Abstract
Purpose
Host response to polytrauma occasionally has unpredictable outcomes. Immune response is a major factor influencing patient's outcome. This study evaluated the interaction of two main cytokines in immune response after major trauma, specifically interleukin-6 (IL-6) and interleukin-10 (IL-10). Plasma level of these cytokines is determined by mRNA expression of these cytokines genes which may decide the outcome of polytrauma patients.
Methods
This prospective multicenter trial held at four trauma centers enrolled 54 polytrauma patients [Injury Severity Score (ISS) ≥ 16]. Plasma levels and mRNA expression of IL-6 and IL-10 were measured for 5 days after trauma. Clinical evaluation was conducted to observe whether patients endured multiple organ dysfunction syndrome (MODS) and death. MODS evaluation was performed using sequential organ failure assessment (SOFA). Trauma load which in this study is represented with ISS, plasma level, expression of cytokine genes and patient's outcome were examined with correlation test and statistical analysis.
Results
The elevated IL-6/IL-10 ratio indicated increased activity of systemic inflammation response, especially pro-inflammation response which bears higher probability of progressing to MODS and death. The decline of IL-6/IL-10 ratio with heavy trauma load (ISS > 30) showed that compensatory anti-inflammation response syndrome (CARS) state was more dominant than systemic inflammatory response syndrome (SIRS), indicating that malfunction and failure of immune system eventually lead to MODS and deaths. The statistical significance in plasma level of cytokines was found in the outcome group which was defined as bearing a low trauma load but mortality.
Conclusion
The pattern of cytokine levels in inflammation response has great impact on the outcome of polytrauma patients. Further study at the genetic level is needed to investigate inflammation process which may influence patient's outcome.
Keywords: Polytrauma, Interleukine-6, Interleukine-10, mRNA expression, Multiple organ dysfunction syndrome
Introduction
Host response to polytrauma and major trauma as a complicated process sometimes leads to unpredictable outcomes despite proper and adequate treatment the traumatic patients receive. Immune response is a major factor that influences patient's outcome. Mediators and cellular elements work in harmony to restore homeostasis for host survival and among these molecules, the main regulator is cytokines.1
Interleukin-6 (IL-6), released as a response to tissue injury, has local and systemic effect to create physiological response as needed. IL-6 concentration will increase if trauma and inflammation occur. This proinflammatory process is followed by anti-inflammatory response and one of the potent anti-inflammation cytokines is interleukin-10 (IL-10).2
This study evaluated the interaction of two main cytokines in immune response after major trauma, specifically interleukin-6 and interleukin-10 which comes from the mRNA expression of these cytokine genes and outcome of polytrauma patients.
Materials and methods
This is a case control study, a multicenter trial held at 4 level-1 academic hospitals, enrolling 54 patients from emergency department. At the age of 16–60 years old, the patients suffered polytrauma with the Injury Severity Score (ISS) ≥ 16, and endured systemic inflammatory response syndrome (SIRS). ISS was determined using the highest Abbreviated Injury Scale applied to three body regions according to the scale's latest manual.3 Exclusion criteria were enduring severe head injury with Glasgow coma scale (GCS) < 9, and suffering from chronic illness before trauma such as diabetes mellitus, chronic heart, kidney, or lung problems. Polytrauma is defined as injuries in at least two body regions with ISS ≥ 16 accompanied with SIRS for at least more than one day within the first 72 h after trauma.4, 5
Proinflammation cytokines (IL-6) and anti-inflammation cytokines (IL-10) level were measured with Enzyme-Linked Immunosorbent Assay to calculate the ratio between these two cytokines. Blood samples for IL-6 measurement were drawn into pre-cooled glass tubes containing Ethylene diamine tetra acetic acid (EDTA). The tubes were spun immediately at 2200 g for 15 min at 4 °C. The plasma was stored at −80 °C until analyses were performed. For IL-6 we used Abcam® (ab46027-interleukin-6 human ELISA kit) designed for the quantitative measurement of IL-6 human serum, plasma, buffered solutions and supernatants. A monoclonal antibody specifically for IL-6 was coated onto the wells of the microtiter strips provided. Samples, including standards of known IL-6 concentrations, control specimens or unknowns were pipetted into these wells according to manufacture protocol. During the first incubation, the standards or samples and a biotinylated monoclonal antibody specifically for IL-6 were simultaneously incubated. After washing, the enzyme Streptavidin-Horseradish peroxidase (HRP) that binds the biotinylated antibody was added, incubated and washed. A 3,3′,5,5 tetramethylbenzidine (TMB) substrate solution which acts on the boundenzyme was added to induce a colored reaction product. The intensity of this colored product is directly proportional to the concentration of IL-6 present in the samples. For IL-10, we used Abcam® (ab46034–interleukin-10 human ELISA kit) and performed the same procedures. A monoclonal antibody specific for IL-10 has been coated onto the wells of the microtiter strips provided. Samples, including standards of known IL-10 concentrations, control specimens or unknowns are pipetted into these wells. During the first incubation, the standards or samples and a biotinylated monoclonal antibody specific for IL-10 are simultaneously incubated. After washing, the enzyme Streptavidin-HRP, that binds the biotinylated antibody is added, incubated and washed. A TMB substrate solution is added which acts on the bound enzyme to induce a colored reaction product. The intensity of this colored product is directly proportional to the concentration of IL-10 present in the samples.
The mRNA expression level of patients genes was also examined by real time quantitative polymerase chain reaction (RT-PCR). As the most sensitive and accurate way, quantitative RT-PCR becomes the method of choice to quantify the mRNA expression of cytokines which are often at very low levels.6 All PCR reagents were obtained from Kapa Sybr® Fast One Step qRT PCR. IL-6 gene primer sequence was For: GAGGATACCACTCCCAACAGACC and Rev: AAGTGCATCATCGTTGTTCATACA with a PCR product being 141bp. For IL-10, a 138 base pairs length was amplified using the primer IL-10 For: GTGATGCCCCAAGCTGAGA and Rev: CACGGCCTTGCTCTTGTTTT. Housekeeping gene of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) For: TCACCACCATGGAGAAGGC and Rev: GCTAAGCAGTTGGTGGTGCA with PCR product being 168bp (Macrogen, Korea).
We quantified gene expression using a multiplex comparative critical threshold (CT) method (CFX Connect, Biorad, USA).
During hospitalization, polytrauma patient management was given according to Advanced Trauma Life Support and Definitive Surgery Trauma Care. Further evaluation was conducted to ascertain whether the patients develop multiple organ dysfunction syndrome (MODS) and multiple organ failure (MOF) using sequential organ failure assessment (SOFA) score. Mortality rate was also noted.7 SOFA identifies organ dysfunction or failure across six organ systems. It consists of measures of PaO2/FIO2 ratio (respiration system), platelet count (hematology system), GCS (central nervous system), mean arterial pressure and inotropic (cardiovascular system), urine output and creatinine level (renal system), and total bilirubin (liver-digestive system). The occurrence of MODS is defined as a SOFA score of 1 or greater in more than one system, and MOF as a SOFA score of 3 or more in at least 2 organ systems (see appendix 1).7
Afterwards, we analyzed the correlation between IL-6/IL-10 ratio, mRNA expression level of both cytokine genes, the occurrence of MODS and mortality. Outcomes of this study were comprised of 4 groups: (1) high ISS and not survived; (2) high ISS but survived; (3) low ISS but not survived and (4) low ISS and survived.
Statistical analysis was done through box plot graphic, one way Anova test and least significance difference methods to analyze the differences among outcome group. Data were analyzed with statistical package for the social science (SPSS) version 17 (SPSS inc, Chicago, IL) and the significance level was set at p < 0.05.
To minimize bias, observation process was done by surgeon on duty (member of authors) at each hospital.
Results
We enrolled 54 participants, 42 males (78%) and 12 females (22%). Severity of trauma was measured with ISS, ranging from 16 to 50 (average 24.1). There were 41 (76%) polytrauma patients suffering from less severe trauma (ISS: 17–30) and 13 (24%) from severe trauma (ISS ≥ 31). MODS developed in 23 participants (42.6%) while 31 participants (57.4%) experienced no MODS. In-hospital deaths were found in 8 cases (14.8%).
Correlation between IL-6/IL-10 ratio, ISS, and MODS
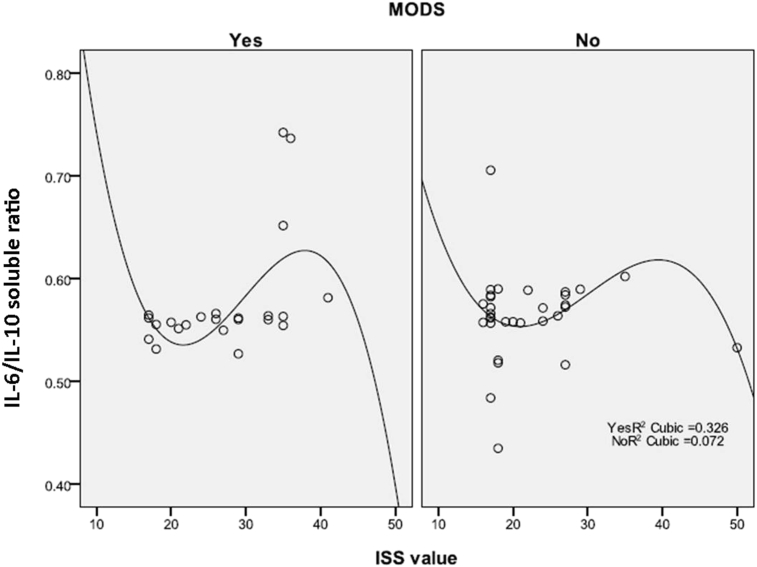
IL-6/IL-10 ratio decreased when trauma load became more severe (ISS > 40). This finding was observed in trauma patients with MODS or without MODS (Fig. 1).
Fig. 1.
Correlation between ISS and IL-6/IL-10 ratio among patients with and without MODS. ISS had a correlation with IL-6/IL10 ratio in cuboid curve shape, with R2 as 0.326 for MODS group and0.072 for non MODS group. Both curve had similar shape.
Correlation between IL-6/IL-10 ratio, ISS, and mortality
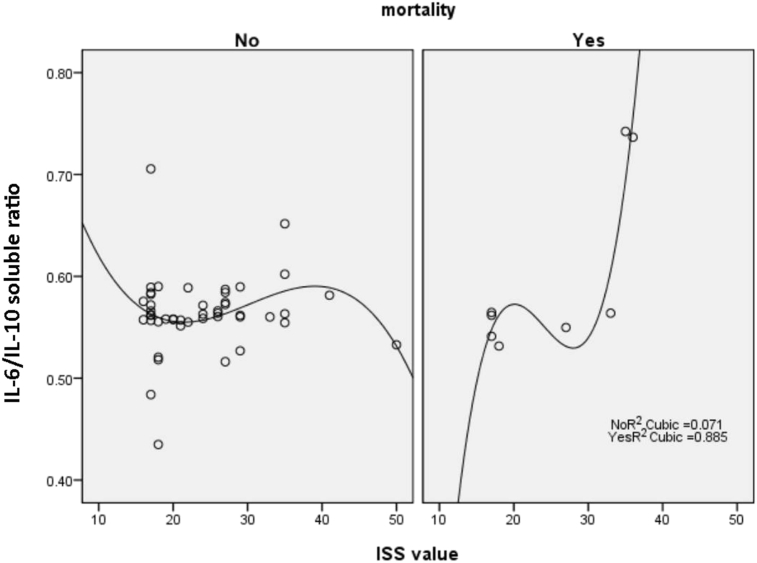
In non-survivor group, if ISS > 30, the IL-6/IL-10 ratio would increase while in survivor groups, the IL-6/IL-10 ratio would decline (Fig. 2).
Fig. 2.
Correlation between ISS and IL /IL-10 ratio among patients who survived and not survived. ISS had a correlation with IL-6/IL10 ratio in cuboid curve shape, with R2 as 0.371 or survivor group and 0.885 in non survivor group.
The mRNA expression of IL-6 and IL-10 gene
In the 4 outcome groups, the average of mRNA expression level of IL-6 was proportionate to its plasma level; low mRNA expression level of IL-6 gene was followed with low plasma level of IL-6, and vice versa. The mRNA expression level and plasma level of IL-6 were highest in outcome group 4 (low ISS and survived), followed by outcome group 2 (high ISS but survived) and they were lowest in outcome group 1 (high ISS and not survived) (Table 1).
Table 1.
mRNA expression level of IL-6 and its plasma level in 4 outcome groups of polytrauma patients.
| Outcome groups | mRNA IL-6 gene |
IL-6 plasma level |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| High ISS survival (n = 3) | 7.110 (0.044) | 15.487 (1.037) |
| High ISS not survival (n = 9) | 13.724 (155) | 101.620 (50.380) |
| Low ISS survival (n = 6) | 11.145 (1.669) | 27.497 (15.609) |
| Low ISS not survival (n = 3) | 16.241 (0.404) | 177.351 (1.127) |
The same result was observed in the mRNA expression and plasma level of IL-10. The mRNA expression level of IL-10 gene was proportionate to IL-10 plasma level; low mRNA expression level of IL-10 gene was followed with low plasma level of IL-10, and vice versa. IL-10 gene mRNA expression level and IL-10 plasma level were highest in outcome group 4 (low ISS and survived), followed by outcome group 2 (ISS high but survived), and they were lowest in outcome group 1 (high ISS and not survived) (Table 2).
Table 2.
mRNA expression level of IL-10 and its plasma level in 4 outcome groups of polytrauma patients.
| Outcome groups | mRNA of IL-10 gene |
IL-10 plasma level |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| High ISS survival (n = 3) | 10.126 (0.083) | 21.0255 (1.125) |
| High ISS not survival (n = 9) | 17.616 (1.506) | 170.849 (76.360) |
| Low ISS survival (n = 6) | 13.479 (1.936) | 49.334 (26.750) |
| Low ISS not survival (n = 3) | 19.661 (0.415) | 340.660 (27.761) |
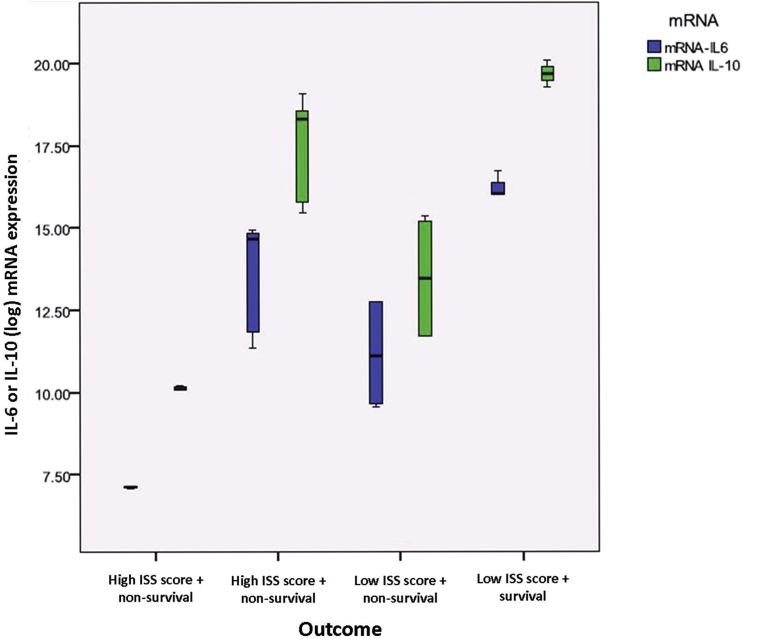
The mRNA expression level of IL-6 and IL-10 gene was highest in outcome group 4 (low ISS and survived) while lowest in outcome group 2 (high ISS and not survived). The mRNA expression level of IL-10 was higher than IL-6 in every outcome groups (Fig. 3). Based on statistic analysis, the significance was found in outcome group 3 (low ISS and not survived).
Fig. 3.
Box plot graphic of IL-6 and IL-10 mRNA expression level and their outcome in polytrauma patients. The mRNA expression level of IL-6 and IL-10 gene were statistically significant in outcome group-3 which had low severity trauma but did not survived.
Discussion
Trauma severity and outcome
Multi-trauma, multiple trauma, polytrauma or major trauma has been used worldwide while without a consensus on its definition and perception. According to Butcher et al4, we used polytrauma which is defined as injuries occurring in several body regions with significant severity (quantified as ISS > 16) and followed by SIRS. Multiple or multi-trauma is more popular among American traumatologist while polytrauma is more common in Europe. As we focused more on a suitable definition for our study, we used polytrauma here. Therefore our inclusion criteria are polytrauma patients who have multiple injuries and SIRS with ISS > 16. These polytrauma patients are more likely to have poor outcomes such as MODS and even death compared with minor trauma patients. Anatomical injury profile has predictive value in forecasting the possibility of MODS.7 A univariate analysis from other studies showed that more severe injury (higher ISS) which tends to cause MODS was defined as ISS ≥ 24.8 Our study identified that severe injury with tendency to endure MODS is trauma case with ISS > 40 and may not survived if their ISS > 30. Brumann et al9 assessed 20 multi-trauma patients using NISS as anatomical injury profile and identified patients with new injury severity score (NISS) > 41 have poorer prognosis, thus defining severe injury as NISS > 41. According to that study, mRNA expression level of IL-6 in polymorphonuclear cell was higher in less severe, moderate or minor trauma patients (NISS<40).9 Trauma load more than this limit would potentially lead to host immune system failure as presented by correlation of cytokine concentration level or ratio and patients outcome. Immune system failure may progress to sepsis or even death if infection or contamination occurs.10, 11
Pattern of cytokines plasma level ratio and patients outcome
From our previous study, the pattern of IL-6 and IL-10 plasma level after polytrauma showed that immune response threshold in severe systemic inflammation is IL-6 50 pg/mL.12 As IL-6 is main proinflammatory cytokines and IL-10 the anti-inflammatory ones, IL-6/IL-10 plasma level ratio correlates to injury severity (ISS score) and mortality induced by polytrauma. Severe trauma patients who survived with ISS > 30 had higher IL-6/IL-10 ratio compared with the same severe trauma patients who did not survive.12
In proportionate correlation between IL-6 and IL-10 plasma level and its ratio is partially explained that IL-6 as a proinflammatory marker is challenged by IL-10 as anti-inflammation markers. Immune response greater than this threshold (IL-6 > 50 pg/mL) lead to immune system failure with the defeat of anti-inflammation response according to Bone theory (SIRS > CARS) and its end of spectrum as multi-organ failure and death.13, 14
Correlation between plasma level and gene mRNA expression level of IL-6 and IL-10
These cytokine plasma levels were proportionate to their mRNA expression level. High level of mRNA expression cytokine gene (IL-6, IL-10) was followed by elevation of both cytokine plasma concentrations. However, there are two matters that need to be noticed from the mRNA expression level of inflammatory cytokine gene in different outcome groups of polytrauma patients. First, mRNA expression level in survivor group was higher than that in non-survivor group. Second, mRNA expression level in less severe trauma patients was higher compared with more severe trauma patients. These two differences can be explained by that higher cytokine genes mRNA expression indicates an adaptive immune reaction which can compensate severe inflammation, successful return to homeostasis state and patient may live. Higher mRNA expression level of IL-10 as anti-inflammation marker in less severe trauma reflects the success of compensation mechanism of the host who obtain homeostasis after trauma.15 This finding is consistent with Bone theory which implied that there exists some kind of competition between SIRS (initial) and compensatory anti-inflammation response syndrome (CARS) (as a compensation). However, SIRS and CARS competition sometimes develops horrendously and causes fatal MODS-MOF.16, 17
Limitation of this study is its smaller sample size due to the shortage of fund.
In conclusion, the IL-6 and IL-10 mRNA expression level and their plasma concentration in survivor group were higher than non-survivor group. The mRNA expression level was higher in less severe trauma patients compared with more severe trauma patients. Higher cytokine genes mRNA expression indicates that adaptive immune reaction can compensate severe inflammation and therefore cytokine pattern in host inflammation response has great impact on the outcome of polytrauma patients.
Further study is needed to focus on genes that regulate main inflammation response cytokine and potentially determine the overall end result.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Mahamid A., Jabarin B., Almogy G. Systemic inflammatory response during laparotomy. Int J Inflamm. 2014;2014:674303. doi: 10.1155/2014/674303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawa R.S., Anillo S., Huntoon K. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26:73–87. doi: 10.1177/0885066610384188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gennarelli T.A., Wodzin E. Barrington; IL, USA: 2008. Abbreviated Injury Scale Update 2008. Association for the Advancement of Automotive Medicine (AAAM)http://www.worldcat.org/title/abbreviated-injury-scale-2005-update-2008/oclc/297171872 [Google Scholar]
- 4.Butcher N., Balogh Z.J. The definition of polytrauma: the need for international consensus. Injury. 2009;40(Suppl 4):S12–S22. doi: 10.1016/j.injury.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Butcher N.E., Enninghorst N., Sisak K. The definition of polytauma: variable interrater versus intrarater agreement–a prospective international study among trauma surgeons. J Trauma Acute Care Surg. 2013;74:884–889. doi: 10.1097/TA.0b013e31827e1bad. [DOI] [PubMed] [Google Scholar]
- 6.Giulietti A., Overbergh L., Valckx D. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. http://www.idealibrary.com Available from: [DOI] [PubMed] [Google Scholar]
- 7.Antonelli M., Moreno R., Vincent J.L. Application of SOFA score to trauma patients. Sequential organ failure assessment. Intensive Care Med. 1999;25:389–394. doi: 10.1007/s001340050863. [DOI] [PubMed] [Google Scholar]
- 8.Rendy L., Sapan H.B., Kalesaran L.T.B. MODS prediction score after multitrauma. J Biomedik. 2016;8:88–95. http://ejournal.unsrat.ac.id/index.php/biomedik/article/view/12669 Available from: [Google Scholar]
- 9.Brumann M., Matz M., Kusmenkov T. Impact of STAT/SOCS mRNA expression levels after major injury. Mediat Inflamm. 2014;2014:749175. doi: 10.1155/2014/749175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoecklein V.M., Osuka A., Lederer J.A. Trauma equals danger-damage control by the immune system. J Leuckoc Biol. 2012;92:539–551. doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huebinger R.M., Rivera-Chavez F., Chang L.Y. IL-10 polymorphism associated with decreased risk for mortality after burn injury. J Surg Res. 2010;164:e141–145. doi: 10.1016/j.jss.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapan H.B., Paturusi I., Jusuf I. Pattern of cytokine (IL-6 and IL-10) level as inflammation and anti-inflammation mediator of multiple organ dysfunction syndrome (MODS) in polytrauma. Int J Burn Trauma. 2016;6:37–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Hietbrink F., Koenderman L., Rijkers G. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15. doi: 10.1186/1749-7922-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brøchner A.C., Toft P. Pathophysiology of the systemic inflammatory response after major accidental trauma. Scand J Trauma Resusc Emerg Med. 2009;17:43. doi: 10.1186/1757-7241-17-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Köller M., Clasbrummel B., Kollig E. Major injury induces increased production of interleukin-10 in human granulocyte fractions. Langenbecks Arch Surg. 1998;383:460–465. doi: 10.1007/s004230050161. [DOI] [PubMed] [Google Scholar]
- 16.Bone R.C. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996;24:163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Bone R.C. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]