Abstract
Background
Background: Bisphenol A (BPA) is an estrogen‐like chemical widely contained in daily supplies. There is evidence that environmental exposure to BPA could contribute to the development of hormone‐related cancers. As is reported in numerous studies, melatonin, an endogenous hormone secreted by the pineal gland, could markedly inhibit estrogen‐induced proliferation of breast cancer (BC) cells. In this study, we intended to reveal the effects of melatonin on BPA‐induced proliferation of estrogen receptor‐positive BC cells.
Methods
Methods: We used methyl thiazolyl tetrazolium, luciferase reporter gene and western blotting assays to testify the effect of melatonin on BPA‐mediated proliferation of MCF‐7 and T47D cells.
Results
Methyl thiazolyl tetrazolium and colony formation assays showed that melatonin could significantly abolish BPA‐elevated cell proliferation. Meanwhile, BPA‐upregulated phosphorylation of ERK and AKT was decreased by melatonin treatment. Mechanistically, we found that BPA was capable of upregulating the protein levels of steroid receptor coactivators (SRC‐1, SRC‐3), as well as promoting the estrogen response element activity. However, the addition of melatonin could remarkably block the elevation of steroid receptor coactivators expression and estrogen response element activity triggered by BPA.
Conclusion
Conclusions: Therefore, these results demonstrated that melatonin could abrogate BPA‐induced proliferation of BC cells. Therapeutically, melatonin could be regarded as a potential medication for BPA‐associated BC.
Keywords: Bisphenol A, breast neoplasms, estrogen response element, melatonin, steroid receptor coactivator
Introduction
Bisphenol A (BPA) is a carbon‐based synthetic compound that has been widely used in many daily supplies, including dental sealants, food packaging, and plastics polycarbonate polyvinyl chloride.1, 2 Under heat, acidic and basic conditions, or constant use, these products could release BPA to the environment.3, 4 Exposure to BPA would jeopardize the human immune system and the female reproductive system.1, 5 Breast cancer (BC) is a malignant carcinoma that severely threatens women's health. Many studies have reported that BPA shows estrogen‐like properties and has a link with BC development.6, 7 Estrogen and estrogen‐like compounds normally interact with estrogen receptor (ER), and then modulate BC progression through two mechanisms: (i) directly bind with estrogen response element (ERE) to regulate gene expression; and (ii) work through a rapid non‐genomic mechanism including activating the MAPK and PI3K/AKT pathways.8, 9 As an estrogen‐like chemical, BPA could imitate estrogen to interact with ER, resulting in abnormal cell proliferation, migration, and apoptosis.6, 10
Melatonin, a secretion from the pineal gland, is an important endogenous hormone in regulating circadian rhythm.11 The synthesis and secretion of melatonin could be disrupted by exposure to light at night.12 However, with the compact modern lifestyle, more and more people are becoming sleep deprived, which leads to disorder of melatonin's serum level. Several studies have substantiated that disruption of the normal circadian rhythm could lead to a higher risk of hormone‐dependent cancer occurrence including BC.11, 13, 14 Multiple in vivo and in vitro studies have demonstrated the effects of melatonin on reducing the incidence and growth rate of BC.15, 16 Further studies show that melatonin could effectively inhibit cell viability and proliferation, and induce apoptosis in ER‐positive (ER+) breast tumors.17 However, whether melatonin could affect BPA‐induced BC cell proliferation remains unknown.
To explore the effect of melatonin on BPA‐mediated survival and proliferation of BC cells, we used MTT, colony formation, and western blotting assays in this study. We found for the first time that melatonin could inhibit BPA‐elevated proliferation of MCF‐7 and T47D cells, through suppressing ERE activity via declined expression of steroid receptor coactivators (SRCs), and downregulating AKT and ERK phosphorylation.
Methods
Materials
We purchased bisphenol A, 17β‐estradiol (E2) and melatonin from Sigma‐Aldrich (St. Louis, MO, USA). BPA and melatonin were dissolved in dimethyl sulfoxide as stock solutions, and 17β‐estradiol was dissolved in ethyl alcohol. These three chemicals were reserved at −20°C. Goat monoclonal antibody against MT1 was purchased from Santa Cruz (Dallas, TX, USA). Rabbit primary monoclonal antibodies against SRCs, AKT, ERK1/2, phospho‐AKTSer473, phospho‐ERK1/2, p21, GAPDH, and anti‐goat and anti‐rabbit immunoglobulin G horseradish peroxidase‐linked secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
MCF‐7 and T47D cell lines were acquired from the American Type Culture Collection (ATCC, Rockville, MD, USA), and cultivated in 10% fetal bovine serum (FBS) supplemented Dulbecco's modified Eagle's medium (DMEM) or RPMI 1640 media (Gibco, Rockville, MD, USA) containing penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37°C with 5% CO2.
Plasmids
The pERE‐E1b‐luc reporter plasmid, which contains the vitellogenin ERE, and pCMV‐[beta]‐galactosidase (pCMV‐[beta]‐gal) were kindly provided by C. Smith (Baylor College of Medicine).
MTT assay
Cell proliferation ability was determined by MTT assay. MCF‐7 and T47D cells were seeded 3000 cells per well in 96‐well plates with five replicates. The cells were incubated for 24 hours to form a monoplayer. Then, DMEM containing no FBS and no phenol‐red was used to substitute for the culture media to starve for 24 hours. Indicated concentrations of BPA (100 nm), E2 (10 nm) or melatonin (1 nm) were added for another four days after starvation. The media were changed every 48 hours during the treatment. Then 10 μL MTT was added to each well for four hours’ incubation. Later, the media were discarded and 150 μL dimethyl sulfoxide was added in each well to solve formazan. The absorption values were determined at OD490nm by use of an absorbance reader (Enspire 2300 multimode reader; Perkin Elmer, Hopkinton, MA, USA).
Colony formation assay
A total of 500 cells for each well were seeded in 12‐well plates containing DMEM with 10% FBS. The media were replaced by FBS and phenol red‐free DMEM with the indicated dose of BPA, E2, and/or melatonin 48 hours later. Cells were maintained in the incubator for 15 days. Distinguishable colonies were stained by crystal violet and calculated.
Protein preparation and western blotting assay
Expression of signaling pathway proteins were quantified by western blotting. We planted indicated cells at a density of 3 × 105 cells for each well. The next day, the media were replaced by DMEM with no phenol red and FBS to starve for 24 hours. Then, indicated BPA and melatonin were added in for another 48 hours. The protein extraction and sodium dodecyl sulfate‐polyacrylamide gel electrophoresis were carried out as described previously.18
Transient transfection assay
Cells were seeded in 12‐well plates and allowed to grow for 24 hours at 37°C. Later, cultural media were substituted by phenol red‐free DMEM containing no FBS. After 12 hours starving, we transfected the cells with 1 μg of pERE‐E1b‐luc plasmid and pCMV‐[beta]‐gal packaged by Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) as the manufacture's protocols instructed. Twelve hours later, media were renewed by which containing BPA or melatonin as indicated in previous experiment assays.
Luciferase reporter gene assay
To implement luciferase assay, we planted MCF‐7 and T47D cells in 12‐well plates, and then transfected them with pERE‐E1b‐luc reporter plasmid and pCMV‐[beta]‐gal (as control). After transfection for 12 hours, the media were replaced by DMEM with no FBS and no phenol red to starve for 12 hours. Then cells were treated with dimethyl sulfoxide, BPA, E2, and/or melatonin for 24 hours. The reporter gene activity was detected by a Luciferase Reporter Assay Kit (K801‐200; BioVision, Mountain View, CA, USA) according to the manufacturer's specification. The fluorescence signal was measured using Enspire 2300 multimode reader (Perkin Elmer). The assay was processed in triplicate and at least three independent assays were carried out.
Statistical analysis
Student's t‐test or one‐way ANOVA were used to process the results in this study by SPSS13.0 software (SPSS, Chicago, IL, USA), among which the P‐value <0.05 was regarded as significant.
Results
Melatonin could block the survival and proliferation of ER+ BC cells induced by BPA
To investigate whether melatonin could abolish the survival and proliferation of ER+ cell lines induced by BPA, we used DMEM media with phenol red‐free and FBS to perform MTT assay. As shown in Figure 1a, the administration of 100 nm BPA could enhance the survival ratio of MCF‐7 and T47D cells, which was similar to the effect reached by 10 nm E2. Another finding worth noting was that this rise could be significantly inhibited by the addition of 1 nm melatonin (Fig 1a). We also performed colony formation assay to testify the effect of melatonin on MCF‐7 and T47D cell survival under BPA treatment. Cells exposed to BPA were able to form larger and more colonies, and melatonin could reverse this change (Fig 1b,c). Thus, we conclude that melatonin could block the survival and proliferation of ER+ BC cell lines induced by BPA.
Figure 1.
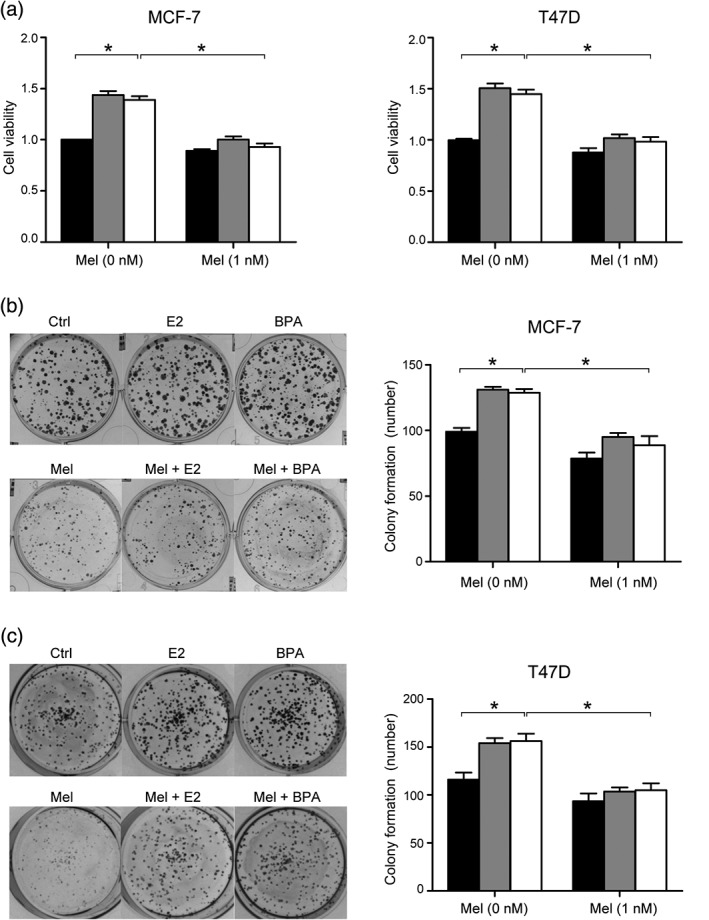
Melatonin could block the survival and proliferation of estrogen receptor‐positive breast cancer cells induced by bisphenol A (BPA). (a) MTT assay was performed to evaluate cell proliferation. Cells were incubated for 96 hours and the value of optical density 490 nm was measured as described in the MTT method. The bars represent the average value and the standard deviation of at least three independent experiments; *P < 0.05. The survival and proliferation of MCF‐ 7 (b) and T47D (c) cells were detected by colony formation assay as described above, the proliferative ability was enhanced by BPA (100 nM) and E2 (10 nM), while this enhancement could be blocked by melatonin (1 nM). The bar graph showed statistical analysis of colony numbers for at least three experiments; *P < 0.05. Ctrl, E2, BPA.
Melatonin is able to modulate the levels of ER‐related key proteins under treatment of BPA
To further investigate the mechanism by which melatonin inhibits BPA‐induced BC cell proliferation, we detected the levels of several proteins reported to be involved in ER‐related cell proliferation. Phosphorylation of ERK and AKT were both obviously elevated when treated by BPA in MCF‐7 and T47D cells (Fig 2a,b). However, melatonin could significantly abolish the upregulation of phosphorylated ERK and AKT mediated by BPA. Meanwhile, the effect of BPA on p21 could also be abrogated under melatonin treatment (Fig 2a,b). To explore whether the impacts of melatonin on ER+ cells are related to melatonin receptor 1 (MT1), we detected the protein level of MT1.The result showed an upregulation of MT1 under treatment of melatonin, indicating that MT1 might be connected with melatonin‐mediated abrogation of BC cell proliferation.
Figure 2.
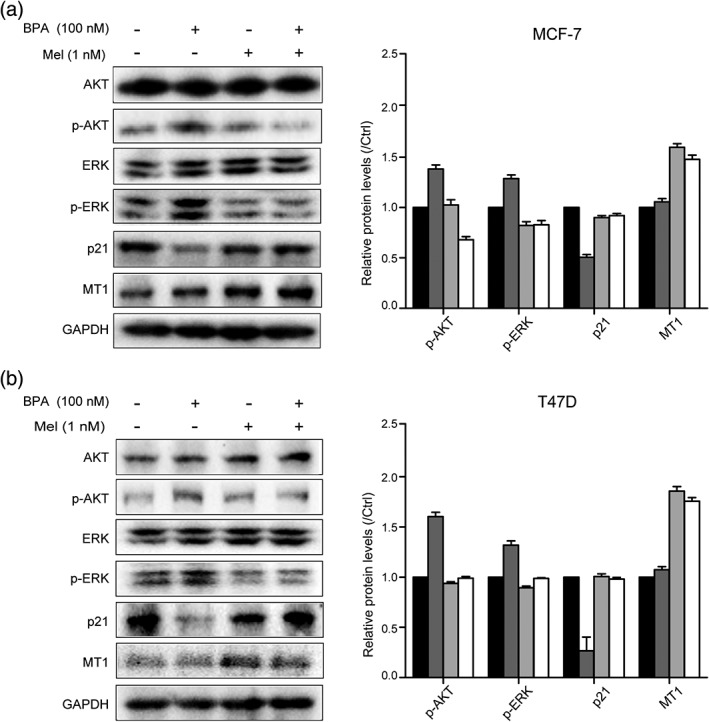
Melatonin is able to modulate the levels of estrogen receptor‐related key proteins under treatment of bisphenol A (BPA). (a) MCF‐7 and (b) T47D cells were starved for 24 hours, and then treated with BPA at a dose of 100 nm or melatonin at a dose of 1 nm for 48 hours. The protein levels of p‐AKT, p‐ERK, p21, and MT1 were detected by western blotting assay. The histogram showed relative levels changed under treatment. The bars represent the average value and the standard deviation of at least three independent experiments. Ctrl, BPA, Mel, BPA+Mel.
Melatonin is capable of inhibiting BPA‐elevated SRC‐1 and SRC‐3
It was reported that BPA induced cell proliferation via its estrogen‐like property.6 Based on this property, we next investigated whether melatonin treatment affected BPA‐induced proliferation by changing the expression of ER coactivators, SRC‐1 and SRC‐3. As shown in Figure 3, the upregulation of SRCs induced by BPA could be counteracted by addition of melatonin.
Figure 3.
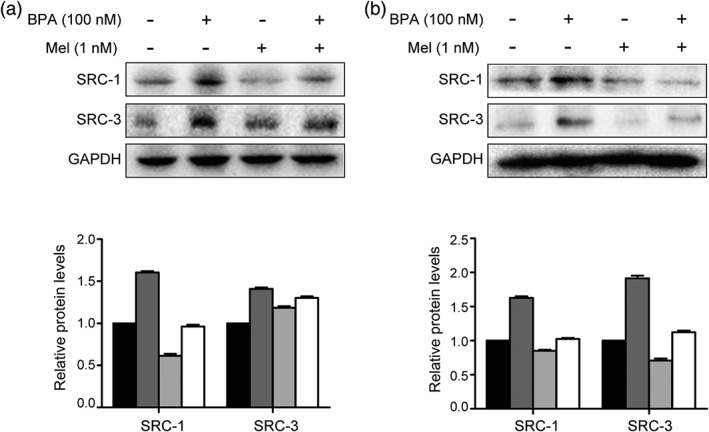
Melatonin is capable of inhibiting bisphenol A (BPA)‐elevated steroid receptor coactivator (SRC)‐1 and SRC‐3. (a) MCF‐7 and (b) T47D cells were treated with BPA (100 nm) and melatonin (1 nm) for 24 hours after starved by Dulbecco's modified Eagle's medium without phenol red and fetal bovine serum. Western blotting assay was performed to detect a change of proteins. The histogram shows relative levels changed under treatment. The bars represent the average value and the standard deviation of at least three independent experiments. Ctrl, BPA, Mel, BPA+Mel.
Melatonin decreases the activity of ERE stimulated by BPA
The change of ER coactivators indicates the alteration of ERE activity.19 Thus, we considered whether ERE activity was also involved in the modulation of melatonin on BPA‐induced proliferation. To verify this hypothesis, we performed luciferase reporter gene assay in MCF‐7 and T47D cells (Fig 4). The cells were transiently transfected with pERE‐E1B‐luc plasmid. Luciferase reporter activity demonstrated that melatonin could effectively inhibit ERE activity promoted by BPA.
Figure 4.
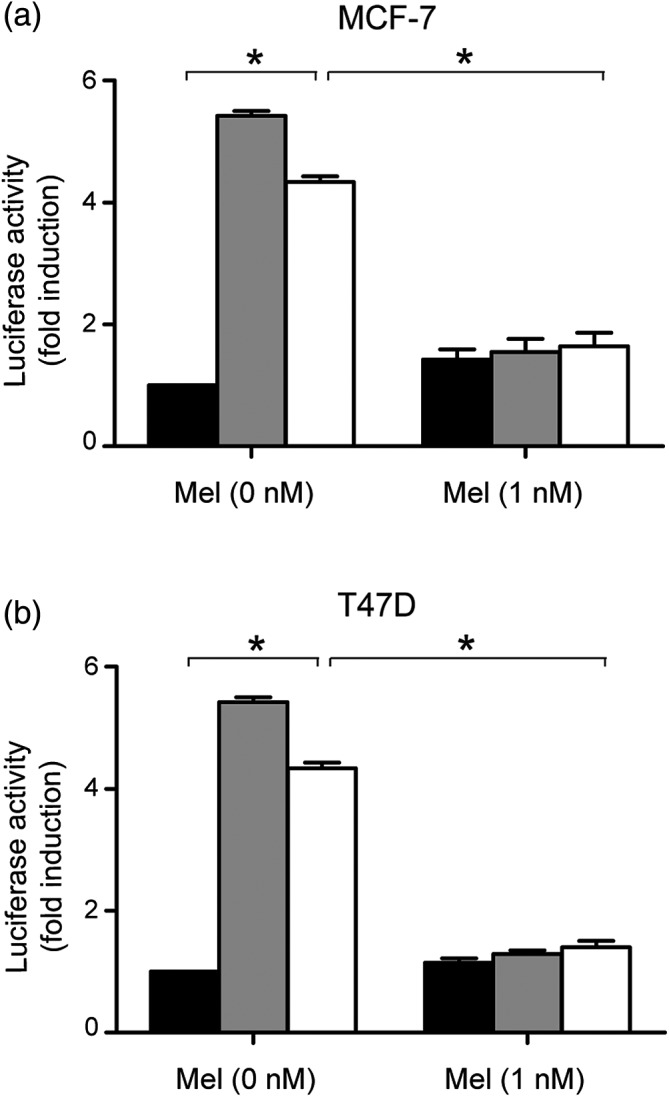
Melatonin decreased the activity of estrogen response element stimulated by bisphenol A. (a) MCF‐7 and (b) T47D cells were transfected with 1 μg of pERE‐E1b‐luc reporter plasmid (estrogen response element‐driven reporter plasmid). After 12 hours’ starvation, cells were treated with 10 nm E2, 100 nm BPA, and 1 nm melatonin. The luciferase activity was measured as indicated and the data were showed as fold change compared with untreated cells (control group), which was arbitrarily indicated as 1. The bars represent the average value and the standard deviation of at least three independent experiments; *P < 0.05. Ctrl, E2, BPA.
Discussion
BPA is a food contact material that is used as composition of plastics for the manufacture of food packaging, beverage bottles, kitchenware, wall of cans, and so on. Exposure to BPA could be constant in daily life, and severely threatens female and male health.1, 6, 10 In vitro and in vivo investigations have revealed the connection between BPA with BC, which was found to be caused by the estrogen‐like properties of BPA including interacting with ER and activating ERE.20, 21, 22 Notably, BPA is competent to raise the levels of ERα and progesterone receptor, recruiting ERα to the promoter of estrogen‐responsive genes in a dose‐dependent pattern, which was similar to the effect attained by E2 treatment.23 It is reported that oral exposure to BPA could induce mammary carcinoma in rats through the activation of the AKT pathway.24 However, whether there exists a potential agent that suppresses BPA‐induced cell proliferation in BC remains unknown.
As an endogenous hormone, melatonin not only works as a regulatory factor for circadian rhythm, but is also involved in angiogenesis and tumor growth.25, 26, 27 Amongst the characteristics of melatonin, we focus on its function as an anti‐neoplastic substance on hormone‐associated tumors. Our data demonstrated that melatonin was capable of suppressing BPA‐induced proliferation in ER+ BC cells. We have known that BPA could mimic estrogen to form a complex with ER and then activate ERE, as well as the MAPK and PI3K/AKT signaling pathways.28, 29, 30, 31, 32 Here, we found that melatonin could abolish BPA‐elevated phosphorylation of ERK and AKT. Numerous studies have shown the crucial role of melatonin receptor MT1 in melatonin‐induced anticancer events.33, 34, 35, 36 In the present study, we observed an obvious elevation of MT1 after treatment of melatonin, which may be involved in the abolishment of BPA‐associated cell proliferation.
Given the estrogen‐like properties of BPA, we are concerned as to whether BPA promoted BC cell proliferation through ER coactivators, SRCs. Strikingly, we for the first time found that BPA was capable of elevating the expression of SRC‐1 and SRC‐3. Furthermore, the activity of ERE could be efficiently increased by BPA treatment. However, melatonin could significantly disrupt the BPA‐elevated SRCs expression and ERE activity, which could be regarded as the mechanisms of melatonin in the suppression of BPA‐induced BC cell proliferation.
In the present study, we find that melatonin could reverse BPA‐induced proliferation of BC cells via reducing the phosphorylation of ERK and AKT, as well as upregulating the level of cell cycle progression blocker, p21. Most importantly, melatonin blocks the activation of ERE triggered by BPA, possibly through downregulating ER coactivator, SRC‐1 and SRC‐3. Thus, we propose that melatonin could be used as a promising medication for BPA‐associated BC progression.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by funds from the National Basic Research Program of China (973 Program, Nos. 2015CB553905); National Natural Science Foundation of China (No.510575, 81272218 and 81372186); the Fundamental Research Funds for the Central Universities, Project of Prevention and Control of Key Chronic Non Infectious Diseases (No. 2016YFC130340); and Liaoning Province Natural Science Foundation of China (No.2013023043). The authors who received the funding: Lihong Ye and Yuhui Yuan. The funders had no part in the experimental design, data acquisition and analysis, determination to publish, or the arrangement of the paper.
Contributor Information
Yuhui Yuan, Email: yuhuiyuan@dlmedu.edu.cn, Email: yuhuiyuan@hotmail.com.
Lihong Ye, Email: yelihong@nankai.edu.cn.
References
- 1. Konieczna A, Rutkowska A, Rachon D. Health risk of exposure to Bisphenol A (BPA). Rocz Panstw Zakl Hig 2015; 66: 5–11. [PubMed] [Google Scholar]
- 2. Valentino R, D'Esposito V, Ariemma F, Cimmino I, Beguinot F, Formisano P. BPA environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J Endocrinol Invest 2016; 39: 259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheng ZG, Zhu BZ. Low concentrations of bisphenol a induce mouse spermatogonial cell proliferation by G protein‐coupled receptor 30 and estrogen receptor‐alpha. Environ Health Perspect 2011; 119: 1775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rochester JR, Bisphenol A. Human health: A review of the literature. Reprod Toxicol 2013; 42: 132–55. [DOI] [PubMed] [Google Scholar]
- 5. Richter CA, Birnbaum LS, Farabollini F, Vandenbergh JG, Walser‐Kuntz DR, vom Saal FS. Vivo effects of bisphenol a in laboratory rodent studies. Reprod Toxicol 2007; 24: 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao H, Yang BJ, Li N, Liu SJ. Bisphenol a and hormone‐associated cancers: Current progress and perspectives. Medicine (Baltimore) 2015; 94: e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mlynarcikova A, Macho L, Fickova M, Bisphenol A. Alone or in combination with estradiol modulates cell cycle‐ and apoptosis‐related proteins and genes in MCF7 cells. Endocr Regul 2013; 47: 189–99. [DOI] [PubMed] [Google Scholar]
- 8. Burns KA, Li Y, Arao Y, Petrovich RM, Korach KS. Selective mutations in estrogen receptor alpha D‐domain alters nuclear translocation and non‐estrogen response element gene regulatory mechanisms. J Biol Chem 2011; 286: 12640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: From concept to therapeutic targeting. Mol Interv 2005; 5: 343–57. [DOI] [PubMed] [Google Scholar]
- 10. Paulose T, Speroni L, Sonnenschein C, Soto AM. Estrogens in the wrong place at the wrong time: Fetal BPA exposure and mammary cancer. Reprod Toxicol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zamfir Chiru AA, Popescu CR, Gheorghe DC. Melatonin and cancer. J Med Life 2014; 7: 373–4. [PMC free article] [PubMed] [Google Scholar]
- 12. Brainard GC, Hanifin JP, Greeson JM, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci 2001; 21: 6405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: The neuroendocrine/circadian melatonin signal. Endocrine 2005; 27: 179–88. [DOI] [PubMed] [Google Scholar]
- 14. Cardinali DP, Pevet P. Basic aspects of melatonin action. Sleep Med Rev 1998; 2: 175–90. [DOI] [PubMed] [Google Scholar]
- 15. Sanchez‐Barcelo EJ, Cos S, Fernandez R, Mediavilla MD. Melatonin and mammary cancer: A short review. Endocr Relat Cancer 2003; 10: 153–9. [DOI] [PubMed] [Google Scholar]
- 16. Grant SG, Melan MA, Latimer JJ, Witt‐Enderby PA. Melatonin and breast cancer: Cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med 2009; 11: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopes JR, Maschio LB, Jardim‐Perassi BV et al Evaluation of melatonin treatment in primary culture of canine mammary tumors. Oncol Rep 2015; 33: 311–9. [DOI] [PubMed] [Google Scholar]
- 18. Yuan Y, Qin L, Liu D et al Genetic screening reveals an essential role of p27kip1 in restriction of breast cancer progression. Cancer Res 2007; 67: 8032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yi P, Wang Z, Feng Q et al Structural and functional impacts of ER Coactivator sequential recruitment. Mol Cell 2017; 67: 733–43 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews JB, Twomey K, Zacharewski TR. Vitro and in vivo interactions of bisphenol a and its metabolite, bisphenol a glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol 2001; 14: 149–57. [DOI] [PubMed] [Google Scholar]
- 21. Hiyama M, Choi EK, Wakitani S et al Bisphenol‐A (BPA) affects reproductive formation across generations in mice. J Vet Med Sci 2011; 73: 1211–5. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine‐disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro. Environ Health Perspect 2012; 120: 1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sengupta S, Obiorah I, Maximov PY, Curpan R, Jordan VC. Molecular mechanism of action of bisphenol and bisphenol a mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol 2013; 169: 167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to bisphenol a increases dimethylbenzanthracene‐induced mammary cancer in rats. Environ Health Perspect 2009; 117: 910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jardim‐Perassi BV, Arbab AS, Ferreira LC et al Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE 2014; 9: e85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim KJ, Choi JS, Kang I, Kim KW, Jeong CH, Jeong JW. Melatonin suppresses tumor progression by reducing angiogenesis stimulated by HIF‐1 in a mouse tumor model. J Pineal Res 2013; 54: 264–70. [DOI] [PubMed] [Google Scholar]
- 27. Talib WH, Saleh S. Propionibacterium acnes augments antitumor, anti‐angiogenesis and Immunomodulatory effects of melatonin on breast cancer implanted in mice. PLoS ONE 2015; 10: e0124384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ptak A, Gregoraszczuk EL, Bisphenol A. Induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol Lett 2012; 210: 332–7. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Jenkins S, Lamartiniere CA. Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol a and genistein. BMC Cancer 2014; 14: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buteau‐Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot‐Applanat M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor‐dependent mechanism. J Endocrinol 2008; 196: 399–412. [DOI] [PubMed] [Google Scholar]
- 31. Lee HS, Park EJ, JH O et al Bisphenol a exerts estrogenic effects by modulating CDK1/2 and p38 MAP kinase activity. Biosci Biotechnol Biochem 2014; 78: 1371–5. [DOI] [PubMed] [Google Scholar]
- 32. Qi S, Fu W, Wang C et al BPA‐induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod Toxicol 2014; 50: 108–16. [DOI] [PubMed] [Google Scholar]
- 33. Xiang S, Mao L, Yuan L et al Impaired mouse mammary gland growth and development is mediated by melatonin and its MT1G protein‐coupled receptor via repression of ERalpha, Akt1, and Stat5. J Pineal Res 2012; 53: 307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuan L, Collins AR, Dai J, Dubocovich ML, Hill SM. MT(1) melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol 2002; 192: 147–56. [DOI] [PubMed] [Google Scholar]
- 35. Collins A, Yuan L, Kiefer TL, Cheng Q, Lai L, Hill SM. Overexpression of the MT1 melatonin receptor in MCF‐7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett 2003; 189: 49–57. [DOI] [PubMed] [Google Scholar]
- 36. Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994; 13: 1177–85. [DOI] [PubMed] [Google Scholar]


