Abstract
Background
There is debate regarding the use of stereotactic ablative radiotherapy (SABR) or surgery for patients with early stage non‐small cell lung cancer (NSCLC). This meta‐analysis compared the clinical efficacy of SABR and lobectomy in stage I NSCLC patients.
Methods
An online search identified eight eligible articles (including 2 trials and 7 cohort studies) for inclusion. The odds ratio (OR) was used as a summary statistic. Overall survival (OS), cause‐specific survival (CSS), and recurrence‐free survival (RFS) were selected to calculate ORs with 95% confidence intervals (CI). Fixed‐effects or random‐effects models were conducted according to study heterogeneity.
Results
There were no significant differences between SABR and lobectomy in terms of one‐year OS or CSS. Significant benefits of surgery were observed in three‐year OS (OR 2.11, 95% CI 1.55–2.86), three‐year CSS (OR 1.94, 95% CI 1.05–3.57), three‐year RFS (OR 1.63, 95% CI 1.12–2.36), and five‐year OS (OR 2.40, 95% CI 1.71–3.36). In addition, lobectomy demonstrated a beneficial trend in one‐year RFS, five‐year RFS, and CSS.
Conclusion
Meta‐analyses of current evidence suggested that lobectomy provides better long‐term survival outcomes for stage I NSCLC patients.
Keywords: Lobectomy, non‐small cell lung cancer (NSCLC), stereotactic ablative radiotherapy (SABR), surgery
Introduction
Lung cancer remains the most common cause of cancer death globally. Non‐small cell lung cancer (NSCLC) accounts for 80–85% of lung cancers.1 Surgery has been the most promising treatment option for patients diagnosed with early stage non‐small cell lung cancer (NSCLC).2 Presently, the standard treatment for operable stage I NSCLC is lobectomy with mediastinal lymph node dissection or sampling. However, many patients with stage I NSCLC do not undergo surgery because of comorbidities or patient preference. Surgical approaches carry risks, and findings from research of video‐assisted thoracoscopic surgery (VATS) and open thoracotomy lobectomy suggests that complications occur in 16.4% and 31.2% of patients, respectively.3 Even with minimally invasive surgical techniques, operative mortality rates within 30–90 days after surgery are still 2–5.4%.4, 5
Stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiotherapy (SBRT), is a non‐invasive radiation therapy that is considered to be a favorable option for inoperable early stage patients.6 Two retrospective studies and one phase II prospective study demonstrated that SABR is associated with similar overall survival as surgery for potentially operable NSCLC patients.7, 8, 9 When SABR is performed with the planning volume receiving a biologically equivalent dose of > 100 Gy, local control is achieved in > 90% of tumors with little acute or chronic severe toxicity. However, other comparative retrospective studies have yielded conflicting results, revealing benefits of SABR10, 11 or a lack thereof.12, 13 Debate over the efficacy of surgery compared to SABR has been ongoing for several years.
To compare the therapeutic effects between lobectomy and SABR, we conducted a meta‐analysis including seven retrospective cohort studies and two randomized trials. The patients in each cohort study were matched by propensity score. Propensity score matching (PSM) can reduce bias from confounding variables when estimating treatment effects and generates two similar groups, as in a randomized controlled trial (RCT).14 Because no relevant RCTs have been conducted, two terminated RCTs (STARS NCT00840749 and ROSEL NCT00687986) were also included in the meta‐analysis. These two RCTs were closed early as a result of slow enrollment; however, Chang et al. combined and analyzed the existing data.15 Therefore, confounding factors, such as age, performance score, and comorbidity score were minimized in this meta‐analysis. In addition, we removed potential duplicated data by investigating research from the same community during the process of identification.
Methods
Search strategy
A comprehensive search of online databases, including PubMed, Embase, and Google Scholar, was conducted. The disease term “lung cancer” and the therapy terms “SABR,” “SBRT,” “Stereotactic Body Radiotherapy,” “Surgery,” and “Surgical Method” were used to locate studies for comparison. The search was limited to articles published prior to September 2016. Two reviewers independently evaluated the titles and abstracts of the identified papers. Only studies published in English language with full‐text articles were included in the meta‐analysis.
Criteria for inclusion and exclusion
Studies were included in our meta‐analysis and systematic review if they met the following criteria: (i) retrospective cohort studies (RCS) or RCTs; (ii) the differences between patients who underwent SABR or surgery were minimized, and RCS was balanced or adjusted by PSM; (iii) the study focused on lobectomy, regardless of the use of VATS or thoracotomy; (iv) patients included were diagnosed with stage I NSCLC; and (v) the study outcomes included at least overall survival (OS).
When patient samples overlapped in two or more studies, only the article that best satisfied the criteria was selected. During screening, we found that the patient sample in a study by Puri et al. overlapped that of a study by Crabtree et al. published in 2010, therefore the results of survival analyses in these two studies were pooled.12, 16
Data extraction and quality assessment
Data extraction was carefully performed on all of the eligible studies. Two reviewers independently extracted the following items from each included study: general information (first author, publication year, type of study, and patient source); sample size (number of patients who underwent surgery or SABR); details of NSCLC stage distribution; treatment details (surgical method, total radiation dose, and dose per fraction); and propensity score matched clinical characteristics (age, gender, World Health Organization [WHO] performance score [PS], comorbidity score, and details of pulmonary function testing). All data was tabulated to facilitate quantitative data synthesis. To extract survival outcomes from the identified articles, we surveyed associated survival curves at one, three, and five‐years using Engauge Digitizer version 10.0 (https://markummitchell.github.io/engauge-digitizer/). The number of events and survival rates were applied to calculate odds ratios (ORs).11, 12, 15, 17, 18, 19, 20, 21
The quality of identified studies was assessed using the validated Newcastle–Ottawa Scale (NOS) for cohort studies. The NOS awards a maximum of eight points to each cohort study (4 for quality of selection, 1 for comparability, and 3 for quality of outcome). We considered studies with scores of > 7 as high quality, and those with scores of ≤ 7 as low‐quality studies. All cohort studies included in this systematic review were estimated at > 7, and therefore could be considered high quality.
Statistical analysis
Overall survival, cause‐specific survival (CSS), and recurrence‐free survival (RFS) were calculated separately. ORs with 95% confidence intervals (CIs) were used as a summary statistic. Subgroup meta‐analyses of one, three, and five‐year survival rates were then conducted.
During meta‐analyses of OS, we found that a study by Crabtree et al. published in 2014 contained a possible overlap with part of the patient sample of a study by Robinson et al. published in 2013.18, 19 Because the study by Crabtree et al. demonstrated fewer differences in characteristics between patients who underwent SABR and surgery, the one and three‐year OS rates were extracted from this study. However, the study by Crabtree et al. did not include five‐year follow‐up rates, therefore these rates were extracted from the study by Robinson et al. Additionally, during meta‐analyses of RFS, locoregional and distant control rates in a study by Mokhles et al. were pooled to calculate cumulative one, three, and five‐year RFS.21
Statistical heterogeneity between studies was assessed using the Q statistic, while the I2 statistic was used to estimate the percentage of total variation across studies. Heterogeneity was considered as P < 0.05. The measure effect OR with 95% CI was calculated using a fixed‐effect (using the Mantel–Haenszel method) or random‐effect (using the inverse variance method) model according to the heterogeneity.22, 23 Begg's funnel plot and Egger linear regression were used to assess potential publication bias.24 P > 0.05 was considered to indicate the absence of potential publication bias.
All P values were two sided. All analyses were conducted using Stata version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Search results and study characteristics
Our predefined search strategy yielded a total of 103 articles. After screening the titles and abstracts, 18 studies were selected for further review. Finally, eight unique studies were selected as potentially appropriate for inclusion, including five single‐center RCSs, two two‐center RCS, and two RCTs. Our search strategy is presented in Figure 1 and the details of the eligible studies are summarized in Table 1.
Figure 1.
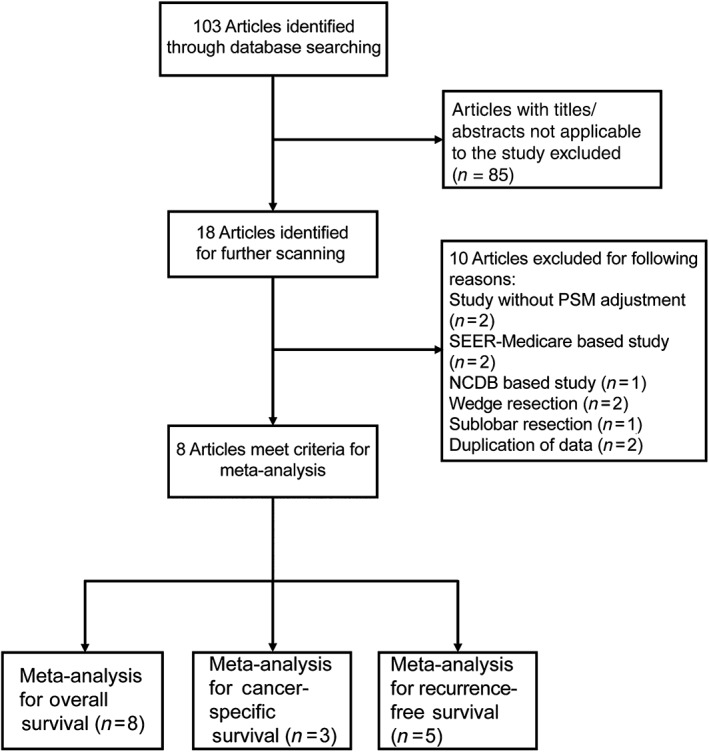
Flow diagram of articles screened and selected for this meta‐analysis. NCBD, National Cancer Database; PSM, propensity score matching; SEER, Surveillance Epidemiology and End Results.
Table 1.
Study characteristics
| Study | Year | Design | Data Source | No. of patients | Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SABR | NSCLC stage | Surgery | SABR | ||||||||
| Surgery | Total | Operable (%) | Surgery | SABR | Total | Lobectomy (%) | Dose/fractions (median) | ||||
| Crabtree et al. 12 | 2010 | OC | WUSTL | 57 | 57 | 0 (0%) | C IA + B | C IA + B | 46 lobectomy; 11 sublobar resection | 80.70% | 54 Gy/3F |
| Palma et al. 11 | 2011 | OC | ACR | 60 | 60 | 11 (18.3%) | C IA + B | C IA + B | 49 lobectomy; 2 pneumonectomy; 9 sublobar | 81.70% | 60 Gy/5F |
| Robinson et al. 18 | 2013 | OC | WUSTL | 76 | 76 | 0 (0%) | C IA + B | C IA + B | 72 lobectomy; 2 biolobectomy; 2 pneumonectomy | 94.70% | 54 Gy/3F |
| Varlotto et al. 17 | 2013 | OC | PSH | 72 | 72 | 0 (0%) | C IA + B | C IA + B | 72 lobectomy | 100% | 60 Gy/3F |
| Crabtree et al. 19 | 2014 | OC | WUSTL | 56 | 56 | 0 (0%) | C IA + B | C IA + B | 1 bilobectomy; 44 lobectomy; 6 segmentectomy; 5 wedge | 80.40% | 54 Gy/3F |
| Mokhles et al. 21 | 2015 | OC | VUMC & EMC | 73 | 73 | Abbr50%† | C IA + B | C IA + B | 32 VATS lobectomy; 41 thoracotomy lobectomy | 100% | 60 Gy/5F |
| Chang et al. 15 | 2015 | RCT | STARS & ROSEL | 27 | 31 | 100% | P IA + B | P IA + B | 19 lobectomy; 5 VATS lobectomy; 1 VATS biopsy; 1 open wedge resection; 1 aborted resection | 88.90% | 54 Gy/3F |
| Hamaji et al. 20 | 2015 | OC | Kyoto‐U | 41 | 41 | 100% | C IA + B | C IA + B | VATS lobectomy | 100% | 48 Gy/4F |
Data source Verstegen et al. 25
ACR, Amsterdam Cancer Registry; C IA + B, clinical stage IA + B; EMC, Erasmus Medical Center; Kyoto‐U, Kyoto University Hospital; NSCLC, non‐small cell lung cancer; OC, cohort study; P IA + B, pathological stage IA + B; PSH, Penn State Hershey; RCT, randomized controlled trial; ROSEL, Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer (NCT00687986); SABR, stereotactic ablative radiotherapy; STARS, Randomized Study to Compare CyberKnife to Surgical Resection in Stage I Non‐small Cell Lung Cancer (NCT00840749); VUMC, VU‐University Medical Center; WUSTL, Washington University School of Medicine, St Louis.
Propensity score matching adjustment was performed in each of the seven retrospective cohort studies between SABR and surgery patients to reduce their physical differences. Adjusted factors included patient age, gender, WHO PS, Adult Comorbidity Evaluation/Charlson Comorbidity Index score, and forced expiratory volume in 1 second/diffusing capacity of the lung for carbon monoxide percentage (FEV1%/DLCO%). A total of 870 patients (SABR n = 435, surgery n = 435) were included in this systematic review. In addition, data of 27 operative patients and 31 SABR patients were obtained from two incomplete trials (NCT00940749 and NCT00687986). Patient characteristics are summarized in Table 2.
Table 2.
Patient characteristics
| Study | Year | Age (year) | P | Male | P | WHO PS | P | Comorbidity Score | P | Pulmonary function test | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | SABR | Surgery | SABR | Surgery | SABR | Surgery | SABR | Surgery | SABR | |||||||
| Crabtree et al. 12 | 2010 | 73 (mean) | 71 (mean) | NS | 59.6% | 40 & | 0.039 | — | — | — | ACE 2 (mean) | ACE 2 (mean) | NS | FEV1% (mean) 77%; DLCO% (mean) 81% | FEV1% (mean) 50%; DLCO% (mean) 50% | < 0.01/0.01 |
| Palma et al. 11 | 2011 | 79 (mean) | 79 (mean) | NS | 67% | 67% | NS | — | — | — | — | — | — | — | — | — |
| Robinson et al. 18 | 2013 | 65 (median) | 76 (median) | < 0.01 | 48.7% | 55.4% | NS | — | — | — | ACE 1 (median); CCI 3 (median) | ACE 2 (median); CCI 4 (median) | < 0.01 | FEV1% (median) 51%; DLCO% (median) 62% | FEV1% (median) 79%; DLCO% (median) 79% | < 0.01/< 0.01 |
| Varlotto et al. 17 | 2013 | ND | ND | NS | ND | ND | NS | — | — | — | ND | ND | NS | — | — | — |
| Crabtree et al. 19 | 2014 | 70 (mean) | 70.7 (mean) | NS | 57.1% | 51.8% | NS | — | — | — | ACE 2 (median) | ACE 2 (median) | NS | FEV1% (mean) 62%; DLCO% (mean) 73% | FEV1% (mean) 64%; DLCO% (mean) 55% | NS/< 0.01 |
| Mokhles et al. 21 | 2015 | 67 (mean) | 67 (mean) | NS | 60.3% | 57.5% | NS | 1 (median) | 1 (median) | NS | CCI 1 (median) | CCI 1 (median) | NS | FEV1% (mean) 80.37% | FEV1% (mean) 81.38% | NS |
| Chang et al. 15 | 2015 | 67.3 (mean) | 67.3 (mean) | NS | 41% | 45% | NS | 0 (median) | 0 (median) | NS | — | — | — | — | — | — |
| Hamaji et al. 20 | 2015 | 74 (median) | 73 (median) | NS | 78.0% | 75.6% | NS | — | — | — | CCI 1 (median) | CCI 1 (median) | NS | FEV1% (mean) 76.5% | FEV1% (mean) 70.1% | NS |
ACE, Adult Comorbidity Evaluation; CCI, Charlson Comorbidity Index; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; ND, no detail; NS, not significant; SABR, stereotactic ablative radiotherapy.
Assessment of overall survival
All studies reported OS results of patients who underwent SABR or surgery, which produced a total sample size of 928 patients for evaluation. Among these, 462 were treated with surgery, and 466 with SABR. Seven studies included one and three‐year OS results. Only five studies included five‐year OS outcomes. The pooled ORs and 95% CIs of the one, three, and five‐year OS meta‐analyses were 0.98 (0.53–1.82), 2.11 (1.55–2.86), and 2.40 (1.71–3.36), respectively (Fig 2). Significant benefits were observed for lobectomy in the three and five‐year OS meta‐analyses.
Figure 2.
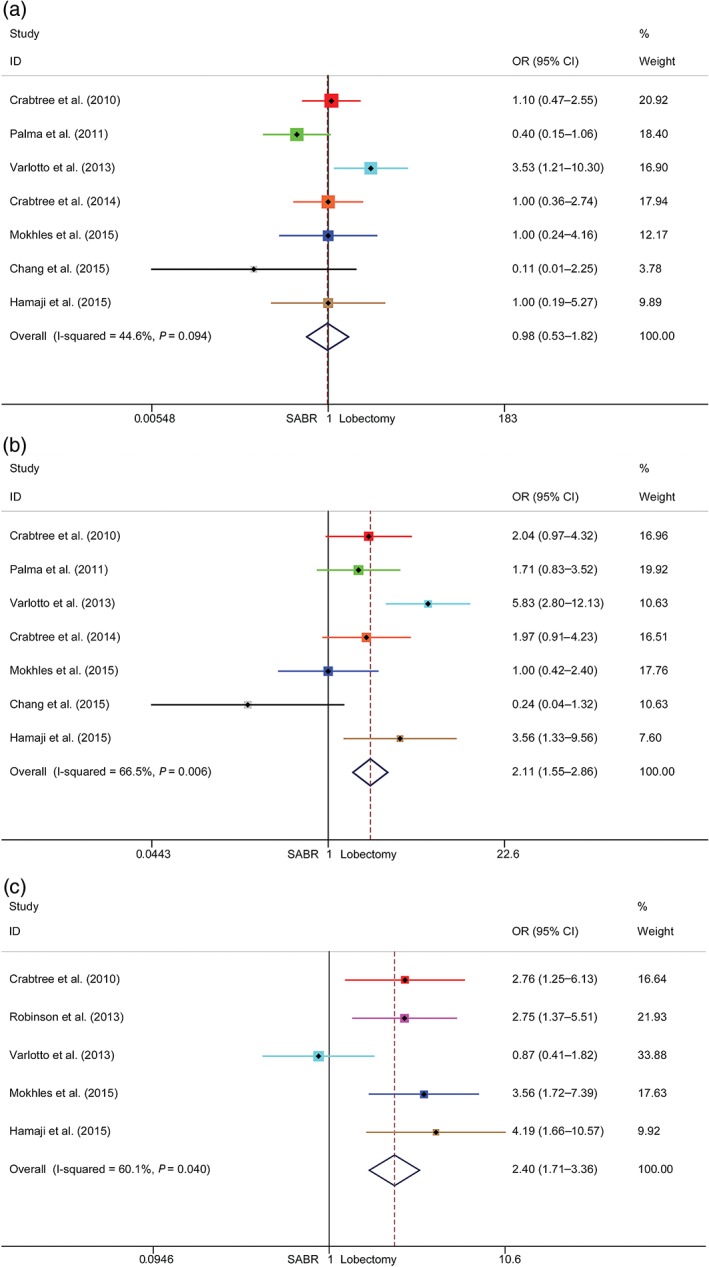
Meta‐analysis of overall survival (OS) following stereotactic ablative radiotherapy (SABR) versus lobectomy for stage I non‐small cell lung cancer. Comparison of (a) one‐year, (b) three‐year, and (c) five‐year OS rates of SABR and lobectomy. CI, confidence interval; OR, odds ratio.
Notably, after sensitivity analysis, significant heterogeneities were observed in the three and five‐year OS rates primarily relating to data from studies by Chang et al. and Varlotto et al., respectively.15, 17 Meta‐analyses of RCS rates were also conducted, and a significant three‐year OS rate was observed for lobectomy (Fig S1).
Assessment of cause‐specific survival
Three identified studies were included in CSS meta‐analyses, and all articles reported one, three, and five‐year CSS. The pooled ORs and 95% CIs of the one, three, and five‐year CSS were 1.20 (0.33–4.36), 1.94 (1.05–3.57), and 1.32 (0.81–2.14), respectively (Fig 3). A significant benefit was observed for three‐year CSS after surgery. No significant heterogeneity was detected between the studies.
Figure 3.
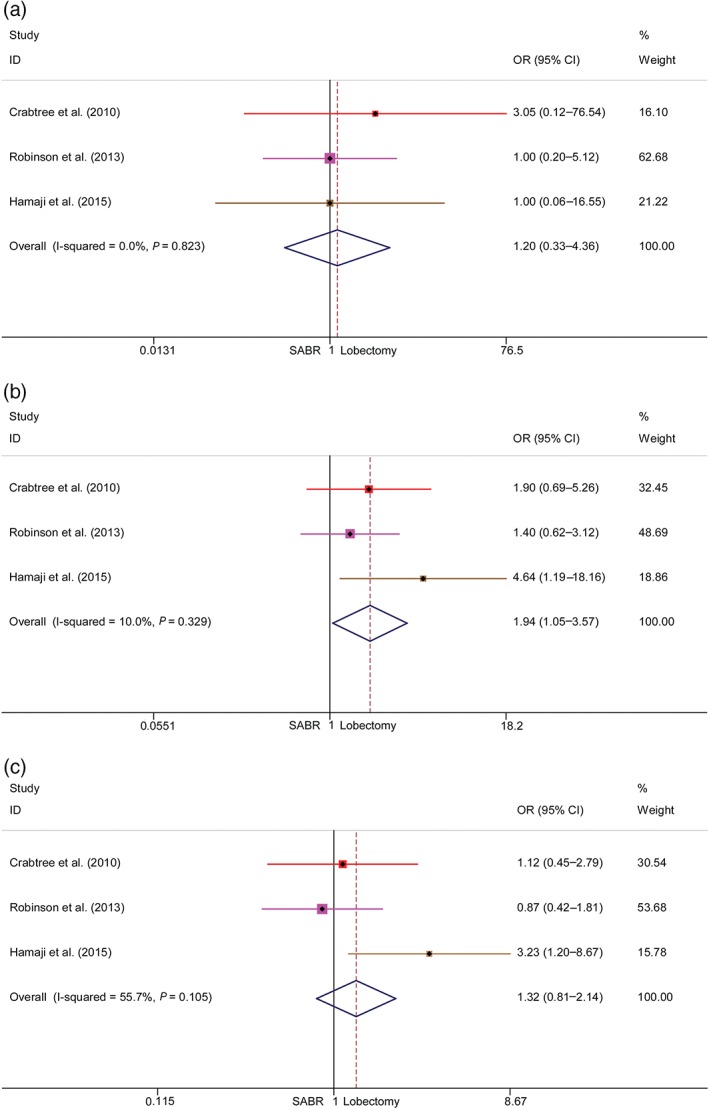
Meta‐analysis of cause‐specific survival (CSS) following stereotactic ablative radiotherapy (SABR) versus lobectomy for stage I non‐small cell lung cancer. Comparison of (a) one‐year, (b) three‐year, and (c) five‐year CSS rates of SABR and lobectomy. CI, confidence interval; OR, odds ratio.
Assessment of recurrence‐free survival
Five studies were included in the meta‐analyses of RFS. All articles demonstrated one and three‐year RFS, but only three reported five‐year RFS. The pooled ORs and 95% CIs of the one, three, and five‐year RFS were 1.48 (0.85–2.54), 1.63 (1.12–2.36), and 1.42 (0.94–2.15), respectively (Fig 4). Additional meta‐analyses on RCS were also conducted and the results of one and three‐year RFS were 1.47 (0.84–2.55) and 1.74 (1.18–2.57), respectively (Fig S2). Significant three‐year RFS benefits were observed after lobectomy. Lobectomy also demonstrated a beneficial trend in the one and five‐year RFS meta‐analyses. Significant heterogeneities were observed in the three and five‐year RFS meta‐analyses.
Figure 4.
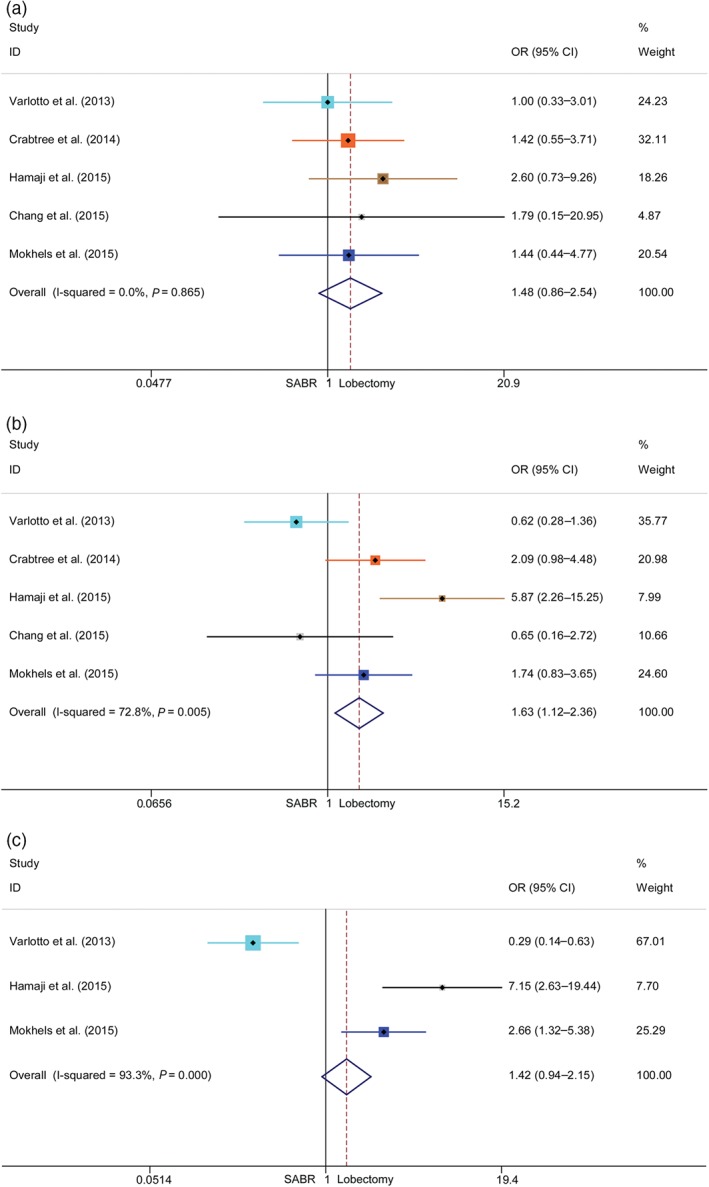
Meta‐analysis of recurrence‐free survival (RFS) following stereotactic ablative radiotherapy (SABR) versus lobectomy for stage I non‐small cell lung cancer. Comparison of (a) one‐year, (b) three‐year, and (c) five‐year RFS rates of SABR and lobectomy. CI, confidence interval; OR, odds ratio.
Publication bias
We found no evidence of publication bias in any analyses using Begg's or Egger tests (all P > 0.05).
Discussion
The choice between SABR and surgery for early stage NSCLC has been debated for years. At present, standard therapy for operable clinical stage I NSCLC is lobectomy with sampling or dissection of mediastinal lymph nodes.2 Although the risks of performing surgery are considered acceptable, use of this procedure has only gradually increased over the past decade. SABR has emerged as a non‐invasive standard treatment alternative to surgery for inoperable patients, such as elderly patients and those with clinically significant comorbidities. Findings from our meta‐analysis demonstrated that surgery (lobectomy) remains a better alternative for stage I NSCLC patients.
Five single‐center RCSs, two two‐center RCS, and two RCTs were included in this systematic review. Seven RCSs contained relevant patient data from six medical centers located in Japan, the Netherlands and the US; the STARS trial enrolled patients from 28 locations in China, France and the US, while the ROSEL trial enrolled patients from four centers in the Netherlands. VATS or thoracotomy lobectomy procedures accounted for > 80% of the surgery group of each study. In regard to radiotherapy, the total SABR dose ranged from 48 Gy to 60 Gy, and the delivered fractions from 3 to 5. All RCSs matched SABR and surgery patients with propensity scores, which dramatically reduced the differences in physical characteristics between the groups. All RCSs matched age and gender between SABR and surgery groups separately; one RCS matched WHO PS; seven RCSs matched comorbidity score; and five RCSs matched pulmonary function test results. The study by Chang et al. that pooled data from these two trials revealed no differences between age, gender, or WHO PS.15 In summary, the differences in physical characteristics between SABR and surgery patients were minimized.
In this systematic review, the evidence was robust for determining the efficacy of SABR and lobectomy for patients with stage I NSCLC. The results (three and five‐year OS, three‐year CSS, and three‐year RFS) indicated better efficacy of lobectomy compared to SABR. In addition, lobectomy demonstrated a beneficial trend in one and five‐year RFS. Notably, heterogeneities were found in three and five‐year OS and RFS rates, primarily resulting from the data used by Chang et al. and Varlotto et al. 15, 17 Two of the RCTs included in the pooled analysis by Chang et al. were terminated because of slow enrollment; therefore, the small patient sample size may induce potential selection bias. Most of the data on deaths occurring after surgery (n = 5) up to the three‐year follow‐up were derived from STARS, which significantly increased overall surgery mortality rates. We observed that data in a study by Mokhles et al. may have overlapped SABR data in a study by Verstegen et al. published in 2013, thus we excluded research by Verstegen et al. because of their shorter follow‐up period.21, 25 However, the two studies came to opposite survival conclusions, including OS, control, and progression‐free survival rates. The patients’ physical characteristics were similar between these studies as a result of PSM; however, surgical data in the study by Mokhles et al. was obtained from the Erasmus Medical Center, while surgical data for the study by Verstegen et al. was obtained from the VU University Medical Center. Notably, a recently published meta‐analysis by Deng et al. focusing on three‐year survival rates between SABR and surgery also included a large number of studies and reached similar conclusions.26 Another earlier systematic review focusing on matched‐pair comparisons between SABR and surgery in early stage NSCLC was published in 2014 by Zhang et al. 27 The original data from this systematic review were extracted from six studies with propensity score matched analyses. Their findings indicated that surgery was associated with better long‐term survival in patients with early stage NSCLC (OR 1.31; 95% CI 0.81–2.12; P = 0.27); however, there were no significant differences between local or distant control. The major deficiencies of the systematical review by Zhang et al. were overlapping data between studies by Robinson et al. and Crabtree et al., and the lack of comparison of five‐year ORs.
In conclusion, our meta‐analysis demonstrated that lobectomy is a better treatment strategy for stage I NSCLC patients. The OS results in this study were promising and the CSS and RFS rates both suggested that lobectomy is associated with better long‐term efficacy. However, we did also observe some heterogeneity, including ratios between VATS and lobectomy, SABR treatment parameters, and proportions of operable SABR patients. Additionally, no tissue diagnosis was made in many of the patients who underwent SABR, and data on whether invasive mediastinal upstaging had been conducted was not available. These confounding factors could impact survival results. Although each retrospective study included in this article matched cases with PSM, some heterogeneities still existed between physical characteristics, thus our results may be impacted by incomparability across studies regarding variables that were not included when matching. Our results can be used as a reference to determine the best treatment strategy for patients with stage I NSCLC.
Disclosure
No authors report any conflict of interest.
Supporting information
Figure S1 Meta‐analysis of overall survival (OS) following stereotactic ablative radiotherapy (SABR) versus lobectomy of retrospective cohort studies (RCSs). Comparison of (a) one‐year and (b) three‐year OS rates of SABR and lobectomy.
Figure S2 Meta‐analysis of recurrence‐free survival (RFS) following stereotactic ablative radiotherapy (SABR) versus lobectomy of retrospective cohort studies (RCSs). Comparison of (a) one‐year and (b) three‐year OS rates of SABR and lobectomy.
Acknowledgments
This study was funded by the Natural Science Foundation of China (81572261, 81672295), the Key Project of Cutting‐edge Clinical Technology of Jiangsu Province (BE2016797), and the Innovation Capability Development Project of Jiangsu Province (BM2015004).
References
- 1. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. (Published erratum appears in N Engl J Med 2009; 360: 1917.) N Engl J Med 2004; 350: 379–92. [DOI] [PubMed] [Google Scholar]
- 2. Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K, American Colleage of Chest Physicians . Treatment of non‐small cell lung cancer stage I and stage II: ACCP evidence‐based clinical practice guidelines. Chest 2007; 132 (3 Suppl): 234S–42S. [DOI] [PubMed] [Google Scholar]
- 3. Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early‐stage non‐small cell lung cancer: A systematic review of the video‐assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008; 86: 2008–16. [DOI] [PubMed] [Google Scholar]
- 4. Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from the Society of Thoracic Surgeons General Thoracic Surgery Database: The surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008; 135: 247–54. [DOI] [PubMed] [Google Scholar]
- 5. Pezzi CM, Mallin K, Mendez AS, Greer Gay E, Putnam JBJ. Ninety‐day mortality after resection for lung cancer is nearly double 30‐day mortality. J Thorac Cardiovasc Surg 2014; 148: 2269–77. [DOI] [PubMed] [Google Scholar]
- 6. Timmerman R, Paulus R, Galvin J et al Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lagerwaard FJ, Verstegen NE, Haasbeek CJ et al Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 83: 348–53. [DOI] [PubMed] [Google Scholar]
- 8. Onishi H, Shirato H, Nagata Y et al Stereotactic body radiotherapy (SBRT) for operable stage I non–small‐cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011; 81: 1352–8. [DOI] [PubMed] [Google Scholar]
- 9. Nagata Y, Hiraoka M, Shibata T et al A phase II trial of stereotactic body radiation therapy for operable T1N0M0 non‐small cell lung cancer: Japan Clinical Oncology Group (JCOG0403). Int J Radiat Oncol Biol Phys 2010; 78 (3 Suppl): S27–8. [DOI] [PubMed] [Google Scholar]
- 10. Grills IS, Mangona VS, Welsh R et al Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non‐small‐cell lung cancer. J Clin Oncol 2010; 28: 928–35. [DOI] [PubMed] [Google Scholar]
- 11. Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: A population‐based matched‐pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011; 101: 240–4. [DOI] [PubMed] [Google Scholar]
- 12. Crabtree TD, Denlinger CE, Meyers BF et al Stereotactic body radiation therapy versus surgical resection for stage I non‐small cell lung cancer. J Thorac Cardiovasc Surg 2010; 140: 377–86. [DOI] [PubMed] [Google Scholar]
- 13. Shirvani SM, Jiang J, Chang JY et al Comparative effectiveness of 5 treatment strategies for early‐stage non‐small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012; 84: 1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 15. Chang JY, Senan S, Paul MA et al Stereotactic ablative radiotherapy versus lobectomy for operable stage I non‐small‐cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol 2015; 16: 630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puri V, Crabtree TD, Kymes S et al A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high‐risk patients: A decision analysis. J Thorac Cardiovasc Surg 2012; 143: 428–36. [DOI] [PubMed] [Google Scholar]
- 17. Varlotto J, Fakiris A, Flickinger J et al Matched‐pair and propensity score comparisons of outcomes of patients with clinical stage I non‐small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013; 119: 2683–91. [DOI] [PubMed] [Google Scholar]
- 18. Robinson CG, DeWees TA, El Naqa IM et al Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non‐small‐cell lung cancer. J Thorac Oncol 2013; 8: 192–201. [DOI] [PubMed] [Google Scholar]
- 19. Crabtree TD, Puri V, Robinson C et al Analysis of first recurrence and survival in patients with stage I non‐small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014; 147: 1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamaji M, Chen F, Matsuo Y et al Video‐assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015; 99: 1122–9. [DOI] [PubMed] [Google Scholar]
- 21. Mokhles S, Verstegen N, Maat AP et al Comparison of clinical outcome of stage I non‐small cell lung cancer treated surgically or with stereotactic radiotherapy: Results from propensity score analysis. Lung Cancer 2015; 87: 283–9. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 23. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies. J Natl Cancer Inst 1959; 22: 719–48. [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verstegen NE, Oosterhuis JW, Palma DA et al Stage I–II non‐small‐cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video‐assisted thoracoscopic surgery (VATS): Outcomes of a propensity score‐matched analysis. Ann Oncol 2013; 24: 1543–8. [DOI] [PubMed] [Google Scholar]
- 26. Deng HY, Wang YC, Ni PZ et al Radiotherapy, lobectomy or sublobar resection? A meta‐analysis of the choices for treating stage I non‐small‐cell lung cancer. Eur J Cardiothorac Surg 2017; 51: 203–10. [DOI] [PubMed] [Google Scholar]
- 27. Zhang B, Zhu F, Ma X et al Matched‐pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non‐small cell lung cancer: A systematic review and meta‐analysis. Radiother Oncol 2014; 112: 250–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Meta‐analysis of overall survival (OS) following stereotactic ablative radiotherapy (SABR) versus lobectomy of retrospective cohort studies (RCSs). Comparison of (a) one‐year and (b) three‐year OS rates of SABR and lobectomy.
Figure S2 Meta‐analysis of recurrence‐free survival (RFS) following stereotactic ablative radiotherapy (SABR) versus lobectomy of retrospective cohort studies (RCSs). Comparison of (a) one‐year and (b) three‐year OS rates of SABR and lobectomy.


