Abstract
Background
Primary pulmonary lymphoepithelioma‐like carcinoma is a rare subtype of lung cancer. Until now, the characteristics of lymph nodes metastases in resectable cases have not yet been reported.
Methods
In this study, a total of 87 consecutive patients with primary pulmonary lymphoepithelioma‐like carcinoma that received surgical treatment were investigated from October 1999 to August 2016. The clinical and radiological data and follow‐up information were extracted from hospital records in detail.
Results
In a univariate analysis, those patients with an early pathological stage (I–II), low rate of lymph node metastases (<30%) and a low number of positive lymph nodes (<5) showed longer recurrence‐free survival and overall survival (all P < 0.05). However, the early pathological stage was identified as the only factor independently associated with recurrence‐free survival by multivariate analysis (P = 0.038). In a preoperative lymph nodes evaluation, the accuracy and specificity of computed tomography alone were 52.9% (46/87) and 88% (302/343), respectively, and 73.2% of these cases with incorrect nodal staging (30/41) were upstaged. Skipping metastases were more frequent in operated stage N2 cases (71.4%), whereas whether or not those patients showed skipping metastasis did not affect their recurrence‐free survival or overall survival (P > 0.05). The highest metastasis frequencies for specific lobes with primary lymphoepithelioma‐like carcinoma are as follows: #5 left upper lobe (21.4%); #7 left lower lobe (40.7%); #2R (28.6%) and/or #4R (14.3%) right upper lobe; #7 (42.9%) right lower lobe; #7 (28%) and/or superior mediastinal nodes (36%) right middle lobe.
Conclusion
Based on accurate staging and uncertain survival benefit, complete mediastinal lymph nodes dissection is still required for curative resection.
Keywords: Lung cancer, lymph nodes, lymphoepithelioma‐like carcinoma, prognostic factors
Introduction
There is a rare classification of non‐small cell lung cancer (NSCLC) called primary pulmonary lymphoepithelioma‐like carcinoma (LELC). Until now, <300 patients have been reported in the worldwide literature, and the majority of these patients were from China.1 Furthermore, the latest World Health Organization classification of lung tumors was published in 2015. Compared with the 2004 version, the criteria of larger cell cancer had changed greatly, including elimination of primary pulmonary LELC, and reclassification as other and unclassified carcinomas.2 Since 1999, this distinct entity was included in the World Health Organization classification of lung tumors. However, the exact classification has been debated over the past 17 years. Obviously, the characteristics of this scarce malignant neoplasm still require further elucidation.
In previous retrospective analyses, many scholars paid attention to etiology, clinical characteristics, detection of target genes, prognostic factors, therapeutic strategy, and so on. As a whole, the Epstein–Barr virus‐associated pulmonary carcinoma often occurred in Southeast Asia, in young and non‐smoker individuals, and there was no sexual predilection. Despite a lack of target agent‐sensitive mutations, overexpressed programmed cell death ligand 1 had been observed in this rare population. At the same time, the serum albumin level, the abovementioned programmed cell death ligand 1 expression level, and the pathological stage might be potential prognostic factors, as reported by Chinese scholars.3, 4 In addition, as with other NSCLC subtypes, surgical resection was also considered as the preferred treatment for LELC of the lung.5 However, because of the limited number of resectable cases, the distributed characteristics of lymph nodes (LNs) in radiology and pathology has not been reported.
Thus, in the present large cohort study, our objectives were to document clinical characteristics and prognostic factors in this Epstein–Barr virus‐associated pulmonary carcinoma. Second, we attempt to explore the pattern of LNs metastasis, which might be helpful for surgeons to determine an operative strategy.
Methods
Patient selection
A total of 132 consecutive patients with primary pulmonary LELC from October 1999 to August 2016 were investigated in our database. All patients were treated at the Sun Yat‐Sen University Cancer Center, Guangzhou, China. Furthermore, written informed consent was signed by all patients on admission to the hospital for the first time. This retrospective analysis was also approved by the Hospital's Research Ethics Committee. We restaged the 132 patients based on the seventh edition of the American Joint Committee on Cancer guidelines, and the detailed individual history data were extracted from hospital records.
Originally, all 98 patients who received surgery were retrieved from our database. Then, patients were eligible to be enrolled if they had received a curative R0 resection and had been diagnosed with LELC of the lung with positive Epstein–Barr encoding regions. In addition, patients in the group should be given nasopharyngoscopy, head and neck computed tomography (CT), or positron emission tomography‐CT to indicate no metastasis from nasopharyngeal carcinoma. Second, patients with N1 and N2 LNs resection and mapping with a minimum of three N2 stations sampled or complete LNs dissection as recommended by the National Comprehensive Cancer Network guidelines contributed an essential condition into the group (eliminated five cases).
The patients who met the following criteria were excluded: death within 30 days of operation, occurrence of a second tumor before/after resection (eliminated one case), and loss to follow‐up (eliminated five cases).
In the final data analysis, 87 patients were ultimately included in this cohort study. The clinical and demographic characteristics of the 87 cases of primary pulmonary LELC are shown in Table 1.
Table 1.
Clinical and demographic characteristics of the 87 resectable primary pulmonary lymphoepithelioma‐like carcinoma, and Kaplan–Meier univariate survival analysis for recurrence‐free survival and overall survival
| Variables | No. of cases, (n) | Five‐year RFS (%) | P † | Five‐year OS (%) | P † |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 53.07 ± 10.22 | ||||
| <60 | 68 | 66.0 | 0.959 | 80.8 | 0.151 |
| ≥60 | 19 | 73.9 | 73.7 | ||
| Sex | |||||
| Female | 44 | 73.2 | 0.768 | 72.9 | 0.379 |
| Male | 43 | 63.6 | 86.7 | ||
| Smoking status | |||||
| Smokers | 20 | 72.6 | 0.574 | 78.4 | 0.693 |
| Non‐smokers | 67 | 57.7 | 95.0 | ||
| Tumor location | |||||
| LUL | 14 | 49.1 | 0.643 | 85.7 | 0.512 |
| LLL | 27 | 76.4 | 91.1 | ||
| RUL | 7 | 85.7 | 100 | ||
| RML | 25 | 82.7 | 54.0 | ||
| RLL | 14 | 61.7 | 54.0 | ||
| Neoadjuvant therapy | |||||
| Yes | 6 | 41.7 | 0.411 | 100 | 0.406 |
| No | 81 | 70 | 78.9 | ||
| Tumor stage | |||||
| T1 | 34 | 75.4 | 0.866 | 71.5 | 0.319 |
| T2 | 47 | 60.0 | 91.1 | ||
| T3 | 6 | 66.7 | 66.7 | ||
| Nodal stage | |||||
| N0 | 32 | 88.9 | 0.000 | 88.9 | 0.056 |
| N1 | 20 | 94.7 | 95.0 | ||
| N2 | 35 | 22.3 | 62.3 | ||
| TNM Stage | |||||
| I | 25 | 87.5 | 0.000 | 85.7 | 0.057 |
| II | 27 | 96.2 | 96.3 | ||
| IIIa | 35 | 22.3 | 62.3 | ||
| Adjuvant therapy | |||||
| Yes | 54 | 53.0 | 0.005 | 80 | 0.936 |
| No | 33 | 94.7 | 81.3 | ||
| Extent of resection | |||||
| Lobectomy | 67 | 68.2 | 0.098 | 81.2 | 0.965 |
| Biloectomy | 8 | 87.5 | 85.7 | ||
| Pneumonectomy | 12 | 55.0 | 66.7 | ||
| Surgical approach | |||||
| Open | 70 | 72.6 | 0.542 | 77.5 | 0.219 |
| VATS | 17 | 35.9 | 100 | ||
| LNR | |||||
| 0% | 31 | 88.2 | 0.006 | 88.9 | 0.019 |
| >0%,<30% | 46 | 62.1 | 90.3 | ||
| ≥30% | 10 | 28.1 | 29.2 | ||
| PLNs | |||||
| 0 | 31 | 88.2 | 0.009 | 88.9 | 0.004 |
| 1–5 | 44 | 61.8 | 84.6 | ||
| ≥5 | 12 | 0 | 35.6 | ||
| Recurrence status | |||||
| Recurrence | 17 | — | — | 82.7 | 0.260 |
| Non‐recurrence | 70 | 75 | |||
The P‐value of <0.05 was regarded as statistically significant.
LLL, left lower lobe; LNR, rate of lymph nodes metastases; LUL, left upper lobe; OS, overall survival; PLNs, the number of positive lymph nodes; RFS, recurrence‐free survival; RML, right middle lobe; RLL, right lower lobe; RUL, right upper lobe; VATS, video‐assisted thoracic surgery.
Computed tomography evaluation of LNs metastasis
Consensus interpretation of CT scanning was carried out by two board‐certified radiologists independently, and suspicion of malignancy of mediastinal or hilar LNs was recorded based on a short‐axis diameter >10 mm.
End‐points and follow‐up
The interval of overall survival (OS) was measured from the date of diagnosis to the date of death by any cause or to the date of the last follow‐up day. Recurrence‐free survival (RFS) was defined as the interval from the date of surgical resection to the date of disease recurrence. The following frequencies of follow‐up are recommended by the National Comprehensive Cancer Network guidelines: 3–4 months postoperatively for one year, every six months for three years, and then every year. The chest CT scan with/without contrast was used to monitor recurrence every 6–12 months for 2 years after operation, and every year thereafter. If patients had symptoms, positron emission tomography‐CT or brain magnetic resonance imaging scan was required. In our group, the median follow‐up time was 34 months (range 1 month–156 months), and the last day of follow‐up was 14 September 2016.
Statistical analysis
All statistical analyses were carried out in SPSS 20.0 (IBM, Armonk, NY, USA). The optimal cut‐off values of the number of positive LNs (PLNs) and rate of LNs metastases (LNR) were calculated by Cox proportional hazards regression analysis. Determined using maximal χ2 score, the optimal cut‐off values were 0/1–5/≥5 for PLNs (χ2 = 8.443, P = 0.004) and 0/0–30%/≥30% for LNR (χ2 = 5.523, P = 0.019).
Survival rates were calculated by the Kaplan–Meier (K‐M) analysis, and were compared by the log–rank test. The χ2‐test or Fisher's exact test was carried out to test for significance in the probability of comparative variables between two or more groups. Significance was determined as a P‐value <0.05.
Results
Patient characteristics
In this retrospective analysis, we found that resected primary pulmonary LELC often occurred in young individuals (78.2%), while the median age at diagnosis was 54 years (range 27–72). Moreover, just 20 patients (23%) were smokers, and the patients with a smoking index ≥400 accounted for 65% (13/20). Furthermore, the proportions of these patients diagnosed at postoperative pathological staging I, II, and IIIa were 28.7%, 31.0%, and 40.2%, respectively.
In these cases, just six pathologically IIIa‐N2 patients received neoadjuvant chemotherapy. After operation, 54 cases were given platinum‐based adjuvant therapy, and four of 54 cases also received adjuvant radiotherapy. The regimens of adjuvant chemotherapy included paclitaxel/docetaxel with cisplatin/carboplatin (28, 51.9%) pemetrexed with cisplatin/carboplatin (18, 33.3%), and gemcitabine with cisplatin/carboplatin (8, 14.8%).
RFS and OS in primary pulmonary LELC
In our cohort, the median follow‐up time was 34 months (range 1–156 months), and the last day of follow‐up was 14 September 2016. According to the OS and RFS curves calculated by K‐M analysis, the one‐, two‐, three‐, four‐, and five‐year OS proportions were 97.7%, 96.4%, 93.3%, 90.1%, and 80.3%, respectively; with the corresponding RFS rates of 93.9%, 86.6%, 82.6%, 72.4%, and 67.6%; however, the medians for all OS and RFS for our cohort were not reached at the last follow‐up.
The K‐M method was used to investigate prognostic factors involved in RFS and OS. The variables listed in Table 1 were analyzed as prognostic factors. Further analysis showed that patients with early pathological stages, without adjuvant therapy, with a LNR and a fewer number of PLNs, and without LNs invasion had better prognosis for RFS. The difference was statistically significant (P < 0.05; Fig 1a,c,e). Meanwhile, for OS, early pathological stages, a low LNR, and fewer PLNs also revealed relevance with a significantly better prognosis (P < 0.05; Fig 1b,d,f). Cox proportional hazard regression analysis was carried out to assess for prognostic factors for RFS and OS. Finally, only a more advanced pathological stage was revealed to be independently associated with inferior RFS (P = 0.038; Table 2).
Figure 1.
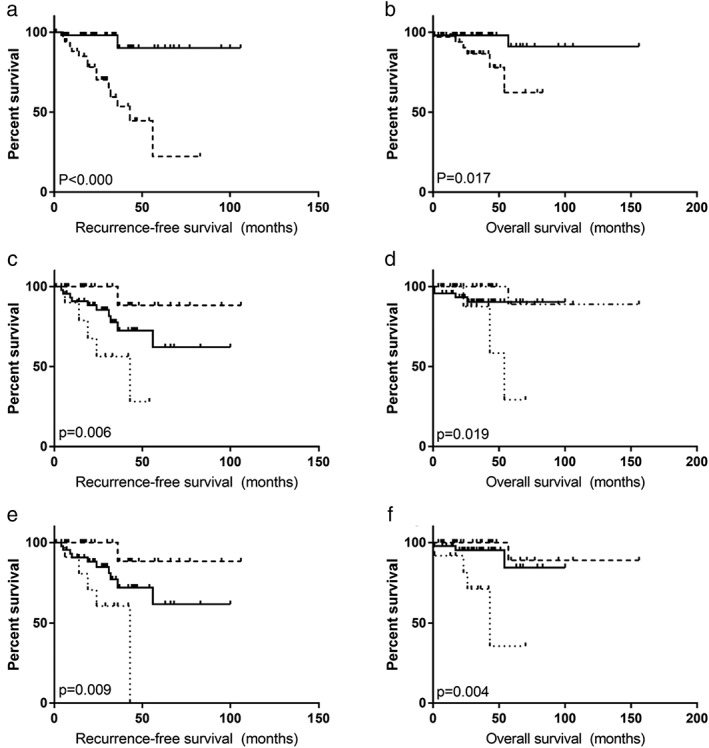
(a,b) Recurrence‐free survival and overall survival curves based on Kaplan–Meier analysis between different pathological stages (I–II/IIIA). (c,d) Recurrence‐free survival and overall survival curves based on Kaplan–Meier analysis according to positive lymph nodes proportion (0%/0–30%/30%). (e,f) Recurrence‐free survival and overall survival curves based on Kaplan–Meier analysis according to positive lymph nodes number (0/1–4/≥5).
Table 2.
Cox multivariate survival analysis for recurrence‐free survival and overall survival of resectable primary pulmonary lymphoepithelioma‐like carcinoma cases
| Variables | Groups | Recurrence‐free survival | Overall survival | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P † | Hazard ratio (95% CI) | P † | ||
| Age (years) | <60/≥60 | 0.900 (0.234–3.456) | 0.878 | 0.781 (0.431–1.416) | 0.416 |
| Sex | Female/male | 1.809 (0.403–8.109) | 0.439 | 1.622 (0.904–2.912) | 0.105 |
| Smoking status | Smokers/non‐smokers | 0.389 (0.066–2.288) | 0.296 | 0.588 (0.298–1.162) | 0.126 |
| Surgical approach | Open/VATS | 0.669 (0.188–2.374) | 0.534 | 0.566 (0.321–0.996) | 0.084 |
| Pathological Stage | I–II/IIIa | 0.103 (0.012–0.879) | 0.038 | 0.882 (0.452–1.720) | 0.713 |
| Nodal stage | N0/N1–N2 | 2.156 (0.185–25.111) | 0.540 | 0.943 (0.492–1.807) | 0.859 |
| Adjuvant therapy | Yes/no | 5.419 (0.635–46.247) | 0.122 | 0.757 (0.450–1.276) | 0.296 |
| LNR | <30%/≥30% | 0.791 (0.183–3.419) | 0.754 | 1.245 (0.408–3.801) | 0.701 |
| PLNs | <5/≥5 | 0.820 (0.199–3.382) | 0.784 | 0.765 (0.246–2.384) | 0.644 |
The P‐value of <0.05 was regarded as statistically significant.
CI, confidence interval LNR, rate of lymph nodes metastases; PLNs, the number of positive lymph nodes.
LNs staging in radiology
The distribution of LNs staging diagnosed at radiology included stage N0 in 36 patients, stage N1 in 13 patients, stage N2 in 36 patients, and stage N3 in two patients. When compared with postoperative pathological diagnosis, the LNs status was correctly evaluated in 46 patients (46/87, 52.9%) by using CT alone. In these incorrect N staging cases, the majority was upstaged (30/41, 73.2%). In addition, all 411 LNs stations, including 346 mediastinal and 65 hilar, were examined under microscopy. Among them, 68 LNs stations (68/411, 16.5%) in 43 patients were confirmed as invasion by malignant cells, and just eight patients were proven to have multiple mediastinal metastases. However, for malignant LNs depiction using preoperative CT acquisition, 98 LNs stations were suspected of malignancy, and 24 patients were determined to have multiple mediastinal metastases, which was an obviously exaggerated result. Finally, the accuracy and specificity of CT in mediastinal and hilar LNs evaluation were 52.9% (46/87) and 88% (302/343), respectively.
Skipping metastasis
Up to 25 of 35 N2 cases (71.4%) showed skipping metastasis. By contrastive analysis, the average number of resected MLNs or ILNs did not differ between the skipping and non‐skipping group (P = 0.341). In addition, five cases with pathological N2 stage in LUL all showed skipping metastasis, followed by right lower lobe (RLL; 5/6, 83.3%), left upper lobe (LLL; 9/12, 75%), right upper lobe (RUL; 1/2, 50%), and right middle lobe (RML; 5/10, 50%). There was no statistically significant difference in the rate of skipping metastasis between upper lobes and lower lobes (P = 0.656), and this comparison between left and right lobes yielded the same result (P = 0.264). In addition, whether or not those patients showed skipping metastasis did not affect their RFS or OS (P = 0.438 and 0.971, respectively).
Distribution of invaded LNs based on different lobes
In the 14 cases with a tumor located in LUL, if concomitant superior mediastinal LNs involvement was not found, there was almost no inferior mediastinal LNs metastasis (0.0%). Similarly, if concomitant hilar or inferior mediastinal LNs invasion was not present, the primary tumor located in LLL rarely metastasized to superior mediastinal LNs (1/27, 3.7%). Based on the above phenomenon, we found that the rate of inferior mediastinal nodes invasion in patients with tumors located in LLL was significantly higher than that of patients with tumors located in LUL (P = 0.015). Similarly, compared with patients who had tumors located in LLL, patients who had tumors located in LUL showed a higher metastatic frequency (P = 0.012) in aortic nodes (Tables 3 and 4).
Table 3.
Number of patients with positive lymph nodes according to different lobes
| Locations of lymph nodes | LUL (n = 14) | LLL (n = 27) | RUL (n = 7) | RML (n = 25) | RLL (n = 14) |
|---|---|---|---|---|---|
| Number (%) | |||||
| 2. Upper paratracheal | — | — | 2 (28.6%) | 2 (8.0%) | 0 (0.0%) |
| 3.Prevascular/retrotracheal | — | — | 0 (0.0%) | 3 (12.0%) | 0 (0.0%) |
| 4. Lower paratracheal | 1 (7.1%) | 2 (7.4%) | 1 (14.3%) | 4 (16.0%) | 0 (0.0%) |
| 5. Subaortic | 3 (21.4%) | 0 (0.0%) | — | — | — |
| 6. Para‐aortic | 0 (0.0%) | 0 (0.0%) | — | — | — |
| 7. Subcarinal | 1 (7.1%) | 11 (40.7%) | 0 (0.0%) | 7 (28.0%) | 6 (42.9%) |
| 8. Paraesophageal | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | 1 (4.0%) | 0 (0.0%) |
| 9. Pulmonary ligament | 0 (0.0%) | 3 (11.1%) | 0 (0.0%) | 5 (2.0%) | 2 (14.3%) |
| 10. Hilar | 1 (7.1%)) | 5 (18.5%) | 1 (0.0%) | 5 (2.0%) | 1 (7.1%) |
| 11. Interlobar | 4 (28.6%) | 10 (37.0%) | 0 (0.0%) | 3 (12.0%) | 5 (35.7%) |
| 12. Lobar | 4 (28.6%) | 2 (7.4%) | 1 (14.3%) | 2 (8.0%) | 3 (21.4%) |
| 13. Segmental | 1 (7.1%) | 4 (14.8%) | 1 (14.3%) | 0 (0.0%) | 0 (0.0%) |
| 14. Subsegmental | — | 1 (3.7%) | — | — | — |
LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Table 4.
Distributions of invaded lymph nodes compared with different lobes
| Stations | LUL vs. LLL (P‐value†) | RUL vs. RML (P‐value†) | RML vs. RLL (P‐value†) | RUL vs. RLL (P‐value†) |
|---|---|---|---|---|
| Superior MLNs (station 2–4) |
1 (7.1%) vs. 2 (7.4%) 0.975 |
2 (28.6%) vs. 7 (28.0%) 0.976 |
7 (28.0%) vs. 0 (0.0%) 0.029 |
2 (28.6%) vs. 0 (0.0%) 0.100 |
| Aortic nodes (station 5–6) |
3 (21.4%) vs. 0 (0.0%) 0.012 |
— | — | — |
| Inferior MLNs (station 7–9) |
1 (7.7%) vs. 12 (42.3%) 0.015 |
0 (0.0%) vs. 7 (28.0%) 0.113 |
7 (28.0%) vs. 6 (42.9%) 0.345 |
0 (0.0%) vs. 6 (42.9%) 0.061 |
| Intrapulmonary nodes (station 10–14) |
8 (61.5%) vs. 16 (57.7%) 0.896 |
2 (28.6%) vs. 9 (36.0%) 0.715 |
9 (36.0%) vs. 5 (35.7%) 0.419 |
2 (28.6%) vs. 5 (35.7%) 0.743 |
The P‐value <0.05 was regarded as statistically significant.
LLL, left lower lobe; LUL, left upper lobe; RML, right middle lobe; RLL, right lower lobe; RUL, right upper lobe.
In the right lobes, the points and characteristics of the upper and lower lobes were extremely similar to the corresponding left lobes. It might be deduced that the paratracheal nodes (3 cases) and the seven‐station (6 cases) were the RUL‐ and RLL‐specific patterns of LNs involvement, respectively. In the 25 cases with the tumor located in RML, the distribution of patients with malignant nodes seemed to be evenly distributed between intrapulmonary, hilar, subcarinal, and superior mediastinal nodes. The seven‐station (7 cases) and superior mediastinal nodes might be considered as the RML‐specific pattern of LNs involvement. In addition, only tumors located in the RML had a greater risk for metastases to the upper mediastinal LNs than those in RLL (P = 0.029; Tables 3, 4).
Generally, the sentinel nodes are intrapulmonary and found in the 11‐station (22 cases), 10‐station (13 cases), and 12‐station (12 cases) regardless of primary tumor location. Route dissection of these stations may not be omitted.
Discussion
In contrast with other subtypes of lung cancer, all results showed that LELC of the lung had a better prognosis.3, 6, 7, 8 Similarly, the above tendency was also obtained from our study. In 87 resectable cases, the five‐year OS and RFS rates were 78.9% and 70.0%, respectively, which were slightly higher than the rates found in a previous review.7 Among our resectable LELC of the lung cases, the K‐M method showed that cases with pathological stages I–II had a better prognosis for RFS and OS than those with stage IIIa (P < 0.000 and 0.017, respectively). Furthermore, patients with LNs involvement had significantly inferior RFS (P = 0.011). For all patients who received radical surgery, adjuvant therapy (CT ± RT) did not improve their RFS (P = 0.005), even in pathological stage IIIa, which was the exact opposite of a finding by Liang et al. 3 Then, through Cox multivariate survival analysis, only pathological stage (I–II vs. IIIa) was confirmed to be an independent prognostic factor for RFS, which was completely consistent with Li Jiang's report.7 In brief, the comprehensive stage for primary pulmonary LELC based on TNM standard has a better clinical application value as a risk factor for RFS.
In addition, in previous reports, the LNs of primary LELC of the lung appeared larger than other subtypes of NSCLC radiologically, and suspicion of LNs invasion by malignant cells was common based on radiological signs (100% by Ooi, 81.3% by Huang, 68.3% by Ma).9, 10, 11 However, due to a lack of pathological confirmation, the sensitivity and specificity of the evaluation of LNs by using radiology alone could not draw a definite conclusion. In our cohort, 30 LNs groups were misdiagnosed as positive at CT scanning (98 groups at radiology vs. 68 groups at pathology), and 16 patients were misclassified as having multiple mediastinal metastases (24 patients at radiology vs. 8 patients at pathology), which was an obviously exaggerated result. Thus, regarding the enlarged LNs observed by radiology, positron emission tomography‐CT scanning, endobronchial ultrasonography‐guided transbronchial needle aspiration, or endoscopic ultrasonography guided fine needle aspiration was used for re‐evaluation to avoid missing an operation chance.
The current eighth edition of NSCLC regarded LNs staging as a creditable prognostic factor, and LNs staging is based on the location of invaded LNs regardless of the number of PLNs.12 However, the updated pathological TNM stage of colorectal and breast cancer had deemed PLNs as novel criteria.13 According to several retrospective studies on NSCLC from 2006 to now, PLNs had been discovered to possibly be a better prognostic factor than station‐based LNs staging.14, 15, 16 In another retrospective study, the LNRs had shown more valuable independent prognostic features.17 In our cohort, efforts were also made to explore the prognostic value of PLNs and LNR in LELC of the lung. Through K‐M analysis, a higher PLN (≥30%) and LNP (≥5) indicated inferior RFS and OS. Specifically, in OS, these two criteria had superiority when compared with LNs staging. Unfortunately, through multivariate analysis, PLNs, LNR, and LNs staging all lost significant prognostic value for this rare NSCLC subtype.
Several studies have tried to explore the involvement pattern in lung cancer based on tumor location. Naruke et al. had explored common metastasis lesions of tumors located in RUL and RLL in 1999, and the distributions of major lesions according to each lobe were as follows: #5‐LUL, #7‐LLL, #3 and/or #4‐RUL, #3‐RML, and #7‐RLL.18 A recent retrospective study carried out by Guo et al. also aimed to explore the distribution of MLNs metastases associated with different primary location, and the highest metastasis frequencies for each lobe were as follows: #5‐LUL, #7‐LLL, #2 and/or #4‐RUL, #7‐RML, and RLL.19 In 2006, Yukinori et al. proposed that 71.4% patients had metastasis to aortic nodes (the 5 and 6 stations) when the tumor was located in LUL. Furthermore, multivariate analysis determined that only aortic nodal involvement was a significant prognostic factor.20 With regard to RML with primary tumor, the superior mediastinal zones (#2–4) and subcarinal zones (#7) were the most frequent sites for metastasis. In addition, LNs dissection had greater therapeutic value for the abovementioned zones, as confirmed by Kuroda et al. 21 The MLNs invaded with high frequency were known as sentinel nodes, and researchers state that if the sentinel MLNs were not invaded, radical systematic dissection can be omitted. The drainage path of the pulmonary LELC is also close to the above results. The highest metastasis frequencies for specific lobes with primary LELC in our 87 cases are as follows: #5‐LUL (21.4%); #7‐LLL (40.7%); #2R (28.6%) and/or #4R (14.3%)‐RUL; #7 (42.9%)‐RLL; #7 (28%) and/or superior mediastinal nodes (36%)‐RML.
There are some limitations to this retrospective analysis. First, as in previous reports, our research cohort was also limited by the scant number of cases, and the majority of the enrolled cases came from South China. Second, the time interval of the study of the patients who were enrolled was from October 1999 to August 2016 (17 years). During this long period, the surgical procedures, the resected range of LNs, the choice of adjuvant chemotherapy, and other factors might have changed; thus, potential selection bias may be unavoidable.
In conclusion, our study is limited by the number of cases and treatment standards for primary LELC of the lung. Pathological N stage, PLNs, LNR, and skipping metastases had not yet been confirmed to be independent predictive factors in our cohort. In addition, in terms of technical aspects, the morbidity or mortality of patients receiving radical MLNs dissection did not increase. Therefore, based on accurate staging and uncertain survival benefit, complete MLNs dissection is still required for curative resection in LELC of the lung.
Disclosure
The study, publication, and preparation of this manuscript were supported by the National Key Research and Development Plan (No. 2016YFC0905400), and the Ministry of Science and Technology of the People's Republic of China. No authors report any conflict of interest.
Acknowledgments
First, we thank the Department of Follow‐up for recording recurrence and death data in detail. In addition, we express our gratitude for the support and suggestions for this study given by the staff at the Department of Thoracic Surgery.
References
- 1. Ho JC, Wong MP, Lam WK. Lymphoepithelioma‐like carcinoma of the lung. Respirology 2006; 11: 539–45. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 3. Liang Y, Wang L, Zhu Y et al Primary pulmonary lymphoepithelioma‐like carcinoma: Fifty‐two patients with long‐term follow‐up. Cancer 2012; 118: 4748–58. [DOI] [PubMed] [Google Scholar]
- 4. Fang W, Hong S, Chen N et al PD‐L1 is remarkably over‐expressed in EBV‐associated pulmonary lymphoepithelioma‐like carcinoma and related to poor disease‐free survival. Oncotarget 2015; 6: 33019–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Long W, Li PF, Lin YB, Liang Y. An elevated peripheral blood monocyte‐to‐lymphocyte ratio predicts poor prognosis in patients with primary pulmonary Lymphoepithelioma‐like carcinoma. PLoS One 2015; 10: e0126269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han AJ, Xiong M, Gu YY, Lin SX, Xiong M. Lymphoepithelioma‐like carcinoma of the lung with a better prognosis ‐ A clinicopathologic study of 32 cases. Am J Clin Pathol 2001; 115: 841–50. [DOI] [PubMed] [Google Scholar]
- 7. Jiang L, Wang L, Li PF et al Positive expression of programmed death ligand‐1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma‐like carcinoma. Onco Targets Ther 2015; 8: 1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin Z, Situ D, Chang X et al Surgical treatment for primary pulmonary lymphoepithelioma‐like carcinoma. Interact Cardiovasc Thorac Surg 2016; 23: 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma H, Wu Y, Lin Y, Cai Q, Ma G, Liang Y. Computed tomography characteristics of primary pulmonary lymphoepithelioma‐like carcinoma in 41 patients. Eur J Radiol 2013; 82: 1343–6. [DOI] [PubMed] [Google Scholar]
- 10. Ooi GC, Ho JC, Khong PL, Wong MP, Lam WK, Tsang KW. Computed tomography characteristics of advanced primary pulmonary lymphoepithelioma‐like carcinoma. Eur Radiol 2003; 13: 522–6. [DOI] [PubMed] [Google Scholar]
- 11. Huang CJ, Chan KY, Lee MY et al Computed tomography characteristics of primary pulmonary lymphoepithelioma‐like carcinoma. Br J Radiol 2007; 80: 803–6. [DOI] [PubMed] [Google Scholar]
- 12. Goldstraw P, Chansky K, Crowley J et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 13. Novak J, Fabian P. Comments on the TNM classification of malignant tumours‐‐7th edition. Klin Onkol 2011; 24: 149–50. [PubMed] [Google Scholar]
- 14. Ito M, Yamashita Y, Tsutani Y et al Classifications of n2 non‐small‐cell lung cancer based on the number and rate of metastatic mediastinal lymph nodes. Clin Lung Cancer 2013; 14: 651–7. [DOI] [PubMed] [Google Scholar]
- 15. Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non‐small cell lung cancer. J Thorac Oncol 2006; 1: 120–5. [PubMed] [Google Scholar]
- 16. Bria E, Milella M, Sperduti I et al A novel clinical prognostic score incorporating the number of resected lymph‐nodes to predict recurrence and survival in non‐small‐cell lung cancer. Lung Cancer 2009; 66: 365–71. [DOI] [PubMed] [Google Scholar]
- 17. Tamura M, Matsumoto I, Saito D, Yoshida S, Takata M, Takemura H. Lymph node ratio as a prognostic factor in patients with pathological N2 non‐small cell lung cancer. World J Surg Oncol 2016; 14: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naruke T, Tsuchiya R, Kondo H, Nakayama H, Asamura H. Lymph node sampling in lung cancer: How should it be done? Eur J Cardiothorac Surg 1999; 16 (Suppl. 1): S17–24. [DOI] [PubMed] [Google Scholar]
- 19. Guo D, Ni Y, Lv X, Zhang Z, Ye P. Distribution and prognosis of mediastinal lymph node metastases of nonsmall cell lung cancer. J Cancer Res Ther 2016; 12: 120–5. [DOI] [PubMed] [Google Scholar]
- 20. Sakao Y, Miyamoto H, Yamazaki A et al The spread of metastatic lymph nodes to the mediastinum from left upper lobe cancer: Results of superior mediastinal nodal dissection through a median sternotomy. Eur J Cardiothorac Surg 2006; 30: 543–7. [DOI] [PubMed] [Google Scholar]
- 21. Kuroda H, Sakao Y, Mun M et al Therapeutic value of lymph node dissection for right middle lobe non‐small‐cell lung cancer. J Thorac Dis 2016; 8: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]


