Abstract
Ghrelin is an appetite-stimulating peptide. Serine 3 on ghrelin must be acylated by octanoate via the enzyme ghrelin-O-acyltransferase (GOAT) for the peptide to bind and activate the cognate receptor, Growth hormone secretagogue receptor type 1a (GHSR1a). Interest in GHSR1a increased dramatically when GHSR1a mRNA was demonstrated to be wide-spread in the brain, including the cortex and hippocampus, indicating that it has multi-faceted functions beyond the regulation of metabolism. However, the source of octanoylated ghrelin for GHSR1a in the brain, outside of the hypothalamus, is not well understood. Here we report the presence of GOAT and its ability to acylate non-octanoylated ghrelin in the hippocampus. GOAT immunoreactivity is aggregated at the base of the dentate granule cell layer in the rat and wild-type mouse. This immunoreactivity was not affected by the pharmacological inhibition of GHSR1a or the metabolic state-dependent fluctuation of systemic ghrelin levels. However, it was absent in the GHSR1a knockout mouse hippocampus, pointing the possibility that the expression of GHSR1a may be a pre-requisite for the production of GOAT. Application of fluorocein isothiocyanate (FITC)-conjugated non-octanoylated ghrelin in live hippocampal slice culture (but not in fixed culture or in the presence of GOAT inhibitors) mimicked the binding profile of FITC-conjugated octanoylated ghrelin, suggesting that extracellularly-applied non-octanoylated ghrelin was acylated by endogenous GOAT in the live hippocampus while GOAT being mobilized out of neurons. Our results will advance the understanding for the role of endogenous GOAT in the hippocampus and facilitate the search for the source of ghrelin that is intrinsic to the brain.
Keywords: Ghrelin-O-acyltransferase (GOAT), organotypic slice culture, GHSR1a KO, immunohistochemistry, fluorescent ghrelin, dentate gyrus
Graphical Abstract
In order to elucidate a cellular mechanism for the ghrelin-mediated regulation of hippocampal function, 0251663360251659264251660288251662336251661312251667456251666432251665408251664384we demonstrate that endogenous ghrelin-O-acyltransferase (GOAT) is present in the dentate gyrus of the hippocampus and biologically active to acylate local des-acyl ghrelin. Failure of detecting GOAT immunoreactive products in the Growth hormone secretagogue receptor type 1a (GHSR1a) KO mouse hippocampus suggests that the expression of GHSR1a is likely a prerequisite for GOAT production in the hippocampus.
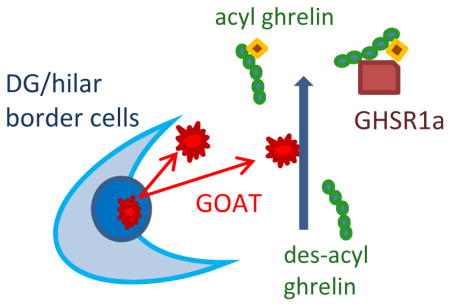
Introduction
Increasing evidence suggests that the stomach hormone ghrelin may have numerous physiological functions in the brain in addition to its primary role in stimulating hypothalamic orexigenic neurons (Massadi et al. 2017). Among these functions is the enhancement of learning and memory (Albarran-Zeckler et al. 2011; Andrews 2010; Ferrini et al. 2009). The intra-hippocampal application of ghrelin improves memory retention (Carlini et al, 2010), increases dendritic spine density (Berrout and Isokawa 2012), phosphorylates the NMDA receptor subunit (Muniz and Isokawa 2015), and stimulates synapse formation (Diano et al. 2006). These reports have aroused interest in the ghrelin-mediated regulation of hippocampal function.
The major active product of the ghrelin gene is the 28-amino acid peptide acylated at Serine 3 (Ser3), which is simply called ghrelin (Kojima et al. 1999). However, the ghrelin gene can generate various molecules in addition to ghrelin, including des-acyl ghrelin, C-ghrelin, obestatin, and des-Gln14 ghrelin (Nishi et al. 2011). Des-acyl ghrelin is a non-acylated form of the ghrelin peptide and exists in far greater amounts in the blood and stomach; over 90% of the immunoreactivity for ghrelin corresponds to this form (Hosoda et al 2000). When ghrelin is acyl-modified, it is referred as n-octanoyl ghrelin (also known as octanoylated ghrelin). This acylation at Ser3 is essential for ghrelin’s activity through GHSR1a. In other words, the post-translational acylation by GOAT is required for ghrelin to activate GHSR1a (Yang et al. 2008a). Des-acyl ghrelin or other ghrelin-associated peptides such as obestatin cannot interact with and directly stimulate GHSR1a.
Ghrelin is produced and released from the stomach. Ghrelin crosses the blood-brain barrier and enter the brain including the hippocampus (Diano et al. 2006). This suggests the possibility that ghrelin-mediated modulation of hippocampal neuron activity and synaptic transmission may be under the regulation of peripherally-circulating ghrelin. However, there is a report in mice that acylated ghrelin was readily transported across the blood-brain barrier in the brain-to-blood direction, but the quantity of its transport in the blood-to-brain direction appeared negligible (Banks et al. 2002). Furthermore, vagotomy prevented peripheral ghrelin’s effect on the hypothalamus (Date et al. 2002). Together they suggest that ghrelin’s direct effect on the brain may be of intrinsic origin (Nakazato et al. 2001; Tschop et al. 2000).
Ghrelin peptides are robustly expressed in axons and associates with dense-core vesicles in presynaptic terminals in the hypothalamus (Cowley et al. 2003). Septal neurons and their axons also express ghrelin peptides (Cowley et al. 2003). There is a well-established septo-hippocampal projection in which septal neurons send their axons to the hippocampus and establish synaptic contacts with hippocampal neurons (Huh et al. 2010). In the hippocampus, the source of ghrelin could be a release from presynaptic septal neurons into hippocampal neuron synapses. The presence of ghrelin in the hippocampal neurons was also reported recently (Russo et al. 2017). In this report, we show the local acylation of ghrelin by endogenous GOAT in the hippocampus, where GHSR1a mRNA (Zigman et al. 2006) and GFP-labeled GHSR1a proteins (Mani et al. 2014) are robustly expressed.
Materials and Methods
Animals
Sprague-Dawley rats (RRID: RGD_5508397), GHSR1a knock-out mice (B6N(Cg)-Ghsrtm1.1(KOMP)Vlcg/J/Stock# 018595, RRID not registered), and the same strain of wild type mice were used. The rats weighing 100–250g were purchased from Charles River (Wilmington, MA), and the transgenic mice weighing 20–40g were purchased from Jackson Laboratory (Bar Harbor, ME). They were housed in a group cage (up to 3 rats per cage and up to 5 mice per case). A nursing female rat with 12–18 pups and a nursing female mouse with 3–8 pups were housed in an individual cage with pups. Food and water were available ad lib. The time-line of the study design/experimental procedure is depicted in Table 1.
Table 1.
The time-line of the study design/experimental procedure
| Immunohistochemistry for ghrelin and GOAT | |
| Day 1 | Placement of P5 rat pups under 18-h fasting |
| Day 2 | Group 1: Preparation of hippocampal slice culture from P6 pups of control and 18-h fasted rats, GHSR1a KO mice, and wild-type mice |
| Group 2: Cardiac perfusion of age-matched pups from control and 18-h fasted rats | |
| Day 3–7 | Group 1: Incubation and culturing of slices prepared in Day 2 |
| Day 8 | Group 1: Overnight fixation of cultured slices (check condition/viability of slices prior to fixation) In some experiments, antagonists/inhibitor will be applied before fixation |
| Group 2: Cryo-sectioning of cardially-fixed brains | |
| Day 9–10 | Application of primary antibody to fixed slices and cryo-sectioned fixed brain specimens |
| Day 11 | Application of secondary antibody and coverslip |
| Day 12 | Imaging and data acquisition with a confocal microscope |
| Fluorescent ghrelin binding in fixed slices | |
| Day 1 | Preparation of hippocampal slice culture from P6 pups of control rats, GHSR1a KO mice, and wild-type mice |
| Day 2–6 | Incubation and culturing of slices prepared in Day 1 |
| Day 7 | Overnight fixation of cultured slices (check condition/viability of slices prior to fixation) In some experiments, antagonists/inhibitor will be applied before fixation. |
| Day 8 | Two-hour application of F302 to one-half of the fixed slices and F203 to the other half of fixed slices |
| Day 9 | Imaging and data acquisition with a confocal microscope |
| Fluorescent ghrelin binding in live slices | |
| Day 1 | Prepare hippocampal slice culture from P6 pups of control rats, GHSR1a KO mice, and wild-type mice |
| Day 2–6 | Incubation and culturing of slices prepared in Day 1 |
| Day 7 | Check condition/viability of slices In some experiments, antagonists/inhibitor will be applied. |
| Day 8 | Overnight application of F302 to one-half of the live slices and F203 to the other half of live slices |
| Day 9 | Imaging and data acquisition from live slices with a confocal microscope |
Hippocampal slice culture
Animals (P6 pups of both sexes) were deeply anesthetized with halothane and decapitated. This protocol was approved by the University of Texas at Rio Grande Valley Institutional Animal Care and Use Committee (IACUC) (Ethical approval reference number: 2016-001-IACUC, 2013-002-IACUC) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). The study was not pre-registered. Both sexes were used because there are no reports to date that indicate the presence of any sexual dimorphism in the expression or function of GOAT in the immature rodent brain. The following measures were taken to minimize animals’ suffering during experiments: restlessness, vocalizing, loss of mobility, failure to groom, open sores/necrotic skin lesions, guarding (including licking and biting) a painful area, and a change in body color. In case any of these signs were observed, they were excluded from further participation and treated appropriately according to the approved protocol.
Brains were quickly immersed in ice cold artificial cerebrospinal fluid (ACSF) consisting of (in mM): 124 NaCl (Cat. #S7653), 3 KCl (Cat. #P9333), 2 CaCl2 (Cat.# C8106), 2 Mg2SO4 (Cat.# M2643), 1.24 NaH2PO4 (Cat.# S5011), 26 NaHCO3 (Cat.# S5761) and 10 glucose (Cat.# D8066) (all from Sigma Chemicals/Merk, MO). Hippocampi from both hemispheres were dissected and sliced into 400 μm thick sections using a chopper (Stoelting, Wood Dale, IL). Slices were placed on Transwell culture insert (Costar/Corning, NY) and incubated in culture media consisted of: 50% MEM (Cat.# 11090-081, Gibco/ThermoFisher, CA), 25% HBSS (Cat.# 14170161, Gibco/ThermoFisher, CA), 24% horse serum (Cat.#26050088, Gibco/ThermoFisher, CA), 0.5% penicillin/streptomycin solution (Cat.# 15140122, Gibco/ThermoFisher, CA), 0.5% glucose solution, and 25 mM HEPES (Cat.# H3375, Sigma-Aldrich/Merk, MO) with 5% CO2 at 35°C for up to 3 weeks (Stoppini et al. 1991).
Slice culture was used because: 1) chemical effect of GOAT and its agonists and antagonists can be assessed directly within the hippocampus in isolation while eliminating multi-synaptic circuit activities projected from extra-hippocampal regions, and 2) acutely-prepared slices may reflect a fluctuating level of systemic ghrelin at the time of decapitation among animals, as systemic ghrelin may cross the blood brain barrier (Muller et al. 2015). On the other hand, slice culture can provide a stable control level in order to test the effect of exogenously-applied ghrelin on hippocampal neurons, independent of systemic ghrelin.
In the present study, following compounds were used: octanoylated ghrelin (1 μM, 10 μM, 50 μM, and 100 μM, Cat.# 031-31, Phoenix Pharmaceuticals, CA), D-Lys3-GHSR6 (10 μM, Cat.#1922, Tocris, Bristol, UK), Go-CoA-Tat (5 μM, Cat.#032-37, Phoenix Pharmaceuticals, CA). Viability was examined in every slice culture before experimentation. Only those slices in which the boundary of individual hippocampal cell layers were clearly identifiable and individual neurons were visible with differential interference contrast (DIC) and water-immersion objectives were used for experiments. Regarding the group assignment, no randomization was performed. No blinding was performed during experiments.
In vivo fasting experiment
A few pups from each litter that was used to prepare the hippocampal slice culture were spared and raised as an age-matched live subject. Some of those pups were placed under an 18 hour fasting condition (in which they were deprived of milk and received an i.p. injection of 1 ml saline every 6 hours, prior to cardiac perfusion).
Fluorescent ghrelin binding
Octanoylated form of FITC-conjugated ghrelin (F302) and non-octanoylated form of FITC-conjugated ghrelin (F203) were provided by Dr. Len Luyt at the University of Western Ontario in Canada (McGirr et al. 2011). We used live and fixed slice culture specimens of rats and mice. In live slices, 1 μM of F302- or F203-containing culture media were prepared, and hippocampal slices were incubated for 2 hours to overnight. At the end of application, slices were imaged live or fixed before imaging with 4% paraformaldehyde in phosphate buffered saline (PBS), rinsed, and glass-mounted. In fixed slices, 1 μM of F302- or F203-containing 0.1 M phosphate buffered saline (PBS) was prepared, and slices were incubated for 2 hours. At the end of the incubation, slices were rinsed and glass-mounted. Results were examined with a confocal microscope (Fluoview 1000, Olympus, Center Valley, PA).
Immunohistochemistry
Two types of preparations were used: 1) Cryo-sectioned whole brain specimens taken from cardially-perfused rats and mice, and 2) immersion-fixed hippocampal slice culture specimens of rats and mice. The fixative was 4% paraformaldehyde in 0.1 M PBS. For both types of preparations, specimens were collected in PBS, treated with 0.2% Triton-X100 and 10% BSA for 1 hour, and incubated in a rabbit polyclonal anti-GOAT (1:1000, Cat.# H032-12, Phoenix Pharmaceuticals, CA; RRID not registered) or a rabbit polyclonal anti-ghrelin (1:500, Cat.# H031-31, Phoenix Pharmaceuticals, CA; RRID not registered) for 48 hours at 4°C with gentle agitation. Slices were then incubated in Alexa 488-conjugated secondary antibody (1:200, Cat.#A-11034, Molecular Probes/Life Technologies, NY) for 1 hour at room temperature, or in an HRP-conjugated secondary antibody (1:200, Cat.# 31460, Molecular Probes/Life Technologies, NY) and diaminobenzidin (DAB) (Cat.# D8001, Sigma chemical, MO). For control, we used a blocking peptide, omitted a primary antibody, or simultaneously applied primary and secondary antibodies. Slices were glass-mounted and imaged at a single cell resolution using a confocal microscope. No dual labeling was attempted. The GOAT antibody that we used (H032-12 by Phoenix Pharmaceutical) has been validated for its specificity by Hopkins and colleagues (2017). The ghrelin antibody that we used (H031-31 by Phoenix Pharmaceutical) has been validated for its specificity by Grönberg et al. (2017) and Wierup et.al. (2004).
Quantification of immunoreactivity and fluorescent ghrelin binding
HRP-DAB reaction products were imaged using a CCD camera (Hamamatsu C-2400, Japan) and the NIH Image-J software. Fluorescent signals were processed with a confocal microscope and the Fluoview-300 imaging software (Olympus, Center Valley, PA). Confocal images were acquired by 10x objectives and 40x objectives, starting with low magnification. In some cases, 40x objectives were combined with a zoom function. Single scanning was used. No repeated exposures, such as Kalman filtering in order to increase the signal-to-background contrast, were attempted. Laser power was kept constant under a given magnification among different experiments till all specimens were scanned completely. Acquired images were stored in the Olympus Fluoview format for off-line analysis.
Fluorescent ghrelin binding signals and immunoreactive products were identified and selected as a region of interest (ROI) using an auto-segmentation tool under the Triangle logic provided by the IPLab image analysis software (Scanalytics/BD Bioscience, San Jose, CA). Area of individual ROIs were summed and used to indicate the magnitude of immunoreactivity or the ligand binding (see Fig. 1b2 and c2, Fig. 3i inset, and Fig. 4i inset).
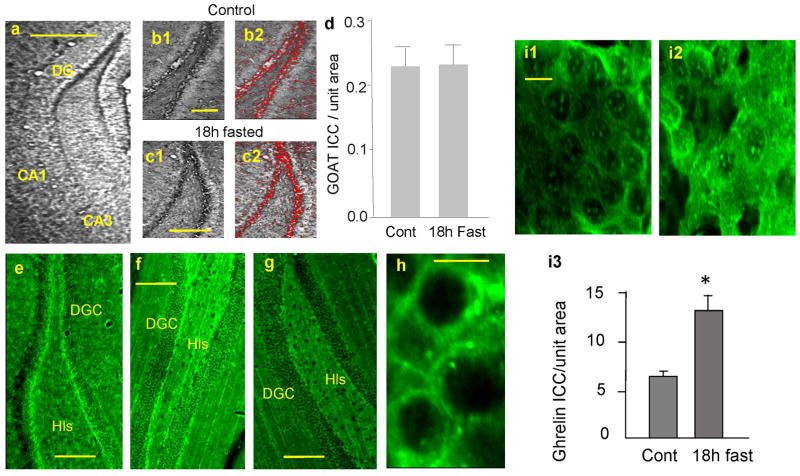
Figure 1.
GOAT immunoreactivity (IR) in cardially-perfused and cryosectioned hippocampus. a. diaminobenzidin (DAB) reaction product in the dentate gyrus (DG), cornu ammonis 3 (CA3) and (cornu ammonis 1) CA1. b1. GOAT-IR in control rat DG. b2. ROIs of b1. c1. GOAT-IR in 18 hour-fasted rat DG. c2. ROIs of c1. d. Summary of GOAT-IR in DG in control (n=10 rats) and 18 hour-fasted (n=10 rats). *p<0.001. e. GOAT-IR with Alexa 488 in control rat DG. f. GOAT-IR in wild type mouse DG. g. GOAT-IR in GHSR1a knockout (KO) mouse DG. h. Localization of GOAT-IR in punctate structures in DG with higher magnification. Calibrations: 500 μm in a, 300 μm in b1 (which is shared with b2, c1, and c2), 300 μm in e, 200 μm in f and g, 10 μm in h. i. Ghrelin immunoreactivity (Ghrelin-IR) in DG in control (i1) and 18 hour fasted (i2) rats. Ghrelin-IR was increased in DG in 18 hour fasted rats (i3: n=8 rats in each experimental group, *p<0.001).
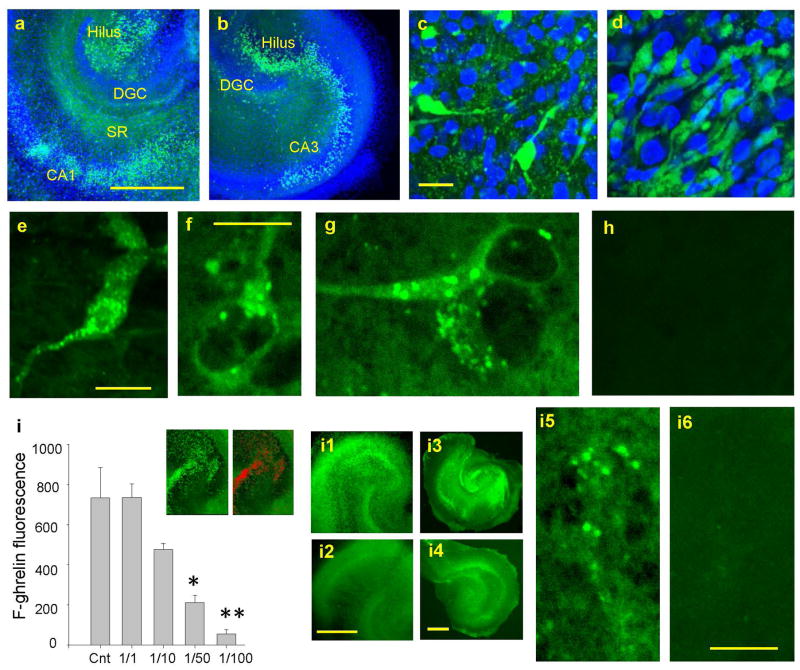
Figure 3.
Fluorescent ghrelin binding in the fixed hippocampal slice culture. a. FITC-conjugated acyl-ghrelin (F302)-binding (in green) in the hilus and CA1 with DAPI staining (in blue). b. F302 binding in CA3 with DAPI staining. c. F302 and DAPI in the hilus. d. F302 and DAPI in CA1. e, f, and g. F-ghrelin binding in small punctate structures on the soma and proximal processes. h. FITC-conjugated non-acyl-ghrelin (F203)-binding. i. Competitive binding of F302 with “cold” acyl-ghrelin in control (F302 alone) (n=10 rats) and with the ratios of F/C = 1/1 (10 rats), 1/10 (10 rats), 1/50 (10 rats), and 1/100 (10 rats). Inset in i: Quantification of F302 signals as red ROI. i1. F302 binding in control live slice. i2. F/C=1/50 in live slices. i3 and i5. F302 binding in control fixed slices. i4 and i6. F/C=1/50 in fixed slices. Calibrations: 500 μm in a (shared with b), 10 μm in c (shared with d), 15 μm in e, 10 μm in f, 500 μm in i2 (shared with i1), 500 μm in i4 (shared with i3), and 10 μm in i6 (shared with i5). *p<0.01, **p<0.0005.
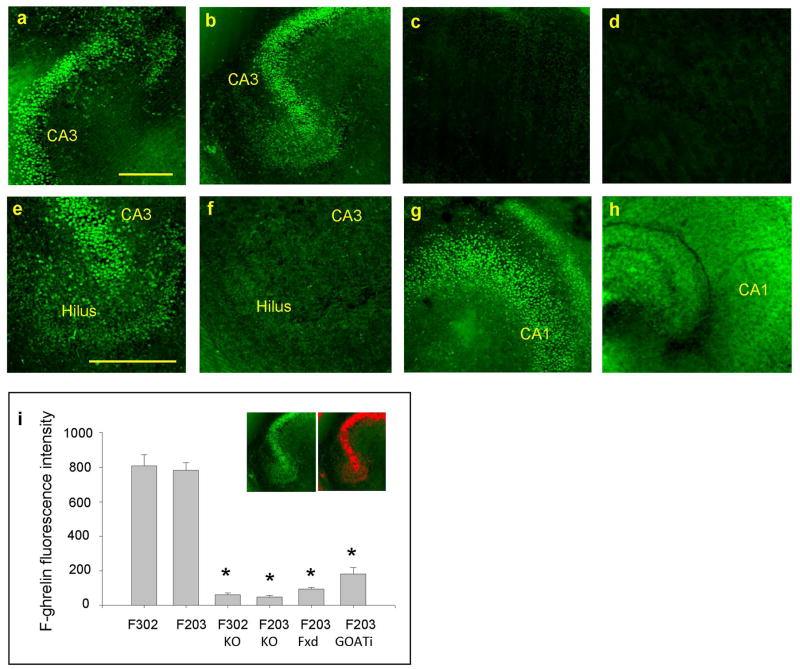
Figure 4.
Non-octanoylated ghrelin (F203) binding in live hippocampal slice culture. a. Overnight incubation of live rat hippocampal slice in F302. b. Overnight incubation of live rat hippocampal slice in F203. c. Overnight incubation of GHSR1a KO live slice in F302. d. Overnight incubation of GHSR1a KO live slice in F203. e. Overnight incubation of fixed rat slice culture in F302. f. Overnight incubation of fixed rat slice culture in F203. g. Overnight incubation of live rat hippocampal slice in GOAT inhibitor with F302. h. Overnight incubation of live rat hippocampal slice in GOAT inhibitor with F203. i. Summary of results: F302 (n=15 rats), F203 (n=15 rats), F302 in GHSR1a KO (n=7 mice), F203 in GHSR1a KO (n=7 mice), F203 in fixed slices (n=7 rats), and F203 in GOAT inhibitor (n=7 rats). Inset in i depicts FITC signals in red ROIs. Calibrations: 300 μm in a (shared with b, c, and d). 400 μm in e (shared with f, g, and h). * p<0.001.
Statistical Analysis
We used Sigma Plot 9.0 and Sigma Stat 4.0 to test statistical significance. Results were presented as mean ± SEM (standard error of the mean). Differences observed between experimental groups and control group were tested with an unpaired student t-test or one-way Analysis of Variance and Kruskal-Wallis One Way Analysis of Variance on Ranks. Normality was checked with the Kolmogorov-Smirnov test. All Pairwise Multiple Comparison Procedures were done with Tukey Test. P < 0.05 was considered significant. No sample calculation was performed.
Results
Localization of GOAT in the hippocampus
In the cardially-perfused and coronally-cryosectioned whole brain of rats and wild-type mice, GOAT immunoreactivity (GOAT-IR) was detected as DAB reaction products (Fig. 1a) and as green fluorescent signals (Fig. 1e and f) at the base of the dentate granule cell layer in the hippocampus. GOAT-IR was localized in small punctate structures surrounding the cell soma (Fig. 1h). GOAT-IR was detected mostly in the dentate gyrus at the hilar border. Interestingly, GOAT-IR was absent in the GHSR1a knockout (KO) mouse hippocampus (Fig. 1g), while the wild-type mouse (Fig. 1f) showed clearly-detectable GOAT-IR at the base of the dentate granule cell layer, similarly to the rat hippocampus (Fig. 1e). GOAT-IR stood above the background level and clearly detectable as ROIs when processed by the IPLab image analysis software (Fig 1b2 and c2).
We tested whether GOAT was affected by the availability of des-acyl ghrelin. It has been shown that both acyl ghrelin and des-acyl ghrelin were elevated in 16 hour-fasted rats (Nakahara et al., 2010). Therefore, GOAT-IR was examined in the hippocampus of control (Fig. 1b) and 18 hour-fasted (Fig. 1c) rats by selecting GOAT-IR as red ROIs (Fig. 1b2 and c2). There was no difference in the magnitude of GOAT expression between control and fasted rat hippocampi (Fig. 1d). Next, we processed alternate sections for ghrelin immunohistochemistry. Ghrelin immunoreactivity (Ghrelin-IR) was localized in the cell soma (Fig. 1i1 and i2). When quantified by selecting and summing ROIs, ghrelin-IR was increased in the hippocampus of 18-hour-fasted rats when compared with control (Fig. 1i3) (t=5.219, p<0.001). It remains unknown whether the increased ghrelin-IR reflected an increase in acyl ghrelin or des-acyl ghrelin because our antibody, which was raised against acyl ghrelin, was 100% cross-reactive to des-acyl ghrelin. Nevertheless, our data suggested that hippocampal GOAT expression was independent of fasting, systemic ghrelin, and the magnitude of ghrelin-IR in the hippocampal neuron.
Localization of GOAT in the hippocampal slice culture
In order to examine whether GOAT was intrinsic to the hippocampus, GOAT immunoreactivity (GOAT-IR) was tested in the hippocampus in isolation by preparing organotypic slice culture. In agreement with our findings in the whole brain sections, GOAT-IR was detected at the base of the dentate granule cell layer in the rat hippocampal slices (Fig. 2a). GOAT-IR was also observed in the hippocampal slice culture that was prepared from WT mouse (Fig. 2d). On the other hand, GOAT-IR was significantly lower in the GHSR1a KO mouse slices (Fig. 2e and f). The result not only supported our finding made in the whole brain sections, but also suggested that the expression of local GHSR1a was critical for the production of GOAT in the hippocampus.
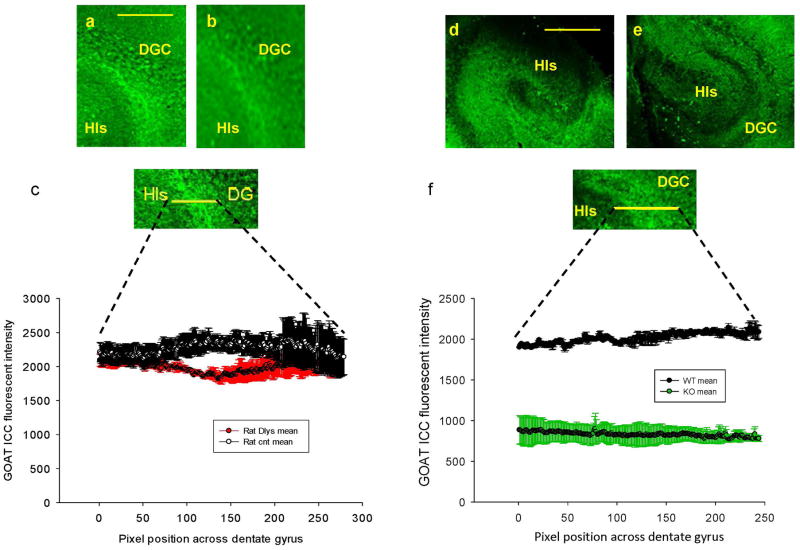
Figure 2.
GOAT immunoreactivity (IR) in organotypic slice culture. a. GOAT-IR in control rat slice. b. GOAT-IR in D-Lys3-GHRP-6 treated rat slice. c. Summary of GOAT-IR in control (n=8 rats) and D-Lys3-GHRP-6 treated (n=8 rats) slices. d. GOAT-IR in wild-type (WT) mouse slice. e. GOAT-IR in GHSR1a KO mouse slice. f. Summary of GOAT-IR in WT (n=8 mice) and GHSR1a KO (n=8 mice) slices. The reduction in IR in GHSR1a KO slices was significant (p<0.001). Calibrations: 300 μm in a and b, 200 μm in c, 150 μm in d, and 70 μm in e.
We asked whether stable GHSR1a signaling was necessary for the production of GOAT. We incubated live slice cultures prepared from rat hippocampus overnight in the inhibitor of GHSR1a, D-Lys3-GHRP-6 (Fig. 2b). Application of GHSR1a antagonist did not reduce GOAT -IR. As shown in Figure 2c, when GOAT-IR was measured across the dentate-hilar axis, the magnitude of GOAT-IR was similar between control and D-Lys3-GHRP-6 treatment. On the other hand, the magnitude of GOAT-IR in GHSR1a KO mouse hippocampus was decreased substantially (p<0.001) (Fig. 2f). Based on this finding, we concluded that, although stable expression of GHSR1a appeared to be a prerequisite for GOAT to be produced in the hippocampus, short-term block of GHSR1a signaling by the overnight application of receptor inhibitor did not disturb GOAT production in the hippocampus.
Fluorescent ghrelin binding in the hippocampal slice culture
We studied binding profiles of FITC-conjugated fluorescent ghrelin in our hippocampal slice culture. It was hypothesized that octanoylated ghrelin (acyl ghrelin) would outline the localization of GHSR1a fluorescently by binding to GHSR1a, while non-octanoylated ghrelin (des-acyl ghrelin) provides no fluorescent signals. First, we applied FITC-conjugated acyl-ghrelin (F302 in 1 μM as a final concentration) to 4% paraformaldehyde-fixed hippocampal slice culture. Robust binding signals were observed in the hilus, CA3 pyramidal cell layer, and in the stratum pyramidale and the stratum radiatum of CA1 (Fig. 3a and b). This result was in agreement with our previous work that reported the localization of GHSR1 using the GHSR1a antibody and the mouse hippocampus that expressed eGFP-tagged GHSR1a, as well as with the use of the identical fluorescent ghrelin molecule in the hippocampal slice (Muniz and Isokawa 2015). F302 (fluorescent acyl-ghrelin) binding profile was accompanied with DAPI staining in blue in order to show the hippocampal structure and subdivisions. With higher magnification, it became clear that only a subset of cells expressed GHSR1a. Examples are shown in the hilus (Fig. 3c) and CA1 (Fig. 3d). It is undetermined whether: 1) only a certain type of cells (either anatomically-defined or neurochemically-defined) expressed GHSR1a, or 2) internalization of GHSR1a limited the binding of F302 (fluorescent acyl-ghrelin) to only a subpopulation of cells. At a single cell level, F302 (fluorescent acyl-ghrelin) binding signal was detected in small punctate structures around the soma and proximal processes (Fig. 3e, f, and g).
We tested the competitiveness of GHSR1a binding between F302 (fluorescent acyl-ghrein) and “cold” (non-fluorescence-tagged) acyl-ghrelin. Hippocampal slice cultures (of both live and fixed) were incubated in the mixture of F302 (fluorescent acyl-ghrelin) and “cold” acyl ghrelin with a concentration ratio of F/C = 1/1, 1/10, 1/50, and 1/100. In each concentration, F302 (fluorescent acyl-ghrelin) signals were selected as red ROIs (inset in Fig. 3i). The area of all ROIs was summed to quantify the magnitude of F302 (fluorescent acyl-ghrelin) binding. FITC fluorescence did not change in the experiments in which F302 (fluorescent acyl-ghrelin) was applied alone or applied with “cold” acyl-ghrelin with the ratio of 1/1. However, when the concentration of “cold” acyl-ghrelin became 10 times higher than the concentration of F302 (fluorescent acyl-ghrelin), FITC fluorescence started to decline. FITC fluorescence decreased significantly with the ratio of 1/50 and greater (Fig. 2i). A competitive decline of F302 (fluorescent acyl-ghrelin) signal was observed in both live hippocampal slices (Fig. 3i1 and i2) and in the fixed slices (Fig. 3i3, i4, i5, and i6). We conclude that F302 (fluorescent acyl-ghrelin) indeed recognizes and binds to GHSR1a specifically in our hippocampal slice culture specimens by competing with exogenously-applied and endogenously-present acyl ghrelin. Finally, paraformaldehyde-fixed hippocampal culture specimens were incubated in F203 (fluorescent des acyl-ghrelin). We did not detect any fluorescent binding signal (Fig. 3h).
Acylation of non-octanoylated ghrelin in the live hippocampal slice culture
It is important to determine whether GOAT is biologically active and capable of octanoylating des acyl-ghrelin in our hippocampal slice culture. We incubated a group of live hippocampal slice cultures overnight in F302 (fluorescent acyl-ghrelin) and the other group of live hippocampal slice cultures in F203 (fluorescent des acyl-ghrelin). At the end of the incubation period, both groups of slices were viewed and imaged using a confocal microscope while keeping them alive. As expected, the slices incubated in F302 (fluorescent acyl-ghrelin) showed intense fluorescent signals in the hilus and CA3 (Fig. 4a). Surprisingly, the slices that were incubated in F203 (fluorescent des acyl-ghrelin) also showed similarly-intense fluorescent signals in the hilus and CA3 by mimicking the binding profile of F302 (fluorescent acyl-ghrelin) (Fig. 4b). This result suggested: 1) F203 (fluorescent des acyl-ghrelin) gained the capability of binding to GHSR1a, or 2) F203 (fluorescent des acyl-ghrelin) bound to something else other than GHSR1a. In order to test the possibility that F203 (fluorescent des acyl-ghrelin) might have bound to something else other than GHSR1a, we prepared hippocampal slice cultures from GHSR1a KO mice. Neither F302 (fluorescent acyl-ghrelin) nor F203 (fluorescent des acyl-ghrelin) in live hippocampal slice cultures of GHSR1a KO mice showed fluorescent signals (Fig. 4c and d). This result confirmed that F203 (fluorescent des acyl-ghrelin) binding that we observed in the live slice cultures of rat hippocampus in Fig. 4b was indeed restricted to GHSR1a.
We next prepared rat hippocampal slice cultures and pre-fixed them with 4% paraformaldehyde before the application of F203 (fluorescent des acyl-ghrelin). In this experiment, we hypothesized that fixation disabled biological activity of GOAT thus F203 (fluorescent des acyl-ghrelin) was not octanoylated. As we predicted, overnight application of F203 (fluorescent des acyl-ghrelin) to pre-fixed slices failed to show FITC signals (Fig. 4f). In contrast, F302 (fluorescent acyl-ghrelin) application in pre-fixed live slice exhibited FITC signals as expected (Fig. 4e).
Finally, we incubated live rat hippocampal slice cultures in the inhibitor of GOAT (GO-CoA-Tat, 5 μM) overnight. These slices were then incubated overnight in F203 (fluorescent des acyl-ghrelin) or F302 (fluorescent acyl-ghrelin). Although those incubated in F302 (fluorescent acyl-ghrelin) showed binding signals in spite of the overnight pre-incubation in the inhibitor of GOAT (Fig. 4g), those incubated in F203 (fluorescent des acyl-ghrelin) failed to show the binding signals after overnight treatment with the inhibitor of GOAT (Fig. 4h). Results are summarized by selecting and summing the FITC signals in red ROIs (the inset in Fig. 4i). We suggest, from these results, that hippocampal GOAT is biologically active, capable of octanoylating des-acyl ghrelin, and makes it available as a ligand for GHSR1a in the hippocampus.
Discussion
In the present study, we demonstrate that endogenous GOAT is present in the dentate gyrus of the hippocampus and biologically active to acylate local des-acyl ghrelin. Furthermore, the failure of detecting GOAT immunoreactive products (GOAT-IR) in the GHSR1a KO mouse hippocampus suggests that the expression of GHSR1a is likely a prerequisite for GOAT production in the hippocampus.
Presence of GOAT in the brain was reported in the human temporal lobes of Alzheimer’s disease patients (Gahete et al. 2010). In the diseased specimen, mRNAs of ghrelin, GHSR1a, and GOAT were all reduced when compared with the temporal lobe specimens of non-Alzheimer’s subjects. Although no cause-result relationships can be addressed from diseased specimens, there was a co-incidental decrease between mRNAs of GOAT and GHSR1a. Our result for the lack of GOAT-IR in GHSR1a KO mouse hippocampus points the possibility that the expression of GHSR1a controls the production of GOAT in the hippocampus. We should also point out that Seim and colleagues (2013) reported that ghrelin regulated GOAT expression in a cell type-specific manner. Further investigations are required for GOAT expression and functions.
GOAT was reported to be expressed highly in gastric cells that are enriched with ghrelin (Sakata et al. 2009). This study indicated that ghrelin and GOAT were likely present in the same cell. Furthermore, local acylation of ghrelin was reported in the rodent bone marrow (Hopkins et al. 2017). However, these reports do not agree with a report by Yang et al (2008a) that the expression of GOAT in cell culture failed to acylate non-octanoylated ghrelin. In the rodent brain, in spite of ample evidence for the ghrelin’s powerful modulation on hippocampal functions, it is still undetermined whether ghrelin and GOAT are co-expressed in any hippocampal neuron. In the present study, the magnitude of GOAT-IR remained unchanged in the 18 hour-fasted rat hippocampus. This observation is in agreement with a report that GOAT mRNA expression remained unaltered in fed and fasted rats (Gonzalez et al. 2008). The localization of GOAT in the hippocampus suggests the possibility that hippocampal neurons may produce ghrelin and acylate it. However, this hypothesis is not supported by a previous work that reported the lack of endogenous ghrelin peptide in the hippocampus (Cowley et al. 2003). It could be that systemic ghrelin entered the hippocampus through the blood-brain-barrier as Diano et al (2006) showed. Alternatively, there is the possibility that ghrelin in the septal neuron axons travelled to the hippocampus. In either case, how endogenous GOAT gained access to extracellular ghrelin may need to be explained.
GOAT is a membrane-bound O-acyltransferase and catalyzes fatty acylation of secreted proteins in the lumen of the secretory pathway (Steinhouser and Treisman 2009). GOAT is located in the endoplasmic reticulum (ER) compartment and mediates the translocation of the octanoyl-CoA from the cytosolic side to the ER lumen through unknown mechanism (Romero et al. 2010). ER is one of the major cytoplasmic organelles in dendritic spines and axon terminals in the hippocampal neuron (Mattson 2004). One possibility is that GOAT might be mobilized from a cell in an activity-dependent manner. There is a report that membrane-bound O-acetyltransferase was associated with cytoplasmic side of the synaptic vesicle and was exposed to the extracellular space at the synaptic cleft during vesicle fusion (Carrol 1994). In the gastric system, dietary lipids are crucial for the activation of GOAT (Romero et al. 2010) although it is not yet determined whether endogenous lipids may have any role on ghrelin acylation. In neurons, synaptic activities stimulate the production of endogenous lipids and recruit them to modulate synaptic transmission and plasticity (Bolshakov and Siegelbaum 1995; Kano et al. 2008). We cannot rule out the possibility that endogenous lipids may initiate the activation of GOAT in the hippocampus. Further studies are needed to unveil underlying mechanisms and functions among neuron activities, endogenous lipids, and activation of GOAT in the hippocampus.
We used fluorescent ghrelin to locate GHSR1a. F302 (fluorescent acyl-ghrelin) and F203 (fluorescent des acyl-ghrelin) are a truncated, 18 amino acid analogue of ghrelin conjugated to a fluorescent molecule, fluorocein isothiocyanate (FITC), through the addition of a lysine at its C-terminus (McGirr et al. 2011). F302 (fluorescent acyl-ghrelin) peptide structure contains a unique n-octanoylated Ser3 residue, while Ser3 of F203 (fluorescent des acyl-ghrelin) is left open (free). The first five amino acids of ghrelin are sufficient for recognition by GOAT (Yang et al. 2008b). Thus, F203 (fluorescent des acyl-ghrelin) peptide structure does not interfere with GOAT to recognize its Ser3 residue for octanoylation. Our discovery of F203 (fluorescent des acyl-ghrelin) binding to GHSR1a after overnight incubation in live hippocampal slice culture provides convincing evidence that endogenous GOAT has access to extracellularly-distributed ghrelin molecules and acylates them. Whether GOAT does so by being released from cells in a neuron activity-dependent manner, or by being mobilized on-demand from cellular compartments like other lipid messengers such as endocannabinoids, needs to be elucidated.
Acknowledgments
This work is support by a National Institute of Health grant 2R15DA021683 to M. Isokawa. Part of this work was presented at the 2nd UTRGV Engaged Scholars Symposium in April 2017 by M. I. Murtuza. The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
A list of abbreviations
- CA1
cornu ammonis 1
- CA3
cornu ammonis 3
- CCD
charge-coupled device
- DAB
diaminobenzidin
- DAPI
4′,6-diamidino-2-phenylindole
- DG
dentate gyrus
- DIC
differential interference contrast
- D-Lys3-GHRP-6
D-Lys3-growth hormone releasing peptide 6
- ER
endoplasmic reticulum
- eGFP
enhanced green fluorescent protein
- FITC
fluorocein isothiocyanate
- Ghrelin-IR
ghrelin immunoreactivity
- GHSR1a
Growth hormone secretagogue receptor type 1a
- GHSR1a KO
GHSR1a receptor knock out mouse
- GOAT
Ghrelin O-acyltransferase
- GOAT-IR
GOAT immunoreactivity
- Go-CoA-Tat
GOAT inhibitor (H-Gly-Ser-α-Dap-β-(2-CoA-Octanoyl)-Phe-Leu-Ser-Pro-Glu-His-Gln-Ahx-Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-OH)
- HRP
horse radish peroxidase
- NIH
National Institute of Health
- PBS
phosphate buffered saline
- ROI
region of interest
- WT
wild-type mouse
Footnotes
Author contributions: MI designed experiments and wrote the paper, MI and LB performed experiments, collected data, and analyzed data.
References
- Albarran-Zeckler RG, Sun Y, Smith RG. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides. 2011;32:2229–2235. doi: 10.1016/j.peptides.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Cell. 2010;34:31–40. doi: 10.1016/j.tins.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Heiman ML. Extent and direction of ghrelin transport across the blood brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Berrout L, Isokawa M. Ghrelin promotes reorganization of dendritic spines in cultured rat hippocampal slices. Neurosci Lett. 2012;516:280–284. doi: 10.1016/j.neulet.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Hippocampal long-term depression: arachidonic acid as a potential retrograde messenger. Neuropharmacolog. 1995;34:1581–7. doi: 10.1016/0028-3908(95)00127-r. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Ghersi M, Schiöth HB, de Barioglio SR. Ghrelin and memory: differential effects on acquisition and retrieval. Peptides. 2010;31:1190–1193. doi: 10.1016/j.peptides.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Carrol PT. Membrane-bound choline-O-acetyltransferase in rat hippocampal tissue is associated with synaptic vesicles. Brain Res. 1994;633:112–118. doi: 10.1016/0006-8993(94)91529-6. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay WC, da Silvo I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7:37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahete MD, Rubio A, Cordoba-Chacon J, Gracia-Navarro F, Kineman RD, Avila J, Luque RM, Castano JP. Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer’s disease patients. J Alzheimer’s Disease. 2010;22:819–828. doi: 10.3233/JAD-2010-100873. [DOI] [PubMed] [Google Scholar]
- Gonzalez CR, Vazquez MJ, Lopez M, Dieguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J Mol Endocrinol. 2008;41:415–421. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- Grönberg M, Ahlin C, Naeser Y, Janson ET, Holmberg L, Fjällskog ML. Ghrelin is a prognostic marker and a potential therapeutic target in breast cancer. PLoS One. 2017;12(4):e0176059. doi: 10.1371/journal.pone.0176059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL, Nelson TAS, Guschina IA, Parsons LC, Lewis CL, Brown RC, Christian HC, Davies JS, Wells T. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R1a activity: evidence for target cell-induced acylation. Scientific Reports. 2017;7:45541. doi: 10.1038/srep45541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Huh CY, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J Neurosci. 2010;30:15951–61. doi: 10.1523/JNEUROSCI.3663-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2008;89:309–80. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Mani BK, Walker AK, Lopez-Soto EJ, Raingo J, Lee CE, Perello M, Andres AB, Zigman JM. Neuroanatomical characterization of a growth hormone secretagogue receptor-gree fluorescent protein reporter mouse. J Comp Neurol. 2014;522:3644–3666. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massadi OA, Lopez M, Tschop M, Dieguez C, Nogueiras R. Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends Neurosci. 2017;40:167–180. doi: 10.1016/j.tins.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr R, McFarland MS, McTavish J, Luyt LG, Dhanvantari S. Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regulatory Peptides. 2011;172:69–76. doi: 10.1016/j.regpep.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Muniz BG, Isokawa M. Ghrelin receptor activity amplifies hippocampal N-methyl-D-aspartate receptor-mediated postsynaptic currents and increases phosphorylation of the GluN1 subunit at Ser896 and Ser897. Eur J Neurosci. 2015;42:3045–3053. doi: 10.1111/ejn.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Okame R, Tetsuro Katayama T, Miyazato M, Kangawa K, Murakami N. Nutritional and environmental factors affecting plasma ghrelin and leptin levels in rats. J Endocrinol. 2010;207:95–103. doi: 10.1677/JOE-10-0062. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yoh J, Hiejima H, Kojima M. Structures and molecular forms of the ghrelin-family peptides. Peptides. 2011;32:2175–2182. doi: 10.1016/j.peptides.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Romero A, Kirchner H, Heppner K, Pfluger PT, Tschop MH, Nogueiras R. GOAT: the master switch for the ghrelin system? Eur J Endocrinol. 2010;163:1–8. doi: 10.1530/EJE-10-0099. [DOI] [PubMed] [Google Scholar]
- Russo C, Russo A, Pellitteri R, Stanzani S. Hippocampal Ghrelin-positive neurons directly project to arcuate hypothalamic and medial amygdaloid nuclei. Could they modulate food-intake? Neurosci Lett. 2017;653:126–131. doi: 10.1016/j.neulet.2017.05.049. [DOI] [PubMed] [Google Scholar]
- Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Colocalization of ghrelin O-aceyl-transferase and ghrelin in gastric mucosal cells. Am J physiol endocrinol Metabol. 2009;297:E134–141. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim I, Jeffery PL, de Amorim L, Walpole CM, Fung J, Whiteside EJ, Lourie R, Herington AC, Chopin LK. Ghrelin O-acyltransferase (GOAT) is expressed in prostate cancer tissues and cell lines and expression is differentially regulated in vitro by ghrelin. Reproductive Biology and Endocrinology. 2013;11:70. doi: 10.1186/1477-7827-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser J, Treisman JE. Lipid-modified morphogens: functions of fats. Current Opinion in Genetics and Development. 2009;19:308–314. doi: 10.1016/j.gde.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52(3):301–10. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin N, Goldstein JL. Identifiation of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008a;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yang J, Shao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin-O-acyltransferase (GOAT) by octanoylated pentaapeptides. Proc Natl Acad Sci USA. 2008b;105:10750–10755. doi: 10.1073/pnas.0805353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–54. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]