Abstract
The Kölliker-Fuse nucleus (KF) is known primarily for its respiratory function as the “pneumotaxic center” or “pontine respiratory group.” Considered part of the parabrachial (PB) complex, KF contains glutamatergic neurons that project to respiratory-related targets in the medulla and spinal cord (Yokota, Oka, Tsumori, Nakamura, & Yasui, 2007). Here we describe an unexpected population of neurons in the caudal KF and adjacent lateral crescent subnucleus (PBlc), which are γ-aminobutyric acid (GABA)ergic and have an entirely different pattern of projections than glutamatergic KF neurons. First, immunofluorescence, in situ hybridization, and Cre-reporter labeling revealed that many of these GABAergic neurons express FoxP2 in both rats and mice. Next, using Cre-dependent axonal tracing in Vgat-IRES-Cre and Vglut2-IRES-Cre mice, we identified different projection patterns from GABAergic and glutamatergic neurons in this region. GABAergic neurons in KF and PBlc project heavily and almost exclusively to trigeminal sensory nuclei, with minimal projections to cardiorespiratory nuclei in the brainstem, and none to the spinal cord. In contrast, glutamatergic KF neurons project heavily to the autonomic, respiratory, and motor regions of the medulla and spinal cord previously identified as efferent targets mediating KF cardiorespiratory effects. These findings identify a novel, GABAergic subpopulation of KF/PB neurons with a distinct efferent projection pattern targeting the brainstem trigeminal sensory system. Rather than regulating breathing, we propose that these neurons influence vibrissal sensorimotor function.
Keywords: respiratory, whisking, FoxP2, trigeminal, parabrachial, PBel, RRID: AB_10015246, RRID: AB_2107107, RRID: AB_2107133
1 | INTRODUCTION
The Kölliker–Fuse nucleus (KF), part of the parabrachial complex (PB), is a major component of the “pneumotaxic center” or “pontine respiratory group.” Manipulations in this region perturb breathing rhythm. KF neurons can produce a respiratory pattern with prolonged inspiration known as apneusis, which is likely related to inspiratory off-switching, that is, the inspiratory-to-postinspiratory phase transition (Chamberlin & Saper, 1992, 1994; Dutschmann & Herbert, 2006; Bonis et al., 2010; Dutschmann & Dick, 2012; Levitt, Abdala, Paton, Bissonnette, & Williams, 2015). The lateral crescent PB subnucleus (PBlc) shares many features with and is likely functionally related to the KF.
Most PB neurons, including those in the KF and PBlc, are glutamatergic and express the type 2 vesicular glutamate transporter, Vglut2 (Niu, Yokota, Tsumori, Qin, & Yasui, 2010; Kaur et al., 2013). Most PB neurons relay ascending sensory information to the forebrain, but KF and PBlc neurons provide descending projections to respiratory and autonomic sites in the lower brainstem and spinal cord (Herbert, Moga, & Saper, 1990; Yokota, Tsumori, Ono, & Yasui, 2001, 2004). Virtually all KF neurons with this projection pattern, and all those responsive to hypercapnia, are glutamatergic (Yokota et al., 2004; Yokota, Oka, Tsumori, Nakamura, & Yasui, 2007; Yokota, Kaur, VanderHorst, Saper, & Chamberlin, 2015).
Although most PB neurons are glutamatergic, putative γ-aminobutyric acid (GABA)ergic neurons (immunoreactive for glutamate decarboxylase) have been reported in rats, primarily in the caudal KF and PBlc (Ford, Holmes, Mainville, & Jones, 1995; Guthmann, Fritschy, Ottersen, Torp, & Herbert, 1998). However, neither their targets nor their anatomical relationship to glutamatergic KF neurons are known. Recently, the expression of a transcription factor, Forkhead box protein 2 (FoxP2), has been detected in some PB neurons, including KF (Gray, 2008; Geerling et al., 2011; Miller et al., 2012) providing an opportunity to revisit PB anatomy with respect to this marker. Across several PB subnuclei, FoxP2-expressing neurons are thought to be glutamatergic (Miller et al., 2012). We hypothesized that a caudal subset of FoxP2-expressing neurons are GABAergic, with distinct axonal projections relative to glutamatergic FoxP2 neurons in this region. As an initial step toward exploring the relationship between inhibitory and excitatory KF neurons, we analyzed the locations and genetic expression patterns of glutamic acid decarboxylase 67 (Gad67 [Gad1], Vgat, and Vglut2 in FoxP2-immunoreactive (-ir) neurons throughout the PB complex in rats and mice. We then performed cell-type–specific axonal tracing from glutamatergic versus GABAergic neurons in and around the KF.
2 | MATERIALS AND METHODS
2.1 | Rodents
All mouse strains used here were heterozygous for the transgenes and maintained on a mixed background. For Cre-dependent axonal tracing, we used adult Vglut2-IRES-Cre and Vgat-IRES-Cre mice (Vong et al., 2011) derived from a colony in the Lowell laboratory (n = 3 male and n = 8 male and female, respectively; 20–33 g). Adult male rats (Wistar, Kobe, Japan; 280–300 g, n = 3) and mice (30–36 g, n = 3 wild-type offspring of a Vglut2-IRES-Cre breeding pair) were used for in situ hybridization (ISH) immunohistochemistry.
To visualize putatively GABAergic and glutamatergic neurons, we also used brain sections from the F1 progeny of adult male Vglut2-IRES-Cre (n = 2) and Vgat-IRES-Cre (n = 2) mice crossed with a R26-lox-STOPlox-L10-GFP “Cre-reporter” (Krashes et al., 2014). In these mice, cells and cell lineages that expressed the Vglut2 or Vgat Cre-driver (in the adult cell, or earlier in development) produce Cre-recombinase, which induces green fluorescent protein (GFP) expression permanently by deleting a floxed STOP sequence. In this particular reporter strain, GFP is conjugated to the ribosomal L10 subunit (Krashes et al., 2014), thus restricting GFP to the soma and proximal dendrites, which results in excellent neuronal cell body visualization. The brain-wide distribution of Vglut2- and Vgat-IRES-Cre was shown in their original publication by the Lowell laboratory (Vong et al., 2011). We bred the mice locally and confirmed the genotype of every mouse via polymerase chain reaction (PCR) assays for Cre and L10-GFP.
All animals were housed on a 12/12-hr light/dark cycle in climate-controlled rooms (21–22 °C; 30–40% humidity) with water and standard rodent chow available ad libitum. The Institutional Animal Care and Use Committees at the Beth Israel Deaconess Medical Center (BIDMC) approved all animal husbandry and experimental protocols, and mouse care met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals. Care of rats and mice also met the Guidelines for Animal Experimentation of the Center for Integrated Research in Science, Shimane University.
2.2 | Stereotaxic AAV injection
Under anesthesia with either isoflurane (1.5%) or ketamine/xylazine (60/7.5 mg/kg i.p.), mice received stereotaxic microinjections into the lateral PB and KF. All injections were made into the right side of the brainstem. The coordinates used for Vgat-IRES-Cre mice (Figure 2) were: 1.85 mm lateral, 5.0 mm caudal to bregma, and 2.95 mm deep to dura. Coordinates for Vglut2-IRES-Cre and additional Vgat-IRES-Cre mice were slightly more rostral: 1.8 mm lateral, 4.6 mm caudal to bregma, and 2.9 mm deep to dura. We delivered picoliter air puffs to a fine-tipped glass micropipette, adjusting the duration and frequency while monitoring the fluid meniscus with a calibrated eyepiece reticule, to deliver 4 to 5 nL (Figure 8a–l) or 30 nL (Figure 8m–y) total adeno-associated virus (AAV) volume. The injection suspension contained 6 × 1012 plaque-forming units of an AAV delivering a DNA vector containing Cre-dependent channelrhodopsin, AAV8-EF1a-DIO-hChR2 (H134R)-mCherry (K. Diesseroth, UNC Vector Core, https://www.med.unc.edu/genetherapy/vectorcore) or a similar construct packaged in AAV10 (gift of Michael Lazarus, WPI-IIIS Lazarus Laboratory, University of Tsukuba, Tsukuba, Japan). Each injection was made slowly, lasting up to 5 min, and was followed by a delay of 3 to 5 min before the pipette was retracted slowly. After closing the scalp wound with Vet-Bond (3M) or vinyl sutures, we monitored mouse recovery on a 36 °C warming pad and administered postoperative analgesia (meloxicam; 5 mg/kg, s.c. or Rimadyl; 5 mg/kg, once per day for 2 days). Mice were then returned to their original cages for at least 4 to 5 weeks, which we and collaborators have found to be optimal for production and axon transport of ChR2-mCherry.
FIGURE 2.

High-magnification images of Gad67-expressing, FoxP2-immunoreactive neurons in the caudal PB in rat. (A, B) Examples from adjacent tissue sections labeled for FoxP2 (brown, DAB) and either Gad67 (magenta arrowheads in A) or Vglut2 (green arrowheads in B). These examples are taken from the regions outlined in red in Figure 1F and F1. Note that colors were adjusted to enhance the contrast between DAB and DIG labeling (see Materials and Methods). Scale bar = 100 μm in B (applies to A and B).
FIGURE 8.


Efferent axonal projections of GABAergic versus glutamatergic neurons in KF/PB. (A–D) Injecting Cre-dependent ChR2-mCherry in a Vgat-ires-Cre mouse (IR72) transduces a restricted distribution of neurons in KF/PB, and some outside the PB complex. (E–L) Axons from these GABAergic KF/PB neurons project primarily within the pons and medulla, primarily targeting the trigeminal sensory nuclei. (M–P) Injecting the same vector in a Vglut2-ires-Cre mouse (SY676) transduces many more neurons in KF/PB (mCherry-ir in brown; Nissl counterstain in blue). (Q–Y) Axons from these glutamatergic KF/PB neurons project caudally to established KF/PBlc target sites, down to the C4 spinal level. For abbreviations, see Figure 1 legend. Additional abbreviations: 7 = facial nucleus; 7n = facial nerve root; 8n = vestibulocochlear nerve root; 10 = dorsal motor nucleus of the vagus nerve; 12 = hypoglossal motor nucleus; Amb = nucleus ambiguus, compact formation; AP = area postrema; Aq = cerebral aqueduct; DCN = dorsal cochlear nucleus; ECu = external cuneate nucleus; Gr = gracile nucleus; IC = inferior colliculus; icp = inferior cerebellar peduncle; IntA = interpositus cerebellar nucleus; IO = inferior olivary nucleus; IRt = intermediate reticular formation; Lat = lateral/dentate cerebellar nucleus; LRt = lateral reticular nucleus; MdD = dorsal medullary reticular nucleus; mlf = medial longitudinal fasciculus; opt = optic tract; PAG = periaqueductal gray matter; Pr5 = principal trigeminal sensory nucleus; py = pyramidal tract; pyx = pyramidal decussation; RPa = raphe pallidus; RM = raphe magnus; s5 = sensory trigeminal nerve root; sl = PB superior lateral subnucleus; SOC = superior olivary nuclear complex; Sp5O = oral part of the spinal trigeminal nucleus; SVe = superior vestibular nucleus; TR = reticulotegmental nucleus; ts = solitary tract; Tz = trapezoid body; V = ventricle; VCN = ventral cochlear nucleus; Vc = pars caudalis of spV; Ve = vestibular nuclei; Vo = spV pars oralis; Vi = pars interpolaris of spinal trigeminal nucleus (spV); VLL = ventral nucleus of the lateral lemniscus; VT = ventral tegmental nucleus; xscp = superior cerebellar peduncle decussation. Scale bar = 200 μm in D (applies to A–D); 400 μm in P (applies to M–P).
2.3 | Perfusions and tissue preparation
We anesthetized mice with a lethal dose of chloral hydrate (700 mg/kg) and perfused them through the ascending aorta with 10 mL of phosphate-buffered saline (PBS) at room temperature followed by 50 mL of 10% formalin in PBS. Each brain was removed immediately and postfixed overnight in formalin-PBS at 4 °C. After cryoprotection in 20% sucrose–PBS, the brain was cut into 30-μm-thick axial sections on a freezing microtome, and the sections were collected in separate 1-in-3 (Figure 8a–l) or 1-in-4 (Figures 5 and 6 and 8m–y) series of brain tissue sections, for a rostrocaudal resolution of 30 μm out of each 120-μm span (90 μm between sections). Sections were stored at 4 °C in PBS with azide until histologic processing.
FIGURE 5.

GFP Cre-reporter labeling in mouse for glutamatergic (A–D) and GABAergic (E–H) neurons in the caudal KF/PBlc, approximately 5.5 mm behind bregma (Paxinos & Franklin, 2008). Immediately caudal to the PBel is a cluster of FoxP2-ir neurons, most of which express GFP reporter for Vgat (vesicular GABA transporter; E, F). The cluster of FoxP2-ir neurons is highlighted by arrowheads. Very few FoxP2-ir neurons here express GFP reporter for Vglut2. For abbreviations, see Figure 1 legend. Additional abbreviations: Bar = Barrington’s nucleus; Cb = cerebellum; LC = locus coeruleus; PBm = medial PB subnucleus; pLC = pre-/peri-locus coeruleus nucleus; Su5 = supratrigeminal nucleus. Scale bar = 200 μm in a (applies to A–C) and E (applies to E–G); 100 μm in D, H.
FIGURE 6.

GFP Cre-reporter labeling for mouse glutamatergic (A–D) and GABAergic (E–H) neurons in the rostral KF, approximately 5.0 mm behind bregma (Paxinos and Franklin, 2008). In contrast to caudal levels, most FoxP2-ir neurons in the rostral KF (highlighted by arrowheads) express GFP reporter for Vglut2, not Vgat. For abbreviations, see Figure 1 legend. Additional abbreviations: mcp = middle cerebellar peduncle; NLL = nucleus of the lateral lemniscus. Scale bar = 200 μm in A (applies to A–C) and E (applies to E–G); 100 μm in D,H.
Rat brain tissue for ISH was obtained after transcardial perfusion as well. Rats were anesthetized with chloral hydrate (700 mg/kg) and perfused with 150 mL saline followed by 500 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). Brains were stored in this overnight at 4 °C, cryoprotected in 20% sucrose–PBS, cut into sections 30 μm thick, and collected in a 1-in-6 series (150 μm between sections).
2.4 | Immunohistochemistry
Antibodies used in this study are listed in Table 1. To visualize FoxP2 in combination with in situ hybridization, we used a rabbit polyclonal raised against a synthetic peptide conjugated to keyhole limpet hemocyanin (KLH) derived from within residues 703 to 715 to the C-terminus of human FoxP2. Preincubation with this peptide prevents staining (Campbell et al., 2009). In tracing and in cre-driver labeling experiments we used an affinity-purified sheep polyclonal antiserum (diluted 1:4–5,000) raised against a recombinant peptide sequence corresponding to Ala640–Glu715 of human FoxP2 (R&D Systems, Minneapolis, MN; AF5647). Both FoxP2 antisera labeled neuronal nuclei in the mouse brainstem in a pattern identical to one another and similar to that in rats, and both labeled a pattern identical to the distribution of Foxp2 mRNA labeled in the Allen Brain Atlas (Lein et al., 2007). Staining in rat tissue was entirely confined to neuronal nuclei, as expected. In mouse tissue the Abcam FoxP2 antibody consistently stained a small subset of fibers in the caudal PB (Figure 3d–g). This was clearly distinguished from the nuclear FoxP2-ir label.
TABLE 1.
Primary antibodies
| Antigen | Immunogen | Source, host species, cat. #, RRID | Concentration |
|---|---|---|---|
| mCherry | DsRed-Express, a variant of Discosoma sp. red fluorescent protein | Clontech, rabbit polyclonal, cat. # 632496, RRID: AB_10015246 | 1:5–10,000 |
| FoxP2 | Synthetic peptide conjugated to KLH derived from within residues 700 to the C-terminus of human FOXP2 (available from Abcam as ab16278). | Abcam, rabbit polyclonal, cat. # ab16046, RRID: AB_2107107 | 1:2,000 |
| FoxP2 | Recombinant peptide sequence corresponding to Ala640–Glu715 of human FoxP2 | R&D Systems, sheep polyclonal, cat. # AF5647, RRID: AB_2107133 | 1:4–5,000 |
FIGURE 3.

Distribution of GABAergic and glutamatergic FoxP2 + neurons in mouse. (A–G) ISH for Gad67 (left column) and for Vglut2 (right column) again revealed complementary clusters of FoxP2-expressing neurons in the dorsolateral pons, including a prominent group of GABAergic neurons in the caudal KF and PBlc (C–G). Magenta dots indicate FoxP2-immunolabeled neurons expressing Gad67 (left) and lacking Vglut2 (right). Green indicates FoxP2 + neurons lacking Gad67 and expressing Vglut2. Brown axonal staining represents unidentified cross-reactivity with the rabbit-anti-FoxP2 antiserum and an unidentified mouse antigen that does not occur in rat tissue. Note the absence of FoxP2-ir in the PBel; the Allen brain atlas also shows Foxp2 mRNA encircling the PBel. Note that DTN neurons, which are GABAergic, contained week FoxP2-ir and we did not mark these neurons with dots. For abbreviations, see Figure 1 legend. DTN = dorsal tegmental nucleus.
To trace axons in Cre-driver mice, we used a rabbit polyclonal antibody (diluted 1:5–10,000) that binds dsRed-derived red-fluorescent proteins, including mCherry (Clontech, Mountain View, CA; #632496). The dsRed antiserum did not label any cells or axons in our control tissue from Cre- and wild-type mice that were not injected with an mCherry construct, and in our experimental mice it never labeled cell bodies outside the injected region or axons outside the established set of regions previously found to receive projections from KF/PB (Herbert et al., 1990) or from brainstem nuclei bordering them (Kudo, 1981).
After washing in PBS, we placed sections in 0.3% H2O2 in PBS for 30 min to inactivate endogenous peroxidase. After additional PBS washes, we incubated the tissue overnight at room temperature (RT) in a primary antibody solution containing 3% normal donkey serum (NDS), Triton X-100 (0.25 or 0.3%), and one or both antisera at the dilutions listed above. The following morning, we incubated sections for 2 to 3 hr in biotinylated donkey anti-rabbit secondary antiserum (Jackson ImmunoResearch, West Grove, PA; #711-065-152) diluted 1:500 in a PBT–NDS solution as above, followed by avidin–biotin peroxidase complex (ABC Elite; Vector, Burlingame, CA), and then labeled mCherry using either diaminobenzidine hydrochloride (DAB) with H2O2 (brown, Figures 1–4 and 7) or nickel-enhanced DAB (black, Figure 8) with H2O2 in 0.2% ammonium nickel (II) sulfate. In a subset of cases (Figure 7), we then labeled FoxP2 to reveal subnuclear patterns in the PB/KF region. In these cases, after additional PBS washes, we incubated the sections for 2 hr in biotinylated donkey anti-sheep secondary antiserum (1:500, Jackson ImmunoResearch; #713-065-147), followed by ABC and then DAB (brown, no nickel) labeling for FoxP2.
FIGURE 1.

Distribution of GABAergic and glutamatergic FoxP2+ neurons in rat. In situ hybridization (ISH) for glutamic acid decarboxylase (Gad67, aka Gad1; blue in left column, A–F) and for vesicular glutamate transporter type 2 (Vglut2; blue in right column, A1–F1) revealed complementary populations of neurons in the dorsolateral pons, including a prominent group of GABAergic neurons in the caudal KF and lateral crescent PB subnucleus (PBlc) (D–F), most of which also are FoxP2-ir. Magenta dots mark FoxP2-ir nuclei in KF/PB neurons expressing Gad67 (left, A–F) and lacking Vglut2 (right, A1–F1). Green indicates FoxP2-ir neurons lacking Gad67 (left, A–F) and expressing Vglut2 (right, A1–F1). Arrows indicate GABAergic neurons scattered in the PBlc, which in rats extends dorsally from the KF and wraps around the outer border of the external lateral PB subnucleus. Red outlines in F and F1 highlight the magnified neuron shown in Figure 2A and B, respectively. Abbreviations: 4n = trochlear nerve root; DLL = dorsal nucleus of the lateral lemniscus; el = external lateral PB subnucleus; exm = external medial PB subnucleus; il = internal lateral PB subnucleus; KF = Kölliker–Fuse nucleus; me5= mesencephalic trigeminal tract; Mo5 = motor trigeminal nucleus; scp = superior cerebellar peduncle; vl= ventral lateral PB subnucleus; vsct= ventral spinocerebellar tract.
FIGURE 4.

High-magnification images of Gad67-expressing, FoxP2-ir neurons in the caudal PB in mouse. (A, B) Examples from adjacent tissue sections labeled for FoxP2 (brown, DAB) and either Gad67 (magenta arrowheads in A) or Vglut2 (green arrowhead in B). These examples are taken from the regions outlined in red in Figure 3G and G1. Note that colors were adjusted to enhance the contrast between DAB and DIG labeling (see Materials and Methods). Scale bar = 100 μm in B (applies to A, B).
FIGURE 7.
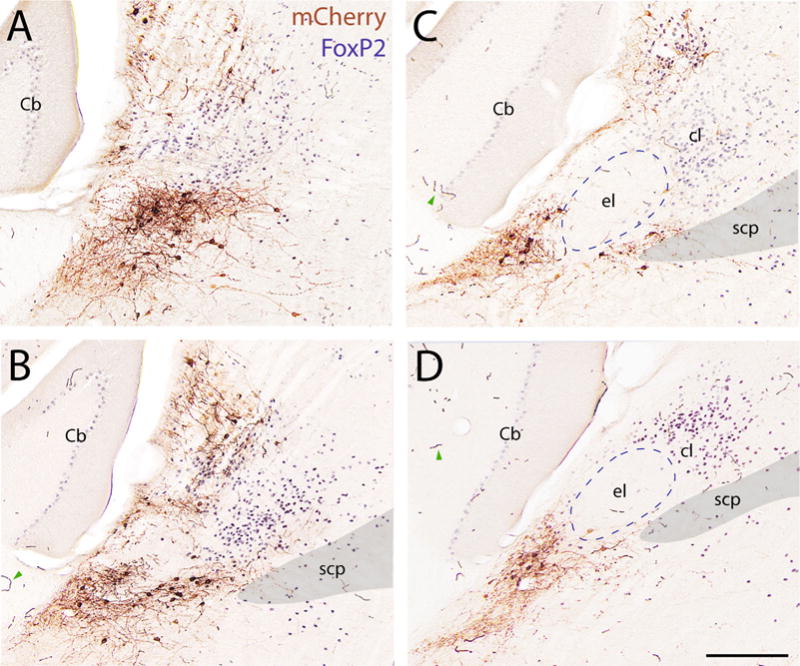
AAV injection site in Vgat-IRES-Cre mouse. (A–D) Rostral to caudal sections containing mCherry-ir neurons (DAB, brown), which express Cre-conditional ChR2-mCherry after AAV injection into the PB-KF region of a Vgat-IRES-Cre mouse (case IR72). FoxP2-ir nuclei are stained in blue. Red blood cells in some capillaries were labeled with DAB due to endogenous peroxidase activity (examples shown by green arrowheads in B–D). For abbreviations, see Figure 1 legend. Additional abbreviations: Cb = cerebellum (Purkinje neurons contain faint nuclear immunoreactivity for FoxP2); cl = central lateral PB subnucleus (dense FoxP2 immunoreactivity). Scale bar = 200 μm in D (applies to A–D).
After final PBS washes, we mounted sections on Superfrost Plus or gelatinized slides and dried them overnight. Slides were then dehydrated in graded ethanols and cleared in xylenes before coverslipping with Permount (Fisher, Pittsburgh, PA) or VectaMount (Vector). In select cases, after initial imaging and analysis (see below), we removed the coverslips from slides by overnight incubation in xylenes, rehydrated them in a reverse ethanol series, and performed a Nissl stain using thionin for cytoarchitectural guidance of figure preparation.
2.5 | Immunofluorescence
To compare FoxP2 labeling in putatively glutamatergic and GABAergic neurons in the PB complex, we immunolabeled brain tissue from off-spring of R26-loxSTOPlox-L10-GFP reporter mice and Vglut2- and Vgat-IRES-Cre mice (see section 2.1 above). After washing sections in PBS, we used the same FoxP2 antiserum as above (sheep polyclonal), diluted 1:4,000 in PBT with 0.2% normal horse serum. After overnight incubation at RT and PBS washes, we incubated the tissue sections in biotinylated anti-sheep (1:500 dilution, as detailed above) followed by streptavidin-Cy5 diluted 1:500 in PBT (Molecular Probes, Eugene, OR; #SA1011). Sections were washed in PBS, mounted on slides as above, dried overnight, coverslipped using Vectashield with 4,6-diamidino-2-phenylindole (DAPI; Vector), and stored in slide folders at 4 °C until imaging.
2.6 | In situ hybridization + immunohistochemistry
The vectors containing a partial, 3.3-kb cDNA template for Vglut2 (Stornetta et al., 2003) and a full-length, 3.2-kb cDNA encoding rat Gad67 (Erlander, Tillakaratne, Feldblum, Patel, & Tobin, 1991) were kindly supplied by Dr. R.L. Stornetta (University of Virginia, Charlottesville, VA) and by Dr. A.J. Tobin (University of California, Los Angeles, CA), respectively. Both sense and antisense riboprobes for ISH of Vglut2 were obtained by linearizing the recombinant plasmids with the restriction enzymes (Takara; XhoI for Vglut2 antisense, SpeI for Vglut2 sense) and transcribing with RNA polymerases (Sp6 for antisense; T7 for sense) in the presence of digoxigenin (DIG)-labeled 11-UTP (Roche, Indianapolis, IN). On the other hand, the vector containing the Gad67 cDNA template was linearized with the restriction enzymes (Takara; NotI for Gad67 antisense, SalI for Gad67 sense), and transcription was done with T7 or T3 RNA polymerase for the antisense or sense, respectively, in the presence of DIG-labeled 11-UTP. The riboprobes were fragmented by limiting alkaline hydrolysis (60 °C, 60 min), to obtain probes of approximately 300 nucleotides in peak length, which were verified by electrophoresis. The efficiency of DIG incorporation was estimated by direct immunological detection on dot blots using an alkaline phosphatase-conjugated anti-DIG antibody (Roche).
Sections were washed in PBS, and then incubated in a hybridization buffer containing Denhardt’s solution (0.02% bovine serum albumin, 0.02% Ficoll, 0.02% polyvinylpyrrolidone; Eppendorf, Happauge, NY), 0.1% Tween 20 (Sigma, St. Louis, MO), 0.5 mg/mL yeast tRNA (Roche), 5 × standard saline citrate (SSC: 150 mM NaCl, 15 mM sodium citrate, pH 7.0), 5% dextran sulfate (Chemicon, Temecula, CA), and 50% formamide in aqueous solution for 1 hr at 55 °C. The sections were transferred to the hybridization buffer containing Vglut2 riboprobe (2 μg/mL) or Gad67 riboprobe (2 μg/mL), which was heat-denatured at 80 °C for 5 min, and left overnight at 55 °C. The sections were washed 3 times in 2 × SSC with 50% formamide at 55 °C, washed twice in 1 × SSC with 50% formamide at 55 °C for 1 hr, and then incubated overnight in Tris-buffered saline (TBS; pH 7.3) containing either sheep (for mouse sections) or mouse (for rat sections) anti-digoxigenin antibody (1:1,000; Roche) and 1% blocking reagent (Roche). Subsequently, the sections were washed in TBS, incubated in TBS containing donkey anti-sheep or anti-mouse IgG (1:500; Jackson ImmunoResearch) for 3 hr, washed in TBS, and then incubated in TBS containing ExtrAvidin-Alkaline Phosphatase (1:4,000; Sigma) for 1 hr. After PBS washes, the sections were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) solution (Roche) for 4 hr. The appropriate reaction time was defined by appearance of signal in the absence of or with minimal trigeminal and hypoglossal motor neuron labeling.
Following ISH, sections were immunolabeled for FoxP2. The sections were incubated in TBS containing avidin blocking solution (Vector) and 1% blocking reagent for 1 hr, washed in TBS, and then incubated overnight in TBS containing rabbit anti-FoxP2 antibody (1:2,000; Abcam, Cambridge, MA), biotin solution (Avidin/Biotin Blocking Kit; Vector) and 1% blocking reagent. After TBS washes, the sections were incubated in TBS with ABC (Vector), and then reacted with 0.05% DAB (Nakarai, Tokyo, Japan) and 0.01% H2O2 in TBS. Subsequently, the sections were mounted onto gelatinized slides, dehydrated in methanol, cleared in xylene, and coverslipped with VectaMount mounting medium (Vector).
2.7 | Image analysis and axon tracing
Fluorescence and brightfield whole-slide imaging was performed at 100× using a slide-scanning microscope (Olympus VS120 or Hamamatsu NanoZoomer). Sections were analyzed at 1× to 1×x in OlyVIA (Olympus) or NDP.View (Hamamatsu). For further analysis and figures, sections were exported as “1×” and “10×” (full-resolution) screen captures, and then imported and manually retiled in Inkscape 0.91 (https://inkscape.org/en). For Vgat projections, we traced axons from the 10 × images, using the pen tool, and outlined anatomical boundaries using 1 × images from sections that had been Nissl counterstained. For Vglut2 projections, we traced axonal projections using a camera lucida attached to an Eclips E-400 microscope (Nikon). For display purposes, to match the orientation of tissue slides in Vgat cases, some drawings and images were flipped to show injections on the left side of the page. For fluorescence figures, gray-scale images from each immunofluorescence color channel were pseudocolored, contrast-adjusted, and cropped in Adobe Photoshop before figure layout. For high-magnification images showing FoxP2 and ISH for Gad67 or Vglut2 (Figures 2 and 4), brown DAB labeling was enhanced in Photoshop to better see double-labeled neurons. To do this, we used the “Replace Color” tool, sampled a brown (DAB-stained, FoxP21) nucleus, with “Fuzziness” set to 200, and increased the saturation to 1100; this procedure highlighted FoxP2 + nuclei without appreciably altering the tissue background or the dark blue ISH labeling.
3 | RESULTS
3.1 | GABAergic and glutamatergic FoxP2-ir neurons in the PB complex in rat
We first investigated the distribution of GABAergic and glutamatergic PB neurons in rats, the species in which most PB anatomic studies have been conducted (Saper & Loewy, 1980; Fulwiler & Saper, 1984; Chamberlin & Saper, 1992, 1994; Miller et al., 2012). As expected, most neurons in the PB complex expressed Vglut2; Figure 1a–f). Relatively few PB neurons expressed Gad67; these were located primarily at the ventral and lateral margins of the PB complex. Within the rostral part of the KF subnucleus, most or all neurons expressed Vglut2 (Figure 1a1 and b1). At progressively more caudal levels of KF and the adjacent PBlc, however, GABAergic neurons became more prominent (Figure 1d–f).
The transcription factor FoxP2 was expressed by many neurons in the PB complex and nearby brainstem nuclei (Figure 1). Their general distribution was similar to that described previously (Geerling et al., 2011; Miller et al., 2012). For example, dense nuclear staining for FoxP2 was clustered in the central and dorsal lateral PB subnuclei (PBcl, PBdl), but largely absent from the external lateral subnucleus (PBel). Most FoxP2-ir neurons in the PB complex, including KF and PBlc, expressed Vglut2 mRNA (Figure 1).
Strikingly, however, a subpopulation of FoxP2-ir neurons expressed the GABA synthetic enzyme GAD67 (aka Gad1; Figure 2a). In adjacent sections, FoxP2-ir neurons with the same distribution lacked Vglut2 mRNA (Figure 2b). Most of these putatively GABAergic FoxP2-ir neurons were located caudally, at the ventrolateral margin of the caudal PB complex, within KF and PBlc (Figure 1c–f). A small number of Gad67-expressing neurons here lacked FoxP2 (Figure 1d–f), and may represent a separate GABAergic population.
3.2 | GABAergic and glutamatergic FoxP2-ir neurons in the PB complex in mice
We next compared the distributions of GABAergic and glutamatergic FoxP2-ir neurons in mice (Figure 3). The distributions of Gad67 mRNA, Vglut2 mRNA, and FoxP2-containing neurons in the mouse PB complex were largely similar to those in rats. In both species, PBel was distinctly void of FoxP2 immunoreactivity (Figures 1 and 3). Rostral to PBel, most FoxP2-ir neurons in and around KF were glutamatergic (Figure 3a1–c1), while at caudal levels most were GABAergic (Figure 3d–g). FoxP2-ir neurons in dorsal lateral PB subnucleus (PBdl) were glutamatergic, even at caudal levels. Finally, as in rat, primarily GABAergic FoxP2-ir neurons occupied the caudal PBlc, a thin, cell-poor layer wrapping around the lateral margin of PBel (Figure 3e,f).
Since some neurons switch neurotransmitter phenotypes during development, and even transient expression of Cre will cause permanent removal of floxed stop sequences, we examined the distribution of Cre-reporters in GABAergic, FoxP2-ir neurons in the caudal KF/PBlc. GFP reporter for the GABA vesicular transporter (Vgat) colocalized with FoxP2 in the same regions that contained Gad67-expressing FoxP2-ir neurons (caudal KF & PBlc; Figure 5e–h). By contrast, most FoxP2-ir neurons in this caudal region did not colocalize with GFP reporter for Vglut2 (Figure 5a–d). The reverse pattern was observed in the rostral KF. There, GFP reporter for Vglut2, but not Vgat was found in most FoxP2-ir neurons (Figure 6). Thus, within the large subpopulation of FoxP2-ir neurons in the PB complex, there were two independent lineages: a glutamatergic majority, and a GABAergic minority, which was clustered caudally in the ventrolateral PBlc and KF. Next, we asked whether these two populations, with separate fast neurotransmitter identities, established different efferent axonal projections.
3.3 | GABAergic efferent projections from KF-PBlc
To selectively label the projections of GABAergic PB neurons, we used Cre-dependent axonal tracing. Genetically encoded tracers produce robust labeling in axons and terminals (Chamberlin, Du, de Lacalle, & Saper, 1998), and a recombinase approach allows us to label selectively those axons projecting from Cre-expressing neurons (Gautron, Lazarus, Scott, Saper, & Elmquist, 2010). Figure 7 shows the distribution of mCherry-ir neurons that were transduced after microinjecting AAV-DIO-ChR2-mCh in the PB–KF region of a representative Vgat-IRES-Cre mouse (case IR72). The subnuclear localization of neurons transduced with ChR2-mCh, based on FoxP2 immunolabeling (blue nuclei in Figure 7) and Nissl cytoarchitecture in adjacent sections (not shown), included primarily the caudal KF and PBlc—the same PB subregions that contained GABAergic FoxP2 + neurons. As expected, many of the transduced neurons contained a FoxP2-ir nucleus. These were clustered in the caudal KF and PBlc, similar to the Gad67-expressing and Vgat-reporting FoxP2-ir neurons described above.
The distribution of mCherry-ir neurons extended dorsally and rostrally as well, with a few neurons labeled in the dorsal nucleus of the lateral lemniscus (DLL). The DLL contained large GABAergic neurons that strongly expressed Gad67 (Figure 1a,b). DLL neurons also expressed GFP reporter for Vgat-IRES-Cre and lacked FoxP2-ir (Figure 6g). The presence of GABAergic neurons here is consistent with prior descriptions of GAD immunoreactivity in cats, rabbits, and rats (Adams & Mugnaini, 1984). The DLL receives auditory information from the cochlear and superior olivary nuclei (Glendenning, Brusno-Bechtold, Thompson, & Masterton, 1981), and projects to the inferior colliculus (Kudo, 1980). One of our injections (IR68), which missed KF dorsally and transduced neurons mainly in the DLL, produced axonal labeling primarily in the IC. DLL axons did not project to any of the regions targeted by descending projections of KF or PB neurons, and KF neurons did not project to the IC or other auditory targets, so in PB/KF cases with any neurons labeled in the DLL, axonal projections to the IC probably originated exclusively in the DLL, not the KF.
The descending axonal projections of GABAergic neurons in PB/KF are shown in Figure 8e–l (IR72). In this and all other Vgat-IRES-Cre cases in which our Cre-dependent AAV injections labeled GABAergic neurons in KF/PBlc (n = 6: IR72, IR99, SY228, 229, 679, 680), the most prominent targets of labeled axonal projections were the sensory trigeminal nuclei. At every level of the pons and medulla, all four subdivisions of the trigeminal sensory complex contained moderate-to-dense axonal arbors with many terminal and en passant boutons. This pattern began in the rostral pons, just ventral to the PB. Labeling here, in the principal sensory trigeminal nucleus, was moderately dense in its lateral and ventral portions. Axons and terminal labeling extended caudally from there, becoming densest in the pars interpolaris (Vi) of the spinal trigeminal nucleus, particularly in its rostral portion (Figure 8j), and then tapering off in the transition zone where the Vi gives way to the pars caudalis (Vc; Figure 8k). Axonal labeling here did not fill the entire subnucleus, preferring lateral to medial sites. Labeling in the Vc increased caudally, in a specific pattern at the medial margin of the Vc bordering the medullary dorsal reticular formation (MdD; Figure 8l). No axons extended caudal to this level.
These projections of GABAergic KF terminals to the trigeminal sensory nuclei were almost exclusively ipsilateral. Outside the trigeminal sensory nuclei, there was light labeling in the regions surrounding the trigeminal, hypoglossal, and facial motor nuclei bilaterally, as well as parts of the reticular formation, the nucleus of the solitary tract, and the ventrolateral medulla. Light axonal labeling in the medullary reticular formation tapered caudally, and there were no labeled axons at or caudal to the pyramidal decussation. Rostral and dorsal to the KF, axons coursed into the IC; as noted above, these axons probably arise exclusively from DLL GABAergic neurons.
Further rostrally (not shown), a few labeled axons ascended through the periaqueductal gray and midbrain reticular formation and into the hypothalamus. The dorsomedial nucleus of the hypothalamus (DMH) contained a moderate plexus of branching and terminating axons, with a few fibers extending slightly into the lateral hypothalamic area (LHA). Similar labeling in the DMH occurred after an injection involving primarily DLL (n= 1, IR68) and after an injection involving primarily the ventral, caudal KF/PBlc and avoiding the DLL (n = 1, IR99), so it is unclear which GABAergic neuron population(s) in this region generate the modest projection to the DMH. Rostral to the DMH, a few axons coursed through the rostral LHA and lateral preoptic area (LPOA). One or two fibers in the LPOA could be followed up to the level of the anterior commissure decussation, without branching or establishing any terminal fields at any level.
3.4 | Glutamatergic efferent projections from KF–PBlc
Injecting AAV10-DIO-ChR2-mCh in Vglut2-IRES-Cre mice (n = 3) transduced many neurons in the lateral PB and KF. The density of labeled glutamatergic neurons and axons precluded plotting labeled neurons individually at the injection site; instead, mCherry-immunoreactivity is shown in Nissl-counterstained sections from a representative case (SY676) in Figure 5m–p. In each Vglut2 case, descending axonal projections from these neurons arborized extensively in the familiar KF targets (Herbert et al., 1990; Yokota et al., 2007).
Descending axonal projections were strongly, but not exclusively, ipsilateral. Rostral to caudal, these included the contralateral PB complex, lateral portion of the facial motor nucleus (VII; Figure 5t–u), the ventrolateral medulla at every level (Figure 5v–x), the hypoglossal motor nucleus, and the ventrolateral and commissural subdivisions of the nucleus of the solitary tract (NTS) (Figure 5w,x). Axonal labeling in the ventrolateral medulla avoided the lateral reticular nucleus (Figure 5w,x) and the compact formation of the nucleus ambiguus (Figure 5v) while continuing caudally through the retroambiguus, past the level of the pyramidal decussation. Axonal labeling in the spinal cord terminated primarily in the intermediolateral cell column (IML) and phrenic motor nucleus, but also in the dorsal horn.
In contrast to these heavy projections to cardiorespiratory nuclei, glutamatergic input to the trigeminal sensory nuclei was minimal, with the exception of light, bilateral terminal fields in Vi (Figure 5v,w), the only brain region receiving inputs from both GABAergic and glutamatergic KF neurons. The principal and other trigeminal sensory nuclei contained sparse axonal labeling at some levels, but the density was strikingly low relative to the GABAergic projections described above, especially given the greater number of neurons transduced in Vglut2-IRES-Cre cases.
4 | DISCUSSION
Our data reveal a previously unidentified, GABAergic subpopulation of PB neurons. These neurons express Gad67, Vgat, and FoxP2 and project to the trigeminal sensory nuclei. Their distinct axonal projections suggest a function distinct from that of glutamatergic KF neurons that participate in respiratory control (Figure 9).
FIGURE 9.

Hypothesized KF inputs to respiratory and whisking sensorimotor circuits.Top: The pre-Botzinger complex (pre-Bot) is the site of respiratory rhythm generation (Feldman, Del Negro, & Gray, 2013). KF glutamatergic neurons target the ventrolateral nucleus of the solitary tract (vlNTS) and the commissural NTS (commNTS), which receive airway mechanical and blood gas sensory information, respectively. The KF also targets motoneurons (Yokota et al., 2001, 2007; Yokota, Niu, Tsumori, Oka, & Yasui, 2011), and likely premotoneurons (rVRG; Ellenberger & Feldman, 1990; Herbert et al., 1990), which innervate the diaphragm (phrenic nucleus), tongue (XII), and larynx (nucleus ambiguous [NA]). All these projections are excitatory. Bottom: GABAergic KF neurons contact areas that receive primary afferent terminals from trigeminal ganglion neurons that innervate vibrissal follicles (PrV, Vi, Vc). In addition to thalamic-projecting sensory neurons, these areas contain premotor neurons that innervate vibrissal motoneurons in lateral VII and through which GABAergic KF neurons may influence motor output, which is driven by neurons in the “vibrissa” subregion of the medullary intermediate reticular formation (vIRt), which is medial to the pre-Bot (Moore et al., 2013). Finally, we hypothesize that the subset of glutamatergic KF neurons that innervate Vi bilaterally and the lateral part of VII are part of the vibrissal system rather than the respiratory system.
4.1 | Technical considerations
Using Cre recombinase to label neurons and trace their axons raises unique technical concerns. First, inefficiencies in the expression of Cre or recombination with various reporters (Schmidt-Supprian & Rajewsky, 2007; Liu et al., 2013) can increase variability and reduce sensitivity. We mitigated these problems by using IRES-Cre knockin mice and an L10-GFP strain with robust and well-documented Cre-reporting (Vong et al., 2011; Krashes et al., 2014; Geerling et al., 2016). In these mice, GFP labeling was consistent and robust, and conformed to established locations of glutamatergic and GABAergic neurons. We did not find any GABAergic or glutamatergic brainstem regions lacking the respective Cre-reporter.
Another consideration is that, at any developmental stage, even transient expression of Cre will result in permanent removal of loxP sites, and thus expression of its reporter, even after the Cre-driver gene is no longer expressed. Indeed, GFP reporter expression is prominent in Vglut2-IRES-Cre mice in several regions where adult neurons express Vglut1 and not Vglut2, including the cerebral cortex, the hippo-campus, and—bordering the PB complex—the mesencephalic trigeminal nucleus (Pang et al., 2006). This property is useful for fate-mapping studies (Machold & Fishell, 2005; Xu, Tam, & Anderson, 2008), but in our study it could lead to the inappropriate identification of some GFP-expressing neurons as glutamatergic or GABAergic, even if they no longer express the respective vesicular transporter. We mitigated this concern in two ways. First, we used a stable marker in both Cre reporter lines (FoxP2), and found complementary patterns of labeling relative to this marker in the caudal KF and PBlc, showing that GABAergic neurons there do not produce Vglut2 at any stage of development (Figures 5 and 6). Second, we directly labeled Vglut2 and Gad mRNA, again in comparison with FoxP2 (Figures 1–4). With the exception of the mesencephalic trigeminal nucleus, the distributions of mRNA and Cre-reporter in this region of the brainstem were similar.
As further evidence that adult PB-KF neurons express Vgat, injecting an AAV vector delivering a FLEXed construct resulted in expression by neurons in the same distribution as mRNA and Cre-reporting for GABAergic marker genes. FLEX constructs (also known as double-inversion orientation [DIO]) have a reverse orientation and require a two-step, Cre-mediated process before expression (inversion, then loxP excision), so our ChR2-mCh expression in PB-KF neurons in Vgat-IRES-Cre mice again shows that Vgat-IRES-Cre is expressed in these neurons in adult mice. After injecting the same AAV into the same region in separate lines of Cre mice (Vglut2 vs. Vgat), the separate patterns of axonal labeling further demonstrates that glutamatergic and GABAergic neurons are mutually exclusive populations in the PB-KF region.
4.2 | Comparison between genetic/conditional and conventional neuroanatomical PB labeling
Our data confirm and extend earlier findings from Loewy’s group showing prominent FoxP2 expression in the KF, PBlc, PBcl, and PBdl and its exclusion from the PBel in rats (Geerling et al., 2011; Miller et al., 2012). We show similar labeling patterns in both rats and mice. Our data strengthen previous assertions that the mouse and rat PB complexes are homologous, with most cell groups in similar positions relative to one another.
Our conditional tracing from GABAergic and glutamatergic PB neurons each labeled a subset of the pathways previously seen with nonselective tracing. It is now clear that both GABAergic and glutamatergic neurons contribute to descending projections from the PB and KF. Together they account for all the descending projection sites previously noted. For example, Yoshida and colleagues (1997) demonstrated PB projections to the trigeminal sensory nuclei and the dorsal horn, arising from the caudal KF and the rostral KF, respectively. This complements our finding of trigeminal projections from caudal GABAergic neurons, and dorsal horn projections from rostral glutamatergic neurons. Our finding of glutamatergic PB inputs to the phrenic motor nucleus confirms our earlier tracing (Yokota et al., 2007, 2015), and we confirmed that KF inputs to the IML are excitatory.
Projections from the KF to the facial motor nucleus, which are excitatory (Figure 5t,u), were first identified in cats and opossums (Takeuchi, Uemura, Matsuda, Matsushima, & Mizuno, 1980; Panneton & Martin, 1983). PB tracing studies in rats also noted facial motor nucleus projections but paid them little attention compared with the respiratory-related targets (Herbert et al., 1990; Dutschmann & Dick, 2012). In a study focused on selective labeling of inputs to vibrissal motoneurons, Hattox, Priest, & Keller (2002) made tracer injections confined to physiologically defined sites in lateral VII, and found retrogradely labeled neurons in the PB complex. Furthermore, vibrissal premotor neurons labeled by transynaptic retrograde rabies virus tracing from the mystacial pad included a discrete cell group in or near the KF (Takatoh et al., 2013). Most of these putative KF neurons were glutamatergic, consistent with the heavy projection we identified from glutamatergic KF neurons to the lateral, vibrissal motoneuron part of VII. Taken together, these data show a robust, excitatory projection from KF neurons to neurons in the lateral facial motor nucleus that control whisking.
4.3 | Developmental origin of GABAergic KF neurons
GABAergic FoxP2 + neurons in the caudal KF may develop from a separate embryonic precursor than other FoxP2-expressing neurons in the PB complex. Most FoxP2-ir neurons in the PB complex were positive for yellow fluorescent protein (YFP) after fate-mapping for Math1/Atoh1 (Gray, 2008), a transcription factor expressed in a subset of embryonic precursor cells in the rhombic lip, specifically those that generate cerebellar granule cells and several brainstem nuclei, including glutamatergic neurons in most PB subnuclei that express FoxP2 (Machold & Fishell, 2005; Wang, Rose, & Zoghbi, 2005; Gray, 2008; Rose, Ahmad, Thaller, & Zoghbi, 2009). In contrast, that same fate-mapping study revealed a caudal, ventrolateral subpopulation of FoxP2-ir neurons that are not YFP + (Gray, 2008), and therefore may not originate from Math1/Atoh1 precursors.
We hypothesize that these neurons in the caudal, ventrolateral PB complex, specifically those that express FoxP2 and are not Math1/Atoh1 derived, correspond to the GABAergic subset we identify here. Furthermore, we predict that these neurons originate from a separate lineage of rhombic lip precursors, which express Ptf1a. Ptf1a precursors give rise to the GABAergic Purkinje cells and interneurons of the cerebellum (Hoshino, 2006), and do, in fact, give rise to a cluster of FoxP2-ir neurons in the caudal, ventrolateral PB complex; this is evident from Figure 4A of the fate-mapping study from Gray (Gray, 2008). It is important to note that Figures 3 and 4 in Gray’s study are mislabeled; in Figures 3D and 4, the ovoid region at the lateral margin of the PB complex, rimmed by FoxP2-ir neurons, is labeled “Pr5.” It is now clear from comparing the distribution of FoxP2 in adult mice and rats here, and from the complementary distributions of FoxP2 and Lmx1b in rats shown by Miller and colleagues (2012), that this region is the PBel, not Pr5. That is, PBel neurons express Lmx1b, and another figure in that study (Gray, 2008) does in fact show Lmx1b-expressing neurons in this position. Also, the arrows in Figures 3F and 4 pointing to “KF” actually point to FoxP2-ir neurons in the PBcl/dl, and we believe that the cell group located ventrolateral to PBel in Figure 4 of Gray (2008) corresponds to the GABAergic, FoxP2-ir neurons we describe here in the caudal KF and PBlc. Most neurons in that position expressed YFP reporter for Ptf1a (Figure 4A) versus none for Math1/Atoh1 (Gray, 2008), so GABAergic KF neurons probably derive from Ptf1a precursors in the rhombic lip, similar to GABAergic cerebellar neurons and separate from the Math1/Atoh1 source of cerebellar granule neurons and FoxP2 + neurons in the PB.
In contrast, the origin of rostral, glutamatergic neurons in the mouse KF is less clear. Glutamatergic, FoxP2-ir neurons in most subnuclei in the lateral PB clearly originate from Math1/Atoh1 precursors in the rhombic lip (Gray, 2008; Rose et al., 2009). Lmx1b-expressing neurons in the PBel develop separately, from precursor cells in the roof plate epithelium that express both Lmx1a and Lmx1b (Zou et al., 2009). In rats, neurons located in the rostral KF and PBlc express both FoxP2 and Lmx1b, and it is unclear whether those rostral, glutamatergic neurons derive from Lmx1a/1b precursors in the roof plate, from Math1/Atoh1 precursors in the rhombic lip, from an intermediate population, or from a combination of the two.
4.4 | Functional implications
To date, the PB complex has been related to control of breathing, blood pressure, blood volume and osmolarity, ingestive behavior, behavioral state, and sensory processing of taste, pain, itch, and skin temperature (Spector, Norgren, & Grill, 1992; Buritova, Besson, & Bernard, 1998; Wu, Clark, & Palmiter, 2012; Davern, 2014; Akiyama, Curtis, Nguyen, Carstens, & Carstens, 2016; Morrison, 2016; Saper & Loewy, 2016). The KF in particular is implicated largely in respiratory control (Dutschmann & Dick, 2012). KF neurons may influence respiratory motor output directly by connections to motor neurons in the medulla and spinal cord or indirectly through premotor neurons in the ventrolateral medulla. Furthermore, KF may modulate mechanical and chemosensory feedback from the airways via projections to the NTS. However, neither the heavy glutamatergic projection to the facial nucleus nor the GABAergic projection to the trigeminal sensory system can be readily integrated into a respiratory model.
Instead, we propose that these connections form part of the mystacial whisker sensorimotor system, in which rodents generate rhythmic vibrissal movements (whisks) to explore objects in their environment. Figure 9 summarizes KF projections that we hypothesize to mediate effects on the respiratory and whisking systems. Motor control of whisking is driven by a dedicated central pattern generator in the rostral medullary reticular formation, near the pre-Botzinger complex (Moore et al., 2013). These neurons control lateral VII motoneurons that innervate intrinsic vibrissal muscles, which drive the whiskers forward, with retraction being mostly passive. The KF glutamatergic projection to lateral VII is well positioned to modulate whisking, while GABAergic input to sensory neurons in Pr5 and spV could modify vibrissal sensory processing.
Despite this circumstantial evidence, it is uncertain whether and how KF and PBlc neurons modulate whisker sensorimotor function. In keeping with a respiratory theme, the KF could help synchronize whisker movements with sniffs (Moore et al., 2013). However, our anatomical data could also reflect an influence on sensory processing. Primary vibrissal afferents terminate topographically in the Pr5 and Vi, which in turn deliver sensory information to the somatosensory barrel cortex via the thalamus. We found heavy projections of GABAergic KF and PBlc neurons to the ventrolateral portions of the Pr5 and Vi, which is where the sensory representations of individual vibrissae are organized in barrelettes (Ma, 1991). KF GABAergic terminal fields also target the ventral part of the Vi (Figure 5j) and the interface of the deep Vc and MdD (Figure 5l), which contain vibrissal premotor neurons (Takatoh et al., 2013), so KF GABAergic afferents could also modulate the motor patterning of whisking.
5 | CONCLUSIONS
We describe a novel GABAergic cell group in the caudal KF subnucleus of the PB complex, with projections targeting primarily the trigeminal sensory nuclei. We suggest a new role for KF in whisking, played by GABAergic neurons and a subset of glutamatergic neurons with targets summarized in Figure 9. Further insight into the physiological role of the GABAergic KF neurons will require determination of their inputs as well as the cellular identity, firing patterns, and functions of postsynaptic targets.
Acknowledgments
The authors thank Sathyajit Bandaru (mouse breeding), Quan Ha (stereotaxic microinjections), Vivian Ling (figure preparation), Paul Gray (discussions on developmental genetics), and Clif Saper (discussions on the neuroanatomy and nomenclature in PB/KF).
Funding information
National Institutes of Health, Grant/Award Numbers: P01 HL095491 (to N.L.C.) and R25 #NS070682 (to J.C.G.).
Footnotes
AUTHOR CONTRIBUTIONS
All authors had full access to all data in this study and take responsibility for the integrity of the data and accuracy of analysis. Study concept and design: NC, JG, IR, SY. Acquisition of data: SY, IR, JG. Analysis and interpretation of data: SY, IR, DR, JG, NC. Drafting of the manuscript: JG, NC. Critical revision of the manuscript for important intellectual content: IR, SY, DR, JG, NC. Obtained funding: NC, JG. Study supervision: NC.
References
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: A nucleus of GABAergic projection neurons. Brain Research Bulletin. 1984;13:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Curtis E, Nguyen T, Carstens MI, Carstens E. Anatomical evidence of pruriceptive trigeminothalamic and trigemino-parabrachial projection neurons in mice. The Journal of Comparative Neurology. 2016;524:244–256. doi: 10.1002/cne.23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Forster HV. Site-specific effects on respiratory rhythm and pattern of ibotenic acid injections in the pontine respiratory group of goats. Journal of Applied Physiology. 2010;109:171–188. doi: 10.1152/japplphysiol.00934.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buritova J, Besson JM, Bernard JF. Involvement of the spinoparabrachial pathway in inflammatory nociceptive processes: A c-Fos protein study in the awake rat. The Journal of Comparative Neurology. 1998;397:10–28. doi: 10.1002/(sici)1096-9861(19980720)397:1<10::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Campbell P, Reep RL, Stoll ML, Ophir AG, Phelps SM. Conservation and diversity of Foxp2 expression in muroid rodents: functional implications. Journal of Comparative Neurology. 2009;512:84–100. doi: 10.1002/cne.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. The Journal of Comparative Neurology. 1992;326:245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. Journal of Neuroscience. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: Use for transgene expression and anterograde tract tracing in the CNS. Brain Research. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern PJ. A role for the lateral parabrachial nucleus in cardiovascular function and fluid homeostasis. Frontiers in Physiology. 2014;5:25. doi: 10.3389/fphys.2014.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Comprehensive Physiology. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker -Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. European Journal of Neuroscience. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Research. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: So near, yet so far. Annual Review of Physiology. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: Codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. The Journal of Comparative Neurology. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Gautron L, Lazarus M, Scott MM, Saper CB, Elmquist JK. Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach. The Journal of Comparative Neurology. 2010;518:2090–2108. doi: 10.1002/cne.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Stein MK, Miller RL, Shin JW, Gray PA, Loewy AD. FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Research. 2011;1375:19–27. doi: 10.1016/j.brainres.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Kim M, Mahoney CE, Abbott SBG, Agostinelli LJ, Garfield AS, Scammell TE. Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. American Journal of Physiology Regulatory, Integrative, and Comparative Physiology. 2016;310:R41–54. doi: 10.1152/ajpregu.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendenning KK, Brusno-Bechtold JK, Thompson GC, Masterton RB. Ascending auditory afferents to the nuclei of the lateral leminscus. The Journal of Comparative Neurology. 1981;197:673–703. doi: 10.1002/cne.901970409. [DOI] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. Journal of Applied Physiology. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Fritschy JM, Ottersen OP, Torp R, Herbert H. GABA, GABA transporters, GABA(A) receptor subunits, and GAD mRNAs in the rat parabrachial and Kölliker-Fuse nuclei. The Journal of Comparative Neurology. 1998;400:229–243. [PubMed] [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. The Journal of Comparative Neurology. 2002;442:266–276. doi: 10.1002/cne.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. The Journal of Comparative Neurology. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hoshino M. Molecular machinery governing GABAergic neuron specification in the cerebellum. Cerebellum. 2006;5:193–198. doi: 10.1080/14734220600589202. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. Journal of Neuroscience. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M. Projections of the nuclei of the lateral lemniscus in the cat: An autoradiographic study. Brain Research. 1980;221:57–69. doi: 10.1016/0006-8993(81)91063-5. [DOI] [PubMed] [Google Scholar]
- Kudo M. Projections of the nuclei of the lateral lemniscus in the cat: An autoradiographic study. Brain Research. 1981;221:57–69. doi: 10.1016/0006-8993(81)91063-5. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Wohnoutka PE, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. The Journal of Physiology. 2015;593:4453–4469. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Willet SG, Bankaitis ED, Xu Y, Wright CVE, Gu G. Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis. 2013;51:436–442. doi: 10.1002/dvg.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PM. The barrelettes—Architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. I. Normal structural organization. The Journal of Comparative Neurology. 1991;309:161–199. doi: 10.1002/cne.903090202. [DOI] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD. Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience. 2012;218:110–125. doi: 10.1016/j.neuroscience.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Deschênes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature. 2013;497:205–210. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF. Central neural control of thermoregulation and brown adipose tissue. Autonomic Neuroscience. 2016;196:14–24. doi: 10.1016/j.autneu.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu JG, Yokota S, Tsumori T, Qin Y, Yasui Y. Glutamatergic lateral parabrachial neurons innervate orexin-containing hypothalamic neurons in the rat. Brain Research. 2010;1358:110–122. doi: 10.1016/j.brainres.2010.08.056. [DOI] [PubMed] [Google Scholar]
- Pang YW, Li JL, Nakamura K, Wu S, Kaneko T, Mizuno N. Expression of vesicular glutamate transporter 1 immunoreactivity in peripheral and central endings of trigeminal mesencephalic nucleus neurons in the rat. The Journal of Comparative Neurology. 2006;498:129–141. doi: 10.1002/cne.21047. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Martin GF. Brainstem projections to the facial nucleus of the opossum. A study using axonal transport techniques. Brain Research. 1983;267:19–33. doi: 10.1016/0006-8993(83)91036-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Cambridge, MA: Academic Press; 2008. [Google Scholar]
- Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proceedings of the National Acadademy of Science of the United States of America. 2009;106:22462–22467. doi: 10.1073/pnas.0911579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Commentary on: Efferent connections of the parabrachial nucleus in the rat. C.B. Saper and A.D. Loewy, Brain Research. 2016;197:291–317. doi: 10.1016/0006-8993(80)91117-8. 1980. Brain Research, 1645, 15–17. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nature Immunology. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behavioral Neuroscience. 1992;106:147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. The Journal of Comparative Neurology. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Takatoh J, Nelson A, Zhou X, Bolton MM, Ehlers MD, Arenkiel BR, Wang F. New modules are added to vibrissal premotor circuitry with the emergence of exploratory whisking. Neuron. 2013;77:346–360. doi: 10.1016/j.neuron.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Uemura M, Matsuda K, Matsushima R, Mizuno N. Parabrachial nucleus neurons projecting to the lower brain stem and the spinal cord. A study in the cat by the Fink-Heimer and the horseradish peroxidase methods. Experimental Neurology. 1980;70:403–413. doi: 10.1016/0014-4886(80)90037-0. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. The Journal of Comparative Neurology. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Phrenic motoneurons receive monosynaptic inputs from the Kölliker-Fuse nucleus: A light- and electron-microscopic study in the rat. Brain Research. 2001;888:330–335. doi: 10.1016/s0006-8993(00)03106-1. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Glutamatergic pathways from the Kölliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Research. 2004;995:118–130. doi: 10.1016/j.brainres.2003.09.067. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kölliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: A combined retrograde tracing and in situ hybridization study in the rat. Neuroscience Research. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yokota S, Niu JG, Tsumori T, Oka T, Yasui Y. Glutamatergic Kölliker-Fuse nucleus neurons innervate hypoglossal motoneurons whose axons form the medial (protruder) branch of the hypoglossal nerve in the rat. Brain Research. 2011;1404:10–20. doi: 10.1016/j.brainres.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Yokota S, Kaur S, VanderHorst VG, Saper CB, Chamberlin NL. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. The Journal of Comparative Neurology. 2015;523:907–920. doi: 10.1002/cne.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Chen K, Moritani M, Yabuta NH, Nagase Y, Takemura M, Shigenaga Y. Organization of the descending projections from the parabrachial nucleus to the trigeminal sensory nuclear complex and spinal dorsal horn in the rat. The Journal of Comparative Neurology. 1997;383:94–111. [PubMed] [Google Scholar]
- Zou HL, Su CJ, Shi M, Zhao GY, Li ZY, Guo C, Ding YQ. Expression of the LIM-homeodomain gene Lmx1a in the postnatal mouse central nervous system. Brain Research Bulletin. 2009;78:306–312. doi: 10.1016/j.brainresbull.2008.12.001. [DOI] [PubMed] [Google Scholar]


