Abstract
We have recently reported that a short course of morphine, starting 10 days after sciatic chronic constriction injury (CCI), prolonged the duration of mechanical allodynia for months after morphine ceased. Maintenance of this morphine-induced persistent sensitization was dependent on spinal NOD-like receptor protein 3 (NLRP3) inflammasomes—protein complexes that proteolytically activate interleukin-1β (IL-1β) via caspase-1. However, it is still unclear how NLRP3 inflammasome signaling is maintained long after morphine is cleared. Here, we demonstrate that spinal levels of the damage associated molecular patterns (DAMPs) high mobility group box 1 (HMGB1) and biglycan are elevated during morphine-induced persistent sensitization in male rats; that is, 5 weeks after cessation of morphine dosing. We also show that HMGB1 and biglycan levels are at least partly dependent on the initial activation of caspase-1, as well as Toll like receptor 4 (TLR4) and the purinergic receptor P2X7R—receptors responsible for priming and activation of NLRP3 inflammasomes. Finally, pharmacological attenuation of the DAMPs HMGB1, biglycan, heat shock protein 90 and fibronectin persistently reversed morphine-prolonged allodynia. We conclude that after peripheral nerve injury, morphine treatment results in persistent DAMP release via TLR4, P2X7R and caspase-1, which are involved in formation/activation of NLRP3 inflammasomes. These DAMPs are responsible for maintaining persistent allodynia, which may be due to engagement of a positive feedback loop, in which NLRP3 inflammasomes are persistently activated by DAMPs signaling at TLR4 and P2X7R.
Keywords: danger signals, priming, opioid-induced hyperalgesia, glia
1. Introduction
Opioids remain a gold-standard analgesic for moderate-to-severe chronic pain. Despite the recent escalation in opioid prescriptions for chronic pain, there are no clinical studies that have rigorously evaluated the long-term consequences of opioid use (Chou et al., 2015). Beyond simply an absence of benefit, there is growing evidence of harm among adults prescribed long-term opioid therapy (Chou et al., 2015; Frank et al., 2017; Hoffman et al., 2017; Sommer, 2017). We have discovered an additional negative consequence for pain in rats: a 5-day course of a moderate morphine dose, starting 10 days after sciatic chronic constriction injury (CCI), prolonged the duration of CCI-allodynia for months after morphine ceased (Grace et al., 2016). Maintenance of this morphine-induced persistent sensitization was dependent on spinal NOD-like receptor protein 3 (NLRP3) inflammasomes—protein complexes that proteolytically activate interleukin-1β (IL-1β) via caspase-1 (Grace et al., 2016). However, an unresolved question from our study is how NLRP3 inflammasome signaling is maintained long after morphine is cleared (Grace et al., 2016).
NLRP3 inflammasomes can be primed and activated by signaling through Toll like receptor 4 (TLR4) and the purinergic receptor P2X7, and both receptors are essential for morphine-induced persistent sensitization (Grace et al., 2016). TLR4 and P2X7R are activated after peripheral nerve injury by endogenous damage associated molecular patterns (DAMPs) that are released by stressed cells in the spinal cord (Grace et al., 2014). As a consequence of inflammasome activation, IL-1β may exacerbate cellular DAMP release (e.g. by disrupting glutamate homeostasis). As morphine-induced persistent sensitization was associated with persistent downregulation of the spinal astrocyte glutamate transporter GLT-1 (Grace et al., 2016), exacerbated DAMP release could be a consequence that leads to continued activation of NLRP3 inflammasomes in a positive feedback loop.
The first of three goals of this study was to determine whether the spinal DAMPs high mobility group box 1 (HMGB1) and biglycan were elevated in the lumbar dorsal spinal cord in our model of morphine-induced persistent sensitization. HMGB1 is a nuclear protein that is expressed by most cells. HMGB1 is released principally by neurons upon peripheral nerve injury, where it can induce an inflammatory response in surrounding cells (Agalave and Svensson, 2015). Biglycan is also expressed in the nucleus of spinal neurons, and in cultured astrocytes, but its function in these cells is not understood (Koops et al., 1996; Liang et al., 1997). Biglycan is upregulated after CNS injury, where it can be expressed by macrophages/microglia, and neurons (Stichel et al., 1995). A potential role for biglycan in pain has not been previously investigated. These representative DAMPs were selected due to their potential to activate NLRP3 inflammasomes: HMGB1 is a TLR4 agonist, while biglycan is an agonist of both TLR4 and P2X7R (Babelova et al., 2009; Frank et al., 2015; Grace et al., 2014). The second goal was to determine whether levels of HMGB1 and biglycan were dependent on TLR4, P2X7R or caspase-1. Finally, we aimed to identify a causal role for spinal DAMPS in the maintenance of morphine-induced persistent sensitization.
2.0. Methods
2.1. Subjects
Pathogen-free adult male Fischer 344 (F344) rats (n = 5–7 rats/group for each experiment; 10–12 wks old on arrival; Harlan Labs, Indianapolis, IN, USA) were used. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado Boulder.
2.2. Drugs
Morphine was obtained from the National Institute on Drug Abuse (Research Triangle Park, NC and Bethesda, MD, USA). (+)-Naloxone was synthesized by Dr. Kenner Rice. BoxA (HMGBiotech, Milan, Italy), fibronectin tetrapeptide (Santa Cruz Biotechnology, Dallas, TX) and 17-dimethylaminoethylamino-17-desmethoxygeldanamycin (17-DMAG; Calbiochem, San Diego, CA) were obtained commercially. Where applicable, drugs were prepared and are reported as free base concentrations.
2.3. RNA interference
Small interfering RNAs (siRNAs) against biglycan (sense: GAUCUCACCUGAUACCACAtt; antisense: UGUGGUAUCAGGUGAGAUCtc) were purchased from Life Technologies (Carlsbad, CA). siRNA or missense control (0.24 µg/µl in DNase-free water) was mixed at a 1:1 ratio with RNAiMAX transfection reagent (6% v/v in PBS; Life Technologies), according to manufacturer instructions.
2.4. Chronic constriction injury (CCI)
Neuropathic pain was induced using the CCI model of sciatic nerve injury (Bennett and Xie, 1988), as described previously (Grace et al., 2016). Four sterile chromic gut sutures (cuticular 4-0 chromic gut, Ethicon, Somerville, NJ) were loosely tied around the gently isolated sciatic nerve. For sham surgery, the sciatic nerve was isolated, but no chromic gut sutures were tied around the nerve.
2.5. Acute and chronic intrathecal catheter implantation
The method of acute intrathecal drug administration and the construction and implantation of the indwelling intrathecal catheters was based on that described previously (Grace et al., 2016). In brief, intrathecal operations were conducted under isoflurane anesthesia by threading sterile polyethylene-10 tubing (PE-10 Intramedic Tubing; Becton Dickinson Primary Care Diagnostics, Sparks, MD, USA) guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheters were attached to a pre-loaded osmotic minipump where appropriate (Alzet, 2001, Cupertino, CA).
2.6. Subcutaneous and intrathecal drug administration
Morphine was administered subcutaneously (s.c.) at 5 mg/kg, twice daily. Intrathecal osmotic minipump infusions were as follows, (+)-naloxone: 60 µg/h; A438079: 30 ng/h; ac-YVAD-cmk: 1 µg/h, based on our previous report (Grace et al., 2016). The DAMP inhibitor cocktail was composed of Bgn siRNA (1.2 µg in 10 µl), BoxA (5 µg in 5 µl), 17-DMAG (10 µg in 1 µl) and fibronectin tetrapeptide (10 µg in 10 µl). The cocktail was administered daily for 7 days, beginning 5 weeks after morphine dosing conclusion. Respective equivolume vehicles were used as controls.
2.7. Western blotting
Western blot analyses were performed on L4/5 spinal ipsilateral dorsal quadrants obtained from rats used in our previous study (Grace et al., 2016), that had been transcardially perfused with saline under sodium pentobarbital anesthesia (100 mg/kg; intraperitoneal). Protein extraction, gel electrophoresis, membrane transfer and antibody incubation was performed as previously described (Grace et al., 2016). Primary antibodies and dilution ratios used were: rabbit HMGB1 1:4000 (Abcam, Cambridge, MA), rabbit biglycan 1:200 (Santa Cruz). Mouse β actin 1:100,000 (Sigma, St. Louis, MO) was used against loading control protein. Secondary antibodies used were: Goat anti-mouse IRDye 680RD 1:15,000 (LI-COR Biosciences), and goat anti-rabbit IRDye 800CW 1:15,000 (LI-COR Biosciences). Bands were quantified using Image Studio (LI-COR Biosciences).
2.8. Mechanical allodynia
Testing was conducted blind with respect to group assignment. The von Frey test (Chaplan et al., 1994) was performed at the distal region of the heel in the hind paws, within the region of sciatic innervation as previously described (Grace et al., 2016). The behavioral responses were used to calculate absolute threshold (the 50% probability of response) as described previously (Grace et al., 2016).
2.9. Statistics
Mechanical allodynia was analyzed as the interpolated 50% thresholds (absolute threshold). One-way ANOVAs followed by Tukey’s post hoc test was used to confirm that there were no baseline differences in absolute thresholds between treatment groups. Differences between treatment groups were determined using repeated measures two-way ANOVA, followed by Sidak’s post hoc test. Integrated Densities for Western blots were analyzed by two-way ANOVA and Sidak’s post hoc test, or unpaired t test, as appropriate. All data are presented as mean ± SEM, and P < 0.05 was considered significant.
3.0. Results
3.1. DAMPs are elevated with morphine-induced persistent sensitization
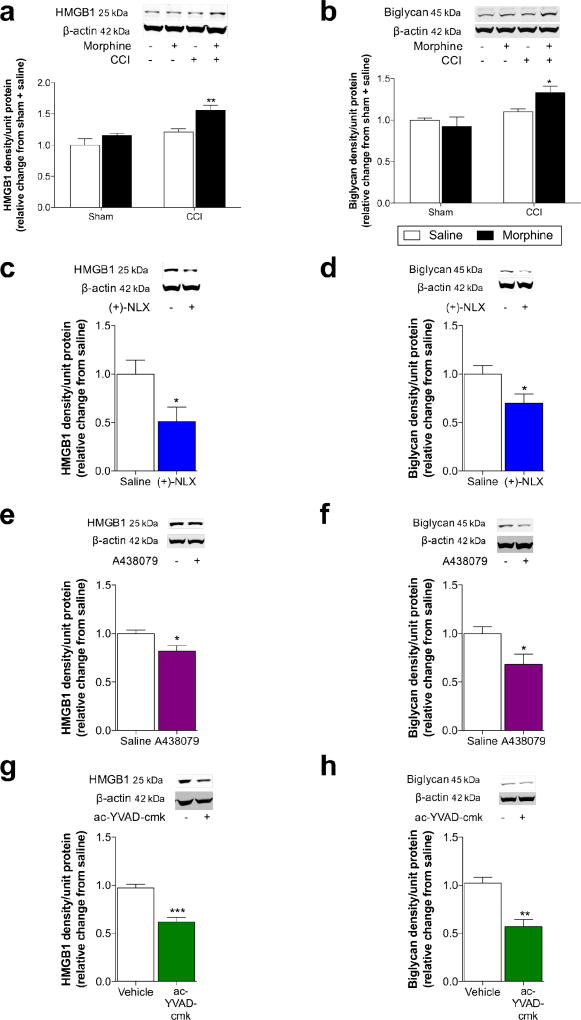
We first tested whether levels of the spinal DAMPs HMGB1 and biglycan were elevated by the CCI and morphine treatment. As illustrated in Fig. S1, CCI or sham surgery was performed, followed ten days later—when neuropathic pain was fully established—by a 5-day course of morphine (5 mg/kg b.i.d., s.c.) or saline vehicle. We have previously shown that CCI-allodynia resolves 5 weeks after saline treatment, while CCI-allodynia persists for 11 weeks after morphine treatment (Grace et al., 2016). Therefore, DAMP levels were assessed in the ipsilateral lumbar dorsal quadrant of the spinal cord at 5 weeks after morphine/saline treatment—the period of persistent sensitization. Levels of HMGB1 (Fig. 1a; Surgery × Drug: F1,20 = 1.92, P = 0.18; Surgery: F1,20 = 19.0, P < 0.001; Drug: F1,20 = 12.4, P < 0.01) and biglycan (Fig. 1b; Surgery × Drug: F1,20 = 4.68, P < 0.05; Surgery: F1,20 = 12.8, P < 0.01; Drug: F1,20 = 1.21, P = 0.2) were elevated during morphine induced persistent sensitization.
Figure 1. Morphine-induced persistent sensitization is associated with increased DAMP levels, which are dependent on TLR4, P2X7R and caspase-1.
(a, b) CCI or sham surgery was performed, followed ten days later by a 5-day course of morphine (5 mg/kg b.i.d., s.c.) or saline vehicle. (a) HMGB1 and (b) biglycan levels were elevated in ipsilateral lumbar dorsal spinal cord quadrants, 5 weeks after morphine/saline administration. (c–h) Inhibitors were continuously intrathecally infused during morphine administration, and DAMP levels was assessed in the ipsilateral lumbar dorsal quadrant of the spinal cord at 5 weeks after morphine/inhibitor treatment. HMGB1 and biglycan levels were decreased by (c, d) the TLR4 antagonist (+)-naloxone (60 µg/h), (e, f) the P2X7R antagonist A438079 (30 ng/h), and (g, h) the caspase-1 inhibitor ac-YVAD-cmk (1 µg/h). (a, b) *P < 0.05, **P < 0.01, relative to Sham+Saline. (c–h) *P < 0.05, **P < 0.01, ***P < 0.001. N = 6/group.
3.2. Blocking TLR4, P2X7R or caspase-1 prevents DAMP elevation with morphine-induced persistent sensitization
The next series of experiments aimed to determine whether inhibition of TLR4, P2X7R or caspase-1 was sufficient to prevent the increase in HMGB1 and biglycan levels. These receptors were targeted due to their role in priming and activation of the NLRP3 inflammasome, while caspase-1 is the enzyme responsible for the proteolytic activation of IL-1β. Inhibitors were continuously intrathecally infused during morphine administration, due to their ability to prevent and reverse persistent sensitization (Grace et al., 2016). Levels of HMGB1 and biglycan were assessed in the ipsilateral lumbar dorsal quadrant of the spinal cord at 5 weeks after morphine/inhibitor treatment. The TLR4 antagonist (+)-naloxone (Hutchinson et al., 2008) prevented increased levels of HMGB1 (Fig. 1c; P < 0.05) and biglycan (Fig. 1d; P < 0.05). Similar results were also found for the P2X7R antagonist A438079 on the levels of HMGB1 (Fig. 1e; P < 0.05) and biglycan (Fig. 1f; P < 0.05), as well as the caspase-1 inhibitor ac-YVAD-cmk on the levels of HMGB1 (Fig. 1g; P < 0.001) and biglycan (Fig. 1h; P < 0.01).
3.3. Inhibiting spinal DAMPs reverses morphine-induced persistent sensitization
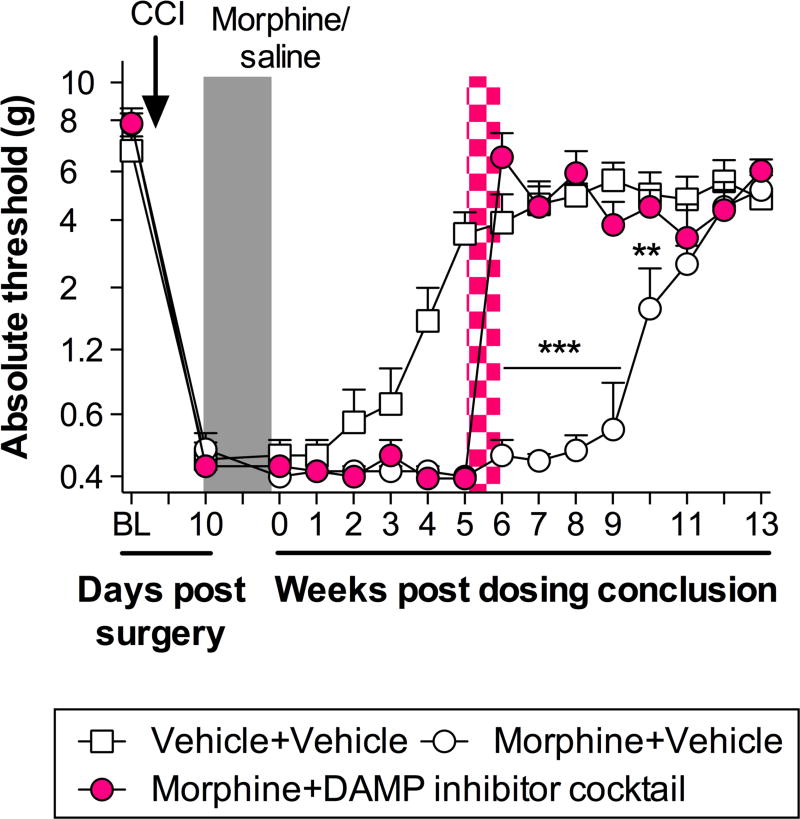
The final experiment sought to determine whether the inflammasome-dependent increase in DAMP levels was causal to morphine-induced persistent sensitization. A cocktail of inhibitors was intrathecally administered for 7 days, beginning five weeks after the conclusion of morphine administration. Given that a range of DAMPs have been implicated in neuropathic pain, it was likely that morphine-induced sensitization was mediated by more than just the representative HMGB1 and biglycan, assessed above. Therefore, heat shock protein 90 (HSP90) and fibronectin were also inhibited due to their activity at TLR4 and known role in pain (Hutchinson et al., 2009; Inoue and Tsuda, 2012; Okamura et al., 2001). Repeated intrathecal injections of BoxA (HMGB1 inhibitor), Biglycan siRNA (knockdown verified in Fig. S2), 17-DMAG (HSP90 inhibitor) and fibronectin tetrapeptide (fibronectin inhibitor) reversed established persistent sensitization in F344s (Fig. 2; Time × Treatment: F28,210 = 15.44, P < 0.001; Time: F14,210 = 103.7, P < 0.001; Treatment: F2,15 = 30.68, P < 0.001). As observed previously, allodynia due to CCI surgery resolved within 5 weeks of saline treatment, in the absence of morphine (Fig. 2) (Grace et al., 2016). These data support a role for the spinal DAMP-mediated signaling in the maintenance of morphine-induced persistent sensitization.
Figure 2. Spinal DAMPs maintain morphine-induced persistent sensitization.
A DAMP inhibitor cocktail against HMGB1 (BoxA; 5 µg in 5 µl), biglycan (siRNA; 1.2 µg in 10 µl), HSP90 (17-DMAG; 10 µg in 1 µl) and fibronectin (fibronectin tetrapeptide; 10 µg in 10 µl) was administered daily for 7 days via intrathecal injection (pink hatch; 7 days). DAMP inhibitor treatment began 5 weeks after morphine administration (5 days, 5 mg/kg b.i.d. administered 10 days after CCI; shaded panel) and absolute thresholds for mechanical allodynia quantified. Saline vehicle treatment (5 days, administered 10 days after CCI; shaded panel) is included for comparison (BL: Baseline). *P < 0.05, **P < 0.01, ***P < 0.001, inhibitor vs. vehicle control. N = 6/group.
4. Discussion
We recently discovered that morphine treatment after CCI induced allodynia that persisted for months after dosing had concluded, and was dependent on activation of spinal NLRP3 inflammasomes (Grace et al., 2016). We postulated that this was due to enduring spinal release of DAMPs that initiate TLR4 and P2X7R signaling (Grace et al., 2014), together with nonstereoselective opioid activation of TLR4 (Grace et al., 2015). However, an outstanding question from our prior work related to the mechanisms by which NLRP3 inflammasomes could remain activated in the absence of morphine. Here, we demonstrate that spinal levels of the DAMPs HMGB1 and biglycan are elevated at 5 weeks after morphine treatment ended, concurrent with persistent allodynia. We also show that these persistent elevations of HMGB1 and biglycan levels are partly dependent on prior activation of TLR4, P2X7R and caspase-1 during morphine administration, which ceased weeks earlier. Finally, pharmacological attenuation of the DAMPs HMGB1, biglycan, HSP90 and fibronectin—known TLR4 and/or P2X7R agonists—persistently reversed morphine-prolonged allodynia. Remarkably, inhibiting DAMP action for 7 days “reset” neuropathic pain to sham levels for over a month after the end of inhibitor delivery, with no return of allodynia. We conclude that morphine treatment after CCI exacerbates cellular stress via NLRP3 inflammasomes, increasing DAMP levels, which maintain NLRP3 inflammasome activation through TLR4 and P2X7R. This newly discovered positive feedback loop may be responsible for maintaining persistent allodynia.
Increased levels of HMGB1 and biglycan were partly dependent on TLR4, P2X7R and caspase-1. Thus, IL-1β may be responsible for DAMP production, as these receptors and the enzyme play a role in the activation of the cytokine. IL-1β can disrupt glutamate homeostasis by downregulating the glutamate transporter GLT-1 (Yan et al., 2014), and we previously found that GLT-1 is downregulated with morphine-induced persistent sensitization (Grace et al., 2016). Such alterations in glutamate homeostasis can lead to excitotoxic release of DAMPs, as well as ATP release from glial cells (Liu et al., 2006; Queiroz et al., 1999). Furthermore, activation of P2X7R can induce release of ATP and reactive oxygen species, while the HMGB1 is also released as a consequence of inflammasome activation (Ficker et al., 2014; Lamkanfi et al., 2010; Suadicani et al., 2006). Therefore, the inflammasome may remain activated due to a positive feedback loop, wherein the initial amplified release of IL-1β after morphine administration creates cellular stress and release of DAMPs that signal back at TLR4 and P2X7R. Future studies may also test whether normalization of DAMP levels correlates with resolution of morphine-induced persistent sensitization.
With the exception of biglycan, the other DAMPs explored here have a known role in pain (Grace et al., 2014), suggesting that morphine exacerbates their increase. HMGB1 is a nuclear protein that is passively released into the extracellular space after necrosis/apoptosis, and is also actively released during inflammatory processes (Frank et al., 2015). We and others have previously shown that nerve injury alone is sufficient to increase spinal levels of HMGB1, and that it functionally contributes to neuropathic pain (Chacur et al., 2001; Liu et al., 2017; Nakamura et al., 2013; Ren et al., 2012). Future studies could assess whether the increase in total protein found herein correlates with extracellular levels, as well as redox state. Fibronectin is an extracellular matrix protein that activates TLR4 and upregulates P2X4R levels by microglia (Inoue and Tsuda, 2012; Okamura et al., 2001). HSPs, such as HSP90, are generally expressed and upregulated to protect cells against damage induced by pathophysiological stress. Intrathecal inhibition of HSP90 reverses allodynia (Hutchinson et al., 2009). Biglycan has not previously been implicated in pain, but is uniquely able to activate both TLR4 and P2X7R (Babelova et al., 2009). This report suggests a previously unexplored role for spinal biglycan signaling in neuropathic pain. Additional studies, perhaps using the DAMP inhibitors independently, will be required to elucidate the individual contribution of each DAMP to morphine-induced persistent sensitization.
In conclusion, we have demonstrated that the DAMPs HMGB1 and biglycan are elevated in a partly inflammasome-dependent fashion in the lumbar dorsal spinal cord during morphine-induced persistent sensitization, measured 5 weeks after morphine dosing has ended. Furthermore, these DAMPs, including HSP90 and fibronectin, may also mediate sustained allodynia with morphine-induced persistent sensitization. As opioids also induce persistent sensitization in other pain models, including inflammatory, postoperative, and spinal cord injury-induced pain (Ellis et al., 2016; Loram et al., 2012; Wilson et al., 2016), it remains to be determined whether DAMP signaling also plays a similar role to that described herein. As neuropathic pain is often intractable to current therapies, targeting DAMP signaling may be a novel strategy to alleviate the enormous burden of pain.
Supplementary Material
Figure S1. Experimental timeline. CCI or sham surgery was performed at Day 0. Beginning on day 10, morphine or saline was administered for 5 days in all experiments. In experiment 2, inhibitors for TLR4, P2X7R, or caspase-1 were administered concurrently with morphine. Tissues was collected 5 weeks after morphine treatment had ended (experiments 1 and 2). DAMP inhibitor cocktail was administered for 7 days, beginning 5 weeks after morphine treatment had ended. *Indicate behavioral timepoints.
Figure S2. Biglycan levels after knockdown. Levels of protein after 2 d Bgn siRNA vs. missense control. *P < 0.05. N = 6 per group.
Morphine administration after peripheral nerve injury increases spinal DAMP levels
Elevation of DAMPs persists through at least 5 weeks after morphine cessation
Increased levels of DAMPs are dependent on TLR4, P2X7R, and caspase-1
These DAMPs are responsible for maintaining persistent allodynia
Acknowledgments
This work was supported by the American Pain Society Future Leaders in Pain Research Grants Program (P.M.G.); National Health and Medical Research Council CJ Martin Fellowship ID 1054091 (to P.M.G.); American Australian Association Sir Keith Murdoch Fellowship (P.M.G.); and, NIH Grants DE021966, DA023132 (to L.R.W.). The work of the Drug Design and Synthesis Section was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agalave NM, Svensson CI. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol. Med. Camb. Mass. 2015;20:569–578. doi: 10.2119/molmed.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne H-J, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J. Biol. Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, Ayala M, Hutchinson MR, Falci S, Rice KC, Maier SF, Watkins LR. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain. Behav. Immun. 2016;58:348–356. doi: 10.1016/j.bbi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker C, Rozmer K, Kató E, Andó RD, Schumann L, Krügel U, Franke H, Sperlágh B, Riedel T, Illes P. Astrocyte-neuron interaction in the substantia gelatinosa of the spinal cord dorsal horn via P2X7 receptor-mediated release of glutamate and reactive oxygen species. Glia. 2014;62:1671–1686. doi: 10.1002/glia.22707. [DOI] [PubMed] [Google Scholar]
- Frank JW, Lovejoy TI, Becker WC, Morasco BJ, Koenig CJ, Hoffecker L, Dischinger HR, Dobscha SK, Krebs EE. Patient Outcomes in Dose Reduction or Discontinuation of Long-Term Opioid Therapy: A Systematic Review. Ann. Intern. Med. 2017 doi: 10.7326/M17-0598. d. [DOI] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain. Behav. Immun. 2015;48:1–7. doi: 10.1016/j.bbi.2015.03.010. d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Maier SF, Watkins LR. Opioid-induced central immune signaling: implications for opioid analgesia. Headache. 2015;55:475–489. doi: 10.1111/head.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E3441–3450. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of Long-term Opioid Therapy With Functional Status, Adverse Outcomes, and Mortality Among Patients With Polyneuropathy. JAMA Neurol. 2017;74:773–779. doi: 10.1001/jamaneurol.2017.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Ramos KM, Loram LC, Wieseler J, Sholar PW, Kearney JJ, Lewis MT, Crysdale NY, Zhang Y, Harrison JA, Maier SF, Rice KC, Watkins LR. Evidence for a role of heat shock protein-90 in toll like receptor 4 mediated pain enhancement in rats. Neuroscience. 2009;164:1821–1832. doi: 10.1016/j.neuroscience.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur. J. Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Purinergic systems, neuropathic pain and the role of microglia. Exp. Neurol. 2012;234:293–301. doi: 10.1016/j.expneurol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Koops A, Kappler J, Junghans U, Kuhn G, Kresse H, Müller HW. Cultured astrocytes express biglycan, a chondroitin/dermatan sulfate proteoglycan supporting the survival of neocortical neurons. Brain Res. Mol. Brain Res. 1996;41:65–73. doi: 10.1016/0169-328x(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti T-D, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. Baltim. Md. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Häring M, Roughley PJ, Margolis RK, Margolis RU. Glypican and Biglycan in the Nuclei of Neurons and Glioma Cells: Presence of Functional Nuclear Localization Signals and Dynamic Changes in Glypican During the Cell Cycle. J. Cell Biol. 1997;139:851–864. doi: 10.1083/jcb.139.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang Z, Qiu Y, Wei M, Li C, Xie Y, Shen L, Huang Y, Ma C. Suppression of MyD88-dependent signaling alleviates neuropathic pain induced by peripheral nerve injury in the rat. J. Neuroinflammation. 2017;14:70. doi: 10.1186/s12974-017-0822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GJ, Kalous A, Werry EL, Bennett MR. Purine release from spinal cord microglia after elevation of calcium by glutamate. Mol. Pharmacol. 2006;70:851–859. doi: 10.1124/mol.105.021436. d. [DOI] [PubMed] [Google Scholar]
- Loram LC, Grace PM, Strand KA, Taylor FR, Ellis A, Berkelhammer D, Bowlin M, Skarda B, Maier SF, Watkins LR. Prior exposure to repeated morphine potentiates mechanical allodynia induced by peripheral inflammation and neuropathy. Brain. Behav. Immun. 2012;26:1256–1264. doi: 10.1016/j.bbi.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Morioka N, Abe H, Zhang FF, Hisaoka-Nakashima K, Liu K, Nishibori M, Nakata Y. Neuropathic pain in rats with a partial sciatic nerve ligation is alleviated by intravenous injection of monoclonal antibody to high mobility group box-1. PloS One. 2013;8:e73640. doi: 10.1371/journal.pone.0073640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kügelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Ren P-C, Zhang Y, Zhang X-D, An L-J, Lv H-G, He J, Gao C-J, Sun X-D. High-mobility group box 1 contributes to mechanical allodynia and spinal astrocytic activation in a mouse model of type 2 diabetes. Brain Res. Bull. 2012;88:332–337. doi: 10.1016/j.brainresbull.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Sommer C. Peripheral neuropathies: Long-term opioid therapy in neuropathy: benefit or harm? Nat. Rev. Neurol. 2017 doi: 10.1038/nrneurol.2017.101. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Kappler J, Junghans U, Koops A, Kresse H, Mu¨ller HW. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Res. 1995;704:263–274. doi: 10.1016/0006-8993(95)01131-5. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NM, Ripsch MS, White FA. Impact of Opioid and Nonopioid Drugs on Postsurgical Pain Management in the Rat [WWW Document] Pain Res. Treat. 2016 doi: 10.1155/2016/8364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Yadav R, Gao M, Weng H-R. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62:1093–1109. doi: 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Experimental timeline. CCI or sham surgery was performed at Day 0. Beginning on day 10, morphine or saline was administered for 5 days in all experiments. In experiment 2, inhibitors for TLR4, P2X7R, or caspase-1 were administered concurrently with morphine. Tissues was collected 5 weeks after morphine treatment had ended (experiments 1 and 2). DAMP inhibitor cocktail was administered for 7 days, beginning 5 weeks after morphine treatment had ended. *Indicate behavioral timepoints.
Figure S2. Biglycan levels after knockdown. Levels of protein after 2 d Bgn siRNA vs. missense control. *P < 0.05. N = 6 per group.