Abstract
Background
Human BarH‐like homeobox 2 (Barx2), a homeodomain factor of the Bar family, plays a critical role in cell adhesion and cytoskeleton remodeling, and has been reported in an increasing array of tumor types except non‐small cell lung carcinoma (NSCLC). The purpose of the current study was to characterize the expression of Barx2 and assess the clinical significance of Barx2 in NSCLC.
Methods
Quantitative real‐time polymerase chain reaction, immunohistochemistry and western blot analysis were used to examine mRNA and protein expression, respectively. The relationships between Barx2 expression and clinicopathological variables were analyzed. Cell Counting Kit‐8 and plate colony formation assay were used to detect cell proliferation. Transwell assay was used to examine cell migration ability. Glucose uptake, lactate, adenosine triphosphate, and lactate dehydrogenase assays were used to detect aerobic glycolysis.
Results
Barx2 is downregulated in NSCLC tissues compared with para‐carcinoma. Furthermore, Barx2 expression shows a negative correlation with advanced TNM stage and a high level of Ki‐67. Survival analysis reveals that Barx2 level is an independent prognostic factor for NSCLC patients. The Barx2 (low) Ki‐67 (high) group had the worst prognosis. Furthermore, the data indicate that downregulation of Barx2 expression promotes cell proliferation, migration, and aerobic glycolysis, including increased lactate dehydrogenase activity, glucose utilization, lactate production, and decreased intracellular adenosine triphospahte level. Furthermore, Barx2 acts as a negative regulator of the canonical Wnt/β‐catenin pathway. Reactivation of Wnt/β‐catenin pathway by LiCl can reverse the inhibiting effect of Barx2.
Conclusions
These findings reveal that Barx2 serving as a tumor suppressor gene could decrease cell proliferation, migration, and aerobic glycolysis through inhibiting the Wnt/β‐catenin signaling pathway, and predicts a good prognosis in NSCLC.
Keywords: Aerobic glycolysis, Barx2, non‐small cell lung carcinoma, prognosis, Wnt/β‐catenin
Introduction
Among all new cancer cases in 2012 worldwide, lung cancer accounted for 13% of them, and lung cancer mortality has remained the highest of all cancer types during the past several decades.1 Lung cancer is categorized into non‐small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC).2 With 85% of all lung cancer cases, NSCLC is further classified into adenocarcinoma, squamous cell carcinoma, and others, which vary in the cell origin and morphology.2 Although new methods for NSCLC diagnosis and treatment have continuously emerged, and the overall therapeutic effect on NSCLC has improved,3, 4, 5 the 5‐year survival rate of NSCLC is still approximately 15%.6 Therefore, improving patient prognosis through a deep understanding of the exact molecular mechanisms of NSCLC development and progression, and identifying specific targets for medication treatment is critical.
BarH‐like homeobox 2 (Barx2), a homeodomain factor of the Bar family, regulates the expression of cell adhesion molecules and influences cellular differentiation in various developmental contexts.7, 8 Recently, downregulation of Barx2 associated with a number of solid tumors, such as gastric cancer,9 ovarian cancer,10 and primary hepatocellular carcinoma,11 has correlated with poor prognosis. However, little is known about Barx2 in NSCLC.
In this study, we investigated Barx2 expression in NSCLC at both the mRNA and protein levels. Furthermore, a tissue microarray with data from 75 patients was to examine the correlation between Barx2 expression and clinicopathological parameters of NSCLC. We also investigated the biological function of Barx2 in NSCLC cells.
Methods
Ethics statement
This study was approved by the Research Ethics Committee of The Affiliated Hospital of Xuzhou Medical University. Written informed consent was obtained from each participant before data collection.
Human NSCLC tissue specimens
A total of 75 cases of NSCLC tissue and adjacent normal tissue samples were collected at The Affiliated Hospital of Xuzhou Medical University between January 2005 and December 2010. All the patients were diagnosed and confirmed with NSCLC by histology, and the patients who had received radiotherapy or chemotherapy were excluded. The follow‐up was ended in January 2017. In total, 75 patients were included, 47 men and 28 women, with a median age of 61 years (range 35–81 years). The overall survival time ranged from 5 to 82 months, with a median of 29 months. Detailed information can be found in Table 1.
Table 1.
Baseline characteristics of 75 analyzed non‐small cell lung carcinoma patients
| Characteristics | Patient cohort (n = 75) |
|---|---|
| Median overall survival, months (range) | 29 (5–82) |
| Median age, years (range) | 61 (35–81) |
| ≤60 years, n (%) | 36 (48.0) |
| >60 years, n (%) | 39 (52.0) |
| Sex | |
| Male, n (%) | 47 (62.7) |
| Female, n (%) | 28 (37.3) |
| Smoking habit | |
| Never, n (%) | 45 (60.0) |
| Ever, n (%) | 30 (40.0) |
| Histological type | |
| Adenocarcinoma, n (%) | 30 (40.0) |
| Squamous cell carcinoma, n (%) | 30 (40.0) |
| Others, n (%) | 15 (20.0) |
| Grade | |
| Well, n (%) | 50 (66.7) |
| Moderate to poor, n (%) | 25 (33.3) |
| T stage | |
| T1, n (%) | 15 (20.0) |
| T2, n (%) | 59 (78.7) |
| T3, n (%) | 1 (1.3) |
| N stage | |
| N0, n (%) | 31 (41.3) |
| N1, n (%) | 25 (33.3) |
| N2, n (%) | 19 (25.3) |
| TNM stage | |
| IA, n (%) | 6 (8.0) |
| IB, n (%) | 8 (10.7) |
| IIA, n (%) | 31 (41.3) |
| IIB, n (%) | 7 (9.3) |
| IIIA, n (%) | 23 (30.7) |
Samples were deparaffinized in xylene and rehydrated in a series of graded alcohol. Endogenous peroxidases were blocked by 3% H2O2, and antigen retrieval was carried out after heating in citrate buffer. The sections were incubated with a rabbit polyclonal antibody against Barx2 (diluted 1:100; Abcam, Cambridge, MA, USA) at 4°C overnight and then with horseradish peroxidase (Gene Tech GTVision III Detection Kit, Shanghai, China) at room temperature for 40 minutes. After three washes with phosphate‐buffered saline, the signal was detected with 3, 3′‐diaminobenzidine solution.
Immunohistochemistry scores
Barx2 expression was scored according to staining intensity and the percentage of positive cells, as previously described.12 The percentage of positive cells was scored as follows: no positive cells (0), ≤10% positive cells (1), 10–50% positive cells (2), and >50% positive cells (3). Staining intensity was scored as follows: no staining (0), faint staining (1), moderate staining (2), and dark staining (3). Comprehensive score = staining percentage × intensity. Barx2 expression was classified as follows: ≤6 (low expression) and >6 (high expression).
Cell culture
Human NSCLC cell lines H1299, H460, A549, LK‐2, and one human bronchial epithelium cell line (HBE) were cultured in RPMI‐1640 (Gibco, Invitrogen, NY, USA) supplemented with 10% fetal bovine serum at 37°C in 5% CO2.
Plasmids construction and cell transfection
Both small interfering RNA (siRNA) specially targeting Barx2 and pcDNA3.1‐Barx2 plasmid for gene overexpression test, and their control sequences were obtained from GeneChem (Shanghai, China). For transient transfection, the cells were transfected with plasmids as indicated for 48 hours before performing the functional assays or western blot assays, respectively.
RNA extraction and quantitative real‐time polymerase chain reaction
RNA extraction and real‐time quantitative polymerase chain reaction (PCR) were performed by the manufacturer's instructions (Takara Bio, Shiga, Japan). The used primers were as follows.
Barx2‐sense, 5′‐CATGATCGACGAGATCCTCTCC‐3′
Barx2‐antisense, 5′‐GTTCCGTCTCTGACTCGCTG‐3′
glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) sense, 5′‐AGAAGGCTGGGGCTCATTTG‐3′
GAPDH antisense, 5′‐AGGGGCCATCCACAGTCTTC‐3′
GAPDH was used as an internal control. Each PCR product was run in triplicate. The relative Barx2 mRNA expression was calculated by 2−ΔΔCt.
Western blotting
Cells were lysed with RIPA lysis buffer supplemented with protease inhibitor cocktail, and nuclear and cytoplasmic protein extraction Kit were obtained from Beyotime Biotechnology (Haimen, China). Protein concentrations were determined using the BCA protein concentration reagent kit (Beyotime Biotechnology). Cell lysates were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Primary antibodies specific to Barx2 (1:200) were purchased from Abcam (Cambridge, USA), and GAPDH (1:1000), c‐MYC (1:1000), β‐catenin (1:1000), and HIF‐1α (1:1000) were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell proliferation and plate colony formation assays
The Cell Counting Kit‐8 (CCK‐8; Beyotime Biotechnology) was used to measure cell proliferation by the manufacturer's instructions. After the addition of CCK‐8 solution (10 μL/well), an additional incubation of two hours was carried out before measuring the absorbance at 460 nm. For plate colony formation assays, transfected cells were seeded in six‐well plates (1000 cells/well) and cultured at 37°C under a humidified atmosphere containing 5% CO2 for 10 days. After fixation by methyl alcohol for 15 minutes, cells were stained with 0.1% crystal violet solution for 20 minutes. Colonies were then counted and photographed.
Transwell assay
The transwell 24‐well Boyden chamber (Millipore, Bedford, MA, USA) with 8.0 μm pore size polycarbonate membrane was used for the cell migration assays as previously described.13 Briefly, each group of cells (1 × 105/chamber) was plated in the upper chambers in 100‐μL serum‐free media for 24 hours, while the lower chambers contained 600 μL media supplemented with 10% fetal bovine serum as a chemoattractant. Cells migrated to the reverse side of chamber inserts were fixed by methyl alcohol and stained with 0.1% crystal violet.
Glucose uptake, lactate, adenosine triphosphate and lactate dehydrogenase assays
Lactate Dehydrogenase Activity Assay Kit (Sigma, St. Louis, MO, USA), Lactate Assay Kit (Sigma, St. Louis, MO, USA), Colorimetric Glucose Assay Kit (BioVision, Milpitas, CA, USA), and firefly luciferase‐based adenosine triphosphate (ATP) assay kit (Beyotime Biotechnology) were used to determine glucose uptake, lactate and ATP production, and activities of lactate dehydrogenase (LDH), respectively, according to the manufacturer's protocols. Briefly, the glucose utilization was estimated using a standard glucose calibration curve prepared under the same conditions and reported in micrograms per microliter. The lactate concentration was estimated using a standard lactate calibration curve prepared under the same conditions and reported in nanograms per microliter. ATP contents were calculated according to a standard curve of the supplied ATP standards and normalized using the cellular proteins. The LDH activity was measured by comparing the initial rate with a calibration curve for a known concentration of standard LDH created at the same time. The LDH activity was reported in milliunits per milliliter.
Statistical analysis
SPSS 13.0 statistical software (SPSS Inc. Chicago, IL, USA) was used to perform the statistical analyses. The three‐group comparisons were conducted using one‐way analysis of variance (ANOVA). The χ2‐test or Fisher's exact test were used to determine the significance of differences between Barx2 and clinicopathological variables. Kaplan–Meier curves with log–rank tests were used to calculate the cumulative survival proportion for overall survival by Barx2 expression level. A Cox proportional hazards model was applied to investigate the univariate and multivariate hazard ratios for the study variables. P < 0.05 was considered to show a statistically significant result.
Results
Decreased expression of Barx2 in NSCLC tissues compared with adjacent tissue
Barx2 mRNA expression was confirmed by the quantitative PCR analysis of 20 pairs of lung cancer tissue and matched adjacent normal mucosa. As shown in Figure 1a, the relative level of Barx2 mRNA in 17 out of 20 (85%) tumor tissues showed a significant decrease in comparisons with that of the adjacent normal mucosa (adenocarcinoma, 6/8; squamous cell carcinoma, 7/8; others, 4/4; P < 0.001). Furthermore, Barx2 mRNA levels in the human NSCLC cell lines H460, H1299, LK‐2, and A549 were lower than in the non‐tumorigenic HBE (Fig 1b,c; P < 0.05). Subsequent western blot analysis also confirmed that Barx2 protein levels were downregulated in tumor cell lines as compared with the paired normal cell (Fig 1d; P < 0.05), consistent with the results of quantitative PCR. In addition, immunohistochemical staining showed Barx2 protein was reduced in NSCLC tissues (Fig 2a,c, Table 2; P < 0.05).
Figure 1.
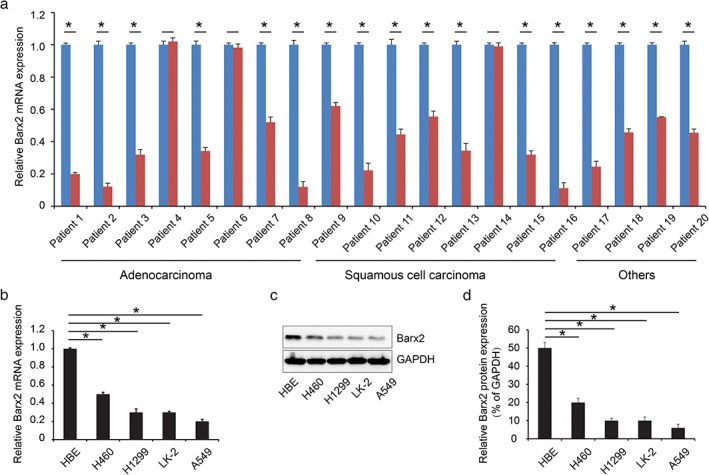
BarH‐like homeobox 2 (Barx2) is overexpressed in non‐small cell lung carcinoma (NSCLC). (a) Quantitative real‐time polymerase chain reaction was performed to analyze mRNA expression of Barx2 in NSCLC tumor tissue and adjacent normal tissue.  Adjacent normal tissue,
Adjacent normal tissue,  Tumor tissue. (b) Quantitative real‐time polymerase chain reaction was used to analyze mRNA expression of Barx2 in NSCLC cell lines and normal epithelial cell line human bronchial epithelium cell line (HBE). (c) Western blotting analysis of protein expression of Barx2 in NSCLC cell lines and normal epithelial cell line HBE. (d) The results of Barx2 protein expression were compared by semi‐quantitative analyses. *P < 0.05.
Tumor tissue. (b) Quantitative real‐time polymerase chain reaction was used to analyze mRNA expression of Barx2 in NSCLC cell lines and normal epithelial cell line human bronchial epithelium cell line (HBE). (c) Western blotting analysis of protein expression of Barx2 in NSCLC cell lines and normal epithelial cell line HBE. (d) The results of Barx2 protein expression were compared by semi‐quantitative analyses. *P < 0.05.
Figure 2.
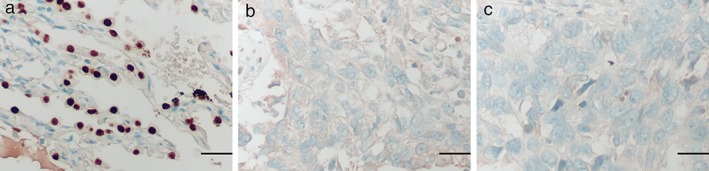
Representative figures of BarH‐like homeobox 2 protein expression in non‐small cell lung carcinoma tumor tissue and adjacent normal tissue using immunohistochemical staining. (a) Adjacent normal tissue. (b) Adenocarcinoma tissue. (c) Squamous cell carcinoma. Scale bar, 25 μm.
Table 2.
Comparisons with BarH‐like homeobox 2 expression between non‐small cell lung carcinoma and paired adjacent normal tissues
| Cases | Barx2 expression | P‐value | ||
|---|---|---|---|---|
| Low | High | |||
| Tumor tissues | 75 | 36 | 39 | <0.001* |
| Adjacent normal tissue | 75 | 15 | 60 | |
P < 0.05.
Total n = 75. Barx2, BarH‐like homeobox 2.
Correlation between Barx2 expression and clinicopathological characteristics
We further evaluated the correlation between Barx2 expression and the clinicopathological characteristics. The patients were divided into two groups (low and high) according to the immunohistochemical scores. As shown in Table 3, Barx2 downregulation was positively correlated with advanced TNM stage (P < 0.0.001) and a high level of Ki‐67 (P < 0.0.001). However, it was not correlated with age (P = 0.426), sex (P = 0.789), smoking habit (P = 0.850), histological type (P = 0.257), grade (P = 1.000), T stage (P = 1.000), and N stage (P = 0.478).
Table 3.
Correlation between the clinicopathological characteristics and BarH‐like homeobox 2 expression in non‐small cell lung carcinoma patients
| Characteristics | N | Barx2 | P‐value | |
|---|---|---|---|---|
| Low (36) | High (39) | |||
| Age (years) | ||||
| ≤60 | 36 | 19 | 17 | 0.426 |
| >60 | 39 | 17 | 22 | |
| Sex | ||||
| Male | 47 | 22 | 25 | 0.789 |
| Female | 28 | 14 | 14 | |
| Smoking habit | ||||
| Never | 45 | 22 | 23 | 0.850 |
| Ever | 30 | 14 | 16 | |
| Histological type | ||||
| Adenocarcinoma | 30 | 11 | 19 | 0.257 |
| Squamous cell carcinoma | 30 | 16 | 14 | |
| Others | 15 | 9 | 6 | |
| Grade | ||||
| Well | 50 | 24 | 26 | 1.000 |
| Moderate to poor | 25 | 12 | 13 | |
| T stage | ||||
| T1 + T2 | 74 | 36 | 38 | 1.000 |
| T3 | 1 | 0 | 1 | |
| N stage | ||||
| N0 | 31 | 13 | 18 | 0.478 |
| N1 + N2 | 44 | 23 | 21 | |
| TNM stage | ||||
| I + II | 51 | 17 | 34 | <0.001* |
| IIIA | 24 | 19 | 5 | |
| Ki‐67 | ||||
| Low | 42 | 10 | 32 | <0.001* |
| High | 33 | 26 | 7 | |
Total n = 75.
Association between Barx2 expression and prognosis
The prognostic effect of Barx2 on the survival rate of NSCLC patients was investigated by Kaplan–Meier survival curves and the log–rank test. The log–rank test showed that patients with NSCLC tumors expressing high Barx2 levels had significantly higher overall survival than those expressing low Barx2 levels (Fig 3a; P < 0.0.001). The cumulative one‐year, three‐year, and five‐year survival rates were only 35% (95% CI 0.193 to 1.507), 10% (95% CI −0.018 to 0.218), and 6% (95% CI −0.038 to 0.158) in the low Barx2 group, whereas it was 97% (95% CI 0.872 to 1.068), 54% (95% CI 0.383 to 0.697), and 14% (95% CI 0.022 to 0.258) in the high Barx2 group (Table 4). In addition, we found that high levels of Ki‐67 had worse prognosis (Fig 3b; P < 0.0.001). To further investigate the association of survival time with Barx2 and Ki‐67 expression, the log–rank test showed that Barx2 (low) Ki‐67 (high) correlated with shorter overall survival of NSCLC patients (Fig 3c; P < 0.001).
Figure 3.
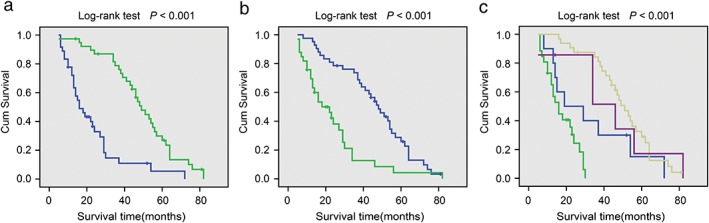
Kaplan–Meier analysis of BarH‐like homeobox 2 (Barx2) and Ki‐67 expression with the prognosis of non‐small cell lung carcinoma patients. (a) Patients with higher Barx2 have a good prognosis.  Barx2 (low),
Barx2 (low),  Barx2 (high). (b) Patients with higher Ki‐67 have a poor prognosis.
Barx2 (high). (b) Patients with higher Ki‐67 have a poor prognosis.  Ki67 (low),
Ki67 (low),  Ki67 (high). (c) Patients with Barx2 (low) Ki‐67 (high) had worst prognosis.
Ki67 (high). (c) Patients with Barx2 (low) Ki‐67 (high) had worst prognosis.  Barx2 (low) Ki67 (low),
Barx2 (low) Ki67 (low),  Barx2 (low) Ki67 (high),
Barx2 (low) Ki67 (high),  Barx2 (high) Ki67 (low),
Barx2 (high) Ki67 (low),  Barx2 (high) Ki67 (high).
Barx2 (high) Ki67 (high).
Table 4.
Comparisons with cumulative survival rate between BarH‐like homeobox 2 low and high groups
| Variables | Cumulative survival rate (95% CI) | ||
|---|---|---|---|
| One year | Three years | Five years | |
| Barx2 | |||
| Low | 35% (0.193–1.507) | 10% (−0.018–0.218) | 6% (−0.038–0.158) |
| High | 97% (0.872–1.068) | 54% (0.383–0.697) | 14% (0.022–0.258) |
Barx2, BarH‐like homeobox 2.
As shown in Table 5, univariate analysis indicated that Barx2 level (P < 0.001), Ki‐67 level (P < 0.001), N stage (P = 0.001), and TNM stage (P < 0.001) were correlated with patient survival. Multivariate analysis showed that Barx2 level was an independent prognostic factor for NSCLC patients (P < 0.001).
Table 5.
Summary of univariate and multivariate Cox regression analysis of overall survival duration in all non‐small cell lung carcinoma patients
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Barx2 | ||||||
| Low | 1 | 1 | ||||
| High | 0.263 | 0.153–0.451 | <0.001* | 0.320 | 0.177–0.577 | <0.001* |
| Ki‐67 | ||||||
| Low | 1 | |||||
| High | 2.789 | 1.643–4.732 | <0.001* | |||
| Age(years) | ||||||
| ≤60 | 1 | |||||
| >60 | 0.680 | 0.408–1.135 | 0.140 | |||
| Sex | ||||||
| Male | 1 | |||||
| Female | 0.925 | 0.545–1.568 | 0.772 | |||
| Smoking habit | ||||||
| Never | 1 | |||||
| Ever | 0.822 | 0.492–1.375 | 0.456 | |||
| Histological type | ||||||
| Adenocarcinoma | 1 | |||||
| Squamous cell carcinoma | 1.041 | 0.598–1.813 | 0.886 | |||
| Others | 0.815 | 0.395–1.681 | 0.580 | |||
| Grade | ||||||
| Well | 1 | |||||
| Moderate to poor | 1.413 | 0.841–2.374 | 0.191 | |||
| T stage | ||||||
| T1 + T2 | 1 | |||||
| T3 | 1.104 | 0.267–4.562 | 0.892 | |||
| N stage | ||||||
| N0 | 1 | |||||
| N1 + N2 | 2.589 | 1.496–4.483 | 0.001* | |||
| TNM stage | ||||||
| I + II | 1 | |||||
| IIIA | 2.922 | 1.661–5.141 | <0.001* | |||
P < 0.05.
95% CI, 95% confidence interval; HR, hazard ratio, Barx2, BarH‐like homeobox 2.
Barx2 inhibits NSCLC cell proliferation, migration, and aerobic glycolysis
To explore the role of Barx2 in NSCLC, we overexpressed Barx2 in A549 cell line (relatively low Barx2 level; Fig 1c). Western blotting was used to confirm the efficiency of Barx2 overexpression (Fig 4a). A CCK‐8 assay and colony formation assay were performed to assess cell proliferation. The results revealed that upregulation of Barx2 expression suppressed cell proliferation significantly (Fig 4b,c; P < 0.05). Transwell assay revealed that overexpression of Barx2 inhibited cell migration (Fig 4d; P < 0.05). In addition, we examined the effect of decreased Barx2 on HBE (non‐tumorigenic) cell proliferation and migration (Fig 5a). The results showed that knockdown of Barx2 expression promoted cell proliferation and migration significantly (Fig 5b,c; P < 0.05).
Figure 4.
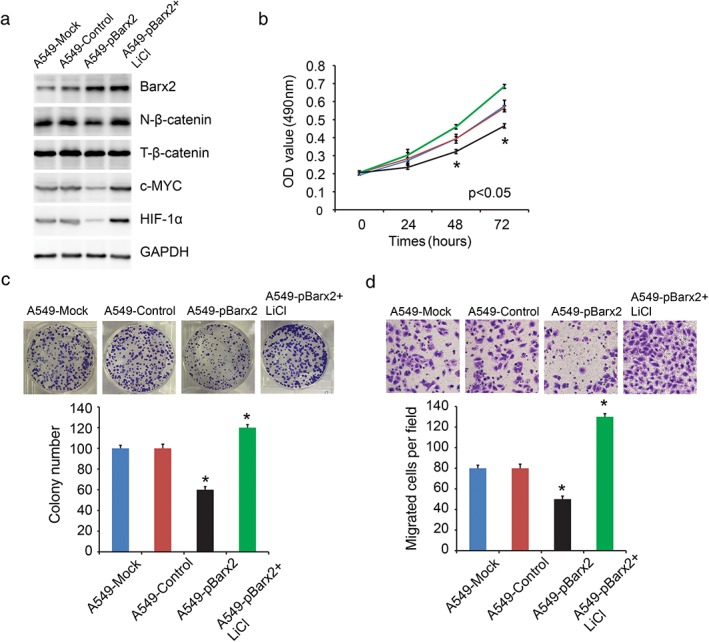
BarH‐like homeobox 2 (Barx2) overexpression inhibits A549 cell proliferation and migration by partially suppressing the Wnt/β‐catenin pathway. (a) Western blotting analysis was used to detect nuclear β‐catenin, total β‐catenin, c‐MYC, and HIF‐1α protein expression. (b) The proliferation curve of cells after transfected with control vector or pBarx2 or pBarx2 + LiCl.  A549‐Mock,
A549‐Mock,  A549‐Control,
A549‐Control,  A549‐pBarx2,
A549‐pBarx2,  A549‐pBarx2+ LiCl. (c) Colony formation assays were performed to assess cell proliferation ability. (d) Transwell assays were performed to detect cell migration ability. *P < 0.05.
A549‐pBarx2+ LiCl. (c) Colony formation assays were performed to assess cell proliferation ability. (d) Transwell assays were performed to detect cell migration ability. *P < 0.05.
Figure 5.
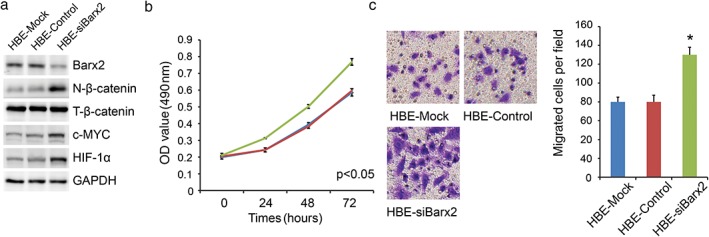
BarH‐like homeobox 2 (Barx2) knockdown promotes human bronchial epithelium cell line (HBE) cell proliferation and migration. (a) Western blotting analysis was used to detect nuclear β‐catenin, total β‐catenin, c‐MYC, and HIF‐1α protein expression. (b) The Cell Counting Kit‐8 assay was used to measure cell proliferation.  HBE‐Mock,
HBE‐Mock,  HBE‐Control,
HBE‐Control,  HBE‐siBarx2 (c) Transwell assays were performed to detect cell migration ability in different groups. *P < 0.05.
HBE‐siBarx2 (c) Transwell assays were performed to detect cell migration ability in different groups. *P < 0.05.
Aerobic glycolysis, also named the Warburg effect, is a common feature of cancer cells, which exhibits aberrant metabolism characterized by high glycolysis even in the presence of abundant oxygen.14, 15 This phenomenon facilitates tumor cell proliferation and migration with elevated glucose uptake and lactate production. Our data showed that Barx2 overexpression markedly decreased LDH activity, glucose utilization, and lactate production, and increased intracellular ATP level in A549 (Fig 6a; P < 0.05). On the contrary, Barx2 knockdown markedly increased LDH activity, glucose utilization, and lactate production, and decreased intracellular ATP level in HBE (Fig 6b; P < 0.05). Taken together, Barx2 inhibits NSCLC cell proliferation, migration and aerobic glycolysis.
Figure 6.
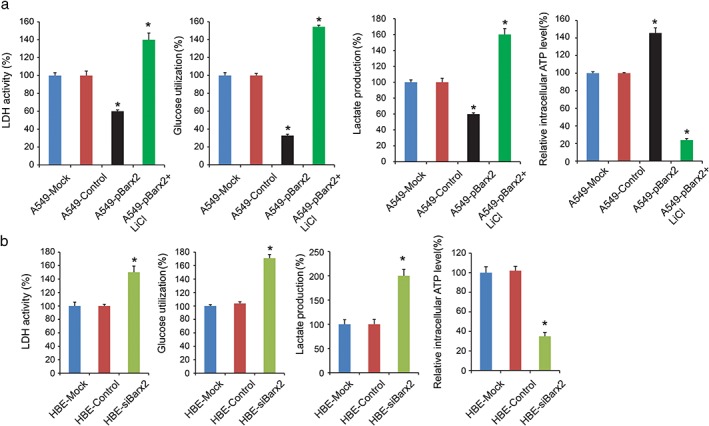
BarH‐like homeobox 2 (Barx2) inhibits aerobic glycolysis through suppressing the Wnt/β‐catenin signaling pathway. (a) The lactate dehydrogenase (LDH) activity, glucose utilization, lactate production, and intracellular adenosine triphosphate (ATP) level were detected in different groups including mock, control, pBarx2, and pBarx2 + LiCl. (b) The LDH activity, glucose utilization, lactate production, and intracellular ATP level were detected in human bronchial epithelium cell line (HBE) cells after transfecting of control vector or siBarx2. *P < 0.05.
Downregulation of Barx2 promotes cell proliferation, migration, and aerobic glycolysis by activating the Wnt/β‐catenin signaling pathway
A previous study revealed that Barx2 inhibited gastric cancer cell proliferation and invasiveness through inhibition of the Wnt/β‐catenin signaling pathway.9 In the present study, Barx2 overexpressing cells (A549‐pBarx2) reduced nuclear β‐catenin, c‐MYC, and HIF‐1α expression (Fig 4a). In comparison, knockdown of Barx2 (HBE‐siBarx2) increased nuclear β‐catenin, c‐MYC, and HIF‐1α expression (Fig 5a). These results supported a role for Barx2 as a negative regulator of the canonical Wnt/β‐catenin pathway in NSCLC cells. Furthermore, we found that activation of the Wnt/β‐catenin pathway by LiCl reversed the suppressing effect of Barx2 on cell proliferation (Fig 4b,c), migration (Fig 4d), and aerobic glycolysis (Fig 6a) in A549 cells.
Discussion
In this study, our data revealed that Barx2 plays an important role in NSCLC proliferation, migration, and aerobic glycolysis. Barx2 is downregulated in NSCLC compared with para‐carcinoma. Barx2 overexpression inhibits NSCLC cell proliferation, migration, and aerobic glycolysis. The underlying mechanism might be involved in suppressing the Wnt/β‐catenin pathway. Survival analysis revealed that the level of Barx2 is an independent prognostic factor for NSCLC patients.
Barx2, located on chromosome 11q24‐25,16 is known to be involved in cytoskeletal organization, cell adhesion, growth factor signaling, and transcriptional regulation, and it acts as a transcription factor.8 Barx2 is also involved in the development of craniofacial structures, salivary glands, hair follicles, and the squamous epithelium of the tongue and esophagus.7, 8, 17, 18 A recent study suggested that it normally acts as a tumor suppressor. Lin et al. reported reduced Barx2 expression, as a miR‐187 target, suppressed the migration, invasion, anchorage‐independent colony formation, and orthotropic tumorigenesis of oral squamous cell carcinoma.19 Mi et al. revealed that low Barx2 expression was associated with colorectal cancer progression and could serve as a potential independent prognostic biomarker for patients with colorectal cancer.20 In gastric cancer, Barx2 is expressed at lower levels than adjacent normal mucosa.9
We observed that Barx2 was downregulated in NSCLC compared with para‐carcinoma. Furthermore, Barx2 expression has a negative correlation with advanced TNM stage and high levels of Ki‐67. Patients with NSCLC tumors expressing high Barx2 levels had significantly higher overall survival than those expressing low Barx2 levels. Measuring Barx2 combined with Ki‐67 expression, we found that the Barx2 (low) Ki‐67 (high) group had the worst prognosis among NSCLC patients. Multivariate analyses showed that Barx2 level was an independent prognostic factor.
Subsequently, we studied the biological function of Barx2 in NSCLC. The results showed that of Barx2 upregulation inhibited cell proliferation and migration significantly. The tumor cells rely mainly on glycolysis, a phenomenon termed the Warburg effect for energy production, even in the presence of sufficient oxygen.21 This metabolic adaptation is believed to be critical for tumor cell growth and proliferation, which is the most outstanding characteristic of energy metabolism in tumor cells.22 Our data indicated that decreased Barx2 expression promoted the Warburg effect, including increased LDH activity, glucose utilization, lactate production, and decreased intracellular ATP level.
The Wnt/β‐catenin signaling pathway has emerged as a key factor during homeostasis and tumorigenesis.23, 24 β‐Catenin, as a central downstream effector of Wnt signaling, plays a critical role in the regulation of tumor growth and development.25 Because Barx2 has been reported to be involved in Wnt signaling, we explored the effect of Barx2 on the Wnt/β‐catenin signaling pathway in NSCLC carcinogenesis.9 Our results reveal that Barx2 inhibits Wnt/β‐catenin signaling in NSCLC cells. Furthermore, reactivation of the Wnt/β‐catenin pathway by LiCl can reverse the inhibiting effect of Barx2 on cell proliferation, migration, and aerobic glycolysis.
In sum, our findings reveal that Barx2 serves as a tumor suppressor gene, and could decrease cell proliferation, migration, and aerobic glycolysis through inhibiting the Wnt/β‐catenin signaling pathway, and predicts a good prognosis in NSCLC patients.
Disclosure
No authors report any conflict of interest.
Acknowledgment
The present study was funded in part by the National Natural Science Foundation of China (81600044).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol 2015; 16: e165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non‐small‐cell lung cancers: A heterogeneous set of diseases. Nat Rev Cancer 2014; 14: 535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch FR, Suda K, Wiens J, Bunn PJ. New and emerging targeted treatments in advanced non‐small‐cell lung cancer. Lancet 2016; 388: 1012–24. [DOI] [PubMed] [Google Scholar]
- 5. Hiley CT, Le Quesne J, Santis G et al Challenges in molecular testing in non‐small‐cell lung cancer patients with advanced disease. Lancet 2016; 388: 1002–11. [DOI] [PubMed] [Google Scholar]
- 6. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 7. Jones FS, Kioussi C, Copertino DW, Kallunki P, Holst BD, Edelman GM. Barx2, a new homeobox gene of the bar class, is expressed in neural and craniofacial structures during development. Proc Natl Acad Sci U S A 1997; 94: 2632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stevens TA, Iacovoni JS, Edelman DB, Meech R. Identification of novel binding elements and gene targets for the homeodomain protein BARX2. J Biol Chem 2004; 279: 14520–30. [DOI] [PubMed] [Google Scholar]
- 9. Mi Y, Zhao S, Zhou C et al Downregulation of homeobox gene Barx2 increases gastric cancer proliferation and metastasis and predicts poor patient outcomes. Oncotarget 2016; 7: 60593–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sellar GC, Li L, Watt KP et al BARX2 induces cadherin 6 expression and is a functional suppressor of ovarian cancer progression. Cancer Res 2001; 61: 6977–81. [PubMed] [Google Scholar]
- 11. Zhang Y, Zhang JX, Huang LL et al Low expression of BARX2 in human primary hepatocellular carcinoma correlates with metastasis and predicts poor prognosis. Hepatol Res 2015; 45: 228–37. [DOI] [PubMed] [Google Scholar]
- 12. Jiao F, Hu H, Han T et al Aberrant expression of nuclear HDAC3 and cytoplasmic CDH1 predict a poor prognosis for patients with pancreatic cancer. Oncotarget 2016; 7: 16505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiao F, Hu H, Yuan C et al Elevated expression level of long noncoding RNA MALAT‐1 facilitates cell growth, migration and invasion in pancreatic cancer. Oncol Rep 2014; 32: 2485–92. [DOI] [PubMed] [Google Scholar]
- 14. Vander HM, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009; 324: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liberti MV, Locasale JW. The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci 2016; 41: 211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hjalt TA, Murray JC. The human BARX2 gene: Genomic structure, chromosomal localization, and single nucleotide polymorphisms. Genomics 1999; 62: 456–9. [DOI] [PubMed] [Google Scholar]
- 17. Naka T, Yokose S. Immunohistochemical localization of barx2 in the developing fetal mouse submandibular glands. Acta Histochem Cytochem 2009; 42: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olson LE, Zhang J, Taylor H, Rose DW, Rosenfeld MG. Barx2 functions through distinct corepressor classes to regulate hair follicle remodeling. Proc Natl Acad Sci U S A 2005; 102: 3708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin SC, Kao SY, Chang JC et al Up‐regulation of miR‐187 modulates the advances of oral carcinoma by targeting BARX2 tumor suppressor. Oncotarget 2016; 7: 61355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mi Y, Zhao S, Zhang W et al Down‐regulation of Barx2 predicts poor survival in colorectal cancer. Biochem Biophys Res Commun 2016; 478: 67–73. [DOI] [PubMed] [Google Scholar]
- 21. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: Therapeutic targeting of the Warburg effect. Acta Pharmacol Sin 2016; 37: 1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daye D, Wellen KE. Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol 2012; 23: 362–9. [DOI] [PubMed] [Google Scholar]
- 23. Kretzschmar K, Clevers H. Wnt/beta‐catenin signaling in adult mammalian epithelial stem cells. Dev Biol 2017; 428: 273–82. [DOI] [PubMed] [Google Scholar]
- 24. Nusse R, Clevers H. Wnt/beta‐catenin signaling, disease, and emerging therapeutic modalities. Cell 2017; 169: 985–99. [DOI] [PubMed] [Google Scholar]
- 25. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta‐catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev 2017; 62: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


