Abstract
The emergence of bone as an endocrine organ able to influence whole body metabolism, together with comorbid epidemics of obesity, diabetes, and osteoporosis, have prompted a renewed interest in the intermediary metabolism of the osteoblast. To date, most studies have focused on the utilization of glucose by this specialized cell, but the oxidation of fatty acids results in a larger energy yield. Osteoblasts express the requisite receptors and catabolic enzymes to take up and then metabolize fatty acids, which appears to be required during later stages of differentiation when the osteoblast is dedicated to matrix production and mineralization. In this article, we provide an overview of fatty acid β-oxidation and highlight studies demonstrating that the skeleton plays a significant role in the clearance of circulating lipoproteins and non-esterified fatty acids. Additionally, we review the requirement for long-chain fatty acid metabolism during post-natal bone development and the effects of anabolic stimuli on fatty acid utilization by osteoblasts. These recent findings may help to explain the skeletal manifestations of human diseases associated with impaired lipid metabolism while also providing additional insights into the metabolic requirements of skeletal homeostasis.
Keywords: Bone, Osteoblast, Fat, Lipid Metabolism, Energy homeostasis
1. Introduction
The osteoblast, derived from a mesenchymal progenitor present in marrow and the periosteum, is the specialized cell tasked with the synthesis, secretion and assembly of the mineralized, collagen-rich matrix that composes bone tissue. Essential to this function is the development of an abundant and well-defined rough endoplasmic reticulum that can produce and package extracelluar matrix proteins during osteoblast maturation (1, 2) as well as the capacity to harvest significant amounts of chemical energy to fuel this intensive process (3). Hierarchical analyses of ATP consumption indicate that protein synthesis, even at levels much lower than would be expected of an active osteoblast, is the most energetically demanding cellular process. As much as 30% of oxygen-coupled ATP production may be dedicated to protein production with the equivalent of 4 ATP molecules required to form each new peptide bond in a growing polypeptide (4, 5). Synthesis of the 3 polypeptides that compose a single type I collagen molecule would therefore be expected to require more than 17,000 ATP equivalents. Surprisingly, detailed investigation of the interplay between intermediary metabolism and bone cell activity has only recently regained the field’s attention (6); likely a result of worldwide epidemics of obesity, diabetes, and osteoporosis and the recognition of comorbidities among these conditions (7–9) as well as the emergence of bone as an endocrine organ that can influence whole body metabolism (10–12).
Studies conducted more than 50 years ago suggested that the osteoblast harvests energy via the metabolism of glucose. In vitro comparisons of glucose uptake by metaphyseal bone slices and liver explants revealed greater uptake in bone samples but a much lower level of oxygen consumption, which implied that glycolytic metabolism is dominant to oxidative metabolism of glucose in the osteoblast (13–16). Neuman and others suggested that acid production via this metabolic paradigm could facilitate the solubility of calcium and phosphate ions in bone’s extracellular fluid by lowering the pH (17–20). More recent studies, utilizing more carefully characterized osteoblast cell models and cultures of primary cells, have confirmed the conversion of glucose to lactate by osteoblasts (21–25). Additionally, molecular genetic studies demonstrated that glucose uptake via the Glut1 transporter is required for early osteoblast commitment and regulates the turnover of the master osteogenic transcription factor, Runx2 (26).
By comparison, the utilization of fatty acids by osteoblasts to fuel bone formation has received relatively little attention. The catabolism of fatty acids yields more energy than the metabolism of glucose as each cycle of β-oxidation (described in detail below) has the potential to generate 17 ATP. The complete oxidation of palmitate (C16), the most common fatty acid in animals, can therefore be used to produce ~131 ATP, whereas the complete oxidation of a glucose molecule can yield just 38. Osteoblasts can oxidize fatty acids and early studies suggested that this might account for as much as 40% to 80% of the energy yield of glucose utilization (27). More recently, we (28) demonstrated that fatty acid oxidation increases dramatically as osteoblasts mature in vitro such that the level of catabolic activity in mineralizing osteoblasts was 3-fold higher than proliferating cells. Moreover, pharmacological inhibition of β-oxidation in vitro impairs osteoblast differentiation (28), while carnitine supplementation to enhance lipid oxidation capacity is associated with an increase in collagen synthesis (29).
In this article, we provide a review of the experimental evidence that osteoblasts utilize fatty acids. We begin with a brief overview of the oxidative pathways by which cells catabolize fatty acids and then highlight potential mechanisms of fatty acid acquisition by osteoblasts. We describe the effect of genetic inhibition of long-chain fatty acid oxidation on bone mass and the regulation of lipid utilization by osteo-anabolic signals. Finally, we conclude with a brief summary of defects in bone metabolism associated with human conditions related to impaired lipid metabolism. For the sake of focus on potential anabolic effects of fatty acid metabolism in bone, we do not discuss the complex effects of high fat diets or oxidized lipids on the skeleton.
2. Overview of fatty acid metabolism
The cellular and physiological metabolism of lipids is critical for maintaining energy balance, membrane dynamics, and the generation of lipid signaling molecules among others. This is accomplished by both the generation of lipids de novo and the ingestion of dietary lipids, some of which mammals do not have the enzymatic machinery to synthesize. Genetic defects in many of the genes encoding this machinery are associated with human disease (described below) that represent stark reminders of the importance of lipid homeostasis
Dietary lipids are packaged in the intestinal epithelial cells as chylomicrons. These large lipoprotein particles are trafficked first through the lymphatic system, which bypasses the liver, and then engage lipoprotein lipase on the endothelial surfaces of target tissues. Very low-density lipoproteins generated and secreted from the liver are metabolized in a similar manner. Lipoproteins can be taken up by receptor-mediated endocytosis at the target cell or alternatively fatty acids liberated by lipoprotein lipase at the endothelial cell surface can be taken up by the target tissue. While the detailed biophysical uptake and shuttling of free fatty acids remains controversial, fatty acids must be first activated by their ligation to Coenzyme A (CoA) by one of 26 Acyl-CoA Synthetases (ACS) encoded by the human genome to be utilized by further metabolic pathways (30, 31). Nonesterified fatty acids have low solubility within cells. The activation of fatty acids to acyl-CoAs traps the fatty acids in cells, makes them more soluble in aqueous solution and generates the high energy thioester to enable downstream acyltransferase reactions. The ACS reaction generates PPi and AMP, therefore every fatty acid activated requires the replenishment of 2 ATP. Following their activation into acyl-CoAs they can then be shuttled into a multitude of anabolic or catabolic pathways. When the incorporation of fatty acids exceeds cellular requirements they can be shuttled and stored in lipid droplets in almost all cells as triglyceride or cholesterol ester for later use.
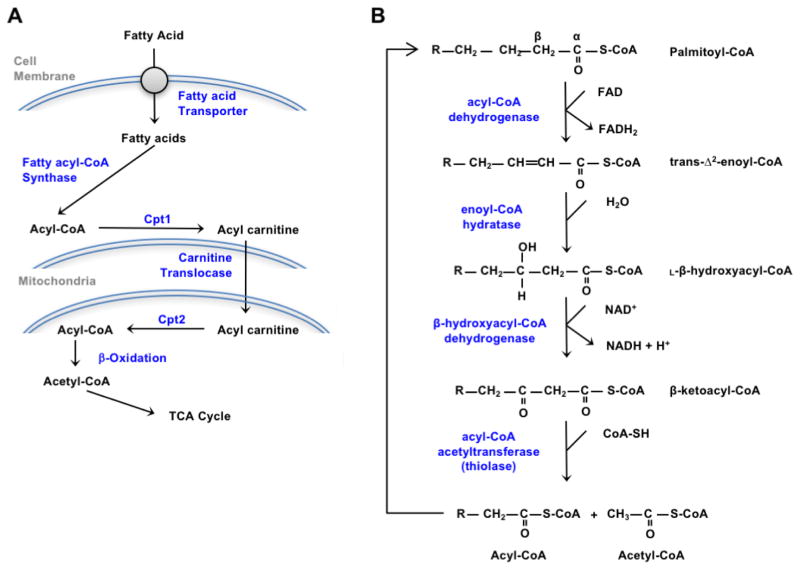
ATP generation is largely accomplished by the β-oxidation of fatty acids within mitochondria, with access to the mitochondrial matrix where the enzymatic machinery is located providing regulatory control over utilization. The transport of long chain fatty acids, the most abundant species that are made and ingested by humans, is mediated by the duel Carnitine Palmitoyltransferase (Cpt) enzymes located on the outer and inner mitochondrial membranes (Figure 1A). Cpt1 located on the outer mitochondrial membrane generates acyl-carnitine from cytoplasmic acyl-CoAs. The acylcarnitines then traverse the inner membrane via the Carntine-Acylcarnitine Translocase. Within the matrix, Cpt2 located on the inner membrane regenerates acyl-CoAs from the acylcarnitines. The regulation of import is mediated in large part from the allosteric inhibition of Cpt1 by malonyl-CoA, the committed step in fatty acid synthesis (32). Cytoplasmic malonyl-CoA is generated by carboxylation of acetyl-CoA via highly regulated acetyl-CoA carboxylase (ACC) and utilized by Fatty Acid Synthase (FASN) to generate palmitate. The carbon for de novo fatty acid synthesis is largely from TCA cycle derived citrate. Fatty acid synthesis, therefore, is an efficient method to store carbohydrates. Excessive carbohydrate intake, particularly fructose, is highly lipogenic.
Figure 1.
Cellular utilization of fatty acids. (A) After entering the cells, fatty acids are activated by fatty acyl-CoA synthase and then converted to acyl-carnitine by carnitine palmitoyltransferase I (Cpt1), the rate-limiting enzyme in fatty acid catabolism. After import into the mitochondrial matrix, Cpt2 converts acyl-carnitine back to acyl-CoA that undergoes β-oxidation. (B) Each cycle of the 4 step β-oxidation process shortens acyl-CoAs by 2 carbons that are then transferred to the TCA cycle in the form of acetyl-CoA. Metabolism of palmitate, with a 16-carbon backbone, requires 7 β-oxidation cycles to generate 8 acetyl-CoA.
Acyl-CoAs are used to generate ATP and reducing equivalents (NADH and FADH2) by the β-oxidation machinery in the mitochondrial matrix. In each cycle of the β-oxidation reactions, 2 carbons are removed from the carboxyl end of the acyl-CoA to form acetyl-CoA (Figure 1B). The four step β-oxidation process involves: 1) the α, β dehydrogenation of the acyl-CoA to yield an enoyl-CoA with a trans double bond between the α and β carbons and FADH2; 2) hydration of the α, β-unsaturated acyl-CoA to β-hydroxyacyl-CoA; 3) the oxidation of β-hydroxyacyl-CoA to form β-ketoacyl-CoA and NADH; and 4) the thiolysis of β-ketoacyl-CoA to yield acetyl-CoA and an acyl-CoA shortened by 2 carbons. One molecule of C16-palmitate undergoes 7 cycles of the oxidative process to generate 8 acetyl-CoA molecules, 7 NADH, and 7 FADH2. Acetyl-CoA enters the TCA cycle, while NADH and FADH are utilized in the electron transport chain to generate ATP. Mono- and polyunsaturated fatty acids must be isomerized and reduced before they can be fully processed by the β-oxidation machinery, but several cycles of β-oxidation may proceed before these reactions. For instance, the monounsaturated fatty acid oleate may undergo 3 cycles of β-oxidation to yield 3 acetyl-CoA before the isomerase enzyme converts the cis double bond to trans.
Short and medium chain fatty acids are also primarily oxidized in mitochondria, but very long chain fatty acids must first be chain shortened in the peroxisome. The peroxisomal β-oxidation machinery cannot fully oxidize fatty acids but will generate acetyl-CoA and octanoyl-CoA. These metabolites can be transferred to mitochondria for further oxidation by both carnitine-dependent and carnitine-independent mechanisms. Defects in peroxisomal fatty acid oxidation often cause devastating disease due in large part to an accumulation of inappropriately long or branched fatty acids. Peroxisomes are also important for the metabolism of branch-chained fatty acids as well as collaborating with the ER in the generation of dicarboxylic fatty acids via ω-oxidation.
3. Fatty acid uptake by osteoblasts
Skeletal lipid uptake has only recently been examined as the in vitro studies highlighted above that identified fatty acids as a potential energy source for osteoblasts did not examine uptake in the context of an intact organism. Using 125I-labelled chylomicron remnants, Neimeier and colleagues (33) compared skeletal uptake of intravenously administered lipoproteins to other lipid-avid tissues and demonstrated a significant role for bone in lipoprotein clearance. Chylomicron remnant uptake by the femoral diaphyses was second only to liver and reached a level that was greater than that in the heart, kidney, and muscle. Additional studies using fluorescent-labeled remnants and electron-microscopy studies revealed that uptake was localized to osteoblasts. These data accord with in vitro findings that demonstrated a requirement for serum lipoproteins for normal osteoblast proliferation (34).
Kim and colleagues (35) performed similar analyses after the delivery of radiolabelled bromo-palmitate (a non-hydrolyzable isoform of the fatty acid) or oleate by gavage. Uptake of both tracers was evident in the femur, tibia, and calvaria at levels comparable to the quadriceps femoris muscle, but was only one-tenth the amount taken up by the liver. As these 3 bones represent only a fraction of the total biomass of the skeleton, it is reasonable to assume that bone also plays a role in free fatty acid clearance. Similar results were obtained by Bartelt et al (36). Kim et. al. (35) (37) predicted that fatty acids taken up by the skeleton are likely to be processed for the generation of ATP as in vivo measures of de novo lipid synthesis by bone were low (approximately a third of gonadal adipose tissue).
Osteoblasts import glucose using the Glut1 or Glut4 transporters (26, 38), but the cellular mechanism by which osteoblasts take up fatty acids has not been well described. Based on gene expression analyses described to date, two potential groups of receptors could fulfill this function. First and inline with Neimeier’s (33) study, osteoblasts express the low-density lipoprotein receptor and low-density lipoprotein related receptor-1 (39, 40) as well as apoE (41, 42), which likely facilitate lipoprotein uptake. It is also plausible that the low-density lipoprotein related receptor-5 (Lrp5) or Lrp6, which are more closely associated with the propagation of Wnt signaling (43), could facilitate lipoprotein uptake. Both receptors have been implicated in lipoprotein trafficking in other cell types (44–47), but data from Frey and colleagues (28) suggests that the contribution of Lrp5 to lipoprotein uptake by osteoblasts is likely to be minimal. Second, osteoblasts express scavenger receptors, like CD36, that could allow for the direct import of free fatty acids and further enable lipoprotein uptake (48, 49). In support of this idea, Kevorkova et al (50) reported that CD36 null mice have a low bone mass phenotype secondary to a reduced bone formation rate and impaired osteoblast differentiation. Lipid uptake by the skeleton of CD36 null mice or osteoblasts isolated from these animals remains to be examined.
It is possible that the Slc27 family of fatty acid transporters, also referred to as FATP1-FATP6 (51), is also used by osteoblasts to acquire fatty acids for oxidation. We have detected expression of FATP1 in cultures of primary calvarial osteoblasts (unpublished data), but the functions of this protein has not yet been tested. To definitively determine the role of any of these receptors in osteoblast function and bone acquisition, as well as their role in the regulation of whole body fatty acid metabolism via the osteoblast will require the development of tissue specific knockout models to eliminate secondary effects in the skeleton due to their actions in other tissues.
4. Long chain fatty acid oxidation is required for normal bone acquisition
Direct evidence of a role for fatty acid oxidation in fueling bone formation can be drawn from Kim et al.’s (35) analysis of mice lacking the gene encoding carnitine palmitoyltransferase-2, an obligate enzyme in fatty acid catabolism, in the osteoblast. As indicated above, Cpt2 regenerates acyl-CoA from acyl-carnitine after it is shuttled into the mitochondrial matrix and is encoded by a single gene, which makes it the ideal target for tissue-specific ablation (52–54). By contrast, the mammalian genome encodes 3 isoforms of Cpt1 (Cpt-1a, Cpt-1b, and Cpt-1c) that exhibit overlapping tissue expression patterns (55–59) and the potential for functional compensation (60).
Cpt2 ablation in the mature osteoblast produced a sexually dimorphic bone phenotype (35). Male Cpt2 mutants exhibited only transient reductions in trabecular bone volume during the rapid growth phase associated with the onset of puberty. The phenotype was much more severe in female mutants, which failed to attain peak trabecular bone volume and exhibited defects in bone volume in the distal femur and L5 vertebrae at all time points examined. Histological analyses performed in the female mutants disclosed a mineralization defect as the numbers of osteoblasts were normal but the mineral apposition rate and as a result the bone formation rate were reduced and the mineralization lag time was dramatically increased. This defect late in the osteoblast differentiation program accords with Frey’s (28) finding that long chain fatty acid oxidation peaks during the matrix deposition and mineralization phase. An explanation for the sexually dimorphic phenotype can be drawn from in vitro studies that demonstrated reductions in fatty acid catabolism due to Cpt2 gene recombination impair in vitro mineralization and that the defect can be exacerbated by the addition of exogenous estrogen. Estrogen favors fatty acid catabolism in a number of tissues (61–64), and when applied to Cpt2 deficient osteoblasts the hormone prevented a switch to glycolytic metabolism. Importantly, a similar phenomenon was evident in vivo where an increase in the uptake of glucose analogs and the expression of lactate dehydrogenase and Glut1 was evident in the bone of male Cpt2 mutants but not female mutants.
Kim’s (35) analysis of osteoblast-specific Cpt2 mutants also confirmed the contribution of the osteoblast to whole-body lipid metabolism. Both male and female mutants exhibited an increase in serum free fatty acid levels, though it remains unclear at present if this was due to a reduction in skeletal lipid uptake or an increase in lipolysis in the adipocyte meant to provide bone with fatty acids to be oxidized. Additionally, male Cpt2 mutants fed a high fat diet develop an increase in hepatic lipid deposition as well as glucose intolerance and insulin resistance in both adipose tissue and bone. Thus, adequate fatty acid utilization by the osteoblast is necessary to maintain skeletal mass, especially in female mice, and for normal lipid metabolism.
Peroxisomal β-oxidation of very long-chain fatty acids appears to be required for normal ossification during development. Brites and colleagues (65) generated mice globally deficient for Pex7, a gene implicated in the Zellweger spectrum disorder (described below) rhizomelic chondrodysplasia punctate and required for the import of oxidative enzymes into peroxisomes (66). In addition to the accumulation of very long chain fatty acids and reduced β-oxidation of C26:0, Pex7−/− mice exhibited a delay in the ossification of a number of skeletal elements including parts of the skull, coccygeal vertebrae, and distal elements of the hind-limbs. Mineralization was also reduced in long bones like the femur, but the numbers of osteoblasts, osteocytes, and osteoclasts were similar in the control and knockout mice. Braverman et al (67) observed a similar phenotype in Pex7−/− mouse line as well as an overall reduction in the size of the skeleton. Direct effects of loss of function mutations in Pex7 or other Pex genes on osteoblast function have not been completed.
A small number of studies have examined how fatty acid utilization by other tissues affects the skeleton. Lee and colleagues (68) queried the role of fatty acid catabolism by the liver in the response to high fat diet-induced obesity and glucose intolerance by abolishing the expression of Cpt2 in hepatocytes. Trabecular bone volume in the distal femur and cortical bone architecture were examined because the liver-specific mutants exhibited dramatic increases in serum Igfbp-1 and Fgf-21, the effects of the later on skeletal homeostasis remaining a matter of debate (69–72). Bone structure in these mice was normal but this study leaves open the more interesting question of how de novo lipid synthesis and VLDL generation in the liver might impact skeletal homeostasis. Bartelt (36) examined the effect of abolishing lipoprotein lipase expression in adipocytes on skeletal structure and lipid composition. Again, this genetic manipulation did not result in an overt bone phenotype but the study did suggest an increase in de novo lipid synthesis in cortical bone that might represent a compensatory mechanism associated with the loss of lipoprotein processing by marrow adipose. This idea is in agreement with the hypothetical role of marrow adipocytes serving as an energy storage site that can be liberated during periods of bone formation (73).
5. Regulation of fatty acid oxidation by anabolic signaling
If fatty acid catabolism is necessary to fuel normal bone formation than it would be expected that osteo-anabolic signals should enhance β-oxidation. Consistent with this idea, Adamek (27) demonstrated that the two quintessential hormones acting on the skeleton, parathyroid hormone and 1,25-dihydroxyvitamin D3, increase palmitate oxidation in cultures of calvarial osteoblasts. By contrast, insulin suppressed the β-oxidation of palmitate. This study is fully compatible with more recent findings that cAMP, a primary signaling response to parathyroid hormone, stimulates β-oxidation and the expression of catabolic genes in other cell types (74–76).
In addition to quantifying fatty acid oxidation at various stages of osteoblast differentiation, Frey and colleagues (28) identified Wnt signaling through the Lrp5 co-receptor as a regulator of fatty acid utilization in osteoblasts. Osteoblast-specific Lrp5 mutants exhibited the expected reductions in bone mass and also accumulated body fat with increases in serum triglycerides and free fatty acids. Microarray studies and subsequent confirmation by quantitative PCR analyses demonstrated that the expression of the genes encoding key catabolic enzymes, including the rate limiting enzyme Cpt1b, are reduced in Lrp5 deficient osteoblast but not those lacking Lrp6. Exogenous Wnt ligands or the forced expression of β-catenin enhanced oleate oxidation in cultured osteoblasts, suggesting that Wnt-mediated regulation of fatty acid metabolism proceeds via a canonical mechanism. Intriguingly, the Long laboratory demonstrated that Wnts stimulate glycolysis in osteoblasts by stimulating mTOR activity (22), indicating that Wnts utilize different signaling mechanisms to differentially affect cellular metabolism.
Mechanical loading as a result of exercise or the various in vivo models used to stimulate bone formation represents another possible anabolic signal that may enhance fatty acid oxidation in the osteoblast. In the skeletal muscle surrounding bone, exercise increases fatty acid oxidation as a result of a switch in substrate utilization away from glucose catabolism (77). However, a direct evaluation of the effects of loading on fuel selection in bone has not been extended beyond Skerry’s (78) initial examination of the effects of loading on the activity of glucose 6-phosphate dehydrogenase activity in periosteal cells and osteocytes. Styner and colleagues have recently provided some indirect evidence of increased fatty acid utilization after voluntary exercise via a running wheel (79). In these studies, exercise reduced marrow adiposity concomitant to an increase in bone volume and induced an increase in the expression of perilipin3, which has been associated with increased lipid catabolism in muscle (80, 81). Additional genetic studies will be required to further our understanding of the fuel requirements of load-induced bone formation.
6. Human Disease
Inborn errors in lipid metabolism due to defects in the genes encoding enzymes in both anabolic or catabolic fatty acid metabolic pathways are associated with human disease (82). In some cases, clinical therapies, with variable effectiveness, have been developed to reset lipid imbalances such as Lorenzo’s oil for adrenoleukodystrophy or statin therapies for hypercholesteremia. In others, the disorders may be lethal within the first few days after birth. We have focused the discussion here on those associated with disorders of fatty acid catabolism. It is important to note that the severity of many of these conditions precludes a determination of whether the skeleton is impacted or if a skeletal phenotype is a direct result of altered bone cell function.
Mutations in the CPT1A and CPT2 genes result in autosomal recessive conditions in which long-chain fatty acid oxidation is dramatically reduced or abolished. Carnitine palmitoyltransferase I deficiency (OMIM #255120, (83)) due to loss of CPT1A function, usually presents in infancy or early childhood. Affected individuals experience severe hypoketotic hypoglycemia that can be brought on or exacerbated by fasting or illness and results in seizures or coma and may lead to death (84–86). Hepatomegaly, muscle weakness and neurological defects are also common. Dietary supplement with medium chain triglycerides alleviates some symptoms because fatty acid from these molecules are able to cross the mitochondrial membrane without the CPT enzymes and can also be used as source of acetyl-CoA for gluconeogenesis. Carnitine palmitoyltransferase II deficiency, due to loss of CPT2 function, results in similar symptoms but may be lethal shortly after birth (lethal neonatal, OMIM #608836), present between 6 and 24 months of age (infantile, OMIM #600649), or onset may be delayed until early adulthood (myopathic, stress-induced, OMIM #255110). Overt skeletal manifestations have not been described for either condition, but Falik-Borenstein (84) described rapid catch-up growth in a 14-month-old girl with carnitine palmitoyltransferase I deficiency after 2 months of medium chain triglyceride therapy, suggestive of some skeletal defect.
Mutations in genes necessary for peroxisome biogenesis and the import of enzymatic machinery to metabolise very long chain fatty acids and branched chain fatty acids are associated with skeletal disorders (87). Mutations in the PEX genes that encode the peroxin proteins result in Zellweger spectrum disorders (88), wherein very long chain fatty acids accumulate in both the serum and cells (89). Severe cases of this disorder usually present in the neonatal period and are associated with a number of developmental malformations (90). Skeletal manifestations include craniofacial abnormalities, like an enlarged fontanelle, and chondrodysplasia punctate. Low bone mineral density and non-traumatic fractures is also a common feature in Zellweger disorders. Bisphosphonate therapy has shown some promise in the treatment of osteopenia in these patients (91).
Niemann-Pick disease and Gaucher’s disease result from mutations in the enzymatic machinery that catabolizes sphinogolipids and are associated with skeletal phenotypes. However, the skeletal defects in these lysosomal storage disorders are likely to be due to secondary effects and the conditions do not involve the metabolism of lipids that are typically used to fuel cellular function.
7. Conclusions
While much still remains to be learned, the experimental evidence highlighted in this review suggests an essential role for fatty acid catabolism in osteoblast function and normal bone formation. When placed in context with our current understanding of glucose utilization by the osteoblast (21, 26, 92), a paradigm emerges wherein glucose fuels fate-specification and osteoblast proliferation and the importance of fatty acid utilization increases during the energetically-costly processes of matrix deposition and mineralization. Developmental and anabolic signals serve as cues to modify intermediary metabolism according the demands of osteoblast function (6). Moreover, the skeleton contributes to the clearance of circulating lipids and disturbances in skeletal fatty acid utilization are sufficient to alter whole body lipid homeostasis in the same way that reductions in insulin receptor signaling or ablation of Glut1 or Glut4 in osteoblasts influence glucose metabolism (26, 38, 93, 94).
Future studies should be directed towards understanding the mechanism by which osteoblasts acquire fatty acids to be used for ATP production. To date, the vast majorities of these studies have used knockout mouse models (ie CD36−/−, Ldlr−/−, and ApoE−/−) with underlying metabolic dysfunction to examine skeletal homeostasis. Emerging evidence suggests that the skeletal phenotype evident in these models does not accurately reflect function in the osteoblast. Additionally, it will be important to determine how changes in nutrient availability alter osteoblast function and need for energy sources can be communicated to fat and other tissues. These data should lend additional credence to the notion that bone is an endocrine organ in tune with and instrumental to the metabolic status of the rest of the body.
Highlights.
The osteoblast contributes to the clearance of lipoproteins and circulating fatty acids
Fatty acid oxidation is required for normal bone acquisition, especially in female mice.
Osteo-anabolic stimuli, like Wnt/β-catenin signaling promotes fatty acid oxidation.
Acknowledgments
Grant Support: NIH DK099134, VA BX001234
Work in the corresponding author’s laboratory is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK099134) and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (BX001234). Additional support is provided by the JHU-UMD Diabetes Research Center (DK079637).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cameron DA. The fine structure of osteoblasts in the metaphysis of the tibia of the young rat. J Biophys Biochem Cytol. 1961;9:583–595. doi: 10.1083/jcb.9.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley HR, Spiro D. The Fine Structure of Bone Cells. J Biophys Biochem Cytol. 1961;11:627–649. doi: 10.1083/jcb.11.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro I, Haselgrove J. Energy Metabolism in Bone. In: Hall B, editor. Bone. CRC Press; 1991. pp. 99–140. [Google Scholar]
- 4.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 5.Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995;312(Pt 1):163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddle RC, Clemens TL. Bone Cell Bioenergetics and Skeletal Energy Homeostasis. Physiol Rev. 2017;97:667–698. doi: 10.1152/physrev.00022.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, Bone IOF, Diabetes Working G. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res. 2008;23:1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh JS, Vilaca T. Obesity, Type 2 Diabetes and Bone in Adults. Calcif Tissue Int. 2017 doi: 10.1007/s00223-016-0229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiGirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nat Rev Rheumatol. 2012;8:674–683. doi: 10.1038/nrrheum.2012.157. [DOI] [PubMed] [Google Scholar]
- 13.Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960;235:1211–1214. [PubMed] [Google Scholar]
- 14.Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:1206–1210. [PubMed] [Google Scholar]
- 15.Cohn DV, Forscher BK. Aerobic metabolism of glucose by bone. J Biol Chem. 1962;237:615–618. [PubMed] [Google Scholar]
- 16.Peck WA, Birge SJ, Jr, Fedak SA. Bone Cells: Biochemical and Biological Studies after Enzymatic Isolation. Science. 1964;146:1476–1477. doi: 10.1126/science.146.3650.1476. [DOI] [PubMed] [Google Scholar]
- 17.Neuman WF, Neuman MW, Brommage R. Aerobic glycolysis in bone: lactate production and gradients in calvaria. Am J Physiol. 1978;234:C41–50. doi: 10.1152/ajpcell.1978.234.1.C41. [DOI] [PubMed] [Google Scholar]
- 18.Neuman MW, Neuman WF. Emerging concepts of the structure and metabolic functions of bone. Am J Med. 1957;22:123–131. doi: 10.1016/0002-9343(57)90343-1. [DOI] [PubMed] [Google Scholar]
- 19.Dixon TF, Perkins HR. Citric acid and bone metabolism. Biochem J. 1952;52:260–265. doi: 10.1042/bj0520260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engel MB, Laskin DM. Bone metabolism and bone resorption after parathyroid extract. AMA Arch Pathol. 1956;62:296–302. [PubMed] [Google Scholar]
- 21.Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155:1589–1595. doi: 10.1210/en.2013-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell metabolism. 2013;17:745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esen E, Lee SY, Wice BM, Long F. PTH Promotes Bone Anabolism by Stimulating Aerobic Glycolysis via IGF Signaling. J Bone Miner Res. 2015;30:1959–1968. doi: 10.1002/jbmr.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc Natl Acad Sci U S A. 2014;111:8673–8678. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279:C1220–1229. doi: 10.1152/ajpcell.2000.279.4.C1220. [DOI] [PubMed] [Google Scholar]
- 26.Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Lezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell. 2015;161:1576–1591. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamek G, Felix R, Guenther HL, Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. Biochem J. 1987;248:129–137. doi: 10.1042/bj2480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, Wolfgang MJ, Clemens TL, Riddle RC. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35:1979–1991. doi: 10.1128/MCB.01343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu KM, Keller ET, Crenshaw TD, Gravenstein S. Carnitine and dehydroepiandrosterone sulfate induce protein synthesis in porcine primary osteoblast-like cells. Calcif Tissue Int. 1999;64:527–533. doi: 10.1007/s002239900644. [DOI] [PubMed] [Google Scholar]
- 30.Watkins PA. Fatty acid activation. Prog Lipid Res. 1997;36:55–83. doi: 10.1016/s0163-7827(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 31.Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. Journal of lipid research. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr. 2008;28:253–272. doi: 10.1146/annurev.nutr.28.061807.155434. [DOI] [PubMed] [Google Scholar]
- 33.Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PC, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43:230–237. doi: 10.1016/j.bone.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Catherwood BD, Addison J, Chapman G, Contreras S, Lorang M. Growth of rat osteoblast-like cells in a lipid-enriched culture medium and regulation of function by parathyroid hormone and 1,25-dihydroxyvitamin D. J Bone Miner Res. 1988;3:431–438. doi: 10.1002/jbmr.5650030410. [DOI] [PubMed] [Google Scholar]
- 35.Kim SP, Li Z, Zoch ML, Frey JL, Bowman CE, Kushwaha P, Ryan KA, Goh BC, Scafidi S, Pickett JE, Faugere MC, Kershaw EE, Thorek DLJ, Clemens TL, Wolfgang MJ, Riddle RC. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartelt A, Koehne T, Todter K, Reimer R, Muller B, Behler-Janbeck F, Heeren J, Scheja L, Niemeier A. Quantification of Bone Fatty Acid Metabolism and Its Regulation by Adipocyte Lipoprotein Lipase. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SP, Li Z, Zoch ML, Frey JL, Bowman CE, Kushwaha P, Ryan KA, Goh BC, Scafidi S, Pickett JE, Faugere MC, Kershaw EE, Thorek DL, Clemens TL, Wolfgang MJ, Riddle RC. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex and diet-dependent manner. JCI Insight. 2017 doi: 10.1172/jci.insight.92704. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kim JK, Riddle RC, Clemens TL. Glucose Transporter-4 Facilitates Insulin-Stimulated Glucose Uptake in Osteoblasts. Endocrinology. 2016;157:4094–4103. doi: 10.1210/en.2016-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U, Heeren J. Expression of LRP1 by human osteoblasts: a mechanism for the delivery of lipoproteins and vitamin K1 to bone. J Bone Miner Res. 2005;20:283–293. doi: 10.1359/JBMR.041102. [DOI] [PubMed] [Google Scholar]
- 40.Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, Ross J, Naot D, Reid IR, Cornish J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol. 2004;18:2268–2278. doi: 10.1210/me.2003-0456. [DOI] [PubMed] [Google Scholar]
- 41.Bachner D, Schroder D, Betat N, Ahrens M, Gross G. Apolipoprotein E (ApoE), a Bmp-2 (bone morphogenetic protein) upregulated gene in mesenchymal progenitors (C3H10T1/2), is highly expressed in murine embryonic development. Biofactors. 1999;9:11–17. doi: 10.1002/biof.5520090103. [DOI] [PubMed] [Google Scholar]
- 42.Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–282. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 43.Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 2003;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–11336. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Mani S, Davis NR, Sarrafzadegan N, Kavathas PB, Mani A. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res. 2008;103:1280–1288. doi: 10.1161/CIRCRESAHA.108.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye ZJ, Go GW, Singh R, Liu W, Keramati AR, Mani A. LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. J Biol Chem. 2012;287:1335–1344. doi: 10.1074/jbc.M111.295287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodeur MR, Brissette L, Falstrault L, Luangrath V, Moreau R. Scavenger receptor of class B expressed by osteoblastic cells are implicated in the uptake of cholesteryl ester and estradiol from LDL and HDL3. J Bone Miner Res. 2008;23:326–337. doi: 10.1359/jbmr.071022. [DOI] [PubMed] [Google Scholar]
- 49.Martineau C, Martin-Falstrault L, Brissette L, Moreau R. The atherogenic Scarb1 null mouse model shows a high bone mass phenotype. Am J Physiol Endocrinol Metab. 2014;306:E48–57. doi: 10.1152/ajpendo.00421.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kevorkova O, Martineau C, Martin-Falstrault L, Sanchez-Dardon J, Brissette L, Moreau R. Low-bone-mass phenotype of deficient mice for the cluster of differentiation 36 (CD36) PLoS One. 2013;8:e77701. doi: 10.1371/journal.pone.0077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Choi J, Scafidi S, Wolfgang MJ. Hepatic Fatty Acid Oxidation Restrains Systemic Catabolism during Starvation. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J, Ellis JM, Wolfgang MJ. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 2015;10:266–279. doi: 10.1016/j.celrep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Choi J, Aja S, Scafidi S, Wolfgang MJ. Loss of Adipose Fatty Acid Oxidation Does Not Potentiate Obesity at Thermoneutrality. Cell Rep. 2016;14:1308–1316. doi: 10.1016/j.celrep.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britton CH, Schultz RA, Zhang B, Esser V, Foster DW, McGarry JD. Human liver mitochondrial carnitine palmitoyltransferase I: characterization of its cDNA and chromosomal localization and partial analysis of the gene. Proc Natl Acad Sci U S A. 1995;92:1984–1988. doi: 10.1073/pnas.92.6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamazaki N, Shinohara Y, Shima A, Yamanaka Y, Terada H. Isolation and characterization of cDNA and genomic clones encoding human muscle type carnitine palmitoyltransferase I. Biochim Biophys Acta. 1996;1307:157–161. doi: 10.1016/0167-4781(96)00069-3. [DOI] [PubMed] [Google Scholar]
- 57.Price N, van der Leij F, Jackson V, Corstorphine C, Thomson R, Sorensen A, Zammit V. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 2002;80:433–442. doi: 10.1006/geno.2002.6845. [DOI] [PubMed] [Google Scholar]
- 58.Wolfgang MJ, Lane MD. Control of energy homeostasis: role of enzymes and intermediates of fatty acid metabolism in the central nervous system. Annu Rev Nutr. 2006;26:23–44. doi: 10.1146/annurev.nutr.25.050304.092532. [DOI] [PubMed] [Google Scholar]
- 59.Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T, Lane MD. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci U S A. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab. 2014;16:757–760. doi: 10.1111/dom.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell SE, Febbraio MA. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E803–808. doi: 10.1152/ajpendo.2001.281.4.E803. [DOI] [PubMed] [Google Scholar]
- 62.Hatta H, Atomi Y, Shinohara S, Yamamoto Y, Yamada S. The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm Metab Res. 1988;20:609–611. doi: 10.1055/s-2007-1010897. [DOI] [PubMed] [Google Scholar]
- 63.Herrero P, Soto PF, Dence CS, Kisrieva-Ware Z, Delano DA, Peterson LR, Gropler RJ. Impact of hormone replacement on myocardial fatty acid metabolism: potential role of estrogen. J Nucl Cardiol. 2005;12:574–581. doi: 10.1016/j.nuclcard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Kendrick ZV, Ellis GS. Effect of estradiol on tissue glycogen metabolism and lipid availability in exercised male rats. J Appl Physiol (1985) 1991;71:1694–1699. doi: 10.1152/jappl.1991.71.5.1694. [DOI] [PubMed] [Google Scholar]
- 65.Brites P, Motley AM, Gressens P, Mooyer PA, Ploegaert I, Everts V, Evrard P, Carmeliet P, Dewerchin M, Schoonjans L, Duran M, Waterham HR, Wanders RJ, Baes M. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hu Mol Genet. 2003;12:2255–2267. doi: 10.1093/hmg/ddg236. [DOI] [PubMed] [Google Scholar]
- 66.Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 67.Braverman N, Zhang R, Chen L, Nimmo G, Scheper S, Tran T, Chaudhury R, Moser A, Steinberg S. A Pex7 hypomorphic mouse model for plasmalogen deficiency affecting the lens and skeleton. Mol Genet Metab. 2010;99:408–416. doi: 10.1016/j.ymgme.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J, Choi J, Selen Alpergin ES, Zhao L, Hartung T, Scafidi S, Riddle RC, Wolfgang MJ. Loss of Hepatic Mitochondrial Long-Chain Fatty Acid Oxidation Confers Resistance to Diet-Induced Obesity and Glucose Intolerance. Cell Rep. 2017;20:655–667. doi: 10.1016/j.celrep.2017.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, Stanislaus S, Asuncion F, Niu QT, Chinookoswong N, Villasenor K, Wang J, Wong P, Boyce R, Dwyer D, Han CY, Chen MM, Liu B, Stolina M, Ke HZ, Ominsky MS, Veniant MM, Xu J. FGF21 Is Not a Major Mediator for Bone Homeostasis or Metabolic Actions of PPARalpha and PPARgamma Agonists. J Bone Miner Res. 2017;32:834–845. doi: 10.1002/jbmr.2936. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Wei W, Krzeszinski JY, Wang Y, Wan Y. A Liver-Bone Endocrine Relay by IGFBP1 Promotes Osteoclastogenesis and Mediates FGF21-Induced Bone Resorption. Cell metabolism. 2015;22:811–824. doi: 10.1016/j.cmet.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, MacDougald O, Baron R, Lotinun S, Karsenty G, Wei W, Ferron M, Kovacs CS, Clemmons D, Wan Y, Rosen CJ. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014;155:3516–3526. doi: 10.1210/en.2014-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, Wan Y. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 74.Lv S, Qiu X, Li J, Liang J, Li W, Zhang C, Zhang ZN, Luan B. Glucagon-induced extracellular cAMP regulates hepatic lipid metabolism. J Endocrinol. 2017;234:73–87. doi: 10.1530/JOE-16-0649. [DOI] [PubMed] [Google Scholar]
- 75.Lim JH, Gerhart-Hines Z, Dominy JE, Lee Y, Kim S, Tabata M, Xiang YK, Puigserver P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1alpha complex. J Biol Chem. 2013;288:7117–7126. doi: 10.1074/jbc.M112.415729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerhart-Hines Z, Dominy JE, Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+) Mol Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res. 1989;4:783–788. doi: 10.1002/jbmr.5650040519. [DOI] [PubMed] [Google Scholar]
- 79.Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, Xie Z, Zong X, Styner MA, Rubin CT, Rubin J. Exercise Decreases Marrow Adipose Tissue Through ss-Oxidation in Obese Running Mice. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Covington JD, Noland RC, Hebert RC, Masinter BS, Smith SR, Rustan AC, Ravussin E, Bajpeyi S. Perilipin 3 Differentially Regulates Skeletal Muscle Lipid Oxidation in Active, Sedentary, and Type 2 Diabetic Males. J Clin Endocrinol Metab. 2015;100:3683–3692. doi: 10.1210/JC.2014-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramos SV, Turnbull PC, MacPherson RE, LeBlanc PJ, Ward WE, Peters SJ. Changes in mitochondrial perilipin 3 and perilipin 5 protein content in rat skeletal muscle following endurance training and acute stimulated contraction. Exp Physiol. 2015;100:450–462. doi: 10.1113/expphysiol.2014.084434. [DOI] [PubMed] [Google Scholar]
- 82.Wanders RJ, Vreken P, den Boer ME, Wijburg FA, van Gennip AH, LIJ Disorders of mitochondrial fatty acyl-CoA beta-oxidation. J Inherit Metab Dis. 1999;22:442–487. doi: 10.1023/a:1005504223140. [DOI] [PubMed] [Google Scholar]
- 83.Online Mendelian Inheritance in Man, OMIM®. Baltimore, MD: McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University; Available from: https://omim.org/ [Google Scholar]
- 84.Falik-Borenstein ZC, Jordan SC, Saudubray JM, Brivet M, Demaugre F, Edmond J, Cederbaum SD. Brief report: renal tubular acidosis in carnitine palmitoyltransferase type 1 deficiency. N Engl J Med. 1992;327:24–27. doi: 10.1056/NEJM199207023270105. [DOI] [PubMed] [Google Scholar]
- 85.Bougneres PF, Saudubray JM, Marsac C, Bernard O, Odievre M, Girard J. Fasting hypoglycemia resulting from hepatic carnitine palmitoyl transferase deficiency. J Pediatr. 1981;98:742–746. doi: 10.1016/s0022-3476(81)80834-7. [DOI] [PubMed] [Google Scholar]
- 86.Bonnefont JP, Haas R, Wolff J, Thuy LP, Buchta R, Carroll JE, Saudubray JM, Demaugre F, Nyhan WL. Deficiency of carnitine palmitoyltransferase I. J Child Neurol. 1989;4:198–203. doi: 10.1177/088307388900400310. [DOI] [PubMed] [Google Scholar]
- 87.Wanders RJ. Peroxisomes, lipid metabolism, and peroxisomal disorders. Mol Genet Metab. 2004;83:16–27. doi: 10.1016/j.ymgme.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 88.Reuber BE, Germain-Lee E, Collins CS, Morrell JC, Ameritunga R, Moser HW, Valle D, Gould SJ. Mutations in PEX1 are the most common cause of peroxisome biogenesis disorders. Nat Genet. 1997;17:445–448. doi: 10.1038/ng1297-445. [DOI] [PubMed] [Google Scholar]
- 89.Poulos A, Singh H, Paton B, Sharp P, Derwas N. Accumulation and defective beta-oxidation of very long chain fatty acids in Zellweger’s syndrome, adrenoleukodystrophy and Refsum’s disease variants. Clin Genet. 1986;29:397–408. doi: 10.1111/j.1399-0004.1986.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 90.Braverman NE, Raymond GV, Rizzo WB, Moser AB, Wilkinson ME, Stone EM, Steinberg SJ, Wangler MF, Rush ET, Hacia JG, Bose M. Peroxisome biogenesis disorders in the Zellweger spectrum: An overview of current diagnosis, clinical manifestations, and treatment guidelines. Mol Genet Metab. 2016;117:313–321. doi: 10.1016/j.ymgme.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rush ET, Goodwin JL, Braverman NE, Rizzo WB. Low bone mineral density is a common feature of Zellweger spectrum disorders. Mol Genet Metab. 2016;117:33–37. doi: 10.1016/j.ymgme.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Esen E, Long F. Aerobic glycolysis in osteoblasts. Curr Osteoporos Rep. 2014;12:433–438. doi: 10.1007/s11914-014-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]