al. Mitochondrial dysfunction induced by disease relevant AβPP and tau protein mutations. J. Alzheimers Dis. 2006;9:139–146. doi: 10.3233/JAD-2006-9206. [DOI] [PubMed] [Google Scholar]
- 103.Rhein V, et al. Amyloid-β leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell. Mol. Neurobiol. 2009;29:1063–1071. doi: 10.1007/s10571-009-9398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schaefer PM, von Einem B, Walther P, Calzia E, von Arnim CA. Metabolic characterization of intact cells reveals intracellular amyloid beta but not Its precursor protein to reduce mitochondrial respiration. PLoS ONE. 2016;11:e0168157. doi: 10.1371/journal.pone.0168157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mossmann D, et al. Amyloid-β peptide induces mitochondrial dysfunction by inhibition of preprotein maturation. Cell Metab. 2014;20:662–669. doi: 10.1016/j.cmet.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 106.Iijima-Ando K, et al. Mitochondrial mislocalization underlies Aβ42-induced neuronal dysfunction in a Drosophila model of Alzheimer’s disease. PLoS ONE. 2009;4:e8310. doi: 10.1371/journal.pone.0008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Area-Gomez E, et al. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 2009;175:1810–1816. doi: 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mamada N, Tanokashira D, Ishii K, Tamaoka A, Araki W. Mitochondria are devoid of amyloid β-protein (Aβ)-producing secretases: evidence for unlikely occurrence within mitochondria of Aβ generation from amyloid precursor protein. Biochem. Biophys. Res. Commun. 2017;486:321–328. doi: 10.1016/j.bbrc.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 109.Wada S, et al. γ-Secretase activity is present in rafts but is not cholesterol-dependent. Biochemistry. 2003;42:13977–13986. doi: 10.1021/bi034904j. [DOI] [PubMed] [Google Scholar]
- 110.Vetrivel KS, et al. Association of γ-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Urano Y, et al. Association of active γ-secretase complex with lipid rafts. J. Lipid Res. 2005;46:904–912. doi: 10.1194/jlr.M400333-JLR200. [DOI] [PubMed] [Google Scholar]
- 112.Cordy JM, Hooper NM, Turner AJ. The involvement of lipid rafts in Alzheimer’s disease. Mol. Membr. Biol. 2006;23:111–122. doi: 10.1080/09687860500496417. [DOI] [PubMed] [Google Scholar]
- 113.Zheng YZ, Berg KB, Foster LJ. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 2009;50:988–998. doi: 10.1194/jlr.M800658-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lue LF, et al. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/S0002-9440(10)65184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schon EA, Area-Gomez E. Is Alzheimer’s disease a disorder of mitochondria-associated membranes? J. Alzheimers Dis. 2010;20(Suppl. 2):S281–S292. doi: 10.3233/JAD-2010-100495. [DOI] [PubMed] [Google Scholar]
- 116.Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 117.Newman M, et al. Differential, dominant activation and inhibition of Notch signalling and APP cleavage by truncations of PSEN1 in human disease. Hum. Mol. Genet. 2014;23:602–617. doi: 10.1093/hmg/ddt448. [DOI] [PubMed] [Google Scholar]
- 118.Schreiner B, Hedskog L, Wiehager B, Ankarcrona M. Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 2015;43:369–374. doi: 10.3233/JAD-132543. [DOI] [PubMed] [Google Scholar]
- 119.Del Prete D, et al. Localization and processing of the amyloid-β protein precursor in mitochondria-associated membranes. J. Alzheimers Dis. 2017;55:1549–1570. doi: 10.3233/JAD-160953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Annaert WG, et al. Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J. Cell Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hayashi T, Su TP. Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Subcell. Biochem. 2010;51:381–398. doi: 10.1007/978-90-481-8622-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Area-Gomez E, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31:4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hedskog L, et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc. Natl Acad. Sci. USA. 2013;110:7916–7921. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pinho CM, Teixeira PF, Glaser E. Mitochondrial import and degradation of amyloid-β peptide. Biochim. Biophys. Acta. 2014;1837:1069–1074. doi: 10.1016/j.bbabio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Contino S, et al. Presenilin 2-dependent maintenance of mitochondrial oxidative capacity and morphology. Front. Physiol. 2017;8:796. doi: 10.3389/fphys.2017.00796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Area-Gomez E, Schon EA. Mitochondria-associated ER membranes and Alzheimer disease. Curr. Opin. Genet. Dev. 2016;38:90–96. doi: 10.1016/j.gde.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Area-Gomez E, Schon EA. On the pathogenesis of Alzheimer’s disease: the MAM Hypothesis. FASEB J. 2017;31:864–867. doi: 10.1096/fj.201601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krols M, et al. Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol. 2016;131:505–523. doi: 10.1007/s00401-015-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paillusson S, et al. There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simmen T, Lynes EM, Gesson K, Thomas G. Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM) Biochim. Biophys. Acta. 2010;1798:1465–1473. doi: 10.1016/j.bbamem.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giorgi C, et al. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2015;22:995–1019. doi: 10.1089/ars.2014.6223. [DOI] [PubMed] [Google Scholar]
- 133.Giacomello M, Pellegrini L. The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ. 2016;23:1417–1427. doi: 10.1038/cdd.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- 135.Puglielli L, et al. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 136.Puglielli L, Ellis BC, Ingano LA, Kovacs DM. Role of acyl-coenzyme A:cholesterol acyltransferase activity in the processing of the amyloid precursor protein. J. Mol. Neurosci. 2004;24:93–96. doi: 10.1385/JMN:24:1:093. [DOI] [PubMed] [Google Scholar]
- 137.Lopez Sanchez MIG, et al. Amyloid precursor protein drives down-regulation of mitochondrial oxidative phosphorylation independent of amyloid beta. Sci. Rep. 2017;7:9835. doi: 10.1038/s41598-017-10233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patergnani S, et al. Calcium signaling around mitochondria associated membranes (MAMs) Cell. Commun. Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tasseva G, et al. Phosphatidylethanolamine deficiency in mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 2013;288:4158–4173. doi: 10.1074/jbc.M112.434183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Theurey P, et al. Mitochondria-associated endoplasmic reticulum membranes allow adaptation of mitochondrial metabolism to glucose availability in the liver. J. Mol. Cell Biol. 2016;8:129–143. doi: 10.1093/jmcb/mjw004. [DOI] [PubMed] [Google Scholar]
- 142.Passer B, et al. Generation of an apoptotic intracellular peptide by γ-secretase cleavage of Alzheimer’s amyloid β protein precursor. J. Alzheimers Dis. 2000;2:289–301. doi: 10.3233/JAD-2000-23-408. [DOI] [PubMed] [Google Scholar]
- 143.Pera M, et al. Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. EMBO J. 2017;36:3356–3371. doi: 10.15252/embj.201796797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br. Med. Bull. 1997;53:539–553. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 145.Yu J, et al. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J. Biol. Chem. 2007;282:25940–25949. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- 146.Monette JS, et al. R)-α-Lipoic acid treatment restores ceramide balance in aging rat cardiac mitochondria. Pharmacol. Res. 2011;63:23–29. doi: 10.1016/j.phrs.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trounce IA, Crouch PJ, Carey KT, McKenzie M. Modulation of ceramide-induced cell death and superoxide production by mitochondrial DNA-encoded respiratory chain defects in Rattus xenocybrid mouse cells. Biochim. Biophys. Acta. 2013;1827:817–825. doi: 10.1016/j.bbabio.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 148.Zigdon H, et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 2013;288:4947–4956. doi: 10.1074/jbc.M112.402719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kogot-Levin A, Saada A. Ceramide and the mitochondrial respiratory chain. Biochimie. 2014;100:88–94. doi: 10.1016/j.biochi.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 150.Behbahani H, et al. Differential role of presenilin-1 and -2 on mitochondrial membrane potential and oxygen consumption in mouse embryonic fibroblasts. J. Neurosci. Res. 2006;84:891–902. doi: 10.1002/jnr.20990. [DOI] [PubMed] [Google Scholar]
- 151.Wang Y, et al. Lost region in amyloid precursor protein (APP) through TALEN-mediated genome editing alters mitochondrial morphology. Sci. Rep. 2016;6:22244. doi: 10.1038/srep22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tambini MD, et al. ApoE4 upregulates the activity of mitochondria-associated ER membranes. EMBO Rep. 2016;17:27–36. doi: 10.15252/embr.201540614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. β-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 155.Li R, et al. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl Acad. Sci. USA. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pera M, et al. Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol. 2013;125:201–213. doi: 10.1007/s00401-012-1062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heilig EA, Gutti U, Tai T, Shen J, Kelleher RJ., 3rd Trans-dominant negative effects of pathogenic PSEN1 mutations on γ-secretase activity and Aβ production. J. Neurosci. 2013;33:11606–11617. doi: 10.1523/JNEUROSCI.0954-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kepp KP. Alzheimer’s disease due to loss of function: a new synthesis of the available data. Prog. Neurobiol. 2016;143:36–60. doi: 10.1016/j.pneurobio.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 159.Sun L, Zhou R, Yang G, Shi Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl Acad. Sci. USA. 2017;114:E476–E485. doi: 10.1073/pnas.1618657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou R, Yang G, Shi Y. Dominant negative effect of the loss-of-function γ-secretase mutants on the wild-type enzyme through heterooligomerization. Proc. Natl Acad. Sci. USA. 2017;114:12731–12736. doi: 10.1073/pnas.1713605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kretner B, et al. Generation and deposition of Aβ43 by the virtually inactive presenilin-1 L435F mutant contradicts the presenilin loss-of-function hypothesis of Alzheimer’s disease. EMBO Mol. Med. 2016;8:458–465. doi: 10.15252/emmm.201505952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the β-secretase-cleaved C-terminal fragment of APP in Alzheimer’s disease transgenic mice. Neurobiol. Dis. 2012;45:417–424. doi: 10.1016/j.nbd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leal NS, et al. Mitofusin-2 knockdown increases ER-mitochondria contact and decreases amyloid β-peptide production. J. Cell. Mol. Med. 2016;20:1686–1695. doi: 10.1111/jcmm.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Watanabe S, et al. Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol. Med. 2016;8:1421–1437. doi: 10.15252/emmm.201606403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hattori N, Arano T, Hatano T, Mori A, Imai Y. Mitochondrial-associated membranes in Parkinson’s disease. Adv. Exp. Med. Biol. 2017;997:157–169. doi: 10.1007/978-981-10-4567-7_12. [DOI] [PubMed] [Google Scholar]