Abstract
Amyloidosis refers to a range of protein misfolding disorders that can cause organ dysfunction through progressive fibril deposition. Cardiac involvement often leads to significant morbidity and mortality and increasingly has been recognized as an important cause of heart failure. The two main forms of cardiac amyloidosis, light chain (AL) and transthyretin (ATTR) amyloidosis, have distinct mechanisms of pathogenesis. Recent insights have led to the development of novel pharmacotherapies with the potential to significantly impact each disease. This review will summarize the preclinical and clinical data for these emerging treatments for AL and ATTR amyloidosis.
Keywords: Amyloidosis, Light chain, Transthyretin, Cardiomyopathy, Neuropathy, Therapy
1. Introduction
Amyloidosis is characterized by the aggregation of misfolded proteins that form fibrillar deposits in various organs. Progressive fibril deposition leads to worsening and sometimes irreversible organ dysfunction. In particular, cardiac amyloidosis is a main driver of morbidity and mortality in patients with amyloidosis, and manifests as restrictive cardiomyopathy, atrial and ventricular arrhythmias, conduction disease, and microvascular ischemia (Dubrey, Hawkins, & Falk, 2011).
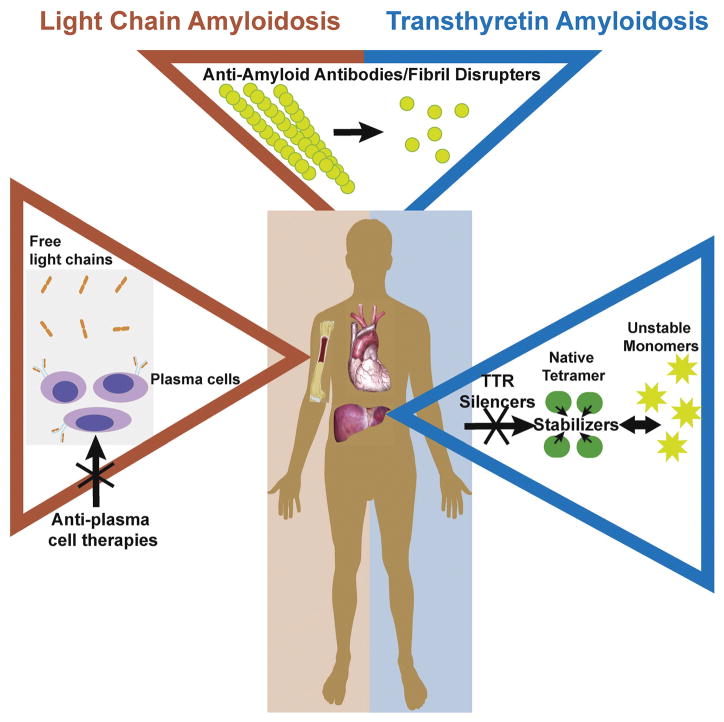
The major forms of cardiac amyloidosis are due to immunoglobulin light chain (AL) or transthyretin (ATTR) precursors. Therapy for these types of cardiac amyloidosis has, until recently, focused on inhibiting the underlying source of amyloid production (i.e., by treatment of plasma cell dyscrasia in AL amyloidosis and liver transplantation in mutant TTR amyloidosis). Recently, additional strategies have been developed, aimed at blocking misfolded proteins from forming fibrils and promoting the disruption and clearance of existing fibrils (Fig. 1). This review will concentrate on the current therapies, available and under investigation, for cardiac amyloidosis (Table 1). Issues related to the clinical diagnosis of cardiac amyloidosis or general heart failure management will not be discussed here.
Fig. 1.
Therapeutic Targets for Amyloidosis. Treatment for AL amyloidosis focuses on eliminating the bone marrow-derived B cell clone responsible for producing toxic light chains. ATTR therapies aim to significantly reduce hepatic production of TTR or to stabilize the native TTR homotetramer to prevent dissociation into proamyloidogenic monomers. Several drugs have been developed to disrupt and clear already deposited AL and/or TTR amyloid fibrils.
Table 1.
Potential pharmacotherapies for amyloidosis.
| Anti-plasma cell therapies | Anti-amyloid antibodies | Amyloid fibril disruptors | TTR stabilizers | TTR silencers |
|---|---|---|---|---|
|
|
|
|
|
Abbreviations: light chain amyloidosis, AL; antisense oligonucleotide, ASO; R-1-[6-[R-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid, CPHPC; epigallocethechin-3-gallate, (EGCG); serum amyloid P component, SAP; small-interfering RNA, siRNA; tauroursodeoxycholic acid, TUDCA.
2. AL amyloidosis
AL amyloidosis is due to amyloid fibrils that consist of misfolded monoclonal immunoglobulin light chains produced from a plasma cell dyscrasia. Although similar to multiple myeloma in etiology, the amount of paraprotein produced is usually less, and the clinical manifestations differ. AL amyloidosis often affects multiple organ systems with approximately half of patients presenting with cardiac involvement. The presence of cardiomyopathy is a crucial distinction as these patients have a significantly worse prognosis (Falk, Alexander, Liao, & Dorbala, 2016; Kyle, Greipp, & O’Fallon, 1986). Cardiac biomarkers, specifically troponin-T and N-terminal pro-B-type natriuretic peptide (NT-proBNP), reflect severity of cardiac involvement and, as such, are important factors in prognostic staging systems used for AL. The widely-used revised Mayo staging system assigns 1 point to the following variables to stratify patients into 4 stages (I–IV, with stage 1 having no adverse features): troponin-T ≥ 0.025 ng/mL, NT-proBNP ≥ 1800 pg/mL, and involved versus uninvolved free light chain difference ≥ 18 mg/dL (Kumar, Dispenzieri, et al., 2012; Table 2). Although there have been significant advances in the development of effective therapies for AL amyloidosis, most clinical trials for AL amyloidosis have excluded or limited the number of patients with advanced disease (i.e., significant cardiac involvement). Therefore, more data are needed to identify specific treatments for those with cardiac AL amyloidosis, a group that continues to have a poor prognosis. The following sections will discuss early and more recent treatment strategies for AL amyloidosis that focus on the underlying plasma cell dyscrasia as well as highlight novel therapies that directly target the amyloid fibrils.
Table 2.
Revised Mayo staging system for AL amyloidosis.
| Revised Mayo staging system for AL amyloidosis, Kumar, Hayman, et al., 2012 | ||
|---|---|---|
|
| ||
| Stage | Total score | Median survival (months) |
| 1 | 0 | 94.1 |
| 2 | 1 | 40.3 |
| 3 | 2 | 14 |
| 4 | 3 | 5.8 |
| One point is assigned for each of the following: cTnT ≥ 0.025 ng/mL, NT-proBNP ≥ 1800 pg/mL, and FLC-diff ≥ 18 mg/dL | ||
Revised prognostic staging system for light chain amyloidosis incorporating cardiac bio-markers and serum free light chain measurements Shaji Kumar, Angela Dispenzieri, Martha Q. Lacy, Suzanne R. Hayman, Francis K. Buadi, Colin Colby, Kristina Laumann, Steve R. Zeldenrust, Nelson Leung, David Dingli, Philip R. Greipp, John A. Lust, Stephen J. Russell, Robert A. Kyle, S. Vincent Rajkumar, and Morie A. Gertz Journal of Clinical Oncology 2012 30:9, 989–995.
3. AL amyloidosis: anti-plasma cell-directed therapy
3.1. Melphalan
Melphalan, an alkylating agent, combined with a corticosteroid (prednisone) was first shown to be effective in AL amyloidosis over 4 decades ago (Jones, Hilton, Tighe, & Hobbs, 1972). With the advent of stem cell transplantation, protocols using high-dose melphalan with a stem cell rescue were developed. A subsequent randomized trial compared this regimen with standard-dose melphalan and dexamethasone (Jaccard et al., 2007). Among high-risk patients, there was no difference in survival, and for low-risk patients, there was a non-significant difference (58% in the high-dose melphalan group versus 80% in the melphalan plus dexamethasone group; p = 0.13). This was a multicenter trial and included centers that recruited small numbers of patients, and thus may not have been experienced in treating higher-risk patients. For this reason, several major centers continue to use high-dose intravenous melphalan therapy with stem cell transplant in carefully selected patients (Dispenzieri et al., 2015).
Only a small proportion of patients (<20%) are eligible for stem cell transplantation due to existing comorbid conditions, including renal impairment, advanced heart failure, advanced age, and poor functional status (Gertz, 2012). The use of oral melphalan with dexamethasone in higher risk patients has had variable results. In a study of melphalan and dexamethasone in patients ineligible for stem cell transplantation, 67% had a hematologic response, defined as a 50% or greater decrease in serum or urine monoclonal light chains, and 33% had a complete remission with complete resolution of the monoclonal light chains for at least 3 months (Palladini et al., 2004). However, a subsequent study using this regimen had disappointing rates of hematologic response and complete remission (44% and 11%, respectively) (Dietrich et al., 2010). Notably, the latter study had more patients with significant cardiac involvement, highlighting the importance of considering the underlying degree of cardiac dysfunction when assessing treatment efficacy. In a study examining melphalan and dexamethasone in a stem cell transplant ineligible population, patients received either standard-dose therapy or reduced-dose dexamethasone if advanced cardiac disease was present (Palladini et al., 2014). These patients with advanced cardiac involvement had much lower hematologic response and complete remission rates compared with the group with less severe cardiac disease (51% and 12% versus 76% and 31%, respectively).
3.2. Bortezomib
The introduction of proteasome inhibitors for multiple myeloma represented a major step forward in treatment compared to the more slowly acting alkylating agents, such as melphalan. Bortezomib was the first such agent approved by the FDA for treating multiple myeloma. Given the role of proteasomes in degrading misfolded proteins, it has been hypothesized that their function is needed for the stability of plasma cells producing proteotoxic light chains; thus, inhibiting this important protective mechanism could lead to selective apoptosis of the plasma cell dyscrasia (Sitia, Palladini, & Merlini, 2007). Indeed, toxic light chains have recently been shown to cause cellular stresses that sensitize plasma cells to proteasome inhibitors (Oliva et al., 2017). An initial investigation of bortezomib in a small number (n = 18) of previously untreated AL amyloidosis patients showed a complete response rate of 47% (Kastritis et al., 2010). Two subsequent retrospective studies using a regimen of cyclophosphamide, bortezomib, and dexamethasone demonstrated a rapid, favorable hematologic response rate at 2 months with a 2-year overall survival of 94% in advanced cardiac stage patients (Mayo Stage III) in the Venner et al. study (Mikhael et al., 2012; Venner et al., 2012).
A larger study (n = 230) showed lower overall hematologic and complete response rates of 60% and 23%, respectively (Palladini et al., 2015). Patients in the most advanced stage (Mayo Stage III with NT-proBNP > 8500 ng/L, n = 43) comprised a large proportion of the study population and were drivers of the observed high early mortality. Even in this very high-risk group, those with at least a partial hematologic response had an improved 2-year survival (67%). Moreover, a recent retrospective study of 103 patients with symptomatic heart failure receiving initial treatment with bortezomib, dexamethasone, and an alkylating agent showed significantly improved survival (hazard ratio 0.209) (Sperry et al., 2016). Interpretation of these studies must be considered in the context of the inherent limitations of retrospective studies. Randomized studies, such as the ongoing phase 3 trial comparing treatment with melphalan and dexamethasone versus bortezomib, melphalan, and dexamethasone, should provide further insights (NCT01277016).
3.3. Other proteasome inhibitors
In the wake of bortezomib, other proteasome inhibitors have been studied for treating AL amyloidosis. In a phase 1/2 study of relapsed/refractory AL patients, ixazomib, a potent oral proteasome inhibitor, had a hematologic response rate of 52% with diarrhea, nausea, fatigue, and soft tissue disorders as the most common adverse effects (Sanchorawala et al., 2017). Ixazomib is currently being studied in a phase 3 trial of ixazomib plus dexamethasone in patients with relapsed or refractory AL amyloidosis (TOURMALINE-AL1, NCT01659658). This study includes patients with significant cardiac involvement (up to Mayo stage III but NT-proBNP < 8000 pg/mL). One of the primary endpoints includes the 2-year cardiac deterioration rate defined as the need for heart failure hospitalization. Another proteasome inhibitor, carfilzomib, has been shown to be effective in a phase 1/2 trial of previously treated AL amyloidosis patients with overall hematologic and complete response rates of 63% and 12.5%, respectively. Cardiac adverse events were common with 18% of patients reporting dyspnea and heart failure noted in 10%. Other cardiac adverse events included hypertension (10%) and symptomatic ventricular tachycardia (10%, including a cardiac arrest requiring defibrillation), and rarely chest pain and hypotension (1 each) (Cohen et al., 2016, NCT01789242). Close monitoring of left ventricular function and dose-reduction has suggested for patients who experience dyspnea or have pre-existing cardiac disease (Mikhael, 2016). Thus, the high frequency of adverse cardiac events may limit its utility in patients with AL cardiomyopathy.
3.4. Anti-CD38 monoclonal antibody
Recently, the use of daratumumab, a human anti-CD38 immunoglobulin G1k monoclonal antibody that shows considerable promise in refractory myeloma, was described in 2 patients with refractory AL amyloidosis, including one with advanced cardiac disease (Sher, Fenton, Akhtar, & Gertz, 2016). Both patients had failed treatment with several agents, including carfilzomib, and remission was achieved with daratumumab, with no reported serious adverse events. Furthermore, in a retrospective study, daratumumab led to overall hematologic and complete response rates of 76% and 36, respectively, in a cohort of 25 pretreated AL amyloidosis patients (Kaufman et al., 2017). Minor infusion-related reactions were observed in 15 patients. There is an ongoing phase 1/2 trial of daratumumab for AL amyloidosis that includes patients with cardiac involvement (up to Mayo stage III but NT-proBNP < 8500 pg/mL) (NCT02841033).
3.5. Immunomodulators
Lenalidomide, a thalidomide derivative and immunomodulator, has been shown to be effective in multiple AL amyloidosis trials. A study using a regimen of lenalidomide, cyclophosphamide, and dexamethasone in 35 patients with AL amyloidosis demonstrated an overall hematologic response rate of 60% (Kumar, Dispenzieri, et al., 2012). Notably, 43% of patients in this trial had advanced cardiac disease (Mayo stage III). In another study of lenalidomide and dexamethasone in AL amyloidosis patients (n = 84) with prior treatment with bortezomib or thalidomide showed an overall hematologic response rate of 61% (Mahmood et al., 2014). At 6 months, 12% had a cardiac improvement defined as a >30% or >300 ng/L decrease in baseline NT-proBNP or ≥2 class decrease in patients with baseline NYHA class 3 or 4 heart failure. Another immunomodulator, pomalidomide, has activity in AL amyloidosis. A study of pomalidomide in previously treated AL amyloidosis (82% with cardiac involvement) had a 48% hematologic response rate and 76% 1-year overall survival (Dispenzieri et al., 2012). Another ongoing phase 1/2 trial of pomalidomide in AL amyloidosis patients (n = 27, 67% with cardiac involvement) found a 50% hematologic response rate (Sanchorawala et al., 2016). Both studies observed an increase in NT-proBNP with treatment, which did not appear to be associated with deterioration of clinical condition, but which warrants further attention.
4. AL amyloidosis: amyloid-directed therapy
4.1. NEOD001
NEOD001 is a humanized monoclonal antibody that binds to a cryptic light chain epitope that is exposed in misfolded but not normally folded light chains (Wall et al., 2012). This antibody was shown to promote the clearance of AL amyloid fibrils via macrophages and neutrophils and thus represents a potential amyloid-targeted adjunct therapy for patients receiving chemotherapy for the underlying plasma cell dyscrasia. Recently, the results of a phase 1/2 trial in AL amyloidosis were reported (Gertz et al., 2016). In this dose-escalation trial with 27 AL amyloidosis patients, who had received at least one anti-plasma cell therapy and had at a least a partial hematologic response, received monthly infusions of NEOD001. The investigators found 57% cardiac and 60% renal response rates, as measured by improvement in NT-proBNP and proteinuria, respectively. These response rates compare favorably with historical cohorts, but there was no concomitant control group. NEOD001 is currently being studied in a phase 3 trial as adjunct treatment to standard of care chemotherapy for AL amyloidosis and in a phase 2b study of previously treated patients with refractory cardiac dysfunction (The VITAL Amyloidosis Study, NCT02312206; The PRONTO Study, NCT02632786; respectively).
4.2. Monoclonal anti-serum amyloid P antibody
Serum amyloid P component (SAP) is a glycoprotein found in all types of amyloid fibrils and is important for fibril formation. Therefore, SAP represents an attractive target for promoting fibril degradation. In both mice and humans, the drug R-1-[6-[R-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC), has been demonstrated to effectively remove circulating SAP by crosslinking SAP molecules and promoting hepatic clearance (Gillimore et al., 2010; Pepys et al., 2002). Although CPHPC was highly effective at depleting serum SAP, it had minimal or no effect on existing amyloid deposits in humans. To address this, the same investigators developed a humanized monoclonal immunoglobulin G anti-SAP antibody (Bodin et al., 2010). This antibody facilitates amyloid removal via a complement-dependent, macrophage-mediated pathway. Using CPHPC in mice to deplete circulating SAP, subsequent anti-SAP antibody infusion promoted amyloid clearance from the liver and spleen (Bodin et al., 2010).
These results led to an open-label trial in 15 patients with systemic amyloidosis, in which participants initially received CPHPC followed by a single infusion of anti-SAP antibody (Richards et al., 2015). The types of amyloidosis included AL, serum amyloid A, apolipoprotein AI, and fibrinogen A alpha chain. Notably, patients with significant cardiac involvement were excluded from this study. Six weeks after infusion, patients had less hepatic amyloid burden by 123I SAP scintigraphy. There were no serious adverse events, and the minor reactions included flushing, headache, nausea, and abdominal pain. These findings are promising for the tolerability and efficacy of CPHPC/anti-SAP antibody for amyloid fibril clearance. Further studies are being planned to determine whether similar benefits will be apparent in amyloid patients with significant cardiac involvement (NCT03044353).
5. ATTR amyloidosis: TTR stabilizers
The rate limiting step for fibril formation in ATTR amyloidosis is dissociation of the tetramer into unstable, amyloidogenic monomers. Multiple investigators have studied small molecules capable of interacting with the thyroxine binding sites, thereby stabilizing the TTR tetramer and preventing dissociation. The subsequent sections will explore several of the promising TTR stabilizers, which are at various stages of development.
5.1. Tafamidis
Tafamidis is a small molecule transthyretin stabilizer that was identified after screening a library of benzoxazoles in an in vitro amyloid fibril formation assay (Razavi et al., 2003). Tafamidis potently stabilizes the TTR tetramer in a dose-dependent manner, under both denaturing and physiologic conditions (Bulawa et al., 2012). Furthermore, tafamidis is effective in vitro for a variety of TTR mutations (Bulawa et al., 2012). The safety and efficacy of tafamidis has been predominantly studied in patients with ATTR polyneuropathy. A phase 2/3 trial was conducted with 128 patients with early-stage ATTR polyneuropathy caused by the Val30Met point mutation (Coelho et al., 2012). This double-blind trial randomized patients to tafamidis 20 mg daily or placebo and followed them for 18 months. The primary endpoint was change in the Neurology Impairment Score-Lower Limbs and the Norfolk Quality of Life-Diabetic Neuropathy total score. The tafamidis group had better preserved neurologic function compared with the placebo group. The 12-month, open-label extension study showed continued reduction in the rate of neurologic decline (Coelho, Maia, et al., 2013). As a result, tafamidis has been approved in Europe, Japan, Mexico, and Argentina for use in patients with stage 1 ATTR polyneuropathy (Scott, 2014). Of note, in the above-mentioned trial of tafamidis in ATTR polyneuropathy patients, there was no statistically significant difference in the primary endpoint using the prespecified intention-to-treat analysis (Coelho et al., 2012). A greater than expected number of patients underwent liver transplantation during the trial (21% observed vs. 10% estimated), which they attribute to donor availability rather than the need for salvage therapy for treatment failure. Consequently, the Food and Drug Administration (FDA) declined approval in the United States, citing inadequate data on drug efficacy and the need for an additional trial (FDA, 2012).
The efficacy of tafamidis was subsequently evaluated in patients with ATTR cardiomyopathy. A phase 2, open-label, multicenter trial enrolled 35 patients with either wild-type (n = 31) or Val122Ile (n = 4) ATTR cardiomyopathy to receive tafamidis 20 mg daily (Maurer et al., 2015). Most patients (96.7%) had New York Heart Association class I or II heart failure at baseline with a median duration of symptoms of 55.6 months. The majority of patients (96.8%) achieved TTR tetramer stability based on an in vitro assay. At 12 months, 48.4% of patients had a clinical progression, defined as death, hospitalization for a cardiovascular etiology, increase in serum creatinine > 0.5 mg/dL, increase in NT-proBNP > 1000 pg/mL, or decrease in 6-minute walk test > 50 m. This rate of disease progression is lower compared with similar patients in the small, observational Transthyretin Amyloidosis Cardiac Study (TRACS), where 72.4% of patients experienced disease progression (Ruberg et al., 2012). Overall, tafamidis was well tolerated with few patients (22.6%) complaining of diarrhea. The results of this phase 2 trial are encouraging, but conclusions from the data are limited by the lack of a placebo arm and small sample size. Thus, a multicenter, randomized-controlled, phase 3 trial (NCT01994889) investigating the use of low (20 mg) or high (80 mg) dose tafamidis is ongoing, and results are anticipated by the end of 2017.
5.2. Diflunisal
Unlike tafamidis, which was specifically developed for treating ATTR amyloidosis, diflunisal is a nonsteroidal anti-inflammatory drug (NSAID) that has been repurposed as a potential TTR stabilizer. Several in vitro studies have demonstrated the modest TTR-binding ability of diflunisal, and a phase 1 clinical trial showed diflunisal-mediated TTR stabilization in human serum (Adamski-Werner, Palaninathan, Scchettini, & Kelly, 2004; Miller, Sekijima, & Kelly, 2004; Sekijima, Dendle, & Kelly, 2006; Tojo, Sekijima, Kelly, & Ikeda, 2006). These findings led to a randomized, placebo-controlled, multicenter trial of diflunisal in 130 patients with TTR-FAP. At 2 years, the diflunisal group had less neurological deterioration compared with the placebo group based on the Neuropathy Impairment Score plus 7 nerve tests (Berk et al., 2013). Of note, more than half of the patients (n = 67, total; n = 27, diflunisal group; n = 40, placebo group) did not complete the trial, mainly due to disease progression or liver transplantation. Regarding patients with ATTR cardiomyopathy, only a small, single arm study has been conducted, in which 13 patients with wild-type or mutant ATTR cardiomyopathy received diflunisal (Castaño, Helmke, Alvarez, Delisle, & Maurer, 2012). Over a mean follow up close to a year, there was no statistically significant deterioration/change in left ventricular ejection fraction or dimensions, BNP, or troponin-I. There was a non-significant trend toward a decrease in glomerular filtration rate. Given the risks of bleeding, kidney injury, and cardiovascular events with long-term NSAID use, particularly in a heart failure population, a large, long-term, randomized-controlled trial would be needed to assess the safety and utility of diflunisal in ATTR cardiomyopathy patients, but this is unlikely to happen as the drug is generic and available in the USA (Gislason et al., 2009; Gooch et al., 2007).
5.3. Tolcapone
Tolcapone is drug, approved by the FDA for use in Parkinson disease, that recently shown to be a potent TTR stabilizer (Sant’Anna et al., 2016). Tolcapone is a catechol-O-methyltransferase inhibitor used as adjunct therapy to augment the bioavailability of levodopa and carbidopa in patients with Parkinson’s disease. Using the Symyx Comprehensive Medicinal Chemistry database of approved and study drugs, the investigators selected 30 compounds with predicted TTR affinity and discovered tolcapone strongly inhibited TTR aggregation in vitro (Sant’Anna et al., 2016). Moreover, tolcapone bound with higher affinity to wild-type and Val122Ile ATTR amyloidosis than tafamidis (Sant’Anna et al., 2016). Unlike the other known TTR stabilizers, tolcapone can cross the blood-brain barrier, suggesting a unique role for tolcapone in treating ATTR amyloidosis patients with leptomeningeal involvement. It should be considered that, in the absence of liver transplantation, ATTR amyloidosis patients would require long-term treatment with tolcapone to maintain disease stability, and the FDA issued a “black box” warning for tolcapone concerning a risk for fulminant liver failure and recommended close monitoring of hepatic function. An open-label, phase 2A trial of tolcapone in ATTR polyneuropathy patients, asymptomatic mutant TTR carriers, and healthy volunteers was recently completed and will provide data information on drug safety and tolerance (NCT02191826).
5.4. AG10
The above-mentioned TTR stabilizers have been studied predominantly in patients with TTR-FAP, and there are very little data in patients with ATTR cardiomyopathy, for which there is no FDA-approved treatment. Potentially significant drug toxicities with prolonged use (i.e., gastrointestinal bleeding and cardiovascular events with diflunisal and hepatotoxicity with tolcapone) are important considerations. Recent investigations have focused on designing a TTR stabilizer specifically for ATTR cardiomyopathy patients (Alhamadsheh et al., 2011; Penchala et al., 2013). In this population, two TTR variants of interest include wild-type TTR, which manifests as senile systemic amyloidosis with restrictive cardiomyopathy in the elderly, and Val122Ile TTR, a mutation seen in 3–4% of African Americans that increases the risk of heart failure (Quarta et al., 2015). By high-throughput, fluorescence polarization assay to screen a library of compounds that exhibit significant binding to wild-type TTR, several molecules that abrogated Val122Ile TTR-mediated toxicity in human cardiomyocytes were identified (Alhamadsheh et al., 2011). One of the promising compounds, AG10, was tested further and demonstrated to have superior binding selectivity to serum TTR compared with tafamidis and diflunisal (Penchala et al., 2013). While the stabilizing effect of tafamidis significantly decreased for homozygous Val122Ile TTR compared with heterozygous Val122Ile TTR or wild-type TTR, AG10 maintained a high level of stability for homozygous Val122Ile TTR. In addition, AG10 had excellent oral bioavailability and no apparent toxicity when administered to rats. Therefore, its favorable affinity and selectivity make AG10 a promising candidate for treating patients with ATTR cardiomyopathy, particularly those with the Val122Ile mutation.
5.5. Curcumin
In vitro and preliminary animal studies report using curcumin, which is the active ingredient in turmeric, in ATTR amyloidosis. Curcumin may stabilize the TTR protein, by binding to the active site to prevent dissociation (Pullakhandam, Srinivas, Nair, & Reddy, 2009). Curcumin may also increase macrophage-mediated degradation of amyloid deposits (Ferreira, Goncalves, Saraiva, & Almeida, 2016). This dual action of stabilizing TTR and disrupting fibrils is promising. Currently, there are no trials registered studying curcumin for amyloidosis.
6. ATTR amyloidosis: TTR silencers
TTR silencers are another emerging drug class aimed at preventing amyloid formation and subsequent deposition. Both small-interfering RNAs (siRNAs) and antisense oligonucleotides have been engineered to localize to the liver as intact compounds and suppress TTR expression, representing a potential target for ongoing and future ATTR amyloidosis trials.
6.1. RNA interference
Investigators have designed a siRNA sequence to target the 3′ untranslated region of TTR messenger RNA, which is conserved among wild-type and mutant TTRs, to induce mRNA degradation and decrease protein expression. Also important is the carrier used to prevent siRNA degradation, ensuring sufficient drug delivery to the target organ. Lipid nanoparticles have been shown to be very effective in delivering siRNAs to hepatocytes (Akinc et al., 2010; Zimmermann et al., 2006). ALN-TTR02 (later called patisiran) consists of such a lipid nanoparticle vehicle and a TTR-lowering siRNA. Effective TTR suppression in small animal studies led to a single dose, phase 1 trial in ATTR polyneuropathy patients with mild to moderate neuropathy and healthy volunteers (Coelho, Adams, et al., 2013).
Patisiran at a dose of 0.3 mg per kilogram significantly decreased TTR levels by 86.8% in 17 healthy volunteers. There were no significant changes in hepatic, renal, hematologic, and thyroid studies. The main adverse event was infusion-associated reactions (7.7%).
Patisiran was then evaluated in a phase 2 trial with 29 TTR-FAP patients, most of whom received 0.3 mg per kilogram doses either every 3 or 4 weeks (Suhr et al., 2015). Administering patisiran every 3 weeks, achieved a 96% reduction in TTR level. Of note, a considerable proportion of the study population was also taking tafamidis (48%) or diflunisal (24%) during the study, and there was no apparent effect on the ability of patisiran to suppress TTR levels. This dosing regimen is currently being investigated in the phase 3, randomized-controlled APOLLO trial studying ATTR polyneuropathy patients (NCT01960348). The primary endpoint is disease progression at 18 months as measured by the modified Neuropathy Impairment Score plus 7.
Another TTR siRNA drug, ALN-TTRSC or revusiran, utilizes an N-acetylgalactosamine conjugate to target the asialoglycoprotein receptor on hepatocytes (Akinc et al., 2010). This formulation has the benefit of subcutaneous rather than intravenous administration. Unfortunately, a recent phase 3, randomized-controlled study investigating the use of revusiran in patients with mutant ATTR cardiomyopathy was discontinued due to an increased mortality rate in the treatment group (Endeavour Trial, NCT02319005). Examination of the data in the concurrently ongoing patisiran trial showed no signal of increased mortality, suggesting that the adverse effect of revusiran was most likely due to the drug formulation rather than a class effect of TTR-lowering agents. Therefore, if the APOLLO trial demonstrates a benefit for patisiran in ATTR polyneuropathy patients, future studies could investigate the efficacy of this drug for ATTR cardiomyopathy.
6.2. Antisense oligonucleotides
Antisense oligonucleotides are another method by which expression can be suppressed (Benson et al., 2006). IONIS-TTRRx is a second-generation antisense oligonucleotide that reduced serum TTR levels by 80% in a transgenic mouse model and in cynomolgus monkeys (Ackermann et al., 2012). These encouraging data and good tolerance in a phase 1 study of healthy volunteers led to a phase 2/3 trial of IONIS-TTRRx in patients with ATTR polyneuropathy (NCT01737398). The primary outcomes are change in the modified Neuropathy Impairment Score plus 7 and the Norfolk Quality of Life Diabetic Neuropathy questionnaire. This study has completed enrollment, and an open label extension study is ongoing (NCT02175004). This study excluded patients with New York Heart Association class 3 or 4 heart failure. Thus, future studies in ATTR cardiomyopathy patients are needed.
7. ATTR amyloidosis: TTR fibril disrupters
The TTR stabilizers and silencers discussed above exert their primary action on upstream targets in the pathogenesis of amyloidosis, namely TTR tetramer instability and TTR production, respectively. Therefore, these therapies likely have a role in halting or slowing disease progression. However, their ability to ameliorate end-organ damage from already established fibril deposition remains uncertain. Indeed, tafamidis, which is used for early-stage ATTR polyneuropathy, is less effective in preventing disease progression in advanced stage Val30Met TTR-FAP patients (Lozeron et al., 2013). Therefore, strategies to disrupt existing amyloid fibrils are needed and will likely be a key component of future treatment regimens.
7.1. Doxycycline/tauroursodeoxycholic acid
Doxycycline, a tetracycline derivative, was discovered to have activity in disrupting amyloid fibrils in vitro and in a Val30Met ATTR transgenic mouse model (Cardoso, Merlini, & Saraiva, 2003; Cardoso & Saraiva, 2006). However, doxycycline is less effective at degrading pre-fibrillar TTR deposits, which are also found in abundance along with amyloid fibrils in elderly ATTR cardiomyopathy patients. Tauroursodeoxycholic acid (TUDCA), a bile acid used to treat cholelithiasis, is effective in degrading these pre-fibrillar deposits. A subsequent study using Val30Met ATTR transgenic mice demonstrated the synergistic effect of co-administration of doxycycline and TUDCA on fibril removal and prevention (Cardoso, Martins, Ribeiro, Merlini, & Saraiva, 2010).
The safety and efficacy of this combination was further studied in a phase 2, open-label trial of 20 patients with hereditary or wild-type TTR (Obici et al., 2012). At 12 months, there was relative stability in neurologic and cardiac status, as assessed by the NIS-LL and BNP, respectively, and no serious adverse events, but poor tolerability led to treatment discontinuation in 2 subjects. In another, recently completed phase 2 trial of doxycycline plus TUDCA, ATTR amyloidosis patients were treated for 12 months and then monitored for a 6-month withdrawal period for signs of disease progression on neurologic and cardiac assessments (NCT01171859). A study of doxycycline plus TUDCA in ATTR cardiomyopathy is ongoing and will determine change in longitudinal left ventricular strain, as a marker of cardiac function, at 18 months (NCT01855360). In addition, doxycycline is currently being studied in a phase 2 trial as adjunctive therapy for patients receiving plasma cell-directed therapy for AL amyloidosis (NCT02207556). A modified form of doxycycline covalently linked to poly-L-glutamic acid has been shown to further improve bio-distribution and fibril degradation (Conejos-Sánchez et al., 2015). Further clinical studies will be necessary to determine whether doxycycline shows any benefit, and whether the modified doxycycline has any additional therapeutic benefit.
7.2. Green tea extract
Epigallocathechin-3-gallate (EGCG), the active ingredient in green tea, interacts with several kinds of amyloid fibrils. In vitro, ECGC has been shown to bind to amyloidogenic light chains, preventing fibril formation (Hora et al., 2017). In observational studies, daily treatment with EGCG led to decreased left ventricular mass and improved NYHA functional class and left ventricular ejection fraction (Mereles, Buss, Hardt, Hunstein, & Katus, 2010). Studies have also examined its role in ATTR amyloidosis. In a small study of 7 patients with wild-type ATTR, after 12 months of treatment with green tea extract, the left ventricular mass decreased by 10% as measured by cardiac magnetic resonance imaging (Aus dem Siepen et al., 2015). These studies are observational and restricted to small sample sizes. There are two ongoing phase 2 trials studying ECGC treatment for AL amyloidosis (TAME-AL, NCT02015312; EpiCardiAL, NCT01511263).
7.3. Monoclonal anti-serum amyloid P antibody
As discussed earlier for AL amyloidosis, anti-SAP antibody is a promising therapy, and its safety and efficacy in amyloidosis patients with significant cardiac involvement, such as ATTR amyloidosis patients, will be the objective of future studies (NCT03044353).
8. Conclusion
Recent years have seen great advances in potential therapies for cardiac amyloidosis. Many treatments aim to specifically block the source of amyloid production, but drugs that target the disruption of already formed amyloid fibrils are also being developed. Many patients have signs of significant end organ involvement on initial presentation; thus, strategies that simply prevent new fibril formation will likely only achieve partial treatment at best. It is likely that therapies intervening at multiple points in the pathogenesis of amyloidosis will be necessary for optimal therapy. Unfortunately, many of the recent studies have a small sample size and/or uncontrolled study design. Larger, randomized-controlled studies, some of which are ongoing, with rigorous clinical endpoints have the potential to provide data to improve clinical practice. In addition, further studies of the mechanisms of disease in amyloidosis will be crucial for identifying additional novel targets for therapy. Table 3 lists some of the key studies discussed in this report.
Table 3.
Key studies for amyloidosis pharmacotherapies.
| Reference | Title | Type | Intervention | Control | Population | N | Primary endpoint | Results/status |
|---|---|---|---|---|---|---|---|---|
| Melphalan & bortezomib | ||||||||
| NCT01277016 | EMN-03 | Phase 3 RCT | Bortezomib + melphalan + dexamethasone | Melphalan + dexamethasone | Treatment-naive AL amyloidosis | 110 | Number of patients with complete or partial response | Completed |
| Other proteasome inhibitors | ||||||||
| NCT01659658 | TOURMALINE-AL1 | Phase 3 open label | Ixazomib + dexamethasone | Physician’s choice of oral chemotherapy | Relapsed or refractory AL amyloidosis with renal or cardiac involvement | 248 | Percentage of participants with overall hematologic response and 2-year vital organ deterioration and mortality rate | Recruiting |
| NCT01789242 | A safety study of carfilzomib in patients with previously-treated systemic light chain amyloidosis | Phase 1 | Carfilzomib + dexamethasone | N/A | Relapsed or refractory AL amyloidosis | 32 | Adverse events as measures of safety and tolerability | Ongoing |
| Anti-CD38 monoclonal antibody | ||||||||
| NCT02841033 | Daratumumab for the treatment of patients with AL amyloidosis | Phase 1/2 | Daratumumab | N/A | Relapsed or refractory AL amyloidosis | 25 | Frequency and severity of side effects and number of patients with a treatment response | Recruiting |
| Immunomodulators | ||||||||
| NCT01570387 | A Phase I/II trial of pomalidomide and dexamethasone in subjects with previously-treated AL amyloidosis | Phase 1/2 | Pomalidomide + dexamethasone | N/A | Previously treated AL amyloidosis | 27 | Dose-limiting toxicity and maximum tolerated dose | Pomalidomide is well tolerated in patients with AL amyloidosis and combined with dexamethasone has a hematologic response rate of 50% in previously treated patients. |
| NEOD001 | ||||||||
| NCT01707264 | Phase 1/2 | NEOD001 | N/A | Previously treated AL amyloidosis with persistent organ dysfunction | 69 | Safety and tolerability | Treatment was safe and well tolerated with favorable organ responses compared with historical cohorts. | |
| NCT02312206 | VITAL | Phase 3 RCT | NEOD001 | Placebo | Treatment-naive cardiac AL amyloidosis | 236 | Time to composite of all-cause mortality or cardiac hospitalization | Recruiting |
| NCT02632786 | PRONTO | Phase 2 RCT | NEOD001 | Placebo | AL amyloidosis with hematologic response and persistent cardiac dysfunction | 100 | Best cardiac response as measured by NT-proBNP at 1 year | Ongoing |
| Monoclonal anti-serum amyloid P antibody | ||||||||
| NCT01777243 | Phase 1 | GSK2135698 + GSK2398852 | N/A | Systemic amyloidosis involving spleen or liver | 15 | Safety as assessed by number of subjects with adverse events | Treatment led to clearance of amyloid deposits from the liver and other organs. No serious adverse events were observed. | |
| NCT03044353 | Multiple treatment session study to assess GSK2398852 administered following and along with GSK2315698 | Phase 2 open label | GSK2135698 + GSK2398852 | N/A | ATTR cardiomyopathy, AL cardiac amyloidosis in remission. or newly diagnosed AL amyloidosis with hematologic response | 50 | Change in left ventricular mass by cardiac magnetic resonance at 8-week follow up | Not yet recruiting |
| Tafamidis | ||||||||
| NCT00694161 | Phase 2 open label | Tafamidis 20 mg | N/A | V122I or wild-type ATTR cardiomyopathy | 35 | Percentage of patients with stabilized TTR tetramer at week 6 | Tafamidis resulted in TTR stabilization in patients with ATTR cardiomyopathy. | |
| NCT01994889 | ATTR-ACT | Phase 3 RCT | Tafamidis 20 mg or tafamidis 80 mg | Placebo | ATTR cardiomyopathy | 400 | All-cause mortality and frequency of cardiovascular-related hospitalizations at month 30 | Ongoing |
| Tolcapone | ||||||||
| NCT02191826 | Study of SOM0226 in familial amyloid polyneuropathy | Phase 1/2 open label | SOM0226 (tolcapone) | N/A | Familial ATTR polyneuropathy or asymptomatic carriers | 17 | TTR stabilization | Completed |
| RNA interference | ||||||||
| NCT01617967 | Phase 2 open label | Patisiran | N/A | Familial ATTR polyneuropathy | 29 | Safety and tolerability | Patisiran was well tolerated and effective in reducing both mutant and wild-type TTR levels | |
| NCT01960348 | APOLLO | Phase 3 RCT | Patisiran | Placebo | Familial ATTR polyneuropathy | 225 | Changed in modified Neuropathy Impairment Score | Ongoing |
| NCT02319005 | ENDEAVOUR | Phase 3 RCT | Revusiran | Placebo | Familial ATTR cardiomyopathy | 206 | Change in 6 min walk distance at 18 months and percent reduction in serum TTR level over 18 months | Discontinued |
| Antisense oligonucleotides | ||||||||
| NCT01737398 | Efficacy and safety of IONIS-TTR Rx in familial amyloid polyneuropathy | Phase 3 RCT | IONIS-TTR Rx | Placebo | Familial ATTR polyneuropathy | 172 | The change from baseline in the modified Neuropathy Impairment Score and Norfolk Quality of Life Diabetic Neuropathy questionnaire at 65 weeks | Ongoing |
| NCT02175004 | Phase 3 open label extension | IONIS-TTR Rx | N/A | Familial ATTR polyneuropathy enrolled in the phase 3 RCT | 172 | Adverse events during the 78 week treatment period | Ongoing | |
| Doxycycline/tauroursodeoxycholic acid | ||||||||
| NCT01171859 | Safety, efficacy and pharmacokinetics of doxycycline plus tauroursodeoxycholic acid in transthyretin amyloidosis | Phase 2 open label | Tauroursodeoxycholic acid and doxycycline | N/A | ATTR amyloidosis | 40 | Changes in Neurologic Impairment Score-Lower Limbs and NTproBNP at 1 year | Completed |
| NCT01855360 | Tolerability and efficacy of a combination of doxycycline and TUDCA in patients with transthyretin amyloid cardiomyopathy | Phase 1/2 open label | Tauroursodeoxycholic acid and doxycycline | N/A | ATTR cardiomyopathy | 40 | Change in longitudinal echocardiographic strain at 18 months | Ongoing |
| NCT02207556 | Oral doxycycline administered as an adjunct to plasma cell directed therapy in light chain amyloidosis | Phase 2 open label | Doxycycline + chemotherapy | N/A | Treatment-naive AL amyloidosis | 30 | Amyloid organ response at 6 and 12 months | Recruiting |
| Green tea extract | ||||||||
| NCT01511263 | EpiCardiAL | Phase 2 open label randomized | EGCG + standard therapy | Standard therapy | Cardiac AL amyloidosis in remission | 86 | Cardiac response at 6 months | Recruiting |
| NCT02015312 | TAME-AL | Phase 2 RCT | EGCG | Placebo | Cardiac AL amyloidosis in remission | 38 | Change in LV mass measured by cardiac MRI at 1 year | Ongoing |
Acknowledgments
Funding support
Dr. Alexander is supported by the National Institutes of Health T32 training grant (T32HL007604). Dr. Singh is supported by the David Shapira Cardiac Amyloidosis Fellowship. Dr. Falk has received support from the Friends of Burt Glazov Cardiac Amyloidosis Fund and the Demarest Lloyd Jr. Foundation.
Abbreviations
- cTnT
Cardiac troponin T
- CPHPC
R-1-[6-[R-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid
- EGCG
epigallocethechin-3-gallate
- FLC-diff
difference between involved and uninvolved serum free light chain
- FDA
Food and Drug Administration
- AL
immunoglobulin light chain amyloidosis
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- RCT
randomized controlled trial
- SAP
serum amyloid P component
- siRNA
small-interfering RNA
- TUDCA
tauroursodeoxycholic acid
- ATTR
transthyretin amyloidosis
Footnotes
Conflict of interest statement
Dr. Alexander has received an investigator-initiated research grant from Pfizer. Dr. Singh has nothing to declare. Dr. Falk serves as a consultant for Ionis Pharmaceuticals and Alnylam Pharmaceuticals and has received research support from GlaxoSmithKline.
References
- Ackermann EJ, Guo S, Booten S, Alvarado L, Benson M, Hughes S, et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19:43–44. doi: 10.3109/13506129.2012.673140. [DOI] [PubMed] [Google Scholar]
- Adamski-Werner SL, Palaninathan SK, Scchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. Journal of Medicinal Chemistry. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Molecular Therapy. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamadsheh MM, Connelly S, Cho A, Reixach N, Powers ET, Pan DW, et al. Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Science Translational Medicine. 2011;3:97ra81. doi: 10.1126/scitranslmed.3002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aus dem Siepen F, Buss SJ, Andre F, Seitz S, Giannitsis E, Steen H, Katus HA, et al. Extracellular remodeling in patients with wild-type amyloidosis consuming epigallocatechin-3-gallate: Preliminary results of T1 mapping by cardiac magnetic resonance imaging in a small single center study. Clinical Research in Cardiology. 2015;104:640–647. doi: 10.1007/s00392-015-0826-3. [DOI] [PubMed] [Google Scholar]
- Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle & Nerve. 2006;33:609–618. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]
- Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: A randomized clinical trial. JAMA. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93–97. doi: 10.1038/nature09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9629–9634. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso I, Martins D, Ribeiro T, Merlini G, Saraiva MJ. Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: Studies in FAP mouse models. Journal of Translational Medicine. 2010;8:74. doi: 10.1186/1479-5876-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso I, Merlini G, Saraiva MJ. 4′-Iodo-4′-deoxydoxorubicin and tetracyclines disrupts transthyretin amyloid fibrils in vitro producing noncytotoxic: Screening for TTR fibril disrupters. The FASEB Journal. 2003;17:803–809. doi: 10.1096/fj.02-0764com. [DOI] [PubMed] [Google Scholar]
- Cardoso I, Saraiva MJ. Doxycycline disrupts transthyretin amyloid: Evidence from studies in a FAP transgenic mice model. The FASEB Journal. 2006;20:234–239. doi: 10.1096/fj.05-4509com. [DOI] [PubMed] [Google Scholar]
- Castaño A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congestive Heart Failure. 2012;18:315–319. doi: 10.1111/j.1751-7133.2012.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. The New England Journal of Medicine. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- Coelho T, Maia LF, da Silva AM, Cruz MW, Planté-Bordenueve V, Suhr OB, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. Neurology. 2013;260:2802–2814. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordenueve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology. 2012;79:785–792. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Landau H, Scott EC, Liedtke M, Kaufman JL, Rosenzweig M, et al. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. American Society of Hematology 58th annual meeting & exposition; December 5, 2016.2016. [Google Scholar]
- Conejos-Sánchez I, Cardoso I, Oteo-Vives M, Romero-Sanz E, Paul A, Sauri AR, et al. Polymer-doxycycline conjugates as fibril disrupters: An approach towards the treatment of a rare amyloidotic disease. Journal of Controlled Release. 2015;198:80–90. doi: 10.1016/j.jconrel.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schönland SO, Benner A, Bochtler T, Kristen AV, Beimler J, et al. Treatment with intravenous melphalan and dexamethasone is not able to overcome the poor prognosis of patients with newly diagnosed systemic light chain amyloidosis and severe cardiac involvement. Blood. 2010;116:522–528. doi: 10.1182/blood-2009-11-253237. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Buadi F, Kumar SK, Reeder CB, Sher T, Lacy MQ, et al. Treatment of immunoglobulin light chain amyloidosis: Mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus statement. Mayo Clinic Proceedings. 2015;90:1054–1081. doi: 10.1016/j.mayocp.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Buadi F, Laumann K, Laplant B, Hayman SR, Kumar SK, et al. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–5404. doi: 10.1182/blood-2012-02-413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: Assessment, diagnosis, and referral. Heart. 2011;97:75–84. doi: 10.1136/hrt.2009.190405. [DOI] [PubMed] [Google Scholar]
- Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: A review of diagnosis and therapy. Journal of the American College of Cardiology. 2016;68:1323–1341. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- Ferreira N, Goncalves NP, Saraiva MJ, Almeida MR. Scientific Reports. Vol. 6. Food and Drug Administration; 2016. May, [Accessed March 1, 2017]. Curcumin: A multi-target disease-modifying agent for late-stage transthyretin amyloidosis; p. 26623. 2013 http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020697s004lbl.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration Center for Drug Evaluation and Research. [Accessed March 1, 2017];Peripheral and central nervous system drugs advisory committee: Vyndaqel (tafamidis meglumine) 2012 May 24; http://www.fda.gov/downloads/AdvisorycommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM304830.pdf.
- Gertz MA. How to manage primary amyloidosis. Leukemia. 2012;26:191–198. doi: 10.1038/leu.2011.219. [DOI] [PubMed] [Google Scholar]
- Gertz MA, Landau H, Comenzo RL, Seldin D, Weiss B, Zonder J, et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. Journal of Clinical Oncology. 2016;34:1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillimore JD, Tennent GA, Hutchinson WL, Gallimore JR, Lachmann HJ, Goodman HJ, et al. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. British Journal of Haematology. 2010;148:760–767. doi: 10.1111/j.1365-2141.2009.08036.x. [DOI] [PubMed] [Google Scholar]
- Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbøl EL, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Archives of Internal Medicine. 2009;169:141. doi: 10.1001/archinternmed.2008.525. [DOI] [PubMed] [Google Scholar]
- Gooch K, Culleton BF, Manns BJ, Zhang J, Alfonso H, Tonelli M, et al. NSAID use and progression of chronic kidney disease. The American Journal of Medicine. 2007;120:280e1–280.e7. doi: 10.1016/j.amjmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Hora M, Carballo-Pacheco M, Weber B, Morris VK, Wittkopf A, Buchner J, … Reif B. Epigallocatechin-3-gallate preferentially induces aggregation of amyloidogenic immunoglobulin light chains. Scientific Reports. 2017;7:41515. doi: 10.1038/srep41515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. The New England Journal of Medicine. 2007;357:1083–1093. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- Jones NF, Hilton PJ, Tighe JR, Hobbs JR. Treatment of “primary” renal amyloidosis with melphalan. Lancet. 1972;2:616–619. doi: 10.1016/s0140-6736(72)93014-0. [DOI] [PubMed] [Google Scholar]
- Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkins PN, Perfetti V, et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. Journal of Clinical Oncology. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]
- Kaufman GP, Schrier SL, Lafayette RA, Arai S, Witteles RM, Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017 Jun 14; doi: 10.1182/blood-2017-01-763599. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. Journal of Clinical Oncology. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SK, Hayman SR, Buadi FK, Roy V, Lacy MQ, Gertz MA, et al. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: Long-term results from a phase 2 trial. Blood. 2012;119:4860–4867. doi: 10.1182/blood-2012-01-407791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: Multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68:220–224. [PubMed] [Google Scholar]
- Lozeron P, Théaudin M, Mincheva Z, Ducot B, Lacroix C, Adams D, et al. Effect on disability and safety of Tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. European Journal of Neurology. 2013;20:1539–1545. doi: 10.1111/ene.12225. [DOI] [PubMed] [Google Scholar]
- Mahmood S, Venner CP, Sachchithanantham S, Lane T, Rannigan L, Foard D, et al. Lenalidomide and dexamethasone for systemic AL amyloidosis following prior treatment with thalidomide or bortezomib regimens. British Journal of Haematology. 2014;166:842–848. doi: 10.1111/bjh.12973. [DOI] [PubMed] [Google Scholar]
- Maurer MS, Grogan DR, Judge DP, Mundayat R, Packman J, Lombardo I, et al. Tafamidis in transthyretin amyloid cardiomyopathy: Effects on transthyretin stabilization and clinical outcomes. Circulation Heart Failure. 2015;8:519–526. doi: 10.1161/CIRCHEARTFAILURE.113.000890. [DOI] [PubMed] [Google Scholar]
- Mereles D, Buss SJ, Hardt SE, Hunstein W, Katus HA. Effects of the main green tea polyphenol epigallocatechin-3-gallate on cardiac involvement in patients with AL amyloidosis. Clinical Research in Cardiology. 2010;99:483–490. doi: 10.1007/s00392-010-0142-x. [DOI] [PubMed] [Google Scholar]
- Mikhael J. Management of carfilzomib-associated cardiac adverse events. Clinical Lymphoma, Myeloma & Leukemia. 2016;16:241–245. doi: 10.1016/j.clml.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB, et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;19:4391–4394. doi: 10.1182/blood-2011-11-390930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Laboratory Investigation. 2004;84:545–552. doi: 10.1038/labinvest.3700059. [DOI] [PubMed] [Google Scholar]
- Obici L, Cortese A, Lozza A, Lucchetti J, Gobbi M, Palladini G, et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: A phase II study. Amyloid. 2012;19:34–36. doi: 10.3109/13506129.2012.678508. [DOI] [PubMed] [Google Scholar]
- Oliva L, Orfanelli U, Resnati M, Raimondi A, Orsi A, Milan E, et al. The amyloidogenic light chain is a stressor that sensitizes plasma cells to proteasome inhibitor toxicity. Blood. 2017 Apr 13;129(15):2132–2142. doi: 10.1182/blood-2016-08-730978. [DOI] [PubMed] [Google Scholar]
- Palladini G, Milani P, Foli A, Obici L, Lavatelli F, Nuvolone M, et al. Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: Long-term results of a risk-adapted approach. Haematologica. 2014;99:743–750. doi: 10.3324/haematol.2013.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladini G, Perfetti V, Obici L, Caccialanza R, Semino A, Adami F, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–2938. doi: 10.1182/blood-2003-08-2788. [DOI] [PubMed] [Google Scholar]
- Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- Penchala SC, Connelly S, Wang Y, Park MS, Zhao L, Baranczak A, et al. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9992–9997. doi: 10.1073/pnas.1300761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys MB, Hebert J, Hutchinson WL, Tennent GA, Lachmann HJ, Gallimore JR, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- Pullakhandam R, Srinivas PN, Nair MK, Reddy GB. Binding and stabilization of transthyretin by curcumin. Archives of Biochemistry and Biophysics. 2009;485:115–119. doi: 10.1016/j.abb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. The New England Journal of Medicine. 2015;372:21–29. doi: 10.1056/NEJMoa1404852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi H, Palaninathan SK, Powers ET, Wiseman RL, Purkey HE, Mohamedmohaideen NN, et al. Benzoxazoles as transthyretin amyloid fibril inhibitors: Synthesis, evaluation, and mechanism of action. Angewandte Chemie (International Ed in English) 2003;42:2758–2761. doi: 10.1002/anie.200351179. [DOI] [PubMed] [Google Scholar]
- Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. The New England Journal of Medicine. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- Ruberg FL, Maurer SM, Judge DP, Zeldenrust S, Skinner M, Kim AY, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: The Transthyretin Amyloidosis Cardiac Study (TRACS) American Heart Journal. 2012;164:222–228. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Sanchorawala V, Palladini G, Kukreti V, Zonder JA, Cohen AD, Seldin DC, et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory light-chain (AL) amyloidosis. Blood. 2017 May 26; doi: 10.1182/blood-2017-03-771220. http://dx.doi.org/10.1182/blood-2017-03-771220. [DOI] [PMC free article] [PubMed]
- Sanchorawala V, Shelton AC, Lo S, Varga C, Sloan JM, Seldin DC. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 1 and 2 trial. Blood. 2016;128:1059–1062. doi: 10.1182/blood-2016-04-710822. [DOI] [PubMed] [Google Scholar]
- Sant’Anna R, Gallego P, Robinson LZ, Pereira-Henriques A, Ferreira N, Pinheiro F, et al. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nature Communications. 2016;7:10787. doi: 10.1038/ncomms10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ. Tafamidis: A review of its use in familial amyloid polyneuropathy. Drugs. 2014;74:1371–1378. doi: 10.1007/s40265-014-0260-2. [DOI] [PubMed] [Google Scholar]
- Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- Sher T, Fenton B, Akhtar A, Gertz MA. First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis. Blood. 2016;128:1987–1999. doi: 10.1182/blood-2016-06-722496. [DOI] [PubMed] [Google Scholar]
- Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: Targeted therapy? Haematologica. 2007;92:1302–1307. doi: 10.3324/haematol.12136. [DOI] [PubMed] [Google Scholar]
- Sperry BW, Ikram A, Hachamovitch R, Valent J, Vranian MN, Phelan D, et al. Efficacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. Journal of the American College of Cardiology. 2016;67:2941–2948. doi: 10.1016/j.jacc.2016.03.593. [DOI] [PubMed] [Google Scholar]
- Suhr OB, Coelho T, Buades J, Pouget J, Conceiao I, Berk J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: A phase II multidose study. Orphanet Journal of Rare Diseases. 2015;10:109. doi: 10.1186/s13023-015-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo K, Sekijima Y, Kelly JW, Ikeda S. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neuroscience Research. 2006;56:441–449. doi: 10.1016/j.neures.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Venner CP, Lane T, Foard D, Rannigan L, Gibbs SD, Pinney JH, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119:4387–4390. doi: 10.1182/blood-2011-10-388462. [DOI] [PubMed] [Google Scholar]
- Wall JS, Kennel SJ, Williams A, Richey T, Stuckey A, Huang Y, et al. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PloS One. 2012;7:e52686. doi: 10.1371/journal.pone.0052686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]