Abstract
Background
Recent evidence suggests that atrial fibrillation (AF) is maintained by high-frequency reentrant sources with a left-to-right–dominant frequency gradient, particularly in patients with paroxysmal AF (pAF). Unequal left-to-right distribution of inward rectifier K+ currents has been suggested to underlie this dominant frequency gradient, but this hypothesis has never been tested in humans.
Methods and Results
Currents were measured with whole-cell voltage-clamp in cardiomyocytes from right atrial (RA) and left (LA) atrial appendages of patients in sinus rhythm (SR) and patients with AF undergoing cardiac surgery. Western blot was used to quantify protein expression of IK1 (Kir2.1 and Kir2.3) and IK,ACh (Kir3.1 and Kir3.4) subunits. Basal current was ≈2-fold larger in chronic AF (cAF) versus SR patients, without RA-LA differences. In pAF, basal current was ≈2-fold larger in LA versus RA, indicating a left-to-right atrial gradient. In both atria, Kir2.1 expression was ≈2-fold greater in cAF but comparable in pAF versus SR. Kir2.3 levels were unchanged in cAF and RA-pAF but showed a 51% decrease in LA-pAF. In SR, carbachol-activated (2 μmol/L) IK,ACh was 70% larger in RA versus LA. This right-to-left atrial gradient was decreased in pAF and cAF caused by reduced IK,ACh in RA only. Similarly, in SR, Kir3.1 and Kir3.4 proteins were greater in RA versus LA and decreased in RA of pAF and cAF. Kir3.1 and Kir3.4 expression was unchanged in LA of pAF and cAF.
Conclusions
Our results support the hypothesis that a left-to-right gradient in inward rectifier background current contributes to high-frequency sources in LA that maintain pAF. These findings have potentially important implications for development of atrial-selective therapeutic approaches.
Keywords: atrium, fibrillation, ion channels, remodeling
There is experimental and clinical evidence suggesting that certain cases of paroxysmal atrial fibrillation (pAF) and chronic atrial fibrillation (cAF) are maintained by high-frequency reentrant sources (rotors) with a consistent left-to-right–dominant frequency gradient, particularly in pAF.1–7 AF-maintaining waves emanating from the left atrium (LA) undergo complex, spatially distributed conduction patterns, with wave front fractionation in the right atrium (RA) manifesting as “fibrillatory conduction.”8 In a large proportion of patients, ablation of dominant frequency sites terminates pAF,6 consistent with the notion that LA sites “drive” AF. LA-to-RA–dominant frequency gradients are much clearer in patients with pAF than those with persistent/permanent cAF.1,2,6
Sustained AF leads to ionic current remodeling, including upregulation of the inward rectifier IK1, downregulation of the muscarinic receptor-activated IK,ACh, and upregulation of a constitutively-active IK,ACh component (“constitutive IK,ACh”).9–12 Inward rectifier K+ currents abbreviate action potential duration and hyperpolarize atrial cardiomyocytes, thereby enhancing sodium channel availability. Therefore, increased IK1 and constitutive IK,ACh may be more effective in stabilizing and accelerating AF-sustaining rotors than changes in other currents (eg, L-type Ca2+ current downregulation) that produce similar action potential duration abbreviation.13–15 In a sheep model of acetylcholine-mediated, pacing-induced AF, the left-to-right–dominant frequency gradient parallels an LA-to-RA IK,ACh gradient,4,16 supporting the hypothesis that an unequal LA-RA distribution of inward rectifier K+ currents contributes to AF maintenance. Although AF-associated changes in IK1 and IK,ACh may be critical for rapidly firing LA driver-sources, there are no published studies directly comparing IK1 and IK,ACh in LA versus RA in AF. The present study was designed to test the hypothesis that AF patients show chamber-specific differences in inward rectifier current function, providing a potential molecular basis for AF-maintaining LA drivers.
Methods
Human Tissue Samples
Experimental protocols were approved by the ethics committees of Dresden University of Technology (No. EK790799) and the Cleveland Clinic (IRB No. 4286). Each patient gave written informed consent. RA and/or LA appendages were obtained from 43 patients with sinus rhythm (SR), 22 patients with pAF, and 30 patients with cAF undergoing open heart surgery for either coronary artery bypass grafting and/or valve replacement (Table), but matched samples of RA and LA appendages were obtained from only 2 SR and 2 cAF patients. After excision, samples were used for myocyte isolation and/or immediately snap-frozen in liquid nitrogen for biochemical measurements. All diseased heart samples were collected in Dresden, except for 6 LA samples from pAF patients collected in Cleveland, used for biochemistry. Anesthesia was performed similarly in both centers; tissue handling followed the same protocol. There were no differences in results for tissues from the 2 sampling centers (Online Figure 1).
Table.
Patient Characteristics
| RA-SR | RA-pAF | RA-cAF | LA-SR | LA-pAF | LA-cAF | |
|---|---|---|---|---|---|---|
| Patients, n | 35 | 13 | 17 | 10 | 9 | 15 |
| Sex, m/f | 24/11 | 7/6 | 10/7 | 5/5 | 6/3 | 6/9 |
| Age, y | 67.9±1.5 | 73.9±2.0 | 70.4±1.8 | 53.5±3.6‡ | 65.6±0.9* | 66.3±1.8* |
| Body mass index, kg/m2 | 27.0±0.6 | 27.5±1.3 | 27.0±0.8 | 26.9±1.4 | 26.4±2.0 | 27.7±1.3 |
| CAD, n | 16 | 7 | 2*† | 3 | 1 | 3 |
| MVD/AVD, n | 14 | 2 | 12*† | 4 | 6 | 10 |
| CAD+MVD/AVD, n | 5 | 4 | 3 | 3 | 2 | 2 |
| Hypertension, n | 28 | 10 | 16 | 5‡ | 5 | 11 |
| Diabetes, n | 11 | 6 | 2† | 1 | 1 | 6 |
| Hyperlipidemia, n | 26 | 9 | 12 | 7 | 3 | 13† |
| LVEF, % | 55.8±1.7 | 45.6±3.5* | 53.5±2.5 | 42.0±4.3† | 53.3±2.9 | 43.1±4.5 |
| LAD, mm | 41.3±0.9 | 47.1±1.8 | 48.9±2.2* | 45.6±2.0 | 48.3±2.7 | 50.8±1.6 |
| LVEDD, mm | 50.2±2.5 | 45.2±6.7 | 52.7±2.4 | 59.4±4.1 | 54.8±2.7 | 54.5±2.2 |
| IVS, mm | 11.6±0.3 | 13.9±1.4 | 13.0±0.7 | 10.2±0.6 | 12.9±0.9 | 11.2±0.6 |
| LVPW, mm | 11.2±0.3 | 12.9±0.9 | 12.3±0.6 | 10.2±0.6 | 11.7±0.6 | 11.2±0.4 |
| Digitalis, n | 1 | 3 | 7* | 4‡ | 1 | 8† |
| ACE inhibitors, n | 22 | 6 | 10 | 10‡ | 5* | 9 |
| AT1 blockers, n | 4 | 4 | 3 | 0 | 0 | 3 |
| β-blockers, n | 28 | 9 | 15 | 9 | 6 | 14 |
| Dihydropyridines, n | 6 | 3 | 3 | 1 | 1 | 2 |
| Diuretics, n | 17 | 7 | 14* | 8 | 5 | 10 |
| Nitrates, n | 9 | 5 | 6 | 1 | 7* | 3† |
| Lipid-lowering drugs, n | 22 | 9 | 9 | 7 | 3 | 10 |
CAD indicates coronary artery disease; MVD/AVD, mitral/aortic valve disease; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LAD, left atrial diameter; IVS, interventricular septum thickness; LVPW, left ventricular posterior wall thickness; ACE, angiotensin-converting enzyme; and AT, angiotensin receptor.
P<0.05 versus SR,
P<0.05 versus pAF, and
P<0.05 versus corresponding values in RA from ANOVA followed by Bonferroni multiple comparisons procedure for continuous variables and from Fisher exact test for categorical variables.
RA and LA samples from nondiseased human hearts that were technically unusable for transplantation based on logistical (not patient-related) considerations were obtained from organ donors to serve as nondiseased controls. Procedures were approved by the ethics committee of the University of Szeged (No. 51 to 57/1997OEJ). Before cardiac explantation, patients did not receive medication except for dobutamine, furosemide, and plasma expanders (Online Table).
Electrophysiological Recordings
Atrial cardiomyocytes were isolated with a standard protocol17 and suspended in storage solution (mmol/L): KCl 20, KH2PO4 10, glucose 10, K-glutamate 70, β-hydroxybutyrate 10, taurine 10, EGTA 10, albumin 1, pH=7.4. Currents were measured with the voltage-clamp technique using ISO-2-Software (MFK, Niedernhausen, Germany) for data acquisition and analysis.9 Borosilicate glass microelectrodes had tip resistances of 2 to 5 MΩ when filled with pipette solution (mmol/L): K-aspartate 80, NaCl 8, KCl 40, CaCl2 2, Mg-ATP 5, EGTA 2, GTP-Tris 0.1, HEPES 10, pH=7.4. Seal resistances were 4 to 8 GΩ. Series resistance and cell capacitance were compensated.
Myocytes were superfused with bath solution containing (mmol/L): NaCl 120, KCl 20, MgCl2 1, CaCl2 2, glucose 10, HEPES 10, pH=7.4. As in previous studies,9–11,17,18 we used high (20 mmol/L) extracellular potassium concentration because this shifts the reversal potential to more positive values and allows us to record much larger (more easily measurable/comparable) inward currents (Figure 1). We recorded the currents at room temperature because current amplitudes at room temperature are comparable to those at 37°C (Online Figure 2) and the success rate of experiments is much higher at room temperature. Drugs were applied via a rapid solution exchange system (ALA Scientific Instruments, Long Island, NY). Agonist-independent basal current was measured with a ramp pulse from −100 to +40 mV (Figure 1A). We analyzed inward currents at −100 mV, as did previous studies.9–11,17,18 Outward currents were analyzed at −10 mV because confounding by other currents is negligible at this potential. Agonist-inducible IK,ACh was stimulated with carbachol (CCh, 2 μmol/L) and defined by total current in the presence of CCh minus basal current. Previous concentration-response experiments showed that 2 μmol/L CCh is a saturating concentration for IK,ACh activation with no differences in CCh sensitivity between SR and AF patients.10 We used a ramp pulse protocol, which is much better tolerated by human atrial myocytes. A direct comparison between results obtained with ramp pulses versus clamp steps (Online Figure 3) indicates their similarity and supports the validity of results obtained with ramp pulse protocols. The basal inward rectifier current and IK,ACh were specifically assessed, based on Ba2+-sensitive (1-mmol/L) currents. The contribution of agonist-independent IK,ACh to basal current was assessed with the selective Kir3-blocker tertiapin (10 nmol/L, Peptides International, Louisville, Ky). Data were not corrected for liquid junction potential (≈−12 mV, JPCalc Software). All drugs were from Sigma Aldrich (St Louis, Mo) unless otherwise stated.
Figure 1.
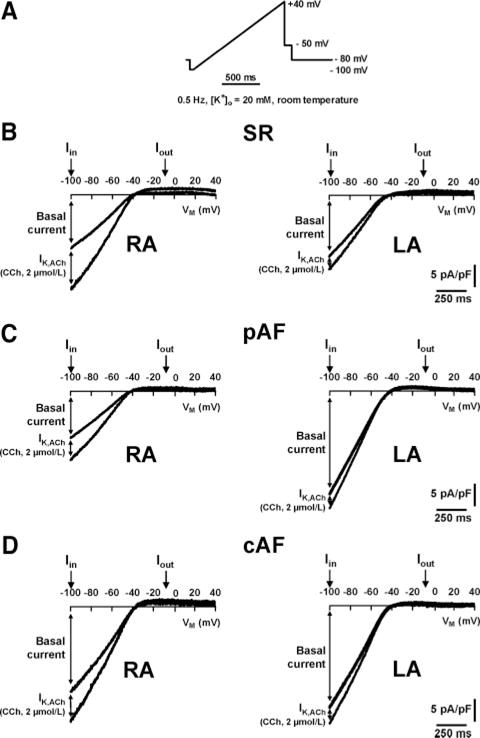
A, Ramp protocol from −100 to +40 mV. B through D, Representative current recordings in RA and LA myocytes of SR, pAF, and cAF. Basal current and CCh-sensitive (2 μmol/L) current increase (IK,ACh) were analyzed at inward (Iin) and outward (Iout) branches at −100 mV and −10 mV, respectively.
Immunoblots
Because of small sample size (usually <200 mg) and low isolated cell yield, immunoblotting is not feasible for isolated atrial cardiomyocyte samples from individual patients. Therefore, primary antibodies against Kir2.1 (1:200; Santa Cruz, Santa Cruz, Calif), Kir2.3 (1:200; Alomone, Jerusalem, Israel), Kir3.1 (1:1000; Alomone), and Kir3.4 (1:200, Santa-Cruz) were used to quantify corresponding proteins in atrial tissue homogenates only.19 Peroxidase-conjugated goat anti-rabbit (1:5000 for Kir2.3, 1:2500 for Kir3.1; Sigma-Al-drich) and donkey anti-goat (1:2000 for Kir2.1, 1:5000 for Kir3.4; Santa Cruz, Calif) were used as secondary antibodies and visualized by chemifluorescence (GE Healthcare, Chalfont St Giles, UK). Quantity One Software (Bio-Rad, Hercules, Calif) was used for quantification. After primary antibody removal with stripping buffer (Carl Roth, Karlsruhe, Germany), calsequestrin expression (1:2500 anti-CSQ, Dianova, Hamburg, Germany; 1:30 000 goat anti-rabbit) was quantified as loading control.
Statistical Analysis
Differences between group means were compared by unpaired Student t test or by 1-way ANOVA with Bonferroni-corrected t test. Two-way ANOVA was applied to assess the independent contribution of atrial chamber and arrhythmia type to ionic current densities. Frequency data were analyzed with the Fisher exact test. Data are mean± SEM. P<0.05 was considered statistically significant.
Results
Patient Characteristics
The pAF group included patients in SR at surgery with a history of at least 1 episode of self-terminating AF lasting <7 days. The cAF group included patients with sustained AF for at least 6 months before surgery. Patient group characteristics are shown in the Table. In general, pAF and cAF patients were older and had larger LA diameters than SR patients. Coronary artery disease was present more often in patients with SR and pAF, whereas cAF patients had higher incidence of valvular heart disease. Patients with cAF more frequently received digitalis than SR and pAF patients. All patients were euthyroid. SR patients who provided LA tissue had lower left ventricular ejection fraction than those providing RA samples but had no clinical evidence of heart failure.
Basal Inward Rectifier Current
There were no significant differences in membrane capacitances between groups, except for a lower membrane capacitance in LA-pAF versus LA-cAF (Online Figure 4). To control for myocyte size variability, currents are expressed as densities (pA/pF).
Representative current recordings in SR, pAF, and cAF are shown in Figure 1B through 1D. Overall, inward basal current in RA was greater in cAF than in SR and pAF (Figure 2A). Inward basal current in LA was 2-fold higher in both pAF and cAF than in SR, with a left-to-right gradient of basal current in pAF only. The greater rotation speed and persistence of rotors associated with increased inward rectifier current have been attributed to larger outward current components.14 Outward basal current differences paralleled inward current differences: pAF showed a left-to-right gradient and cAF had larger currents in both LA and RA. The enhanced basal currents in RA and LA of cAF were associated with a more negative RMP (Online Figure 5), without significant RA-LA differences. Mean RMP was larger in LA cardiomyocytes from pAF versus SR and versus RA cardiomyocytes from pAF, but the differences were not statistically significant.
Figure 2.
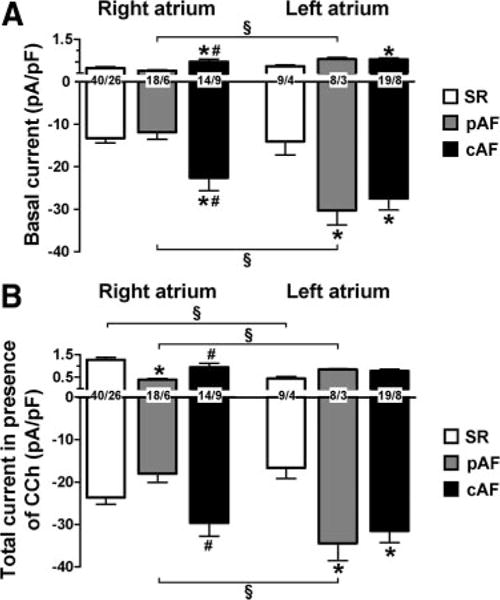
Inward rectifier currents in RA and LA myocytes from SR, pAF, and cAF at −100 mV and −10 mV, respectively. Values are mean±SEM. A, Basal current in absence of CCh (2 μmol/L). B, Total current (basal current+CCh-mediated current increase) in presence of CCh. Numbers indicate myocytes per patient. *P<0.05 and #P<0.05 versus corresponding values in SR and pAF, respectively. §P<0.05 versus corresponding values in RA.
Inward Rectifier Current in the Presence of CCh
Application of the muscarinic receptor agonist CCh led to an increase in total current density (Figure 2B and Figure 3) caused by activation of IK,ACh. Figure 2B shows total inward rectifier current density in the presence of 2 μmol/L CCh. In RA, the inward and outward components of total current were significantly smaller in pAF than in cAF. As for basal current, pAF patients had a significant LA-RA gradient. Compared with SR, cAF cells showed larger total current amplitudes in both atria with no RA-LA difference (Figure 2B).
Figure 3.
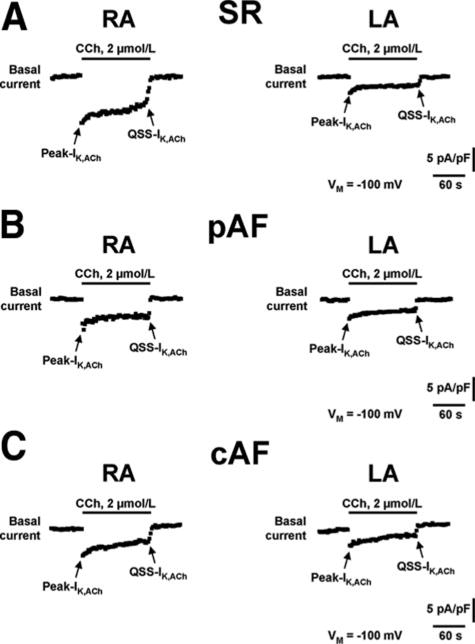
Representative time course recordings of IK,ACh in RA and LA myocytes from patients with SR (A), pAF (B), and cAF (C). Currents recorded during ramp protocol (Figure 1) were analyzed continuously (0.5 Hz) at −100 mV. IK,ACh was activated with the muscarinic receptor agonist CCh (2 μmol/L) and was defined as CCh-sensitive current component. Despite the continuous presence of CCh during 2 minutes, the initial increase (Peak-IK,ACh) faded to a quasi-steady-state level (QSS-IK,ACh) caused by a process termed “desensitization.”
Previous studies in RA showed reduced agonist-activated IK,ACh in pAF and cAF patients.9–11 Figure 4A shows mean current density of maximum CCh-activated IK,ACh in RA and LA of SR, pAF, and cAF. In RA, the inward and outward components were lower for pAF and cAF compared with SR. In LA, however, CCh-induced IK,ACh was similar in SR, pAF, and cAF for both inward and outward components. CCh-induced IK,ACh was greater in SR-RA than in SR-LA, indicating a clear right-to-left gradient. Qualitatively similar but statistically nonsignificant RA-LA differences were present for pAF and cAF (Figure 4A). Consistent with AF-related differences for CCh-induced IK,ACh in RA, the CCh-induced hyperpolarization of RMP was smaller in pAF and cAF versus SR (Online Figure 5).
Figure 4.
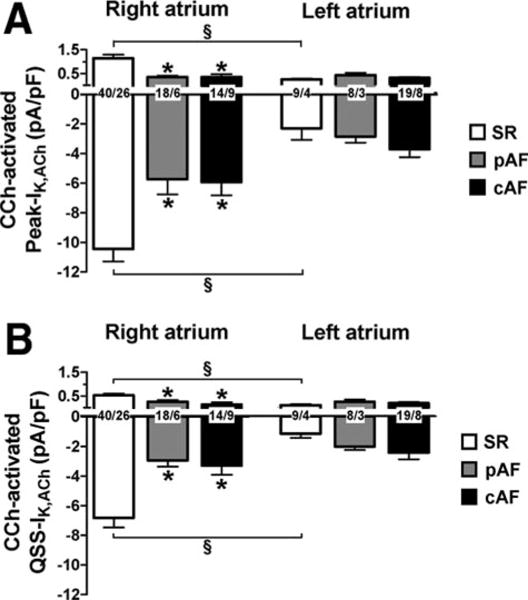
Carbachol-sensitive (2 μmol/L) current increase (IK,ACh) in RA and LA myocytes from SR, pAF, and cAF at −100 mV and −10 mV, respectively. Values are mean± SEM of Peak (A) and quasi-steady-state (QSS, B) current levels. Numbers indicate myocytes per patient. *P<0.05 versus corresponding values in SR. §P<0.05 versus corresponding values in right atrium.
IK,ACh currents are well known to show rapid “desensitization.”9–11,17 Figure 3 illustrates this phenomenon for CCh-induced IK,ACh: Rapid rises to a maximum (Peak-IK,ACh) were followed by decreases to quasi-steady-state levels (QSS-IK,ACh). The ratio of QSS-IK,ACh/Peak-IK,ACh values, an index of desensitization, was not affected by AF (Online Figure 6). Like the Peak-IK,ACh responses illustrated in Figure 4A, QSS-IK,ACh was smaller in RA of pAF and cAF compared with SR, was comparable in each group of LA, and showed a right-to-left gradient in SR (Figure 4B).
To determine potential contributions of underlying clinical conditions and/or medication (Table) to variations in basal current and CCh-activated IK,ACh, we performed univariate ANOVA analysis with rhythm status atrial chamber (left versus right), selected clinical parameters, and medication as independent variables. The density of basal current was significantly associated with rhythm status atrial chamber, body mass index, and valvular heart disease, whereas CCh-activated IK,ACh was significantly associated with rhythm status atrial chamber only. A test of interaction effects between rhythm status atrial chamber versus body mass index and valvular heart disease with 2-way ANOVA showed that rhythm status atrial chamber may independently associate with basal current because no significant interaction was detected (P=0.581 for body mass index; P=0.214 for valvular heart disease).
To assess whether the RA-LA differences seen in the grouped data were consistent with RA-LA differences from individual patients, we analyzed separately the current data available from matched RA-LA samples of SR and cAF patients. Online Figure 7 shows that the results obtained in myocytes from matched RA-LA samples are consistent with the overall trend in these groups (compare Figure 2 and Figure 4), supporting the validity of the mean overall group data.
Agonist-Independent Constitutive IK,ACh in LA of cAF Patients
In RA of cAF patients, agonist-independent constitutive IK,ACh probably contributes to AF maintenance.9 To unmask constitutive IK,ACh in LA, we used the selective IK,ACh blocker tertiapin (Figure 5). In LA, the tertiapin-sensitive component of basal current was higher in cAF compared with SR, indicating that cAF increased constitutive IK,ACh.
Figure 5.
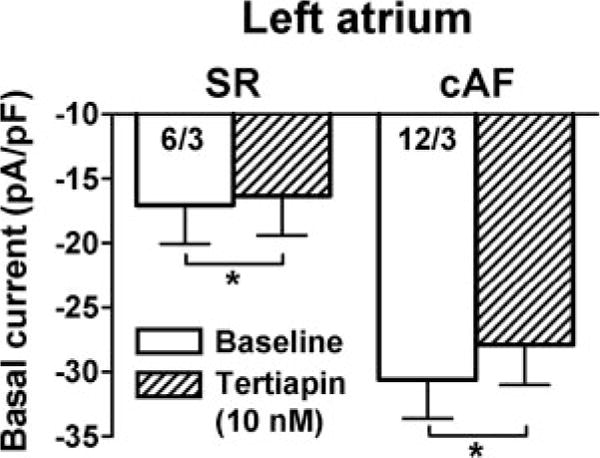
Effect of the IK,ACh channel blocker tertiapin (10 nmol/L) on basal current in LA myocytes. LA basal current at −100 mV before (baseline) and during tertiapin application in SR and cAF. Numbers indicate myocytes per patient. *P<0.05 versus baseline.
Protein Expression of IK1-Channel Subunits
We analyzed expression changes in ion channel subunits as a potential mechanism of chamber-specific differences in current density. Kir2.1 immunoblots showed greater Kir2.1 expression in RA and LA of cAF versus SR, without chamber-specific differences (Figure 6A and Online Figure 8). Compared with SR, pAF showed unchanged Kir2.1 expression in RA and LA. We observed no change in Kir2.3 proteins in RA of either group and in LA from SR versus cAF. However, Kir2.3 expression was 51% lower in LA of pAF versus SR (Figure 6B).
Figure 6.
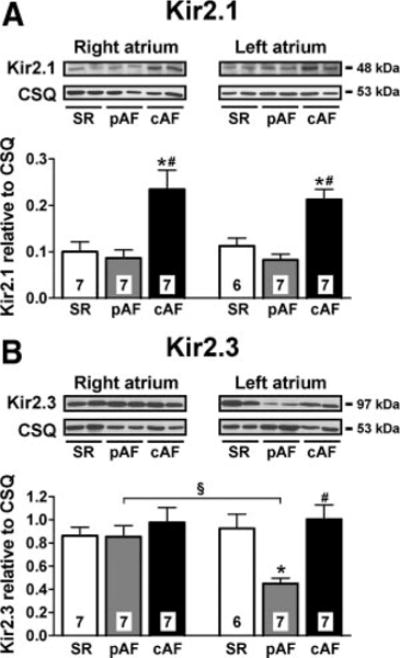
Expression of IK1 channel subunits in RA and LA from SR, pAF, and cAF. Representative immunoblots and densitometric analysis of Kir2.1 (A) and Kir2.3 (B) subunits. Numbers indicate tissue samples. *P<0.05 and #P<0.05 versus corresponding values in SR and pAF, respectively. §P<0.05 versus corresponding values in RA.
Protein Expression of IK,ACh-Channel Subunits
As in previous publications,10,20–22 we found reduced protein levels of Kir3.1 and Kir3.4 in RA of both pAF and cAF (Figure 7 and Online Figure 8). LA expression of Kir3.1 and Kir3.4 was unchanged in either group. Kir3.1- and Kir3.4-subunit expression was significantly greater in RA versus LA from SR patients. LA-RA gradients were absent in pAF and cAF, consistent with functional results shown in Figure 4.
Figure 7.
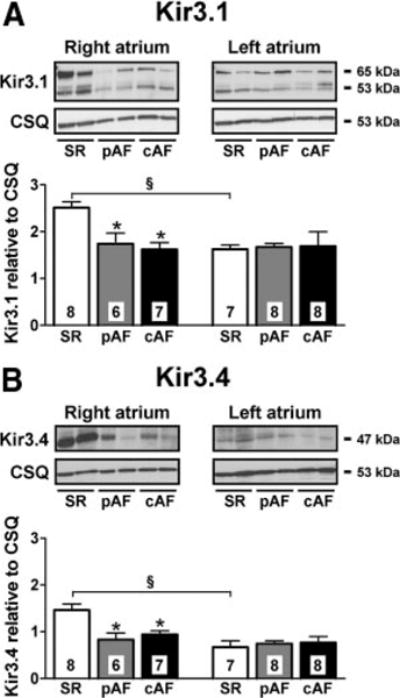
Expression of IK,ACh channel subunits in RA and LA from SR, pAF, and cAF. Format as in Figure 6. *P<0.05 versus corresponding values in SR. §P<0.05 versus corresponding values in RA.
To exclude the possibility that right-to-left gradients of Kir3.1 and Kir3.4 expression in SR were due to heart disease, we analyzed their expression in normal (nondiseased) atria (Online Figure 9). Similar to SR patient results, Kir3.1 and Kir3.4 expression in normal atria was greater in RA compared with LA.
Discussion
Experimental studies and computer simulations point to the importance of inward rectifier currents in AF dynamics, relating the faster rotation and greater stability of high-frequency reentrant sources to the expression levels and function of inward rectifier currents.13,16 Clinical evidence suggests a left-to-right–dominant frequency gradient in AF.1,2,5–7 Differences in inward rectifier K+ current function, which could explain this dominant frequency gradient, have not previously been investigated. Here, we addressed this issue for the first time in the literature. We found that pAF patients had inward rectifier current densities that were 2-fold larger in LA than in RA cardiomyocytes. In cAF patients, despite greater basal currents, there were no significant LA-RA differences. CCh-activated IK,ACh was larger in RA than in LA for SR patients, suggesting a right-to-left gradient in the absence of AF.
Comparison With Previous Studies
Emerging evidence suggests that AF is maintained by high-frequency sources (drivers), causing a hierarchical organization in activation rates for different regions of the atria.2,5,6 The presence of a left-to-right atrial frequency gradient has been demonstrated in a sheep model of AF and in some studies of clinical AF.1–7 Enhanced inward rectifier K+ currents promote reentry by abbreviating action potential duration as well as by accelerating and stabilizing rotors by hyperpolarizing atrial cardiomyocytes (thereby removing voltage-dependent sodium current inactivation).13–15 We and others recently reported larger basal inward rectifier current in RA and LA of cAF compared with SR patients.9–11,23–26 In the current study, we noted greater basal currents in cAF without significant LA-RA differences. These findings are in agreement with the lack of consistent left-to-right–dominant frequency gradients that has been reported in patients with persistent/permanent AF.1,2,6 The inward rectifier basal current increases, observed in both atria, could contribute to arrhythmia persistence by providing multiple potential regions of high-frequency rotor activity or by promoting reentrant wavelets by shortening refractoriness. Basal current of pAF patients was 2-fold larger in LA compared with SR patients but was similar in RA, implicating LA inward rectifier current enhancement as a potential contributor to high-frequency LA rotors underlying AF. In addition, these results suggest that the left-to-right inward rectifier current gradient in pAF may contribute to their left-to-right–dominant frequency gradient.6 A comparable mechanism has been shown in experimental ventricular fibrillation, where larger left ventricular IK1 critically underlies high-frequency sources.14
Previous studies on inward rectifier current remodeling consistently showed that RA Kir2.1 expression is increased in cAF, probably contributing to enhanced basal current.10,22 A recent study reported increased basal current in LA of cAF patients that is associated with enhanced Kir2.1 but unaltered Kir2.3,24 supporting the upregulation of IK1 as a key mechanism. Here, we provide evidence that in addition to increased IK1, agonist-independent constitutive IK,ACh also contributes to the larger basal inward rectifier current in the LA during cAF. These results supplement previous findings9,11 obtained in RA of cAF patients, underscoring the notion that constitutive IK,ACh may contribute, along with increased IK1, to reentrant sources maintaining AF.
In contrast to increased Kir2.1 expression in both atria of cAF patients, Kir2.1 expression was unchanged in the LA of pAF patients, despite their larger basal currents. Although Kir2.3 expression was unchanged in cAF, it decreased in the LA of pAF patients. Whether decreased Kir2.3 expression contributes to the functional increase of basal current in LA of pAF patients is unclear, but heteromeric Kir2.1 channels containing less Kir2.3 subunits would be expected to have higher IK1 amplitude because of reduced tonic Kir2.3 inhibition by βγ-subunits of G-protein–coupled receptors,27 protein kinase C–mediated phosphorylation,28 and transforming growth factor β1 modulation.29 In addition, constitutive IK,ACh may contribute to the larger basal current in LA of pAF patients. Further work is needed to verify these hypotheses and to identify the precise mechanisms of the chamber-specific increases of basal current in pAF.
Chamber-specific differences in agonist-activated IK,ACh show species variation: Whereas experiments in dogs and sheep suggest larger agonist-activated IK,ACh in LA,16,30 murine studies indicate greater agonist-activated IK,ACh in RA.31 To our knowledge, the present study is the first to address this issue in humans, showing a right-to-left gradient of agonist-activated IK,ACh and corresponding Kir3.1/3.4 expression differences. IK,ACh-subunit (Kir3.1/3.4) protein expression was greater in RA versus LA of undiseased atria, supporting the consistency of the finding and indicating that the right-to-left gradient in IK,ACh channel expression in SR patients was not a function of underlying heart disease. Differential posttranslational channel subunit modifications may also contribute to IK,ACh chamber- and disease-related gradients. Because of a selective IK,ACh reduction in RA, AF-related remodeling strongly attenuated the right-to-left gradient of CCh-activated IK,ACh. The lack of RA-dominant, agonist-activated IK,ACh may play a permissive role for LA-dominant drivers in pAF and cAF, particularly in vagal contexts.
Novel Findings and Potential Significance
The precise mechanisms underlying AF maintenance in humans are not fully understood. We noted a left-to-right inward rectifier gradient in pAF patients, consistent with the more rapid AF activity in the LA than in the RA.1–4,6,32 pAF patients with inducible AF after pulmonary vein isolation often have localized high-frequency sources outside the pulmonary veins.6,33,34 This observation may relate to the left-to-right atrial gradient of inward rectifier K+ currents that we observed in pAF. Targeting inward rectifier K+ currents may prove a new therapeutic approach for pAF.
Several studies suggest a complex activation pattern in cAF, with loss of spatiotemporal organization1,6,35 and higher dominant frequencies than pAF in all atrial regions.1,6 The comparable inward rectifier currents in LA and RA of cAF patients may provide a cellular explanation for the lack of left-to-right–dominant frequency gradients in cAF. The larger K+ currents in RA of cAF versus pAF patients may contribute to AF persistence by favoring more widespread driver regions in cAF, or alternatively by promoting reentrant waves via abbreviated refractoriness. In addition, the fibrotic structural changes commonly seen in both atria with cAF36 probably favor AF stabilization, potentially accounting for the greater persistence in cAF patients compared with pAF patients.
Potential Limitations
We studied atrial myocytes from RA and LA appendages only. Therefore, our results cannot be directly extrapolated to other RA and LA regions.
We noted a right-to-left gradient of agonist-activated IK,ACh in SR that was attenuated in pAF and cAF patients. In vivo regulation of IK,ACh is more complex, and chamber-specific regulation of IK,ACh may occur on various levels. The LA-RA distribution of parasympathetic neurons, choline acetyltransferase, acetylcholine content, and muscarinic receptors in human atria is unknown and should be addressed in future work.
We would have liked to examine the mechanistic basis of increased basal current in LA of pAF patients. Unfortunately, because of small sample size and limited access to LA biopsies, we were not able to study channel expression in membrane fractions or to perform single-channel recordings to determine whether enhanced IK1 and/or constitutive IK,ACh contributes to increased basal current in pAF. The data we obtained in LA cardiomyocytes from cAF patients (Figure 5) suggest that constitutive IK,ACh increases in LA of cAF patients but that it accounts for a relatively small part of the large increases in basal current seen in cAF. Thus, most of the increase observed probably is due to IK1 upregulation. Although IK1 is also probably responsible for increased basal current in the LA of pAF patients, this idea remains to be tested in subsequent work.
The present study focused on RA-LA differences in inward rectifier K+ currents, which are only 1 factor contributing to the left-to-right–dominant frequency gradient underlying AF maintenance. LA-RA differences in distribution of sodium currents, delayed rectifier K+ currents, or other atrial currents may also contribute. In addition, we did not measure dominant frequency gradients in the patients in which we recorded properties of inward rectifier currents. Thus, direct correlations between distribution of dominant frequencies and properties of atrial currents in individual patients, which would be desirable to define the putative role of inward rectifier currents for dominant frequency gradients, are unavailable. Nevertheless, inward rectifier currents remain an important determinant of atrial refractoriness and AF-promoting reentrant sources, and the LA increases of inward rectifier currents noted in the present study are likely to shorten LA refractoriness, contributing significantly to the role of the LA in maintaining pAF.
We used CSQ as an internal standard for normalization of protein expression data. Selection of internal standards for protein expression correction in remodeled states must be judicious and requires that the standard is unaffected by the remodeling paradigm studied. In a previous investigation, we found atrial CSQ expression to be slightly but significantly lower in dogs with congestive heart failure compared with control dogs.37 However, this difference is not seen in samples from AF versus SR patients. We used CSQ for normalization because in previous publications, protein levels of human atrial CSQ did not change during cAF,38 and these results were confirmed in samples from the present study (Online Figure 10A). GAPDH is often used as an internal standard. We measured GAPDH in samples from the present study (Online Figure 10B) and found that protein levels of GAPDH are strongly regulated in atria of AF patients, with reduced expression in both atria of cAF patients and higher expression in the LA versus RA of pAF patients. These data confirm the appropriateness of CSQ rather than other alternatives like GAPDH as an internal control for normalization.
Supplementary Material
CLINICAL PERSPECTIVE.
Recent evidence suggests that atrial fibrillation (AF) is maintained by high-frequency reentrant sources (rotors) with a consistent left-to-right–dominant frequency gradient, particularly in patients with paroxysmal AF (pAF). Ablation of left atrial-dominant frequency sites terminates clinical pAF, supporting the notion that left atrial sites “drive” AF. Inward rectifier potassium currents abbreviate refractoriness and hyperpolarize atrial cardiomyocytes, accelerating and stabilizing AF-sustaining rotors. Although inward rectifier currents are believed to contribute to the left-to-right–dominant frequency gradient in AF patients and are upregulated in AF, the relative left atrial versus right atrial distribution of inward rectifier currents in humans is unknown. In the present study, we used right and left atrial appendages from patients undergoing open heart surgery to explore left-to-right inward rectifier current differences in patients with sinus rhythm, pAF, or chronic AF (cAF). We noted a larger left versus right atrial basal inward rectifier current in pAF patients, which explain the left-to-right–dominant frequency gradient typical of pAF patients. In cAF patients, basal inward rectifier currents were increased equally in both atria, potentially accounting for the lack of a consistent left-to-right–dominant frequency gradient in cAF patients. Agonist (carbachol)-activated acetylcholine-gated IK,ACh was larger in right than left atria of sinus rhythm patients; a right-to-left gradient was absent in pAF and cAF patients. The lack of right atrium–dominant, agonist-activated IK,ACh in AF-patients may play a permissive role for left atrium–dominant drivers in vagal contexts. Targeting the molecular mechanisms underlying the large left atrial basal inward rectifier currents that promote AF persistence in pAF may provide a new therapeutic option for pAF treatment.
Acknowledgments
The authors thank Trautlinde Thurm, Annett Opitz, and Sabine Kirsch for excellent technical assistance.
Sources of Funding
These studies were supported by the Deutsche Forschungsgemeinschaft (Do769/1-1-3), the German Federal Ministry of Education and Research through the Atrial Fibrillation Competence Network (01Gi0204), the Canadian Institutes of Health Research (MOP44365), and the European-North American Atrial Fibrillation Research Alliance (ENAFRA) grant of Fondation Leducq (07CVD03).
Footnotes
The online-only Data Supplement is available at http://circep.ahajournals.org/cgi/content/full/CIRCEP.110.954636/DC1.
Disclosures
None.
References
- 1.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 2.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 3.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 5.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 6.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 7.Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL, Kasprowicz K, Bhatta L, Puskas F, Kalifa J, Jalife J. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009;6:1415–1422. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. CircRes. 2002;90:1173–1180. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 9.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 10.Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, Christ T, Schuler S, Ravens U. Molecular basis of downregulation of G-protein–coupled inward rectifying K(+) current I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 11.Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, Strasser RH, Ravens U, Dobrev D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D, Nattel S. Changes in IK,ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res. 2008;77:35–43. doi: 10.1093/cvr/cvm051. [DOI] [PubMed] [Google Scholar]
- 13.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 15.Sekar RB, Kizana E, Cho HC, Molitoris JM, Hesketh GG, Eaton BP, Marban E, Tung L. IK1 heterogeneity affects genesis and stability of spiral waves in cardiac myocyte monolayers. Circ Res. 2009;104:355–364. doi: 10.1161/CIRCRESAHA.108.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarmast F, Kolli A, Zaitsev A, Parisian K, Dhamoon AS, Guha PK, Warren M, Anumonwo JM, Taffet SM, Berenfeld O, Jalife J. Cholinergic atrial fibrillation: I(K,ACh) gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–873. doi: 10.1016/s0008-6363(03)00540-6. [DOI] [PubMed] [Google Scholar]
- 17.Dobrev D, Wettwer E, Himmel HM, Kortner A, Kuhlisch E, Schuler S, Siffert W, Ravens U. G-Protein beta(3)-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents. Circulation. 2000;102:692–697. doi: 10.1161/01.cir.102.6.692. [DOI] [PubMed] [Google Scholar]
- 18.Himmel HM, Meyer Zu Heringdorf D, Graf E, Dobrev D, Kortner A, Schuler S, Jakobs KH, Ravens U. Evidence for Edg-3 receptor-mediated activation of I(K.ACh) by sphingosine-1-phosphate in human atrial cardiomyocytes. Mol Pharmacol. 2000;58:449–454. doi: 10.1124/mol.58.2.449. [DOI] [PubMed] [Google Scholar]
- 19.Greiser M, Halaszovich CR, Frechen D, Boknik P, Ravens U, Dobrev D, Luckhoff A, Schotten U. Pharmacological evidence for altered src kinase regulation of I(Ca,L) in patients with chronic atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:383–392. doi: 10.1007/s00210-007-0174-6. [DOI] [PubMed] [Google Scholar]
- 20.Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, Grandjean JG, Van Gilst WH, Crijns HJ. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- 21.Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AA, Van Gilst WH, Crijns HJ. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001;37:926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 22.Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Leger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S. Human atrial ion channel and transporter subunit geneexpression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 23.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 24.Girmatsion Z, Biliczki P, Bonauer A, Wimmer-Greinecker G, Scherer M, Moritz A, Bukowska A, Goette A, Nattel S, Hohnloser SH, Ehrlich JR. Changes in microRNA-1 expression and IK1 upregulation in human atrial fibrillation. Heart Rhythm. 2009;6:1802–1809. doi: 10.1016/j.hrthm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 26.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 27.Cohen NA, Sha Q, Makhina EN, Lopatin AN, Linder ME, Snyder SH, Nichols CG. Inhibition of an inward rectifier potassium channel (Kir2.3) by G-protein betagamma subunits. J Biol Chem. 1996;271:32301–32305. doi: 10.1074/jbc.271.50.32301. [DOI] [PubMed] [Google Scholar]
- 28.Zhu G, Qu Z, Cui N, Jiang C. Suppression of Kir2.3 activity by protein kinase C phosphorylation of the channel protein at threonine 53. J Biol Chem. 1999;274:11643–11646. doi: 10.1074/jbc.274.17.11643. [DOI] [PubMed] [Google Scholar]
- 29.Perillan PR, Chen M, Potts EA, Simard JM. Transforming growth factor-beta 1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-delta in reactive astrocytes from adult rat brain. J Biol Chem. 2002;277:1974–1980. doi: 10.1074/jbc.M107984200. [DOI] [PubMed] [Google Scholar]
- 30.Zhao QY, Huang CX, Liang JJ, Chen H, Yang B, Jiang H, Li GS. Effect of vagal stimulation and differential densities of M2 receptor and IK,ACh in canine atria. Int J Cardiol. 2008;126:352–358. doi: 10.1016/j.ijcard.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 31.Lomax AE, Rose RA, Giles WR. Electrophysiological evidence for a gradient of G protein-gated K+ current in adult mouse atria. Br J Pharmacol. 2003;140:576–584. doi: 10.1038/sj.bjp.0705474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 33.Haissaguerre M, Hocini M, Sanders P, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Jonsson A, O’Neill MD, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616–625. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama E, Osaka T, Takemoto Y, Suzuki T, Ito A, Kamiya K, Kodama I. Paroxysmal atrial fibrillation maintained by nonpulmonary vein sources can be predicted by dominant frequency analysis of atriopulmonary electrograms. J Cardiovasc Electrophysiol. 2009;20:630–636. doi: 10.1111/j.1540-8167.2008.01376.x. [DOI] [PubMed] [Google Scholar]
- 35.Sih HJ, Zipes DP, Berbari EJ, Adams DE, Olgin JE. Differences in organization between acute and chronic atrial fibrillation in dogs. J Am Coll Cardiol. 2000;36:924–931. doi: 10.1016/s0735-1097(00)00788-9. [DOI] [PubMed] [Google Scholar]
- 36.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation:mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 37.Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 38.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


