Abstract
Background
Jiangsu Province, China, is highly developed economically and culturally, and has a high prevalence of lung cancer. We aimed to evaluate the diagnostic procedures, genetic aberration analysis status, and first‐line treatment models of lung cancer in Jiangsu Province.
Methods
Lung cancer patients diagnosed in 2016 at 22 tertiary care hospitals were evaluated. Demographic characteristics, tumor histology, staging, family history of lung cancer, auxiliary examinations, genetic testing, and first‐line treatment were collected on discharge. Diagnostic and treatment data were analyzed by descriptive statistics.
Results
A total of 928 patients were enrolled. Chest computed tomography was the most frequently used diagnostic method; pathology diagnosis was carried out by transbronchial lung biopsy and transthoracic needle aspiration. Stage T1‐2N0M0 small‐cell lung cancer patients experienced surgical resection, and others received cisplatin and etoposide chemotherapy. Stage I and stage II non‐small cell lung cancer patients experienced surgical resection; stage III and stage IV patients received cisplatin and pemetrexed chemotherapy as first‐line treatment. Detection of epidermal growth factor receptor (EGFR) mutations occurred in 29.9% of non‐selective, 36.5% of locally advanced or metastatic, and 42.1% of advanced non‐squamous non‐small cell lung cancer. The overall EGFR‐positive rates were 49.0%, 52.5%, and 53.9%. A total 72.0% of patients with EGFR mutations were treated with tyrosine kinase inhibitors.
Conclusion
Chest computed tomography was the most commonly performed diagnostic method for lung cancer. First‐line treatment was primarily determined by disease stages and EGFR mutation status, with few expectations.
Keywords: Antineoplastic protocol, cross‐sectional study, cytogenetic aberration, lung cancer, mainland China
Introduction
Lung cancer is the most prevalent cancer and the leading cause of cancer‐related death in China, including Jiangsu Province.1 With the rapid development of precision medicine, molecular targeted therapy and immunotherapy are exhibiting their superiority in the treatment of lung cancer. However, a lack of publicity of new technologies, standard diagnosis, and treatment procedures have hindered the development of precision individualized treatment of lung cancer.
A national survey of doctors in 12 major cities in China for the medical treatment status of non‐small cell lung cancer (NSCLC) in 2012 revealed 53.2% patients received first‐line therapy, and gemcitabine plus platinum was primarily used (27.4%); epidermal growth factor receptor (EGFR) gene mutation was analyzed in just 9.6% of patients, whereas the positive rate was 46.8%; and targeted therapy was used more commonly as salvage rather than upfront therapy.2 Another retrospective study showed the treatment disparity to the national treatment guidelines was 45.3% in China in 2014.3 However, recent a multicenter survey of stage IIIB/IV NSCLC in China showed that chemotherapy was still the most common first‐line regimen (72.5%), but pemetrexed plus platinum was predominant (70.2%); the detection rate of EGFR gene mutation was 71.4% and the positive rate was 46.5%. Furthermore, 66.3% of patients with positive EGFR gene mutation were treated with EGFR‐tyrosine kinase inhibitors.4 This shows the treatment model in China has changed in recent years. However, all these national studies were conducted in major cities with advanced medical technology, which cannot reflect the real situation in some small cities and rural areas.
Jiangsu is a province with great imbalance in medical facilities and clinical practice. In order to obtain the whole picture of diagnosis and treatment of lung cancer in Jiangsu Province, we conducted a multicenter survey to investigate the diagnostic procedures, gene aberration test status, and first‐line treatment models of lung cancer in Jiangsu Province.
Methods
Study design
This retrospective, cross‐sectional, observational study included newly diagnosed, treatment‐naïve lung cancer patients in Jiangsu Province. Patient demographics, and clinical characteristics, tumor histology, disease stage, family history of lung cancer, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), auxiliary examinations, gene aberration testing, and first‐line treatment were retrieved from medical records one to two days after hospital discharge. The protocol was approved by the Research Ethics Committee of the Jinling Hospital, Nanjing, Jiangsu Province, China.
Study population and sample size calculation
Patients with an initial pathological lung cancer diagnosis between 1 March 2016 and 1 May 2016 were enrolled. Patients who were aged ≤18 years or ≥85 years, were outpatients, and had previously been treated for lung cancer were excluded.
The sample size calculation assumed an EGFR mutation rate of 25% for East Asian populations, an allowable error of 10%, and loss of 5–10% of enrolled patients because of missing data or other errors. The requisite cross‐sectional sample size was N = Z α/2 P (1‐P) / d2 = 1.962 × 0.25 × (1 –0.25) / (0.025)2 = 1152, and indicated the requirement of 1300 patients.
Data collection
Patient information was retrieved from the medical records at 22 tertiary care hospitals and recorded on schedules designed in advance. Two copies of each patient schedule were jointly completed by a study investigator and a physician, and were checked for accuracy and completion by a clinical inspector. One copy was used for data entry and management; the other was filed at the research unit. Following Good Clinical Practice guidelines, patient schedules and source records were maintained by the research unit until conclusion of the study.
Statistical analysis
Data were independently entered into a database and checked by an administrator. Discrepancies were corrected and updated by the clinical inspector, a study investigator, and the data administrator. Data analysis and database lock were jointly conducted by the data administrator, study investigators, sponsors, and statistician. Mean ± standard deviation, minimum, and maximum values were reported for continuous variables, and proportional values were reported for categorical variables. Descriptive statistics were performed using SPSS version 20.0 (IBM, Armonk, NY, USA).
Results
Patient demographics and clinical characteristics
A total of 928 patients from 22 comprehensive and specialist hospitals in Jiangsu Province were enrolled. Table 1 shows the patient demographic and clinical characteristics. The mean age at diagnosis was 64.05 ± 10.29 years (range 21–96 years); half the patients were aged 58–71 years. Of these, 71.9% were men, 41.3% were never‐smokers, and 1.5% had family history of lung cancer. Adenocarcinoma was diagnosed in 48.5% of the patients, squamous carcinoma in 22.0%, and small cell lung cancer (SCLC) in 13.9%. The proportion of male patients with SCLC was higher than that in NSCLC (P = 0.04; data was not given), which may be related to smoking. In patients with known TNM stages, 8.2% were stage I, 6.6% stage II, 12.9% stage IIIA, and 72.3% stage IIIB/IV. Symptoms included fever in 11.9% of patients, irritating cough in 56.8%, blood in sputum in 29.8%, shortness of breath in 28.0%, and chest pain in 25.3%. A total of 66.7% had an ECOG PS ≤1, only 1.9% of them were ECOG PS ≥3, and 21.3% had no record of ECOG PS (Table 1).
Table 1.
Demographics and clinical characteristics of patients
| Characteristics | All patients (n = 928) n (%) |
|---|---|
| Age (years) | |
| Mean (min., max.) | 64.05 ± 10.29 (21, 96) |
| <58 | 213 (23.0) |
| ≥58, <71 | 466 (50.2) |
| ≥71 | 249 (26.8) |
| Gender | |
| Male | 667 (71.9) |
| Female | 261 (28.1) |
| Smoking status | |
| Current Smoker | 157 (16.9) |
| Former Smoker | 194 (20.9) |
| Never Smoker | 383 (41.3) |
| Unknown | 194 (20.9) |
| Family history of lung cancer | |
| Yes | 14 (1.5) |
| No | 824 (88.8) |
| Unknown | 90 (9.7) |
| Histological subtypes | |
| Adenocarcinoma | 450 (48.5) |
| Squamous carcinoma | 204 (22.0) |
| Small cell lung cancer | 129 (13.9) |
| Unknown | 114 (12.3) |
| Other | 31 (3.3) |
| Stage | |
| Stage I | 67 (7.2) |
| Stage II | 54 (5.8) |
| Stage IIIA | 103 (11.1) |
| Stage IIIB/IV | 587 (63.3) |
| Unknown | 117 (12.6) |
| ECOG PS | |
| 0 | 401 (43.2) |
| 1 | 218 (23.5) |
| 2 | 93 (10.0) |
| 3 | 18 (1.9) |
| Unknown | 198 (21.3) |
| Symptoms | |
| Irritable dry cough | 527 (56.8) |
| Blood in sputum | 277 (29.8) |
| Chest pain | 235 (25.3) |
| Fever | 110 (11.9) |
| Shortness of breath | 260 (28.0) |
ECOG, Eastern Cooperative Oncology Group; max., maximum; min., minimum; PS, performance status.
Diagnostic procedure for lung cancer
Chest computed tomography (CT), the preferred method for primary diagnosis, was used with 94.7% of patients at high risk of lung cancer. Other diagnostic methods included chest X‐ray in 21.3% of patients, positron emission tomography CT in 14.5%, ultrasound in 25.4%, and radioisotope scanning in 31.8%. Pathological evaluation included bronchoscopic inspection for central lesions, including transbronchial lung biopsy in 24.0%, transbronchial needle aspiration in 2.8%, and endobronchial ultrasonography transbronchial needle aspiration in 4.8% of patients. Transthoracic needle aspiration was used in 24.6% of patients and was preferred for peripheral lesions because it can be performed quickly, is less invasive, and has few complications. Thoracentesis was performed in 13.3% of patients, and superficial lymph node biopsy (3.4%), pleural biopsy (1.1%), and endoscopic procedures, such as mediastinoscopy (0.2%) and thoracoscopy (1.5%), were rarely used (Fig 1).
Figure 1.
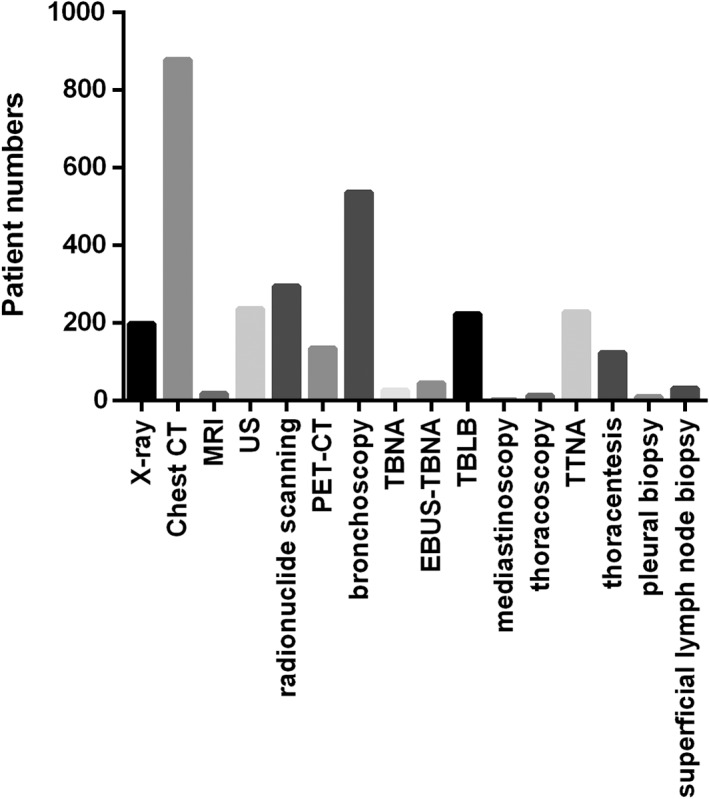
Diagnostic procedure of lung cancer in Jiangsu Province, China. CT, computed tomography; EBUS‐TBNA, endobronchial ultrasonography TBNA; MRI, magnetic resonance imaging; PET‐CT, positron emission tomography‐CT; TBLB, transbronchial lung biopsy; TBNA, transbronchial needle aspiration; TTNA, transthoracic needle aspiration; US, ultrasound.
SCLC
Molecular profiles were rarely reported for SCLC patients, because genetic aberrations are infrequently found in this patient subgroup. Of the 129 SCLC patients, 18 had detection of EGFR mutations and seven of ALK fusion. A total of 10 patients had a positive EGFR mutation, and none had ALK fusion (Table 2). Of the eight patients who took EGFR tyrosine kinase inhibitors (TKIs), seven were EGFR mutation‐positive, one was negative; six took gefitinib, and two took erlotinib.
Table 2.
Genetic aberration analysis
| Total (n = 928) | NSCLC (n = 683) | SCLC (n = 129) | ||||
|---|---|---|---|---|---|---|
| Detection rate (%) | Positive rate (%) | Detection rate (%) | Positive rate (%) | Detection rate (%) | Positive rate (%) | |
| EGFR | 242 (26.1) | 121 (50.0) | 204 (29.9) | 100 (49.0) | 18 (14.0) | 10 (55.6) |
| ALK | 111 (12.0) | 4 (3.6) | 95 (13.9) | 3 (3.2) | 7 (5.4) | 0 |
| c‐MET | 11 (1.2) | 3 (27.3) | 10 (1.5) | 3 (30.0) | 0 | 0 |
| ROS1 | 26 (2.8) | 5 (19.2) | 25 (3.7) | 5 (20.0) | 0 | 0 |
| RET | 3 (0.3) | 0 | 3 (0.4) | 0 | 0 | 0 |
ALK, anaplastic lymphoma kinase; c‐MET, MET proto‐oncogene; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; RET, RET proto‐oncogene; ROS1, c‐ros oncogene 1; SCLC, small cell lung cancer.
Of the 88 patients with known TNM stages, six were T1‐2N0M0, but only one of them had a standard lobectomy plus adjuvant chemotherapy. Two had wedge resection or segment resection. The remaining three did not have surgery, but one had radiotherapy, one was transferred, and one was not treated. Of the 15 patients with limited stage in excess of T1‐2N0M0 (PS 0‐2), 10 had first‐line chemotherapy, and two had first‐line radiotherapy, but with no information of subsequent adjuvant therapy. Two others underwent molecular‐targeted therapy or combined surgery and chemoradiotherapy. Of the 108 patients with extensive stages, 63.0% (68/108) were treated with first‐line chemotherapy, four of whom had concurrent radiotherapy; 21.3% (23/108) refused treatment, possibly because of the cost (Table 3). First‐line chemotherapy was a platinum plus etoposide regimen in 66.7% of patients, and 73.1% of those regimens were cisplatin and etoposide (Fig 2).
Table 3.
First‐line treatment of small cell lung cancer patients
| T1‐2N0M0 (n = 6) (%) | Limited stages in excess of T1‐2N0M0 (n = 15) (%) | Extensive stages (n = 108) (%) | |
|---|---|---|---|
| Surgery | 3 (50.0) | 1 (6.7) | 7 (6.5) |
| Chemotherapy | 0 | 10 (66.7) | 68 (63.0) |
| Radiotherapy | 1 (16.7) | 2 (13.3) | 1 (0.9) |
| Targeted therapy | 0 | 1 (6.7) | 3 (2.8) |
| Untreated/transferred | 2 (33.3) | 1 (6.7) | 28 (21.3) |
SCLC, small cell lung cancer.
Figure 2.
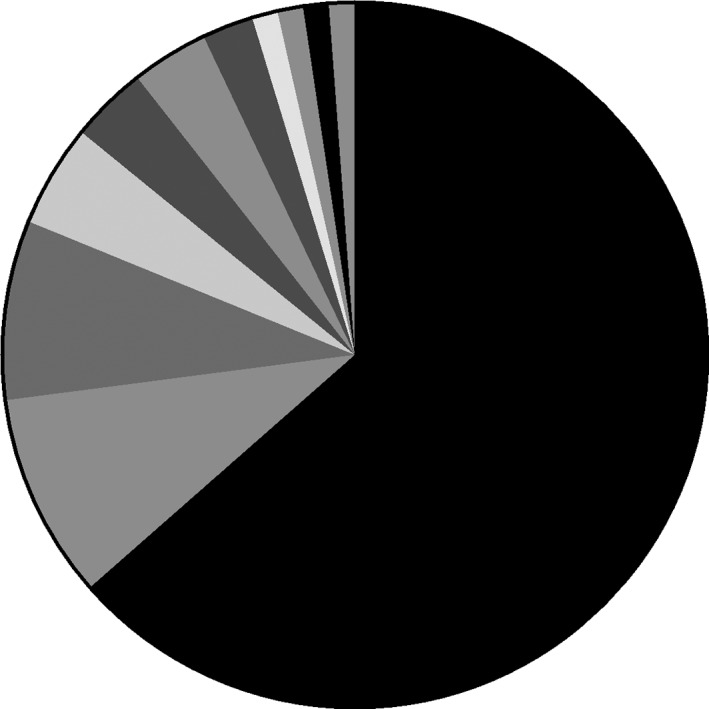
First‐line chemotherapy regimens of small cell lung cancer patients. SCLC, small cell lung cancer.  platinum + etoposide,
platinum + etoposide,  platinum + pemetrexed,
platinum + pemetrexed,  platinum + docetaxel,
platinum + docetaxel,  platinum + gemcitabine,
platinum + gemcitabine,  platinum + irinotecan,
platinum + irinotecan,  etoposide,
etoposide,  platinum + paclitaxel,
platinum + paclitaxel,  etoposide +oxaliplatin,
etoposide +oxaliplatin,  nedaplatin,
nedaplatin,  platinum + fluorouracil,
platinum + fluorouracil,  platinum + topotecan.
platinum + topotecan.
NSCLC
First‐line treatment based on stages
Surgery was the first‐line treatment of 72.5% (37/51) of stage I and 51.2% (22/43) of stage II NSCLC patients. In the stage I subgroup, 17.6% (9/51) of patients, and in the stage II subgroup, 20.9% (9/43) of patients were not treated at diagnosis, and were either transferred to other hospitals or went home due to monetary constraints. The remaining patients were treated with single chemotherapy, radiotherapy, or targeted therapy, and not surgery. In the stage III group, 44.0% (33/75) of patients with stage IIIA disease and 49.1% (28/57) with stage IIIB disease received first‐line chemotherapy. A total of 26.7% (20/75) of stage IIIA patients received first‐line surgery; 25.3% (19/75) of stage IIIA and 35.1% (20/57) of stage IIIB patients were not treated or transferred to other hospitals. Of the stage IV patients, 46.8% (181/387) were treated with first‐line chemotherapy, and 19.9% (77/387) received targeted therapy; 20.9% (81/387) refused treatment, and 5.9% (23/387) were transferred to other hospitals with more advanced medical resources (Table 4).
Table 4.
First‐line treatment of non‐small cell lung cancer patients based on stages
| Stage I (n = 51) (%) | Stage II (n = 43) (%) | Stage IIIA (n = 75) (%) | Stage IIIB (n = 57) (%) | Stage IV (n = 387) (%) | |
|---|---|---|---|---|---|
| Surgery | 37 (72.5) | 22 (51.2) | 20 (26.7) | 1 (1.8) | 10 (2.6) |
| Chemotherapy | 2 (3.9) | 10 (23.3) | 33 (44.0) | 28 (49.1) | 181 (46.8) |
| Radiotherapy | 1 (2.0) | 1 (2.3) | 1 (1.3) | 0 | 2 (0.5) |
| Targeted therapy | 1 (2.0) | 0 | 2 (2.7) | 7 (12.3) | 77 (19.9) |
| Others† | 1 (2.0) | 1 (2.3) | 0 | 1 (1.8) | 13 (3.4) |
| Untreated/transferred | 9 (17.6) | 9 (20.9) | 19 (25.3) | 20 (35.1) | 104 (26.9) |
Mainly referred to traditional Chinese medicine.
First‐line treatment based on EGFR mutation status
EGFR mutations were sequenced in 29.9% (204/683) of patients with non‐selective NSCLC, 36.5% (162/444) with locally advanced or metastatic NSCLC (stage IIIB/IV), and 42.1% (141/335) with advanced non‐squamous NSCLC. The positive mutation rates were 49.0%, 52.5%, and 53.9%. ALK fusion, c‐MET amplification, ROS1 fusion and RET rearrangement occurred in three out of 95, three out of 10, five out of 25, and zero out of three patients (Table 2). Coexisting EGFR mutation, c‐MET amplification, and ROS1 fusion were discovered in one patient, and double mutations including ALK and ROS1 fusion, EGFR mutation and c‐MET amplification, and EGFR mutation and ROS1 fusion were observed in three patients. These four patients were all aged >60 years and did not have underlying diseases, or a family history of lung cancer.
Of the 100 patients with EGFR mutations, 72.0% (72/100) were treated with first‐line EGFR‐TKIs, including gefitinib (70.8%), icotinib (15.3%), and erlotinib (8.3%). Four patients received TKIs, but the drug name was not specified (Table 5). For patients with negative or unknown EGFR mutation status, chemotherapy was the preferred first‐line treatment, given to 44.8% (261/583) of patients. Just 5.7% (33/583) of patients accepted treatment with EGFR‐TKIs.
Table 5.
First‐line treatment of non‐small cell lung cancer patients based on EGFR mutation status
| EGFR mutation | Total (%) | |||
|---|---|---|---|---|
| Negative (%) | Positive (%) | Null (%) | ||
| Targeted therapy | ||||
| Gefitinib | 4 | 51 | 9 | 64 |
| Icotinib | 1 | 11 | 13 | 25 |
| Erlotinib | 0 | 6 | 1 | 7 |
| TKIs† | 0 | 4 | 4 | 8 |
| Crizotinib | 1 | 0 | 0 | 1 |
| Total | 6 (5.8) | 72 (72.0) | 27 (5.6) | 105 (15.4) |
| Chemotherapy | 56 (53.8) | 12 (12.0) | 205 (42.8) | 273 (40.0) |
| Others | 14 (13.5) | 6 (6.0) | 92 (19.2) | 112 (16.4) |
| Untreated/transferred | 28 (26.9) | 10 (10.0) | 155 (32.4) | 193 (28.3) |
| Total | 104 | 100 | 479 | 683 |
TKIs means the drug name was not specified. Others included surgery, radiotherapy, and traditional Chinese medicine.
EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; TKIs, tyrosine kinase inhibitors.
In 93.8% of patients (255/273), first‐line chemotherapy was a platinum‐based doublet regimen; four received a triplet regimen, consisting of platinum‐based doublet regimens plus angiogenesis inhibitors. Pemetrexed constituted the main component of doublet regimens, used in 42.0% of patients (107/255). The most common platinum agent was cisplatin, used in 54.1% (138/255) regimens, carboplatin in 25.5% (65/255), nedaplatin in 18.8% (48/255), and lobaplatin in the remaining 1.6% (4/255) (Fig 3).
Figure 3.
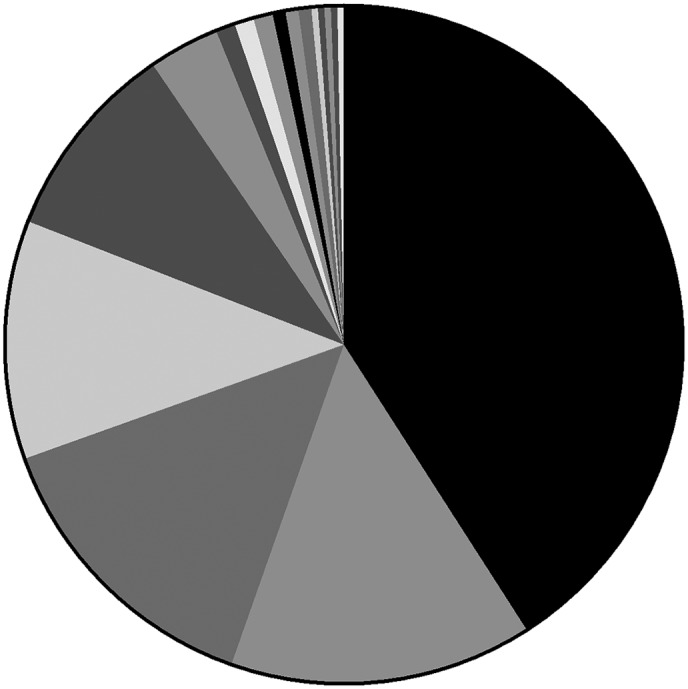
First‐line chemotherapy regimens of non‐small cell lung cancer patients. NSCLC, non‐small cell lung cancer; TGO, tegafur gimeracil oteracil potassium capsule. platinum + pemetrexed,  platinum + docetaxel,
platinum + docetaxel,  platinum + gemcitabine,
platinum + gemcitabine,  platinum + etoposide,
platinum + etoposide,  platinum + paclitaxel,
platinum + paclitaxel,  pemetrexed,
pemetrexed,  platinum + topotecan,
platinum + topotecan,  platinum + docetaxel + endostatin,
platinum + docetaxel + endostatin,  docetaxel,
docetaxel,  platinum + cyclophosphamide,
platinum + cyclophosphamide,  cisplatin,
cisplatin,  paclitaxel,
paclitaxel, 
 platinum + pemetrexed + bevacizumab,
platinum + pemetrexed + bevacizumab,  carboplatin,
carboplatin,  endostatin,
endostatin,  TGO, TGO + endostatin.
TGO, TGO + endostatin.
Characteristics of patients untreated/transferred
In total, 263 patients (28.3%) were untreated/transferred at diagnosis, with 193 being NSCLC, 31 SCLC, and 39 unknown; among patients with NSCLC, 122 had adenocarcinoma and 61 had squamous cell carcinoma; as for stages, 14 patients were stage I, 10 stage II, 56 stage III, 136 stage IV, and 47 stage unidentified. A total of 41 patients had EGFR gene mutation analysis and 12 were positive, whereas 18 and four patients had ALK and ROS1 sequenced, respectively, and none was positive. The χ2‐test revealed no difference of age, sex, histopathology, stages, and gene mutation between patients that received treatment or not, except for the EGFR gene mutation rate (P = 0.004) (Table S1).
Discussion
We investigated the prevalence of widely used auxiliary diagnostic examinations and first‐line treatments of lung cancer patients in Jiangsu Province, China. Chest CT was the most frequently used method for the screening and diagnosis of lung cancer, in 94.7% of patients. Low‐dose CT (LDCT) is recommended for routine screening and follow‐up of lung cancer in high‐risk populations because of its many advantages, including the high rate of detecting small lesions, elimination of overlapping structures, high resolution, low radiation dose, noninvasiveness, and inexpensiveness.5 The US National Lung Screening Trial confirmed 20% reduction in lung cancer‐related mortality in selected high‐risk populations, when screened by LDCT, despite poor specificity, over‐diagnosis, bias, and cost‐effectiveness concerns.6 Many people may be concerned about the radiation dose of CT scanning. However, re‐analysis of the data from a 10‐year LDCT lung cancer screening study revealed that the cumulative radiation dose was 9.3 mSv in men and 13.0 mSv in women. Standard chest CT scanning exposure was 7–8 mSv, and it was 13–14 mSv for an abdomen‐pelvic CT scan. Only one lung cancer was induced by radiation exposure for every 173 diagnosed patients. The safety of LDCT was considered acceptable when compared with the reduced screening‐associated mortality.7 This means that patients at high risk should be routinely screened by LDCT for timely diagnosis of lung cancer, increased likelihood of successful radical surgery, and improved quality of life. Evidence of positive benefits of X‐ray screening, sputum cytology, or bronchoscopy screening on survival of patients with lung cancer is lacking, but many hospitals in China use X‐ray screening of lung cancer, leading to underdiagnosis.8
The overall rate of gene aberration analysis of 26.1% in this study has increased in comparison with previous studies.2, 9 This may be related to current guidelines that recommended testing EGFR mutation status before selecting optimal treatment regimens for patients with advanced NSCLC10 the cost of genetic analysis is falling, and its availability is increasing; also, as the use of targeted therapy increased, more and more patients became aware of it and began to ask for genetic analysis to determine whether TKIs are indicated. However, this was far below the average countrywide rate.4 On one hand, tertiary hospitals in major cities that serve as study sites had higher adherence to guidelines than other hospitals; on the other hand, not all the hospitals were qualified for genetic aberration analysis; moreover, patients in rural areas had more economic stress and were less compliant. It is now widely known that the EGFR mutation rate was approximately 50% in China, which is consistent with the findings of the present study.11, 12 However, just 72.0% of EGFR mutation‐positive patients in this study were first‐line treated with TKIs. Possible reasons include financial strains; TKIs not available for purchase in the study centers; and some patients in rural areas were unaware of it. The ALK gene‐related chromosomal rearrangement was present in 3.6% of patients, which is consistent with the 1.5–6.7% in previous reports.13, 14, 15
Treatment disparities are inevitable because of regional differences and the large size of the population in China, even within a province like Jiangsu.3, 16, 17 Physicians in a town or rural areas might be less well trained, and patients there may be less well educated, and both of these may lead to decreased adherence to guidelines. The Chinese population is mobile, and individuals frequently change hospitals and doctors, which leads to discontinuation of standard therapy or initiation of non‐standard treatments.18, 19 In this study, 22.7% of patients refused active treatment at diagnosis (transferred patients not included), with cost being the predominant reason. Previously, TKIs were self‐paying and many patients could not afford them; however, beginning in 2017, gefitinib and icotinib were covered by medical insurance, which would significantly increase the proportion of patients receiving targeted therapy. Low educational attainment and health awareness also contributed to the high rate of treatment refusal. To improve this, health policy administrators should take steps to reduce social imbalance and improve rural education. Physicians are duty‐bound to continue training and improve skills; also, they should promote and communicate basic knowledge of lung cancer to the public with the assistance of advertising and public relations. Finally, it is important that patients increase their health literacy and work with physicians to obtain optimal individualized therapy.
Study limitations include recruitment at only tertiary care hospitals, which may not be representative of primary and secondary hospitals. Medical care in tertiary hospitals is relatively more normative than that in primary and secondary hospitals. However, patients in China generally understand that lung cancer is such a serious illness that is best treated at “big hospitals.” Some patients diagnosed with lung cancer at primary or secondary hospitals are urged to transfer to tertiary hospitals for better treatment. We thus believe that most lung cancer patients in Jiangsu Province are treated in the tertiary hospitals that served as study sites and that our results are reliable. However, outpatients were not included, which may lead to study bias, and the cross‐sectional study design could describe only the situation at the time of survey. Finally, only treatment status was described, and evaluation of treatment response and survival outcomes are necessary in further study.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1 Characteristics of patients untreated/transferred.
Table S2 Study sites.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81401903, 81572937, and 81572273), and the Natural Science Foundation of Jiangsu province (No. BL2013026, BK20140736, and BK20161386).
Contributor Information
TangFeng Lv, Email: bairoushui@163.com.
Yong Song, Email: yong_song6310@yahoo.com.
References
- 1. Govindan R, Page N, Morgensztern D et al Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24 (28): 4539–44. [DOI] [PubMed] [Google Scholar]
- 2. Xue C, Hu Z, Jiang W et al National survey of the medical treatment status for non‐small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77 (2): 371–5. [DOI] [PubMed] [Google Scholar]
- 3. Yang LL, Zhang XC, Yang XN et al Lung cancer treatment disparities in China: A question in need of an answer. Oncologist 2014; 19 (10): 1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou Q, Song Y, Zhang X et al A multicenter survey of first‐line treatment patterns and gene aberration test status of patients with unresectable stage IIIB/IV nonsquamous non‐small cell lung cancer in China (CTONG 1506). BMC Cancer 2017; 17 (1): 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu J, Qian GS, Bai CX, Lung Cancer Study Group of Chinese Thoracic Society , Chinese Alliance Against Lung Cancer Expert Group . Chinese consensus on early diagnosis of primary lung cancer (2014 version). Cancer 2015; 121 (Suppl 17): 3157–64. [DOI] [PubMed] [Google Scholar]
- 6. Su C, Meyer M, Pirker R et al From diagnosis to therapy in lung cancer: Management of CT detected pulmonary nodules, a summary of the 2015 Chinese‐German Lung Cancer Expert Panel. Transl Lung Cancer Res 2016; 5 (4): 377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rampinelli C, De Marco P, Origgi D et al Exposure to low dose computed tomography for lung cancer screening and risk of cancer: Secondary analysis of trial data and risk‐benefit analysis. BMJ 2017; 356: j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang SY. [The status quo, confusion and prospect of early diagnosis for lung cancer.]. J Xi'an Jiaotong University 2011; 32: 1–5 (In Chinese.) [Google Scholar]
- 9. Yatabe Y, Kerr KM, Utomo A et al EGFR mutation testing practices within the Asia Pacific region: Results of a multicenter diagnostic survey. J Thorac Oncol 2015; 10 (3): 438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015; 121 (Suppl 17): 3165–81. [DOI] [PubMed] [Google Scholar]
- 11. Zhou C. Lung cancer molecular epidemiology in China: Recent trends. Transl Lung Cancer Res 2014; 3 (5): 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi Y, Li J, Zhang S et al Molecular Epidemiology of EGFR Mutations in Asian Patients with Advanced Non‐Small‐Cell Lung Cancer of Adenocarcinoma Histology ‐ Mainland China Subset Analysis of the PIONEER study. PLoS ONE 2015; 10 (11): e0143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soda M, Choi YL, Enomoto M et al Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448 (7153): 561–6. [DOI] [PubMed] [Google Scholar]
- 14. Perner S, Wagner PL, Demichelis F et al EML4‐ALK Fusion Lung Cancer: A Rare Acquired Event. Neoplasia 2008; 10 (3): 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takeuchi K, Choi YL, Soda M et al Multiplex reverse transcription‐PCR screening for EML4‐ALK fusion transcripts. Clin Cancer Res 2008; 14 (20): 6618–24. [DOI] [PubMed] [Google Scholar]
- 16. Goss PE, Strasser‐Weippl K, Lee‐Bychkovsky BL et al Challenges to effective cancer control in China, India, and Russia. Lancet Oncol 2014; 15 (5): 489–538. [DOI] [PubMed] [Google Scholar]
- 17. American Cancer Society . Global Cancer Facts & Figures, 3rd edn. American Cancer Society, Atlanta: 2015. [Google Scholar]
- 18. Ettinger DS, Wood DE, Aisner DL et al Non‐Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15 (4): 504–35. [DOI] [PubMed] [Google Scholar]
- 19. Kalemkerian GP, Akerley W, Bogner P et al NCCN issues 20th Annual SCLC guidelines. Pharmacoecon Outcomes News 2014; 711 (1): 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Characteristics of patients untreated/transferred.
Table S2 Study sites.


