SUMMARY
DPY-30 is a subunit of mammalian COMPASS-like complexes (complex of proteins associated with Set1) and regulates global histone H3 Lys-4 trimethylation. Here we report structural evidence showing that the incorporation of DPY-30 into COMPASS-like complexes is mediated by several hydrophobic interactions between an amphipathic α helix located on the C terminus of COMPASS subunit ASH2L and the inner surface of the DPY-30 dimerization/docking (D/D) module. Mutations impairing the interaction between ASH2L and DPY-30 result in a loss of his-tone H3K4me3 at the β locus control region and cause a delay in erythroid cell terminal differentiation. Using overlay assays, we defined a consensus sequence for DPY-30 binding proteins and found that DPY-30 interacts with BAP18, a subunit of the nucleosome remodeling factor complex. Overall, our results indicate that the ASH2L/DPY-30 complex is important for cell differentiation and provide insights into the ability of DPY-30 to associate with functionally divergent multisubunit complexes.
INTRODUCTION
Histone proteins are essential scaffolding factors contributing to the high level of DNA densification in eukaryotes. Changes in the chromatin landscape by ATP-dependent chromatin remodeling complexes, the loading of histone variants, DNA methylation, and posttranslational modifications (PTMs) of histone proteins represent different means of regulating DNA-based transactions (Banaszynski et al., 2010; Cedar and Bergman, 2012; Goldberg et al., 2007; Sadeh and Allis, 2011). Among these mechanisms, PTMs deposited on histones H3, H4, H2A, or H2B are important factors controlling the structural reorganization of chromatin or forming binding platforms for the recruitment of various multisubunit protein complexes.
Cell-specific transcription patterns are important for relaying the proper cues to determine the timing or fate of cell differentiation, which is in part controlled by specific histone PTMs, such as histone H3 Lys-4 (H3K4) methylation. Members of the SET1 family of histone methyltransferases, which site-specifically methylate H3K4 (Couture and Skiniotis, 2013; Shilatifard, 2008), are closely associated with different differentiation programs. For example, MLL2-containing complexes bind to the β-globin locus control region (LCR) and maintain a high level of expression of the β-globin gene, a marker for terminal erythroid differentiation (Demers et al., 2007). The same enzyme is also important in the establishment of bivalent promoters in embryonic stem cells (Hu et al., 2013). The recruitment of SET1 by USF1 is key for mesoderm specification and lineage differentiation (Deng et al., 2013). Combined with studies showing that deletion of MLL1 leads to embryonic lethality (Yu et al., 1995), these findings demonstrate that members of the SET1 family of methyltransferases play crucial roles during development.
Evolutionary conserved, the members of the SET1 family of methyltransferases reside in multisubunit complexes with a common four-subunit complex composed of WDR5, RbBP5, ASH2L, and DPY-30 (referred to as WRAD). Although WRAD stabilizes and stimulates the enzymatic activity of all SET1 members, each subunit plays distinct roles. WDR5 binds a VDV motif on RbBP5 (Avdic et al., 2011; Chen et al., 2012; Odho et al., 2010) as well as the WDR5-interacting motif region of all SET1 members to fully stimulate the enzymatic activity of these enzymes (Dharmarajan et al., 2012; Zhang et al., 2012). In addition, binding of the ASH2L SPIa and Ryanodine receptor domain to RbBP5 creates a binding platform that directly interacts with and stimulates the enzymatic activity of the MLL1 SET domain (Cao et al., 2010; Chen et al., 2012). Recent cryoelectron microscopy (cryo-EM) analysis of yeast (and human) core complex of proteins associated with Set1 (COMPASS) supported these crystallographic studies, revealing that Cps60 (ASH2L homolog) and Cps25 (DPY-30 homolog) form a globular structure sitting at the base of the Y-shaped COMPASS structure sandwiching, along with the Cps50 (RbBP5 homolog)/Cps35 (WDR5 homolog) heterodimer, the catalytic domain of SET1 (Takahashi et al., 2011). Consistent with the role of SET1 members in developmental biology, the depletion of subunits regulating H3K4 methyltransferases such as WDR5 results in a global loss of histone H3K4 methylation and concomitant loss of Hox gene expression in human embryonic kidney 293 (HEK293) cells (Dou et al., 2006). Similarly, knockdown of DPY-30 and ASH2L leads to a defect in neural lineage formation by embryonic stem cells (Jiang et al., 2011) and incomplete differentiation of myogenic precursors, respectively (Rampalli et al., 2007).
DPY-30 is an important subunit of COMPASS as well as mammalian COMPASS-like complexes. Knockdown of DPY-30 in fibroblasts leads to a senescent phenotype (Simboeck et al., 2013), and deletion of Cps25 in Saccharomyces cerevisiae (also known as Sdc1 or SAF19) results in a global loss of H3K4 trimethylation (H3K4me3) (South et al., 2010). The role of DPY-30 is in part controlled by its ability to bind the C terminus of ASH2L, a motif that was coined as Sdc1 DPY-30 interacting region (SDI) (South et al., 2010). Initial crystallographic studies revealed that residues 45–99 of DPY-30 fold as three antiparallel α helices forming an X bundle showing striking structural homology to the dimerization/docking (D/D) domain of protein kinase A (PKA) regulatory (R) domains (Wang et al., 2009). However, the lack of structural data for DPY-30 bound to ASH2L has hindered our understanding of the molecular basis for binding selectivity, formation of the ASH2L/DPY-30 complex, and structural determinants underlying the role of this heterodimer in cell differentiation.
To understand the structural basis underlying the binding of DPY-30 to ASH2L, we determined the crystal structure of the DPY-30 D/D module (DPY-30D/D) in complex with a fragment corresponding to residues 509–524 of ASH2L (referred to as ASH2LSDI). Mutations of residues forming the DPY-30D/D-ASH2LSDI interface result in a decrease of H3K4 trimethylation at the β-globin gene and concomitant reduction of β-globin expression in differentiated erythroid cells. In parallel, our overlay assays reveal the ability of DPY-30D/D to accommodate different amphipathic α helices and suggest that DPY-30 is a subunit of several protein complexes, including the nucleosome remodeling factor (NURF) complex, an ATP-dependent chromatin remodeler and a bona fide reader of H3K4me3, a mark controlled by DPY-30.
RESULTS
Overall Structure
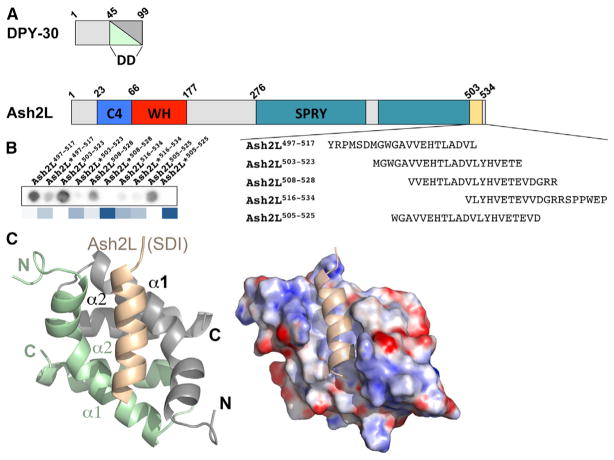
Previous studies have shown that DPY-30D/D binds the C terminus of ASH2L (South et al., 2010; Figure 1A). To first define the minimal structural determinants underlying the formation of the DPY-30/ASH2L complex, GST-DPY-30 was incubated with ASH2L peptides crosslinked to a membrane. As shown in Figure 1B, incubation of GST-DPY-30 with peptides corresponding to the various regions of the ASH2L C terminus reveals that DPY-30 preferably binds ASH2L503–523. Consistent with previous biochemical studies (Chen et al., 2012), replacement of ASH2L hydrophobic residues with aspartic acid residues severely impairs the binding of DPY-30. Taken together, these results show that ASH2L residues 503–523 are important for DPY-30 binding.
Figure 1. ASH2LSDI Binds to the DPY-30D/D Module as an Amphipathic α Helix.
(A) Schematic representation of DPY-30 and ASH2L. Each functional domain is highlighted differently, and the numbering of ASH2L residues is based on the isoform 3 reported in the Uniprotkb database. WH, winged helix.
(B) Optimization of the overlay assay. Indicated are peptide sequences tested in our initial overlay assay. Shown is a processed image of the LICOR analysis of GST-DPY-30 binding to a small array containing peptides corresponding to ASH2LSDI. The star denotes peptides harboring substitutions of each leucine residue for an aspartic acid. A heatmap representation of the quantification is shown below the array. Deviations higher than 1 above the WT are represented by different shades of blue. Dark blue, >10-fold; blue, >5-fold; light blue, >3-fold.
(C) Crystal structure of ASH2LSDI in complex with DPY-30D/D. DPY-30 α helices of protomers A and B are colored in green and gray, respectively, whereas ASH2SDI is highlighted in beige (left). Shown is the electrostatic surface potential of the DPY-30D/D crystal structure in complex with ASH2LSDI (right). Electrostatic potentials are contoured from +10kbTe−1 (blue) to −10 kbTe−1 (red).
To understand how ASH2L binds DPY-30, we solved the crystal structure of DPY-30D/D (residues 45–99) in complex with ASH2L (residues 509–524) at a resolution of 2.4 Å (Figure 1C). The asymmetric unit is composed of eight DPY-30D/D:ASH2LSDI complexes, with each complex presenting a 2:1 stoichiometry of DPY-30 to ASH2L. Overall, each DPY-30D/D protomer consists of three α helices connected by short intersecting loops (Figure 1C). Together, the two DPY-30 protomers form a prototypical X-type, four-helix bundle and a quasi-symmetric dimer. As judged from pairwise structural alignment, each DPY-30 protomer adopts similar conformations with average root-mean-square deviations (rmsds) of 0.3 Å, demonstrating that binding of ASH2L does not induce major structural reorganization of the DPY-30 dimerization/docking module (Figure S1 available online).
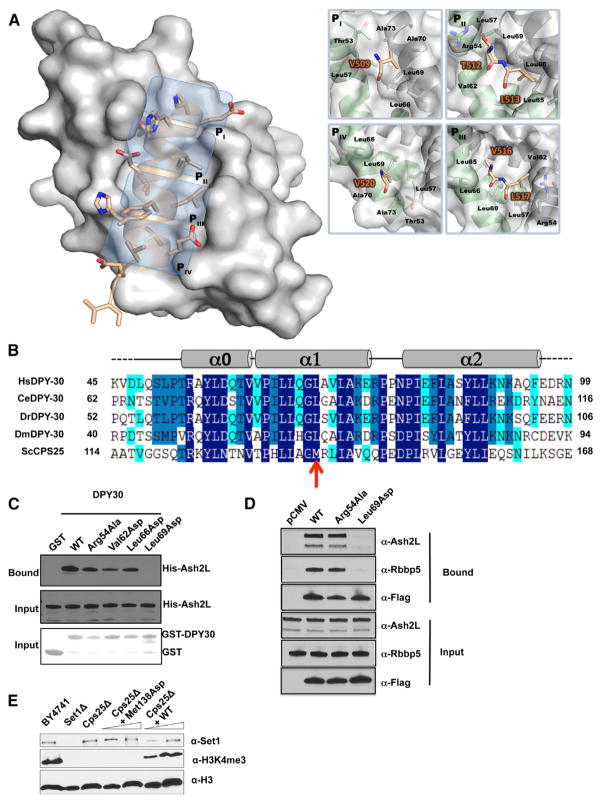
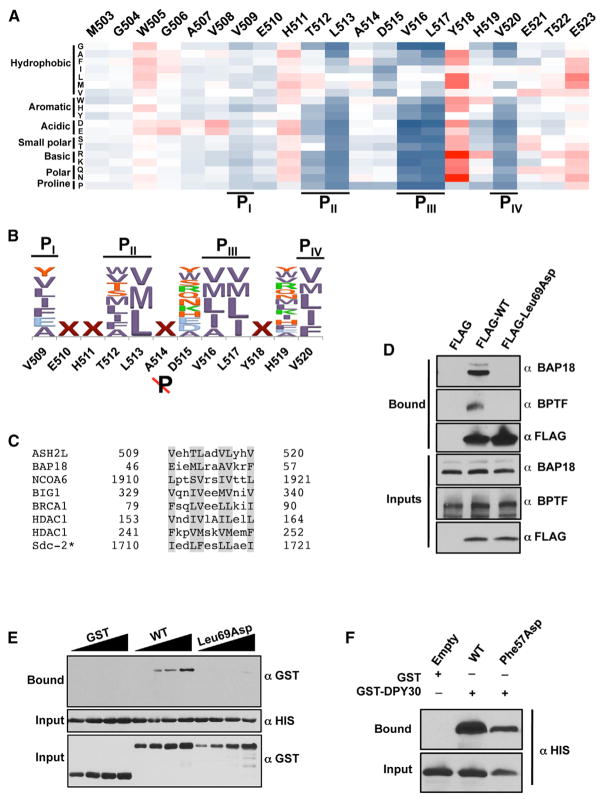
Examination of the density corresponding to ASH2LSDI shows the location of residues 509–524 of ASH2L (Figure S2). ASH2LSDI forms an amphipathic α helix nestled in an elongated cleft formed by both DPY-30 protomers and lies ~45° across one face of the symmetrical homodimer (Figure 1C). Binding of the ASH2L α helix occupies ~680 Å 2 of the DPY-30D/D interface that is formed predominantly by a core of hydrophobic residues. Intriguingly, ASH2LSDI interaction with DPY-30 is symmetric, as revealed by a quasi-two-fold symmetry of the ASH2L primary structure centered on residues A514 and D515. These two residues are located in the position on ASH2LSDI that divides the DPY-30D/D dyad axis within the ASH2L/DPY-30 complex. From this position, a pattern of four hydrophobic pockets (pocket I–IV [PI–PIV]) can be discerned (Figure 2A). These pockets are predominantly formed by residues located in DPY-30D/D α helices α0 and α1, which engage in several hydrophobic interactions with the ASH2L α helix hydrophobic ridge. The PI pocket is mainly formed by Leu57A, Leu66B, and Leu69B side chains and makes several contacts with V509 (residues denoted with a single letter refer to ASH2L, and the superscript indicates the DPY-30 protomer involved in the interaction). At the core of the cradle-like structure, the PII pocket binds T512 and L513 of ASH2L by forming a shallow depression shaped by Arg54A, Leu57A, Val62A, Leu65B, and Leu69B side chains. In proximity of the PII pocket, a pair of hydrophobic residues (V516 and L517) binds in a third pocket (PIII) composed of Val62B, Leu57B, Leu69A, Leu65A, and Leu66A side chains. Similar to the PI pocket, PIV is occupied by a valine residue. In this pocket, V520 is in close proximity to Leu69B and Ala70B side chains. Overall, our observations highlight the pseudosymmetric fold of DPY-30D/D/ASH2LSDI interactions and the extent of hydrophobic contacts underlying the formation of the ASH2L/DPY-30 heterotrimer (Figure 2A).
Figure 2. Four Pockets Define the ASH2L Binding Site in DPY-30.
(A) Overall representation of ASH2LSDI in complex with the DPY-30 dimerization/docking module. Shown is a surface representation (gray) of DPY-30D/D in which cyan boxes highlight the four pockets. On the right, residues forming the four pockets are indicated.
(B) Sequence alignment of DPY-30. Amino acid conservation is illustrated with different shades of blue so that dark, medium, and pale blue correspond to 100%–90%, 90%–80%, and 80%–60% of amino acid conservation, respectively.
(C) Immunoblot analysis of ASH2L after GST pull-down experiments of bacterial extracts containing either DPY-30 WT or mutants. Bound ASH2L was detected using an anti-His antibody (top panel). The center and bottom panels show the input for ASH2L constructs and that an equivalent amount of DPY-30 was bound to glutathione Sepharose beads, respectively.
(D) Western blot analysis of WRAD integrity following immunoprecipitation by M2 Dynabeads of HEK293 cell lysates transiently expressing FLAG-tagged DPY-30 WT and mutants. The top three panels indicate proteins retained by the M2 agarose beads, whereas the bottom three panels show the input. RbBP5 and ASH2L were detected with the indicated antibodies, whereas DPY-30 wild-type or mutants were detected with anti-FLAG antibody.
(E) Monitoring of the impact of a single point mutation in Cps25. The top panel indicates the level of Set1. The center panel shows the overall level of H3K4me3, and the bottom panel shows that an equivalent amount of protein was loaded on the gel. The western blots were performed with ScSet1-, H3K4me3-, and histone H3-specific antibodies.
A Central Hydrophobic Residue in DPY-30D/D Is Critical for ASH2L/DPY-30 Complex Formation and Global H3K4me3 in Yeast
After examining the structure of DPY-30D/D in complex with ASH2L, we sought to probe the role of evolutionarily conserved interfacial residues using a glutathione S-transferase (GST) pull-down approach (Figure 2B). The GST-tagged wild-type (WT) or mutants of DPY-30 were incubated with WT His-tagged ASH2L, and binding was monitored by western blot using an anti-His antibody. As shown in Figure 2C and consistent with recent studies (Chen et al., 2012; South et al., 2010), binding was observed when ASH2L was incubated with GST-DPY-30, whereas no enrichment could be detected when binding reactions were carried out with GST alone. Given the extent of the interactions provided by residues forming the PI–PIV pockets, we reasoned that replacing these hydrophobic residues with an aspartic acid residue would disrupt the binding of ASH2L to DPY-30. Accordingly, substitution of the PII and PIII residues Val62 and Leu66 (Figure 2C) modestly impaired the binding of ASH2L, whereas the same mutation of Leu69, a residue shaping all pockets and making several contacts with the ASH2LSDI hydrophobic ridge, completely abrogated the binding of ASH2L. In contrast to Leu69, substitution of Arg54 to alanine modestly affected binding of ASH2L, indicating that the polar contacts mediated by this residue’s side chain play a minor role in the stabilization of the α helix within the DPY-30D/D module.
To further evaluate the impact of disrupting the interactions between DPY-30 and ASH2L in cells, we transfected FLAG-tagged constructs of DPY-30 corresponding either to the WT, Leu69Asp, or Arg54Ala in HEK293 cells and performed immunoprecipitation using anti-FLAG M2 magnetic beads. As shown in Figure 2D, both DPY-30 WT and the Arg54Ala mutant pulled down ASH2L and RbBP5, whereas the Leu69Asp mutant failed to immunoprecipitate the ASH2L/RbBP5 heterodimer. Overall, these results demonstrate the importance of Leu69 in maintaining the integrity of the ASH2L/DPY-30 complex and suggest that the interaction between DPY-30D/D and ASH2L is predominantly mediated by hydrophobic interactions.
After determining that Leu69 is important for the formation of the ASH2L/DPY-30 complex, we sought to measure the impact of this mutation in vivo. In Saccharomyces cerevisiae, deletion of the DPY-30 yeast homolog Cps25 is nonlethal but results in a complete loss of H3K4me3 (South et al., 2010), and the residue corresponding to Leu69 is Met138 (Figure 2B). We reintroduced either Cps25 WT or a Cps25 Met138Asp mutant in a yeast strain lacking Cps25 (Cps25KO) and probed for global histone H3K4me3. As shown in Figure 2E, deletion of Cps25 leads to a global loss of H3K4me3, and complementation of Cps25 WT restores the level of this PTM to the WT level. However, consistent with the pull-down experiments, Cps25 Met138Asp complementation could not restore H3K4me3, suggesting that this mutation impairs Cps60/Cps25 interaction and the allosteric regulation of Set1 trimethyltransferase activity.
A Mutation of the ASH2L Hydrophobic Ridge Impairs ASH2L/DPY-30 Complex Formation and Decreases β-Globin Transcription during Erythroid Cell Terminal Differentiation
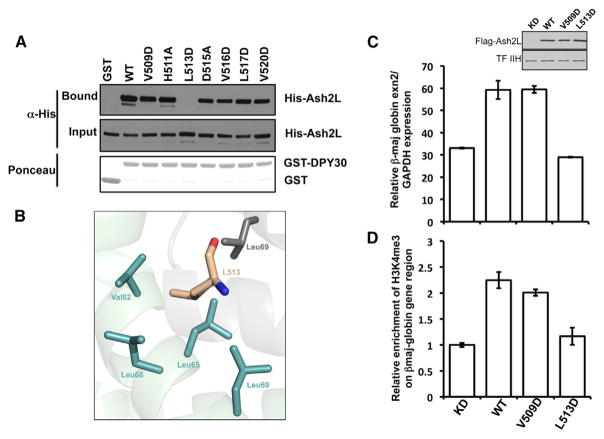
After monitoring the role of residues forming the inner face of DPY-30D/D module, we sought to probe the roles of ASH2L residues important for the interaction with DPY-30. Binding assays show that substitution of polar amino acids such as H511 and D515 to an alanine residue does not impair binding of ASH2L to DPY-30 (Figure 3A). Similarly, replacement of residues interacting with the PI and PIV pockets, such as V509 and V520, to aspartic acid only modestly prevents the formation of the ASH2L/DPY-30 complex (Figure 3A). Conversely, substitution of L513, a residue that fits snugly within the PII pocket (Figure 3B), with an aspartic acid cripples the binding of ASH2L. This result indicates that L513 is a key residue for the formation of the ASH2L/DPY-30 complex and further underlines the importance of hydrophobic residues in the binding of ASH2L to DPY-30.
Figure 3. Impairing the ASH2L/DPY-30 Interaction Blunts Erythroid Terminal Differentiation.
(A) Binding studies of the ASH2L/DPY-30 complex in vitro. Bound ASH2L was detected using an anti-6×His-tag antibody, and the input is indicated below. The Ponceau staining shows that equivalent amount of GST-DPY30 (WT) was used for the pull-down assays.
(B) Zoomed view of the L513 binding pocket. The carbon atoms of DPY-30 residues are highlighted in green and gray (protomers A and B). ASH2L L513 carbon atoms are colored in beige.
(C) Assessment of DPY-30 mutations on β-globin gene expression. Quantitative RT-PCR was performed on two independent experiments performed in triplicate. Levels of ASH2L were detected with an anti-FLAG tag antibody, and TFIIH p89 was used as a loading control. Error bars represent SD.
(D) Measure of the impact of the L513D mutation on H3K4 trimethylation at the LCR of the β-globin gene. Enrichment of H3K4me3 was measured by ChIP as described previously (Sarvan et al., 2011) with either the empty vector (KD) or constructs corresponding to ASH2L-WT, ASH2L-V509D, or L513D mutants. Error bars represent SD.
DPY-30 regulates pathways in cellular senescence (Simboeck et al., 2013) and ESC fate specification (Jiang et al., 2011), but its role during erythroid terminal differentiation has not been examined. The MLL2 complex methylates H3K4 in the LCR of the β-globin gene in murine erythroid leukemia cells and maintains a high level of β-globin gene expression (Demers et al., 2007). To test whether association of DPY-30 to ASH2L is important for MLL2 activity, mouse erythroleukemia (MEL) cells carrying a doxycycline-inducible small hairpin RNA (shRNA) specific for ASH2L were ectopically transfected with FLAG-tagged Sh-resistant constructs corresponding to WT, V509D and L513D mutants of ASH2L. Following induction of differentiation, we performed chromatin immunoprecipitation (ChIP) experiments using H3K4me3 antibodies combined with quantitative RT-PCR to monitor the level of β-globin transcripts. As shown in Figure 3C and consistent with the role of ASH2L in the function of the MLL2 complex (Demers et al., 2007), transfection of WT ASH2L resulted in upregulation of β-globin gene expression and an increased level of H3K4me3 at the LCR of the β-globin gene (Figure 3D). Correlatively, the ASH2L V509D mutant displayed activity similar to the WT, whereas rescue with the ASH2L L513D mutant failed to stimulate β-globin gene expression and H3K4me3 at the LCR of the β-globin gene. Taken together, these results suggest that association of DPY-30 with the MLL2 complex regulates β-globin transcription during terminal erythroid differentiation.
Comparison with the RIα/AKAP Complex
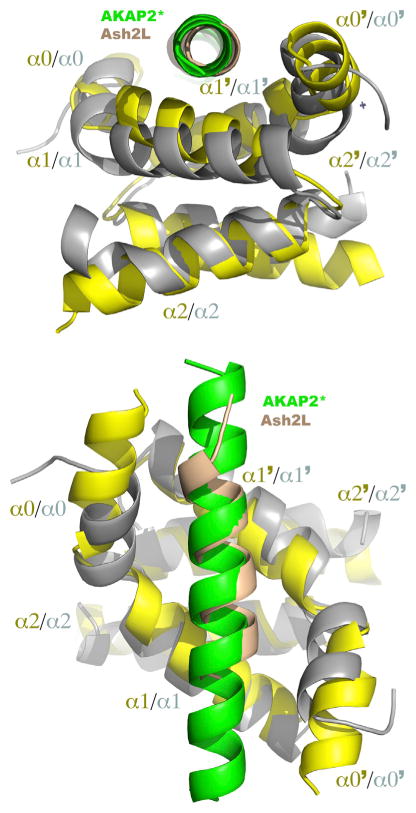
In an attempt to find the best structural homolog of DPY-30, we performed a search using the DALI server (Holm and Rosenström, 2010) and DPY-30D/D/ASH2LSDI coordinates. The search yielded several results, with the regulatory region (RIα) of PKA showing the highest structural similarities. The RIα of PKA plays important roles in restricting the activity of the enzyme in binding to the A kinase-anchoring proteins (AKAPs). Similar to DPY-30, AKAP binds as an amphipathic helix and plays pivotal roles in directing PKA to its suitable cellular localization and targeting the enzyme to its substrates. Initial mapping experiments performed on the α helix of AKAP revealed that substitution of any residues lining the hydrophobic ridge to aspartic acid is detrimental to the formation of the RIα-AKAP complex (Sarma et al., 2010). Given that some mutations introducing acidic residues on ASH2L retained binding to DPY-30, we reasoned that structural differences exist between the binding modes of RIα and DPY-30 dimerization/docking modules. Overall, DPY- 30D/D aligns with RIα with an rmsd of 2.58 Å for all Ca atoms, indicating the presence of some structural differences. A structural comparison with RIα shows that both D/D modules are composed of two homodimers, each comprising three helices (Figure 4). In contrast to DPY-30, RIα α0 is longer by one turn of helix, which extends the cradle-like structure by ~8 Å. In addition, the angle between DPY-30D/D α0 and α1 is more acute than the corresponding α helices of RIα. Because of these structural differences, the base of the DPY-30 cradle-like structure is wider than the RIα binding cleft. However, despite these differences, the ASH2LSDI and AKAP α helices superimpose well (Figure 4). This similar positioning, combined with the structural differences between DPY-30 and RIα, generate a longer distance between the ASH2LSDI and the surface of DPY-30. These comparative analyses suggest that structural differences in DPY-30 control its ability to reside in specific protein complexes.
Figure 4. Differences in the Geometry of Dimerization/Docking Domains Control the Binding of Amphipathic α Helices.
Shown are two orthogonal views of an overlay of RIα/AKAP2 and DPY-30/ASH2LSDI complexes in which DPY-30 and RIα are highlighted in gray and yellow, whereas AKAP2 and ASH2LSDI are colored in green and beige, respectively.
Identification of the Specificity Determinants for Binding to DPY-30
In protein kinases A signaling pathways, differences in recognition of amino acid sequences between different regulatory domains provide a mechanism for introducing specificity to the enzyme (Gold et al., 2006; Kinderman et al., 2006). To systematically evaluate the motif recognized by DPY-30, we developed an overlay assay. Recombinant GST-DPY-30 was incubated with an array of ASH2L peptide derivatives crosslinked to cellulose where each residue in ASH2LSDI (503–523) was replaced by all 20 amino acids. Binding was measured using a horseradish peroxidase (HRP)-coupled anti-GST antibody and quantified by phosphorimaging (Figure S3). As shown in Figure 5A, we found a strong correlation between the overlay assay and the crystal structure of the DPY-30D/D/ASH2LSDI complex. Mutations of residues preceding V508, a residue that does not make a noticeable interaction with DPY-30D/D, have little impact on the binding of DPY-30. Similarly, any substitutions of solvent-exposed residues, with the exception of a proline, do not prevent the binding of ASH2L (Figure 5A). Conversely, mutation of amino acids interacting with DPY-30D/D pockets to residues other than hydrophobic ones is detrimental, to different magnitudes, for the binding of ASH2L to DPY-30. For example, mutation of the PI-interacting residue V509 to an aspartic acid or other bulky charged residues has a mild impact on the binding of DPY-30, whereas the same substitutions on residues interacting with PII and PIII pockets result in a complete loss of binding. Notably, replacement of L513 (a PII pocket binding residue) by any residues other than a methionine or an isoleucine results in a drastic loss of binding to DPY-30. A similar selectivity is observed for the PIV-interacting residues. PIV is highly restrictive and is only able to accommodate hydrophobic residues. Although mutation of residues forming the hydrophobic ridge showed the most noticeable impact upon mutation, replacement of D515 exhibited a peculiar pattern. Substitution of this residue with any nonhydrophobic residues did not impair the binding of DPY-30, whereas its replacement with any hydrophobic residue led to a 4- to 5-fold loss of DPY-30D/D binding, suggesting that the spacing between clusters of hydrophobic residues is important to maintain high-affinity binding (Figure 5A). Interestingly, with the exception of a proline and glycine residues, substitution of Y518 resulted in a significant gain of binding. In sum, these observations show that the hydrophobic residues near the symmetric dyad are critical for binding to DPY-30 and highlight a pattern of residues, which will now be referred to as DPY-30 binding motif (DBM), important for interaction with DPY-30. The DBM consists of [YVLIFEA]-X-X-[ϕTSWM]-[VML]-X-[ψ]-[VILMA]-[VMLI]-X-X-[VMLIF], where ϕ and ψ represent any hydrophobic and nonhydrophobic residues, respectively (Figure 5B).
Figure 5. Two Hydrophobic Pockets Define the Binding Determinants Underlying the Binding of DPY-30 to ASH2L and Integration within the NURF Complex.
(A) Two distinctive sets of hydrophobic residues on ASH2L are important for binding to DPY-30. Shown is a heatmap representation calculated from an array in which each residue of ASH2LSDI was systematically mutated to all other 20 natural amino acids. Deviations are colored as in Figure 1A, with the exception that gain of binding was colored in different shades of red or so that dark red >10-fold, red >5-fold, and pink >3-fold. Raw data are presented in Figure S3.
(B) Identification of the motif recognized by DPY-30. The sequence logo representing the DBMs was derived by the overlay assay. Letters are scaled according to the intensity of the spots and colored according to hydrophobicity, size, and charge.
(C) Sequence alignment of putative DPY-30-interacting proteins. Shown is the sequence alignment of DBMs of various proteins. Residues predicted to bind DPY-30 pockets are shaded in gray.
(D) Binding of DPY-30 with the NURF complex in HEK293 cells. Coimmunoprecipitation of BAP18 and BPTF by DPY-30 WT or Leu69Asp was analyzed by immunoblots with the indicated antibodies. The top three panels show the bound fraction, whereas the bottom three panels show that an equivalent amount of proteins has been loaded.
(E) Validation of BAP18 as a direct DPY-30-interacting protein. Binding of BAP18 to DPY-30 WT or Leu69Asp was performed by GST pull-down experiments using protein expressed in bacteria and measured with the indicated antibody.
(F) Characterization of the BAP18 DBM. Binding assays were performed as in Figure 5E with either BAP18 WT or the Phe57Asp mutant and detected with the indicated antibody.
DPY-30D/D Is a Versatile Protein-Protein Interaction Module
After determining the sequence rules for binding specificity by DPY-30, we sought to explore whether known DPY-30-interacting proteins harbored this motif. We queried a list of DPY-30 interactors (van Nuland et al., 2013) with the DBM using Prosite (Sigrist et al., 2013). Interestingly, we found several proteins harboring this motif, including BIG1, BAP18, NCoA6, and HDAC1. Manual inspection of other DPY-30 interactors characterized in worms revealed that sex determination and dosage compensation 2 (Sdc2) also harbors a DBM (Figure 5C).
To test whether one of the candidates, BAP18, can bind DPY-30, we immunoprecipitated transiently expressed FLAG-tagged DPY-30 WT or the Leu69Asp mutant in HEK293 cells. As shown in Figure 5D, BAP18 and BPTF copurified with the WT, whereas the same interactions were lost with the DPY-30 mutant. To further verify whether DPY-30 directly interacts with BAP18, we performed GST pull-down experiments with proteins expressed in bacteria. Similar to the FLAG immunoprecipitation experiments, we observed binding of BAP18 to DPY-30. Conversely, no binding could be detected in binding reactions carried out with the DPY-30 Leu69Asp mutant (Figure 5E). Finally, to validate our motif, we repeated these binding assays with BAP18 Phe57Asp, a mutation predicted to impair the interaction with DPY-30D/D PI-PII pockets. As shown in Figure 5F, we observed a notable loss of interaction between DPY-30 and the BAP18 mutant, strongly suggesting that DPY-30 directly interacts with BAP18 and is a genuine subunit of the NURF complex. Finally, these results suggest that DPY-30 directly interacts with several proteins (e.g., HDAC1 and NCoA6). However, additional binding studies are needed to support this hypothesis.
DISCUSSION
The DPY30D/D/ASH2LSDI complex structure described here provides a structural framework for understanding the molecular determinants for the recognition of the ASH2LSDI motif by the DPY-30D/D module. This study also presents evidence that the binding elements in DPY-30D/D mainly comprise four shallow pockets, each accommodating hydrophobic residues distanced by one turn of the α helix on ASH2LSDI. Of these pockets, PIII is critical in binding ASH2L because a single Leu69Asp substitution impairs binding to ASH2L in HEK293 cells and because the mutation Met138Asp in Cps25 results in a complete loss of global histone H3K4me3 in budding yeast. Similarly, replacement of the ASH2L residue interacting with the PIII (L513) pocket impairs H3K4me3 at the β-globin gene and results in a loss of β-globin expression. Overall, our mutational analysis indicates that the interaction between ASH2L and DPY-30 is critical for maintaining a high level of histone H3K4me3 and contributes to the allosteric regulation of MLL1 methyltransferase activity. Our findings are also consistent with recent studies showing that knockdown of ASH2L or DPY-30 results in a significant loss of H3K4me3 in mouse embryonic stem cells and in human teratocarcinoma NT2 cells (Jiang et al., 2011; Simboeck et al., 2013). How does DPY-30 contribute to the high level of H3K4me3? The cryo-EM analysis of the core COMPASS architecture showed that the Cps60/Cps25 complex sits at the base of COMPASS and forms a globular structure that is in close proximity to the Set1 catalytic domain (Takahashi et al., 2011). Combined with the crystal structure of the ASH2L/DPY-30 complex, these observations suggest that ASH2LSDI serves as an anchoring platform to recruit DPY-30. This binding event allows the DPY-30 N terminus, the DPY- 30D/D/ASH2L complex, or both to directly contact and stimulate MLL1 trimethyltransferase activity. Consistent with this model, South et al. (2010) have shown recently that mutations impacting the interaction between Cps60 and Cps25 are also detrimental for the binding to ScSET1. Alternatively, association of DPY-30 to ASH2L triggers a structural reorganization of the ASH2L/RbBP5 complex, ultimately leading to the opening of the Set1 catalytic site and resulting in a stimulation of its trimethyltransferase activity.
Binding of A-kinase anchoring proteins to RIα regulates PKA and, consequently, controls a myriad of signaling events in facilitating the localization of the kinase to specific sites in the cell (Smith et al., 2006). The crystal structure of DPY-30D/D in complex with ASH2LSDI showed that, similar to the RIα and D/D domains, DPY-30 binds the hydrophobic ridge of an amphipathic α helix (Gold et al., 2006; Kinderman et al., 2006) and exhibits sequence specificity regarding the hydrophobic residues recognized by its X bundle structure. However, a detailed mutational analysis combined with overlay assays revealed that DPY- 30D/D, in contrast to the RIα D/D domain, recognizes a divergent set of residues. More specifically, the DPY-30D/D PI pocket is far less restrictive than the RIα-analogous pocket. Indeed, although RIα only accommodates an alanine residue in the second position binding to the PI pocket, the DPY-30D/D PI pocket enables the binding of several bulky hydrophobic residues. Analogous to the PI pocket, the other pockets of RIα all accommodate the binding of small side chain residues, whereas overlay assays performed with DPY-30 revealed that none of these pockets are amenable for binding residues with small side chains. These observations are further supported by a comparative analysis between the RIα and DPY-30 X bundle structures (Burns-Hamuro et al., 2003, 2005; Sarma et al., 2010), which shows that the distance between ASH2LSDI and the DPY-30 X bundle surface is greater than the distance between AKAP and RIα. Our data show that the difference in the angle between α0 and α1 of a dimerization/docking module will determine the relative positioning of the binding α helix and contribute to, along with the four binding pockets, the specificity of interactions of this large family of scaffolding domains. It is interesting to note that the effects of substituting hydrophobic residues of ASH2L in pull-down experiments were less detrimental when compared with the results observed with the overlay assays. In fact, although the V516D substitution, a PIII-interacting residue, had a negligible effect on DPY-30 binding using pull-down assays, mutation of the same residue in overlay assays revealed that only hydrophobic residues are accommodated in this pocket. These differences suggest that other subunits binding to COMPASS stabilize the association of DPY-30 to ASH2L.
DPY-30 directly interacts with ASH2L (Chen et al., 2012; South et al., 2010), AKAP95 (Jiang et al., 2013), and BIG1 (Xia et al., 2010; Xu et al., 2009), a protein localized in the trans-Golgi network. In Caenorhabditis elegans, DPY-30 associates with the dosage compensation complex (Pferdehirt et al., 2011), and in Drosophila, the same protein binds to the metal-responsive transcription factor 1 (Vardanyan et al., 2008). In addition, recent proteomics studies showed that DPY-30 immunoprecipitates several proteins, including many subunits of the NURF complex. In combining the motifs established with the overlay assays and the proteomics data, we found that DPY-30 directly interacts with BAP18, suggesting that DPY-30 is a subunit of the BPTF complex and that this protein may associate with several proteins. This idea is further supported by recent studies demonstrating that knockdown of DPY-30 downregulates ID proteins and results in a senescent state (Simboeck et al., 2013). Attempts to rescue the phenotype by ectopic expression of ID proteins only partly rescued the phenotype, suggesting that DPY-30 controls senescence through other pathways or protein complexes. Accordingly, given its association to several functionally different proteins, future studies aimed at characterizing DPY-30 must take into consideration the functional diversity of this protein. Finally, considering the pervasiveness of amphipathic α helices in proteins, the extent of protein complexes in which DPY-30 resides has likely been underestimated, raising the possibility that DPY-30 plays a versatile role in several biological processes.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Using primer-overlapping PCR, a DNA sequence encoding the ASH2LSDI was placed in tandem with the cDNA corresponding to the D/D domain of DPY-30 (residues 45–99 (DPY-30D/D)). The construct, which was engineered so that a tobacco etch virus protease (TEV) cleavage site separates ASH2LSDI and DPY-30D/D, was cloned in a pGEX-based vector for expression of protein in fusion with a TEV-cleavable GST affinity tag. The GST fusion ASH2LSDI-DPY-30D/D protein was overexpressed in Rosetta cells for 3 hr at 37°C with 100 μM isopropyl β-D-1-thiogalactopyranoside. The cells were centrifuged, and the pellet was harvested in PBS buffer (pH 7.3). The cells were lysed by sonication and clarified by centrifugation. The supernatant was applied onto glutathione Sepharose beads for 1 hr. After extensively washing the beads, the fusion protein was incubated with TEV protease in 20 mM Tris (pH 7.0) and 100 mM NaCl for 16 hr at 4°C. Cleaved proteins were concentrated, separated by size exclusion chromatography (Superdex S75) pre-equilibrated in the same buffer.
Crystallization, Data Collection, and Crystal Structure Determination
Following gel filtration, fractions of the main peak were pooled and immediately dialyzed in 20 mM (2-[N-morpholino]ethanesulfonic acid (pH 6.0) and 100 mM NaCl. The complex was subsequently concentrated to 10 mg/ml, and crystallization trials were carried out using various sparse matrix screens. Initial hits were obtained using Index Screen (Hampton Research). Diffraction-quality crystals were obtained by vapor diffusion, with the mother liquor composed of 0.2 M MgCl2, 25% polyethylene glycol (PEG) 3350, 0.1 M Tris (pH 8.5), and 10 mM spermidine. Triangular crystals were harvested, soaked in the mother liquor supplemented with 20% PEG 400, and quickly flash-frozen in liquid nitrogen. A full data set was collected on a Micromax 007-HF (Rigaku), and images were recorded on a RAXIX IV++ instrument. The reflections were indexed and scaled using d*TREK (Pflugrath, 1999).
A homology model of DPY-30D/D-ASH2LSDI was generated using the PKA-RIIα structure in complex with a peptide corresponding to residues 5–19 of D-AKAP2. The DPY-30D/D domain (Protein Data Bank [PDB] ID code 3G36) was overlaid onto the PKA-RIIα structure (PDB ID code 2HWN), and AKAP2 residues were mutated to alanine. The resulting heterotrimer was used as a search model using Phaser (Zwart et al., 2008). Eight heterotrimers were located in the asymmetric unit and were used as model building in Coot (Emsley et al., 2010) and refined with Buster (Blanc et al., 2004). Omit maps were calculated with Phenix (Figure S2), and the quality of the model was assessed using Molprobity (Table 1).
Table 1.
Data Collection and Refinement Statistics
| Dpy-30D/D-Ash2LSDI | |
|---|---|
| Data Collection | |
| Space group | P1 |
| Cell dimensions | |
| a, b, c (Å) | 56.3, 56.8, 95.1 |
| α, β, γ (°) | 90.1, 89.9, 115.4 |
| Resolution (Å) | 28.9–2.40 (2.49–2.40)a |
| Rsym | 7.3 (39.9) |
| I/σI | 8.3 (1.9) |
| Completeness (%) | 95.0 (93.4) |
| Redundancy | 2.1 (2.1) |
| Refinement | |
| Resolution (Å) | 22.9–2.4 |
| No. of reflections | 47655 |
| Rwork/Rfree | 24.6–29.6 |
| No. of atoms | |
| DPY-30 | 5,919 |
| Ash2L | 1,503 |
| Water | 29 |
| Sulfate | 40 |
| B factors (Å2) | |
| Dpy-30 | 75.5 |
| Ash2L | 67.7 |
| Water | 63.3 |
| Sulfate | 113.1 |
| Rmsds | |
| Bond lengths (Å) | 0.01 |
| Bond angles (°) | 1.19 |
| Molprobity score | 1.94 |
| Ramachandran (%) | |
| Most allowed region | 96.6 |
| Allowed region | 3.4 |
The data set was collected on a single crystal.
The highest resolution shell is shown in parentheses.
GST Pull-Down Experiment
ASH2L was overexpressed as described previously (Sarvan et al., 2011). Pellets were resuspended in 50 mM sodium phosphate, 500 mM NaCl, and 5 mM β-mercaptoethanol, and cells were lysed by sonication. Constructs corresponding to full-length DPY-30 (WT and mutants) were expressed in fusion with GST in BL21(DE3) Rosetta cells, and the bacterial extracts were centrifuged, harvested, in PBS, and lysed by sonication. The supernatant was bound to glutathione Sepharose beads (GE Healthcare) for 1 hr at 4°C in PBS and 0.1%Triton X-100. The beads were washed using a binding buffer composed of 50 mM Tris (pH 8.0), 300 mM NaCl, and 0.02% Triton X-100 and incubated immediately for 2 hr at 4°C with bacterial extracts containing His-tagged ASH2L. The beads were subsequently washed with the binding buffer, and bound proteins were eluted in SDS loading buffer. Proteins were separated on SDS page gel and detected by western blot with appropriate antibodies (see below). GST pull-down experiments for BAP18/DPY-30 interactions were performed using similar conditions.
Antibodies
The commercial antibodies used in these studies included H3K4me3 (Abcam, catalog no. ab8580), H3 (Millipore, catalog no. 05-928), FLAG (Sigma, catalog no. F3165), histidine (Santa Cruz Biotechnology, catalog no. sc-803), GST/HRP (Abcam, catalog no. ab3416), and BAP18 (Bethyl Laboratories). ScSet1 and histone H3 antibodies were provided by the A.S. laboratory.
Terminal Differentiation of Erythroid Cells
Sixteen micrograms of constructs corresponding to FLAG-empty (pCMV), FLAG-ASH2L WT, FLAG-ASH2L V509D (retains binding activity on DPY-30), and FLAG-ASH2L L513D (unable to bind DPY-30) were introduced into MEL cells by electroporation. Twelve hours after electroporation, erythroid differentiation was induced by adding 2% final DMSO to the culture medium. At the same time, the knockdown of endogenous Ash2L was induced by adding 5 μg/ml final doxycycline to the culture medium. Forty-eight hours later, MEL cells were centrifuged, harvested, and crosslinked as described previously (Demers et al., 2007). Chromatin extraction, H3K4me3 immunoprecipitation, and real-time quantitative PCR analysis were performed as described previously (Sarvan et al., 2011).
Budding Yeast Strain Engineering
Cps25 WT was cloned into the pRS406 vector, and the Cps25 Met138Asp mutant was generated by site-directed mutagenesis (Agilent Technologies). The vectors containing Cps25 WT or Met138Asp under the control of the URA3 promoter were transformed into Cps25 δ cells and grown on a synthetic defined plate deficient of URA3 (SD-URA), and positive single-colony isolates were selected and confirmed by PCR. Complemented cells were cultured in YPD overnight at 30°C, and nuclear extracts were isolated and analyzed by western blotting using specific antibodies.
Design and Synthesis of the SPOT Arrays
The peptide arrays were synthesized using a SPOT synthesis technique (Frank, 2002; Hilpert et al., 2007) (Kinexus). Briefly, the synthesis was carried out using a MultiPep peptide synthesizer (INTAVIS Bioanalytical Instruments). The peptides were built by subsequent delivery of 0.1 μl of 0.3 M solutions of preactivated amino acids to each spot on a trioxa-tridecanediamine membrane, an amino-functionalized cellulose membrane (Winkler and Hilpert, 2010). The linkage of the peptides to the TODT membrane is very stable and, for that reason, particularly useful for direct probing on the membrane. The distance between centers of two spots was 2.8 mm, and the diameter of each spot was about 2 mm. The density of peptides was about 500 nmol/cm2, which resulted in an amount of approximately 16 nmol of peptide per spot.
Overlay Assays
Binding of GST-DPY-30 with the arrays was performed as follows. The arrays were first blocked for 1 hr in Tris-buffered saline (TBS)-Tween (TBS-T) supplemented with 5% BSA at 22°C. GST-DPY30 was then added at a concentration of 2.5 μg/ml in TBS-T supplemented with 2.5% BSA and incubated with the arrays for 105 min. Following the incubation, the arrays were washed with TBS-T three times for 10 min each. An antibody against GST coupled to HRP was then used at 0.2 μg/ml in 2% BSA/TBS-T for 1 hr, followed by three washes of 10 min each in TBS-T. Finally, a chemiluminescence substrate and a Li-Cor Odyssey Fc imaging system were used to detect and quantify the interaction between the ASH2LSDI peptides and the DPY-30 protein. The assay, in which every amino acid of the ASH2LSDI (positions 503–523) is substituted systematically and individually, was performed in duplicate, and the assay for the optimization of the binding with 15 different SDI peptides was repeated four times.
Immunoprecipitation of BAP18
Cells expressing FLAG-DPY-30 were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer. The supernatant was applied to anti-FLAG M2 magnetic beads (Invitrogen) for 1 hr and washed twice with RIPA buffer. Bound proteins were eluted with RIPA buffer supplemented with the FLAG peptide for 1 hr. Eluted proteins were separated by SDS-PAGE and detected with the indicated antibodies.
Supplementary Material
Acknowledgments
J.F.C. is supported by a CIHR grant (MOP-136816) and acknowledges an Early Research Award from MEDI and a Canada Research Chair in Structural Biology and Epigenetics. P.Z. holds a Banting & Best Ph.D. scholarship. M.B. acknowledges a CIHR grant (MOP-89834). G.S. acknowledges support from the Pew Scholars Program in Biomedical Sciences. The studies performed by the A.S. laboratory are supported by NIH grant R01GM069905.
Footnotes
ACCESSION NUMBERS
Coordinates and structure factor files have been deposited into the PDB with accession number 4RIQ.
Supplemental Information includes three figures and can be found with this article online at http://dx.doi.org/10.1016/j.str.2014.10.002.
References
- Avdic V, Zhang P, Lanouette S, Groulx A, Tremblay V, Brunzelle J, Couture JF. Structural and biochemical insights into MLL1 core complex assembly. Structure. 2011;19:101–108. doi: 10.1016/j.str.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Banaszynski LA, Allis CD, Lewis PW. Histone variants in metazoan development. Dev Cell. 2010;19:662–674. doi: 10.1016/j.devcel.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc E, Roversi P, Vonrhein C, Flensburg C, Lea SM, Bricogne G. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr D Biol Crystallogr. 2004;60:2210–2221. doi: 10.1107/S0907444904016427. [DOI] [PubMed] [Google Scholar]
- Burns-Hamuro LL, Ma Y, Kammerer S, Reineke U, Self C, Cook C, Olson GL, Cantor CR, Braun A, Taylor SS. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc Natl Acad Sci USA. 2003;100:4072–4077. doi: 10.1073/pnas.2628038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns-Hamuro LL, Hamuro Y, Kim JS, Sigala P, Fayos R, Stranz DD, Jennings PA, Taylor SS, Woods VL., Jr Distinct interaction modes of an AKAP bound to two regulatory subunit isoforms of protein kinase A revealed by amide hydrogen/deuterium exchange. Protein Sci. 2005;14:2982–2992. doi: 10.1110/ps.051687305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Chen Y, Cierpicki T, Liu Y, Basrur V, Lei M, Dou Y. An Ash2L/RbBP5 heterodimer stimulates the MLL1 methyltransferase activity through coordinated substrate interactions with the MLL1 SET domain. PLoS ONE. 2010;5:e14102. doi: 10.1371/journal.pone.0014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H, Bergman Y. Programming of DNA methylation patterns. Annu Rev Biochem. 2012;81:97–117. doi: 10.1146/annurev-biochem-052610-091920. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cao F, Wan B, Dou Y, Lei M. Structure of the SPRY domain of human Ash2L and its interactions with RbBP5 and DPY30. Cell Res. 2012;22:598–602. doi: 10.1038/cr.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture JF, Skiniotis G. Assembling a COMPASS. Epigenetics. 2013;8:349–354. doi: 10.4161/epi.24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers C, Chaturvedi CP, Ranish JA, Juban G, Lai P, Morle F, Aebersold R, Dilworth FJ, Groudine M, Brand M. Activator-mediated recruitment of the MLL2 methyltransferase complex to the beta-globin locus. Mol Cell. 2007;27:573–584. doi: 10.1016/j.molcel.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Li Y, Liang S, Cui K, Salz T, Yang H, Tang Z, Gallagher PG, Qiu Y, Roeder R, et al. USF1 and hSET1A mediated epigenetic modifications regulate lineage differentiation and HoxB4 transcription. PLoS Genet. 2013;9:e1003524. doi: 10.1371/journal.pgen.1003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan V, Lee JH, Patel A, Skalnik DG, Cosgrove MS. Structural basis for WDR5 interaction (Win) motif recognition in human SET1 family histone methyltransferases. J Biol Chem. 2012;287:27275–27289. doi: 10.1074/jbc.M112.364125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R. The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports—principles and applications. J Immunol Methods. 2002;267:13–26. doi: 10.1016/s0022-1759(02)00137-0. [DOI] [PubMed] [Google Scholar]
- Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Taskén K, Carlson CR, Scott JD, Barford D. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hilpert K, Winkler DF, Hancock RE. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat Protoc. 2007;2:1333–1349. doi: 10.1038/nprot.2007.160. [DOI] [PubMed] [Google Scholar]
- Holm L, Rosenström P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–9. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Garruss AS, Gao X, Morgan MA, Cook M, Smith ER, Shilatifard A. The Mll2 branch of the COMPASS family regulates bivalent promoters in mouse embryonic stem cells. Nat Struct Mol Biol. 2013;20:1093–1097. doi: 10.1038/nsmb.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Shukla A, Wang X, Chen WY, Bernstein BE, Roeder RG. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell. 2011;144:513–525. doi: 10.1016/j.cell.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lu X, Shimada M, Dou Y, Tang Z, Roeder RG. Regulation of transcription by the MLL2 complex and MLL complex-associated AKAP95. Nat Struct Mol Biol. 2013;20:1156–1163. doi: 10.1038/nsmb.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderman FS, Kim C, von Daake S, Ma Y, Pham BQ, Spraggon G, Xuong NH, Jennings PA, Taylor SS. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odho Z, Southall SM, Wilson JR. Characterization of a novel WDR5-binding site that recruits RbBP5 through a conserved motif to enhance methylation of histone H3 lysine 4 by mixed lineage leukemia protein-1. J Biol Chem. 2010;285:32967–32976. doi: 10.1074/jbc.M110.159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pferdehirt RR, Kruesi WS, Meyer BJ. An MLL/COMPASS subunit functions in the C. elegans dosage compensation complex to target X chromosomes for transcriptional regulation of gene expression. Genes Dev. 2011;25:499–515. doi: 10.1101/gad.2016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugrath JW. The finer things in X-ray diffraction data collection. Acta Crystallogr D Biol Crystallogr. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- Rampalli S, Li L, Mak E, Ge K, Brand M, Tapscott SJ, Dilworth FJ. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol. 2007;14:1150–1156. doi: 10.1038/nsmb1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh R, Allis CD. Genome-wide “re”-modeling of nucleosome positions. Cell. 2011;147:263–266. doi: 10.1016/j.cell.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma GN, Kinderman FS, Kim C, von Daake S, Chen L, Wang BC, Taylor SS. Structure of D-AKAP2:PKA RI complex: insights into AKAP specificity and selectivity. Structure. 2010;18:155–166. doi: 10.1016/j.str.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvan S, Avdic V, Tremblay V, Chaturvedi CP, Zhang P, Lanouette S, Blais A, Brunzelle JS, Brand M, Couture JF. Crystal structure of the trithorax group protein ASH2L reveals a forkhead-like DNA binding domain. Nat Struct Mol Biol. 2011;18:857–859. doi: 10.1038/nsmb.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilatifard A. Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol. 2008;20:341–348. doi: 10.1016/j.ceb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist CJA, de Castro E, Cerutti L, Cuche BA, Hulo N, Bridge A, Bougueleret L, Xenarios I. New and continuing developments at PROSITE. Nucleic Acids Res. 2013;41:D344–D347. doi: 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simboeck E, Gutierrez A, Cozzuto L, Beringer M, Caizzi L, Keyes WM, Di Croce L. DPY30 regulates pathways in cellular senescence through ID protein expression. EMBO J. 2013;32:2217–2230. doi: 10.1038/emboj.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Langeberg LK, Scott JD. The where’s and when’s of kinase anchoring. Trends Biochem Sci. 2006;31:316–323. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- South PF, Fingerman IM, Mersman DP, Du HN, Briggs SD. A conserved interaction between the SDI domain of Bre2 and the Dpy-30 domain of Sdc1 is required for histone methylation and gene expression. J Biol Chem. 2010;285:595–607. doi: 10.1074/jbc.M109.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YH, Westfield GH, Oleskie AN, Trievel RC, Shilatifard A, Skiniotis G. Structural analysis of the core COMPASS family of histone H3K4 methylases from yeast to human. Proc Natl Acad Sci USA. 2011;108:20526–20531. doi: 10.1073/pnas.1109360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nuland R, Smits AH, Pallaki P, Jansen PW, Vermeulen M, Timmers HT. Quantitative dissection and stoichiometry determination of the human SET1/MLL histone methyltransferase complexes. Mol Cell Biol. 2013;33:2067–2077. doi: 10.1128/MCB.01742-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardanyan A, Atanesyan L, Egli D, Raja SJ, Steinmann-Zwicky M, Renkawitz-Pohl R, Georgiev O, Schaffner W. Dumpy-30 family members as determinants of male fertility and interaction partners of metal-responsive transcription factor 1 (MTF-1) in Drosophila. BMC Dev Biol. 2008;8:68. doi: 10.1186/1471-213X-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lou Z, Dong X, Yang W, Peng Y, Yin B, Gong Y, Yuan J, Zhou W, Bartlam M, et al. Crystal structure of the C-terminal domain of human DPY-30-like protein: A component of the histone methyltransferase complex. J Mol Biol. 2009;390:530–537. doi: 10.1016/j.jmb.2009.05.061. [DOI] [PubMed] [Google Scholar]
- Winkler DF, Hilpert K. Synthesis of antimicrobial peptides using the SPOT technique. Methods Mol Biol. 2010;618:111–124. doi: 10.1007/978-1-60761-594-1_8. [DOI] [PubMed] [Google Scholar]
- Xia B, Joubert A, Groves B, Vo K, Ashraf D, Djavaherian D, Awe J, Xiong Y, Cherfils J, Ma D. Modulation of cell adhesion and migration by the histone methyltransferase subunit mDpy-30 and its interacting proteins. PLoS ONE. 2010;5:e11771. doi: 10.1371/journal.pone.0011771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Gong Q, Xia B, Groves B, Zimmermann M, Mugler C, Mu D, Matsumoto B, Seaman M, Ma D. A role of histone H3 lysine 4 methyltransferase components in endosomal trafficking. J Cell Biol. 2009;186:343–353. doi: 10.1083/jcb.200902146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Lee H, Brunzelle JS, Couture JF. The plasticity of WDR5 peptide-binding cleft enables the binding of the SET1 family of histone methyltransferases. Nucleic Acids Res. 2012;40:4237–4246. doi: 10.1093/nar/gkr1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart PH, Afonine PV, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, McKee E, Moriarty NW, Read RJ, Sacchettini JC, et al. Automated structure solution with the PHENIX suite. Methods Mol Biol. 2008;426:419–435. doi: 10.1007/978-1-60327-058-8_28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.