Abstract
While protective, the acute inflammatory response when uncontrolled can lead to further tissue damage and chronic inflammation that is now widely recognized to play important roles in many commonly occurring diseases, such as cardiovascular disease, neurodegenerative diseases, metabolic syndrome, and many other diseases of significant public health concern. The ideal response to initial challenges of the host is complete resolution of the acute inflammatory response, which is now recognized to be a biosynthetically active process governed by specialized pro-resolving mediators (SPM). These chemically distinct families include lipoxins, resolvins, protectins and maresins that are biosynthesized from essential fatty acids. The biosynthesis and complete stereochemical assignments of the major SPM are established, and new profiling procedures have recently been introduced to document the activation of these pathways in vivo with isolated cells and in human tissues. The active resolution phase leads to tissue regeneration, where we’ve recently identified new molecules that communicate during resolution of inflammation to activate tissue regeneration in model organisms. This review presents an update on the documentation of the roles of SPMs and the biosynthesis and structural elucidation of novel mediators that stimulate tissue regeneration, coined conjugates in tissue regeneration. The identification and actions of the three families, maresin conjugates in tissue regeneration (MCTR), protectin conjugates in tissue regeneration (PCTR), and resolvin conjugates in tissue regeneration (RCTR), are reviewed here. The identification, structural elucidation and the pathways and biosynthesis of these new mediators in tissue regeneration demonstrate the host capacity to protect from collateral tissue damage, stimulate clearance of bacteria and debris, and promote tissue regeneration via endogenous pathways and molecules in the resolution metabolome.
Introduction
While Galileo Galilei (1564-1642) was fighting for data arguing for a new view of our solar system, his body’s leukocytes were engaged in a fight of their own with gout crystals causing joint inflammation, as deduced by Gerald Weissmann (2007) from portraits of Galileo. William Harvey (1578-1657) pushed the boundaries of medical knowledge by arguing for the systemic circulation with blood pumping to the brain and organs by the heart as published in his book ‘De Motu Cordis’. He was also a sufferer of uncontrolled inflammation from monosodium urate crystal deposition in his joints resulting in gout, which is a condition that was prevalent in many scholars of his time (Weissmann, 2007). Today, uncontrolled inflammation is a major component of many diseases and in aging. To combat these, new treatments are needed, yet dogma on the mechanism and control of inflammation is deep seated in the medical and scientific literature. For example, blood-borne bacteria can lead to infectious inflammation, sepsis and mortality that are significant public health concerns, placing considerable financial burden that impacts millions in the USA (Epstein et al., 2016; Ward and Bosmann, 2012), thus emphasizing the urgent need for development of new treatments (Epstein et al., 2016; Ward and Bosmann, 2012; Serhan, 2017; Wei and Gronert, 2017; Buechler et al., 2017; Lee and Zeldin, 2015; Russell and Schwarze, 2014). Human phagocytes [neutrophils (PMN) and macrophages (MΦ)] play pivotal roles in host defense, acute inflammatory responses and their timely resolution (Serhan and Savill, 2005; Tabas and Glass, 2013; Delano and Ward, 2016; Nathan, 2012; Leslie, 2015).
In the post-genomic era, understanding inflammation and its intricate mechanisms is the frontier. While ancient physicians recognized inflammation’s cardinal signs as heat, redness, swelling and pain centuries ago, the cellular and molecular players in this vital inflammatory host response have only been elucidated for the most part in the last century (Majno and Joris, 2004). Today, it’s now well appreciated that uncontrolled inflammation and excessive tissue levels of inflammatory mediators play central roles in the pathogenesis of many widely occurring diseases throughout the body and all its organs. Earlier, the study of inflammation and inflammatory diseases was confined to chronic inflammatory diseases such as rheumatoid arthritis, periodontal disease and the like, along with their initiating signals. Today it is widely appreciated that neurodegenerative diseases, cognitive decline, vascular disease, asthma, obesity and many other commonly occurring diseases involve uncontrolled, recurrent bouts of local and systemic inflammation. For us to gain and harness new approaches to treat these diseases and appreciate the complexity of the inflammatory response, it is essential for biomedical scientists and health care practitioners to command a detailed appreciation of the cellular and molecular language of the inflammatory response, the mediators and governance of this vital body defense system.
Uncontrolled inflammation is now widely appreciated as a unifying component in many diseases including vascular diseases, metabolic syndrome, neurological diseases, and many others (Serhan and Savill, 2005; Tabas and Glass, 2013). Since the acute inflammatory response is protective, evolved to permit repair of injured tissues and eliminate invading organisms (Cotran et al., 1999), it is ideally self-limited and leads to complete resolution of leukocyte infiltrates and clearance of cellular debris enabling homeostasis. Although resolution of disease is appreciated by clinicians, resolution was considered a passive process (Cotran et al., 1999; Serhan, 2017), until we and now many others (Fredman et al., 2016; Chattopadhyay et al., 2017; Easley et al., 2016; Jo et al., 2016; Petri et al., 2017; Pope et al., 2016; Gilbert et al., 2014; Perretti et al., 2017; Viola et al., 2016; Bang et al., 2010; El Kebir et al., 2012; Hellmann et al., 2011; Liao et al., 2012; Liu et al., 2012; Lund et al., 2010; Palmer et al., 2011; Qu et al., 2012; Rajasagi et al., 2011; Rogerio et al., 2012; Settimio et al., 2012) have obtained new evidence that resolution of self-limited inflammation is an active process. A weakness of current anti-inflammatories is that they eventually become immunosuppressive (Cotran et al., 1999; Serhan, 2017). With our strategy employing unbiased LM-lipidomics, genetically engineered mice, exudates and human cell systems, we obtained the first evidence that resolution is actively “turned on” and not simply a passive process. Thus, we defined the signs of resolution and its biochemical and cellular code (reviewed in (Serhan, 2014, 2017; Serhan et al., 2015a)).
Local Mediators in Resolution of Inflammation
Central to this paradigm change is the identification of a novel super-family of pro-resolving mediators (SPM) (Fig. 1) that include resolvins, protectins, their aspirin-triggered forms (Serhan, 2014, 2017), and more recently maresins (Serhan et al., 2009; Serhan et al., 2012). These provided evidence that local mediators and their biosynthesis from n-3 precursors EPA and DHA orchestrate resolution in humans (Barden et al., 2016a; Jaudszus et al., 2013; Mas et al., 2016; Morris et al., 2009). Since these mediators stimulate resolution as agonists they are coined immunoresolvents (Serhan, 2017).
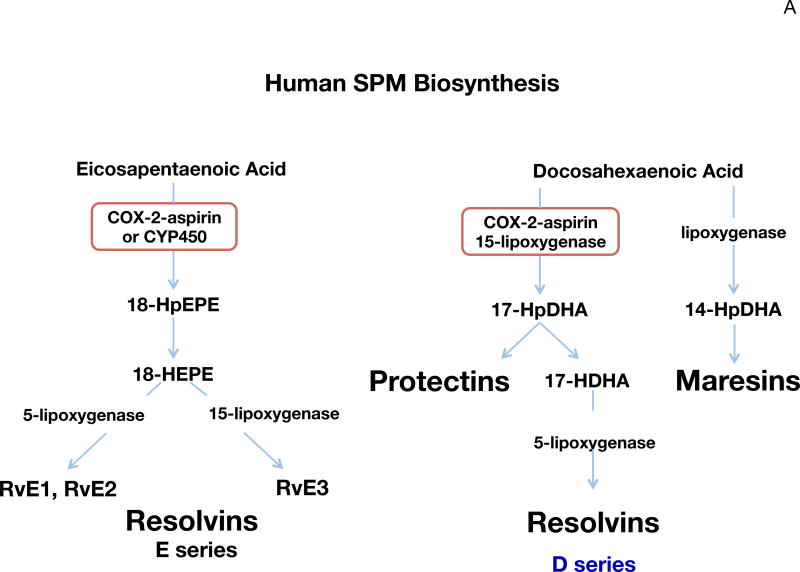
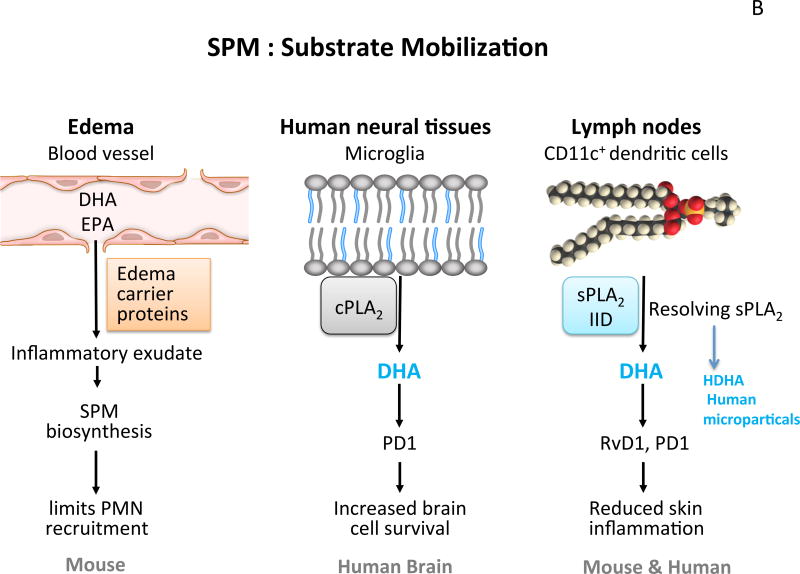
Figure 1. SPM biosynthetic pathways and substrate mobilization.
(A) Human SPM biosynthesis. Biosynthesis of E-series resolvins is initiated with molecular oxygen insertion at carbon-18 position of EPA, which is converted to bioactive E-series members resolvin E1, resolvin E2 and resolvin E3. Biosynthesis of D-series resolvins and Protectins are initiated by molecular oxygen insertion at carbon-17 position of DHA. Maresins are produced via initial lipoxygenation at carbon-14 position. The stereochemistry of each bioactive SPM is established, and SPM biosynthesis in human cells and tissues confirmed.
(B) Substrate mobilization. In murine inflammation, edema carries substrate DHA and EPA from circulating blood to local inflamed site for SPM production. In human neural tissues, phospholipids are the source of DHA, which is released by cPLA2 for PD1 formation. In lymph nodes, sPLA2G2D releases substrate to produce RvD1 and PD1 that reduce inflammation in the skin.
The biosynthesis of E-series resolvins from EPA proceeds via the production of 18-hydroperoxy-eicosapentaenoic acid and subsequently 18-hydroxy-eicosapentaenoic acid, which are biosynthesized from COX (cyclooxygenase)-2 in an aspirin-dependent and independent manner (Oh et al., 2011; Nebert, 2017), where this precursor is also produced by P-450 enzymes in humans and microbial cells. This pivotal intermediate is converted by human leukocytes to RvE1 and RvE2 by 5-lipoxygenase (LOX) reactions (Tjonahen et al., 2006). The potent actions of RvE1 and RvE2 following their complete stereochemical assignments have been confirmed by many independent laboratories (for review, see Serhan, 2014). Resolvin E3 is also produced from EPA and carries potent anti-inflammatory actions (Arita et al., 2005; Isobe et al., 2012a). EPA-rich high-density lipoprotein (HDL) in particular is a source of RvE3 (Tanaka et al., 2017). Like RvE1 and RvE2, RvE3 is a potent anti-inflammatory that stops PMN infiltration. It’s not yet known if RvE3 is also pro-resolving like RvE1 and RvE2 in stimulating increases in phagocytosis of apoptotic PMN, debris and bacteria (Tanaka et al., 2017; Arita et al., 2005; Schwab et al., 2007). Earlier studies demonstrated that COX2-derived PGE2 and LXA4 accelerate resolution of allergic edema (Bandeira-Melo et al., 2000). In addition, inhibition of COX-2 delays resolution of acute inflammation (Schwab et al., 2007). Therefore, COX-2 plays an important role in accelerating resolution via enhancing SPM biosynthesis.
DHA is enriched in specific tissues in humans such as brain and testis (Lands, 2005) and is precursor to three main structurally distinct families of mediators that include D-series resolvins, protectins and maresins (Fig. 1A). The biosynthesis, function and complete stereochemistry of each major bioactive product in each family are established (see Serhan, 2014). The D-series resolvins and protectins are produced via a 17-lipoxygenation reaction carried out predominantly by the human 15-LOX, and the maresins are initiated by 14-lipoxygenation of DHA via the human 12-LOX (Serhan, 2014; Serhan et al., 2012; Serhan et al., 2009).
Dietary n-3 supplements with EPA and DHA are widely used but <25% are directed by health care providers (Bailey et al., 2013). Clinical trials with n-3 fatty acids show mixed, sometimes weak, results (Bisgaard et al., 2016; Ramaswami et al., 2016; Ramsden, 2016; De Caterina, 2011; Lourdudoss et al., 2017; Polus et al., 2016; Uno et al., 2016), likely due to poor product quality (Albert et al., 2015). Since fatty acids themselves are not suitable drugs and widely used, it is paramount for public health to establish mechanisms that underlie their essential health requirements and benefits.
Using a rigorous systems approach with self-resolving exudates, we isolated and elucidated novel n-3-derived SPM (Colas et al., 2014; Dalli et al., 2015a; Serhan et al., 2002). Their biosynthesis and complete stereochemistry of each major resolvin (RvE1, RvD1, RvD2, RvD3, RvD4 and RvD5) conferring their potent actions (reviewed in (Serhan, 2014, 2017)) were the focus of investigations first established from this laboratory and now independently confirmed by many investigators.
Substrate mobilization
In neural systems, DHA is esterified in phospholipids and in cells such as the microglia of the brain. We found that DHA is released from phosphatidylethanolamine in phagocytosis (Hong et al., 2003) and released substrate is converted to (Fig. 1B) Neuroprotectin D1/PD1 that reduces inflammatory cytokine production by these cells (Serhan et al., 2002). Phospholipids are the source of DHA in the human brain (Lukiw et al., 2005); see also the review by Bazan in this series. In lymph nodes, Miki et al. (2013) identified a specific PLA2 denoted sPLA2G2D, which releases substrate to produce RvD1 and PD1 in reducing inflammation in the skin (Fig. 1B). Human microparticles are also a source of hydroxy-containing DHA, i.e. 17-HDHA, 14-HDHA, namely intermediates and markers for release by sPLA and conversion to resolvins and related SPM (Norling et al., 2011). During initiation and resolution of acute inflammatory responses, circulating n-3 in blood is carried by edema proteins such as albumin that shuttle EPA and DHA from blood to inflammatory exudates for conversion to SPM that in turn limit the size of the exudate (Kasuga et al., 2008) and bring the pus to resolution (Fig. 1B).
The structures and pico to nanogram functions of E-series Rv are extended to several systems, e.g. vascular (Miyahara et al., 2013), airway (Seki et al., 2010), dermal (reviewed in (Lee, 2012; Serhan et al., 2008), ocular (Li et al., 2010), pain (Xu et al., 2010; Huang et al., 2011; Feng et al., 2012; Lima-Garcia et al., 2011; Xu et al., 2013), surgery (Uno et al., 2016), fibrosis, wound healing (Vassiliou et al., 2008; Campbell et al., 2010; de Paiva et al., 2012; Hisada et al., 2009; Ishida et al., 2010; Jin et al., 2009; Keyes et al., 2010; Kim et al., 2012; Wan et al., 2011; Lund et al., 2010; El Kebir et al., 2012; Qu et al., 2012; Rajasagi et al., 2011), and tissue regeneration (Serhan et al., 2012).
Also each D-series resolvin, RvD1 (Bang et al., 2010; Rogerio et al., 2012; Settimio et al., 2012; Hellmann et al., 2011; Liao et al., 2012; Liu et al., 2012; Palmer et al., 2011; Tang et al., 2013; Li et al., 2013; Terrando et al., 2013; Lee et al., 2013), RvD2 (Spite et al., 2009; Bohr et al., 2013)) and neuroprotectin/protectin (Schwab et al., 2007; Sheets et al., 2010; Isobe et al., 2012b; Bazan et al., 2010; Kenchegowda et al., 2013; Park et al., 2011) has actions throughout the organ systems of the body. This is because resolvins and SPM act on leukocytes (Fig. 2) that function in blood from head to toe throughout the body. To pinpoint SPM in vivo functions in inflammation, we also defined the first resolution indices permitting identification of SPM and drugs that shorten resolution intervals (Bannenberg et al., 2005; Schwab et al., 2007). These indices are now widely used to monitor resolution (Pruss et al., 2011; Navarro-Xavier et al., 2010; Morris et al., 2010a; Hilberath et al., 2011; Fujieda et al., 2013; Koltsida et al., 2013; Lucas et al., 2014; Luo et al., 2016; Montero-Melendez et al., 2015). Recently, AT-RvD1 was shown to control herpes simplex virus-induced corneal immunopathology, reducing neutrophils as well as Th1 and Th17 cells (Rajasagi et al., 2017). In experimental autoimmune encephalitis, oral administration of RvD1 decreased disease progression via regulating autoreactive T cells, monocytes/macrophages and resident brain microglial cells (Poisson et al., 2015). These results demonstrate potent actions of RvD1 in controlling viral infection and autoimmunity via regulating both innate and adaptive immune systems (vide infra). In addition, D-series Rv regulate pain and depression. For example, RvD1 reduces osteoarthritic pain (Huang et al., 2017) and post-thoracotomy pain (Wang and Strichartz, 2017). RvD1 and RvD2 demonstrate anti-depressant properties in a chronic unpredictable stress model (Ishikawa et al., 2017). The rigor of our structural elucidation enabled organic syntheses that confirmed and reproduced our original SPM assignments (reviewed in (Serhan and Petasis, 2011)), permitting their commercialization, and extend our findings with these mediators to many important disease models and systems.
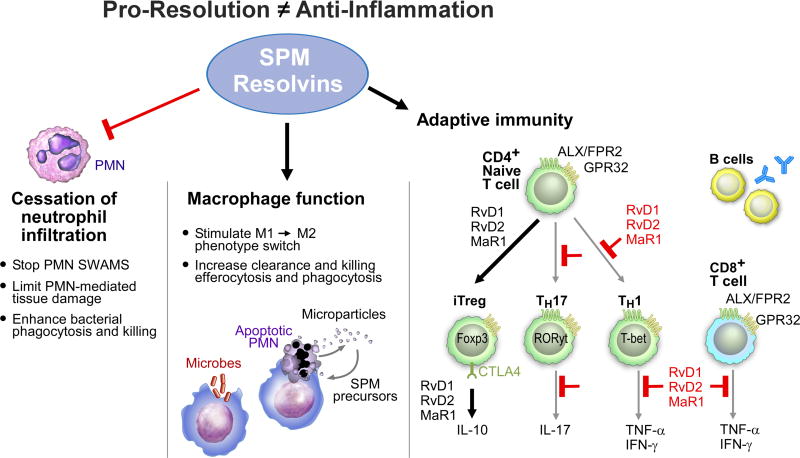
Figure 2. Main Actions of SPM in the Innate Immune System: Pro-Resolution and Anti-Inflammation Are Not Equivalent Mechanisms.
The actions of SPM on innate immune system have been demonstrated with phagocytes including limiting PMN and stimulating macrophage functions. SPM also directly act on the adaptive immune cells, including T cells and B cells (see text for detail).
The acute inflammatory response is protective
While SPM stimulate resolution by limiting PMN infiltration and stimulating uptake and clearance of apoptotic PMN from head to toe (Fig. 2), we unexpectedly found that SPM also stimulate phagocytosis of bacteria as well as their killing and clearance (Chiang et al., 2012; Spite et al., 2009). These counterintuitive results are confirmed by others (Russell and Schwarze, 2014; Lee and Zeldin, 2015) and are associated with the ability of SPM to clear infections in animals (Chiang et al., 2012; Codagnone et al., 2017), and thus a potential new approach to treat sepsis (Buechler et al., 2017; Enkhbaatar, 2016). We monitored SPM profiles in sepsis patients where their temporal profiles correlate to survival and outcomes (Dalli et al., 2017b) (vide infra). In addition to regulating innate immune system phagocytes (Fig. 2), SPM also display potent actions in the adaptive immune system including the regulation of T-cell phenotype and responses (Chiurchiu et al., 2016) and the actions of B-cells to viral infections (Ramon et al., 2012; Kosaraju et al., 2017). Key to SPM actions is their ability to activate specific G-protein coupled receptors (GPCR) (Dalli et al., 2013a; Serhan et al., 2011b). For example, RvE1 acts at two receptors on human PMN and MΦ (Arita et al., 2007; Ohira et al., 2010). RvD1 and RvD5 activate GPR32 (DRV1) in humans (Krishnamoorthy et al., 2010; Recchiuti et al., 2011; Chiang et al., 2012), RvD2 activates GPR18 (DRV2) (Chiang et al., 2015) that mediates the actions of RvD2 on PMN and MΦ as well as sepsis (Chiang et al., 2017). Lipoxin (LX) A4 and 15-epi-LXA4 activated ALX receptor (Cooray et al., 2013; Takano et al., 1998; Libby, 2015; Petri et al., 2015). For a recent review of the resolvin and SPM receptors, see (Chiang and Serhan, 2017).
The actions of SPM were recently reported in human settings where an RvE1 analog entered human clinical trials (Lee, 2012) and was reported to be effective in ocular inflammation (Resolvyx Pharmaceuticals Inc., 2012; Brooks, 2009; Science Blog, 2009). Another SPM identified in the author’s lab, i.e. aspirin-triggered LXA4, effectively reduced infant eczema without side effects in double-blind trial (Wu et al., 2013), and LXA4 proved a marker of metabolic syndrome(Yu et al., 2015). Other SPM are in Phase I and II trials (Birnbaum, 2012; Lee, 2012; Perretti et al., 2017). These first in class, human trial results now open possibilities for resolution agonists and resolution pharmacology.
Human specialized pro-resolving mediator (SPM) experiments: Profiling PUFA bioactive metabolomes
Having established the complete stereochemistry of the E-series and D-series resolvins, protectins and maresins (MaR1 and MaR2) as well as key deuterium-labeled SPM that serve as internal standards for LC-MS-MS-based targeted identification and quantitation, we were ready to begin the operationalization of human tissue profiling of the bioactive SPM metabolomes (Colas et al., 2014). To study LM-SPM in human peripheral blood, we carried out targeted LM metabololipidomics using an LC-MS-MS system. To assess potential losses during processing as well as to normalize RT inter-run variations, we employed deuterated internal standards that marked specific chromatographic regions of interest within each chromatographic profile. With this approach, we profiled human sera, where each sample was a composite of ~100 individual donors. In these, we identified LM from each of the DHA, EPA and AA bioactive metabolomes. The complete LM stereochemistry and annotated biological functions are reported in (Dalli and Serhan, 2012; Colas et al., 2014). In serum, the SPM included endogenous RvD1, RvD2, RvD3, PD1, MaR1 and LX (Colas et al., 2014). Each LM and biosynthetic pathway product was identified in accordance with published criteria (Serhan and Petasis, 2011; Dalli and Serhan, 2012) that included matching RT and at least six characteristic and diagnostic ions. This is illustrated, for example, with tissue-derived endogenous RvD1 to RvD6, protectins, maresins and classic eicosanoids.
Identification and quantification of LM and SPM is achieved using targeted MRM of specific ion pairs Q1 (parent ion) to Q3 (diagnostic daughter ion) without the need for derivatization of the samples (Dalli and Serhan, 2012). In human serum composites, the DHA bioactive metabolome represented ~30.7% of targeted SPM and/or pathway markers (Colas et al., 2014). From these, we identified D-series resolvins, RvD1 (30.9 ± 7.0 pg/mL) and its 17R-epimer (40.7 ± 13.9 pg/mL), from the protectins, PD1 (5.6 ± 3.4 pg/mL) along with its double dioxygenation isomer 10S,17S-diHDHA (227.4 ± 68.2 pg/mL a.k.a. PDx) (Balas and Durand, 2016), and from the maresins, MaR1 (21.2 ± 7.2 pg/mL) and 4S,14S-diHDHA (1,579.7 ± 282.8 pg/mL).
The EPA bioactive metabolome in human serum was ~25.9 % in which we identified RvE2 (2212.6 ± 1587.6 pg/mL). Each SPM was present in amounts commensurate with their known bioactions (Dalli and Serhan, 2012). The AA bioactive metabolome comprised ~43.4% of the targeted LM; we identified lipoxins LXA4 (115.6 ± 45.5 pg/mL) and LXB4 (48.7 ± 25.2 pg/mL). In human serum we also identified the double dioxygenation isomer of LTB4, 5S,12S-diHETE (2,162.6 ± 515.5 pg/mL), PGE2 (72.5 ± 10.9 pg/mL) and PGD2 (271.0 ± 57.7 pg/mL) (Colas et al., 2014). The unesterified precursors and monohydroxy-EFA products that can serve as biosynthetic pathway markers of utilization were also identified and quantitated. In a separate study, LXB4 increases survival of mice with CLP-induced sepsis regulated by NLRP3 inflammasome (Lee et al., 2017).
To provide a benchmark for cross-laboratory validation by our new approach, we assessed human pooled plasma obtained from NIST (www.nist.gov), as a standard reference material (SRM 1950) that is available to all interested investigators. This composite sample contained plasma from 100 individuals with equal number of men and women ages 40–50. The racial distribution reflects US population (for reference, see www.nist.gov). We identified in these the DHA, EPA and AA bioactive metabolomes, which represented ~40.3%, 40.1% and 19.6% respectively of the targeted LM pathways. From DHA metabolome, we identified D-series resolvins RvD1 (2.6 ± 0.1 pg/mL), RvD5 (1.2 ± 0.3 pg/mL) and RvD6 (58.1 ± 5.2 pg/mL) and the maresin 4S,14S-diHDHA. From AA metabolome, we identified the leukotriene 5-LOX pathway product LTB4, as well as the prostaglandins, PGD2 and PGE2. Human plasma contained ~10–100 times less LMs than serum recoveries. This finding suggests that upon activation of peripheral blood bioactive LM-SPM increase. A LM index is obtained by taking the ratio of the SPM summation, i.e. LX, D- and E-series Rv, MaR1, PD1 and related pathway isomers (i.e. epoxide intermediate hydrolase products such as D12-trans-MaR1 and 7-epi, D12-trans-MaR1) divided by the summation of prostanoids and leukotrienes. This value, 5.4, is higher in SRM1950 plasma compared to 2.2 obtained for serum.
Individual signature profiles of LM-SPM from human peripheral blood
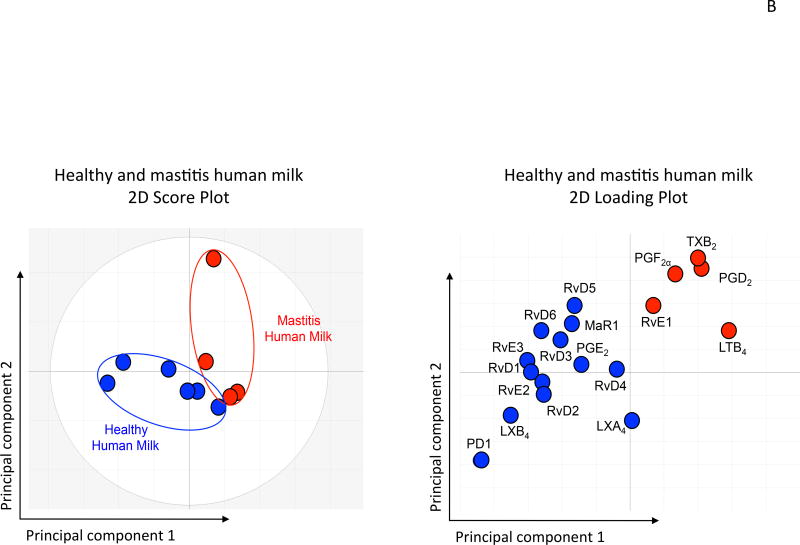
Data-driven modeling such as PCA is useful to interrogate large biological datasets such as metabolomics (Janes and Yaffe, 2006). LM-SPM profiles obtained with SRM 1950 (human plasma composite 100 individuals) and human serum composites (~300 individuals, ~100 subjects in each composite) as well as fresh plasma and serum from healthy individual donors were assessed using PCA (Colas et al., 2014). LM are displayed in three-dimensional space consisting of three principal components, illustrated in Figure 3. They were calculated from the systematic variation in the data matrix consisting of the overall bioactive LM identified in each sample. This included ~75 % of the variation of the entire dataset (n=42, where n=each LC-MS-MS profile). Gray ellipse in the score plot denotes 95 % confidence regions. This region diagnosis strong outliners based on Hotelling’s T squared equation (see http://www.itl.nist.gov/div898/handbook/). Principal component 1 showed distinct separation between the plasma cluster and serum cluster. The loading plot demonstrated that the serum cluster displayed lower LTB4 levels and higher levels of lipoxins, resolvins, protectins, maresins, and prostaglandins compared to plasma for both SRM 1950 reference and fresh plasma. In these, plasma samples showed a tight cluster of LM while serum proved more dispersed (Colas et al., 2014).
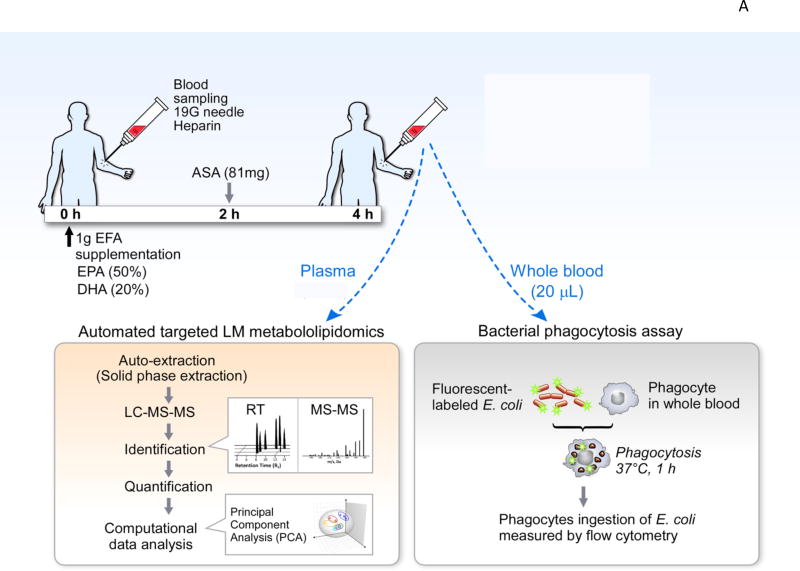
Figure 3. Demonstrations of SPM Production in Humans.
(A) Illustration of functional metabololipidomics: human SPM production and assessment of their function. At time zero, individuals all ingested 1 gram of omega-3, containing 50% EPA and 20% DHA. At 2h, they took low-dose aspirin (81 mg). At 4h, blood was collected to carry out LM-metabololipidomics together with PCA analysis. In parallel, whole blood from the same subjects were used for functional assessment with phagocytosis. Whole blood was incubated with fluorescent-labeled E. coli ex vivo, and phagocyte ingestion of E. coli measured by flow cytometry. A cluster of SPM was elevated with acute n-3 and ASA intake, and correlated with increased phagocyte function in whole blood. This approach provides a tool for functional metabololipidomics.
(B) Principal Component Analysis: Mastitis human milk gives altered LM-SPM profiles with higher levels of PG and LT, while healthy human milk contains higher amounts of SPM, including LX, Rv, PD and MaR1.
As a demonstration of this approach using targeted LM metabololipidomics, we investigated endogenous LM in human lymph nodes and spleens given their roles in immune function. Each bioactive LM, SPM and/or pathway markers is identified. For example, in human axillary lymph nodes, we identified D-series resolvins, RvD1 (5.5–48.6 pg/100 mg tissues), RvD5 (1.9–9.4 pg/100 mg tissues) and RvD6 as well as the E-series resolvin, RvE3 (Isobe et al., 2012a). Human lymph nodes also contained LXA4 (16.4–114.3 pg/100 mg tissues) and LXB4 (9.9–101.1 pg/100 mg tissues), as well as pro-inflammatory LTB4 (0.7–3.8 pg/100 mg tissues) and its related double lipoxygenase product 5S,12S-diHETE (3.0–50.8 pg/100 mg tissues). In these, prostanoids were also identified, with PGE2 at levels significantly higher than other prostanoids. We identified LM from each metabolome in human spleens. These included RvD5 from the D-series resolvins, the protectins (PD1 and 10S,17S-diHDHA) as well as the maresins, MaR1 (22.6 pg/100 mg tissues) and its double dioxygenation product, 7S,14S-diHDHA (46.1 pg/100 mg tissues). In these, we identified E-series resolvins RvE1, RvE2 and RvE3 as well as LXA4 (5.7–122.9 pg/100 mg tissues). Of the prostanoids, we identified PGD2, PGE2 and PGF2µ. Results from these studies clearly demonstrate that omega-3-derived SPM from the 3 main bioactive metabolomes as well as eicosanoids were present in human lymphoid tissues.
Functional metabolomics: LM-SPM signature profiles in human plasma and phagocytosis
We questioned whether EFA and low dose ASA intake could impact circulating LM-SPM in a small group of healthy human volunteers (n=10) (Figure 3). Plasma LM profiles of healthy volunteers after EPA, DHA and ASA intake were investigated using LC-MS-MS metabololipidomics, and the functional impact was assessed in the same whole blood from each subject using bacterial phagocytosis. Since each SPM is known to increase bacterial uptake by phagocytes at concentrations in pM to nM range (Chiang et al., 2012; Dalli and Serhan, 2012), phagocytosis of E. coli was assessed in this human experiment. The fresh plasma from individual healthy volunteers displayed similar LM signature profiles as those present in the NIST standard reference SRM 1950 (Colas et al., 2014).
To investigate whether PUFA substrate supplementation and low-dose aspirin are causally related to changes in LM-SPM profiles, we carried out PLS-DA with results from n = 10 healthy subjects (illustrated in Figure 3). The two principal components, calculated using the data matrix, showed clear separation between the time zero cluster and 4h cluster. In addition, PLS-DA loading plot demonstrated a positive association of n-3 EFA and ASA intake with elevated RvD1, RvD2, 17epi-PD1, RvE2 and RvE3. TxB2 decreased ~75%. This reduction is in accordance with ASA mode and duration of action in humans (Higgs et al., 1987). With PUFA supplementation, prostaglandin and TxB2 were also reduced. Phagocytosis of live E. coli by phagocytes in whole blood increased at 4 h and positively correlated (r2 = 0.77) with increased levels of RvD1, RvD2, RvE2, RvE3 and 17epi-PD1 in human plasma (Colas et al., 2014 and Figure 3). This approach establishes rigorous identification criteria for the resolvin, protectin and maresin families of n-3-derived local SPM from human peripheral blood (serum and plasma) and other tissues employing MS-MS-based profiling of their biosynthetic pathways.
The SPM as well as LTB4 and prostanoids were identified in these tissues at levels commensurate with their known bioactive ranges in vivo (ng levels). For example, results (Colas et al., 2014) indicate that LXA4 was present in human peripheral blood serum and lymphoid tissues (i.e. spleen and lymph nodes); also present were RvD1, RvD2, RvD3 and RvD5, and the E series resolvins RvE1, RvE2 and RvE3. RvE3 is a newly identified bioactive resolvin (Isobe et al., 2012a). Also, D-series resolvins RvD1 and RvD2 were recently identified using mass spectral-based methods in human blood samples (Mas et al., 2012) as well as E-series RvE1 and RvE2 (Oh et al., 2011). With this new approach, the relationship(s) between each of these mediators and their biosynthetic pathways can be assessed. Bioactive mediators from EFA including each of the AA, DHA and EPA bioactive metabolomes are amenable to profiling from tissues; their respective relationships and summation index appears to provide information regarding the potential inflammatory and/or resolution status of a given target tissue.
Lipidomics vs. LM-SPM metabololipidomics: Focus on pathway and function
Biosynthesis of the potent EFA-derived mediators involves epoxide-containing intermediates (Serhan, 2004; Serhan et al., 2000; Haeggstrom and Funk, 2011; Shimizu, 2009; Oh et al., 2011). In the case of leukotriene A4, the epoxide intermediate is precursor to stereochemically-defined LTB4, which reflects its enzymatic formation. In parallel, the non-enzymatic aqueous hydrolysis of the epoxide LTA4 leads to two major isomers, (5,12-dihydroxy-eicosatetraenoic acid) and two minor (5,6-vicinal diols) isomers (Samuelsson, 1983a). Hence, focusing on the evaluation and relationship between LTB4 and its non-enzymatic related isomers gives a signature profile indicative of the central role of 5-LOX in the biosynthesis of LTA4. In this regard, 5-HETE, which is produced from the 5-hydroperoxyeicosatetraenoic acid (5-HpETE) intermediate in this leukotriene pathway, can be used as a biosynthetic pathway biomarker reflecting its reduction from 5-HpETE in the AA metabolome.
In the SPM metabolomes, 17-HDHA serves as a marker of the biosynthetic conversion of DHA via, for example, human 15-LOX type I for D-series resolvins and protectins (Serhan et al., 2002; and reviewed in Serhan and Petasis, 2011) and 14-HDHA, a marker of activation of the maresin pathway (Fig. 1) via the 14-lipoxygenation carried out by the 12-LOX (Serhan et al., 2012; Serhan et al., 2009). Hence, the relative amounts of each individual bioactive LM-SPM, their biosynthetic isomers, namely Δ12-trans-MaR1, Δ15-trans-PD1, and biosynthetic pathway markers including 5-HETE, 12-HETE, 17-HDHA, 14-HDHA provide a LM-SPM signature that reflects the status of a particular organ and/or phenotype.
In addition to resolving inflammatory exudates (Serhan et al., 2002), the SPM are also produced by human peripheral blood leukocytes (Oh et al., 2011; reviewed in Serhan and Petasis, 2011). RvE1 (100–400pg/mL) was identified in human plasma at 4h after EPA (1g) and ASA (160mg) supplementation using MS3 (Arita et al., 2005). We also found that p450 and COX-2 without aspirin can produce RvE1 in human cells if ample substrate is present and both 18S and 18R versions of RvE1 and RvE2 are produced (Oh et al., 2011). Present results demonstrated that without known supplementation, RvE1 was identified in serum (both commercial and fresh) as well as in fresh plasma. Its absence in SRM1950 might reflect a dilution effect with the 100 selected individuals or RvE1 lability in these samples, as it degrades without addition of methanol. Of note, Psychogios et al (2011) reported RvE1 (182pg/mL) in plasma from a cohort of 70 individuals. This value is similar to present values found after EPA supplementation in other healthy subjects. This could reflect differences in diet. Psychogios et al. (2011) also identified RvD1 at 17 pg/mL. Mas and colleagues (2012) identify RvD1, 17epi-RvD1 and RvD2 in both human plasma and serum following EFA supplementation for several weeks at levels commensurate with those we identify here in human serum.
Omega-3 PUFA supplementation in human studies in some cases has given seemingly opposite results. Supplementation was protective in rheumatoid arthritis (Di Giuseppe et al., 2013) and certain cardiovascular diseases (De Caterina, 2011) but also associated with increased risk of prostate cancer (Brasky et al., 2013). In these and similar human studies, the mechanism of n-3 remains a subject of discussion and emphasize the need for rigorous identification and functional LM-SPM profiling approaches (Colas et al., 2014; English et al., 2017). Using functional LM-SPM metabololipidomics and PLS-DA, we identified a cluster of pro-resolving mediators that were elevated with acute essential fatty acids and aspirin intake. The levels of SPM were within their bioactive ranges and positively correlated (r2=0.77) with increased function, e.g. an enhanced E. coli phagocytosis in whole blood from these subjects. These findings are in accordance with recent findings that SPM enhance bacterial killing in mice, lowering antibiotic requirement (Chiang et al., 2012). Results from SPM functional profiling also underscore the utility of this approach in determining the potential resolution status of an individual or a target organ following EFA intake. Diets rich in omega-3 PUFA increase both resolvins and protectins in model organisms (Jones et al., 2013) whereas SPM such as PD1 are reduced in disease as described in human asthma patients (Levy et al., 2007; Miyata et al., 2013), where LM can be regulated with increased omega-3 supplementation of asthmatics (Lundstrom et al., 2013).
Using profiling of specific n-3 and n-6-derived bioactive EFA-metabolomes with reference human tissues that can be used by different laboratories and investigators for instrument and LM-SPM calibration as documented (Colas et al., 2014; English et al., 2017) can provide information of potentially diagnostic and therapeutic value. Widely used assessment of omega-3 fatty acids incorporated in red blood cell membranes is very useful, along with the omega-3 index that correlates with this membrane compartment and functional outcomes to evaluate the availability and intake of n-3 PUFA (Tan et al., 2012). Importantly, rigorous LM-SPM profiling is needed because human resolution phenotypes have only recently emerged in healthy individuals (Morris et al., 2010b) and those undergoing surgery (Pillai et al., 2012). Their biosynthetic pathways give rise to specific stereochemistry for each of these local mediators (Serhan and Petasis, 2011) and thus specific signature profiles in human tissues (Colas et al., 2014; Dalli and Serhan, 2012; English et al., 2017). To this end, we used LC-MS-MS-based LM-metabololipidomics together with authentic biologically derived and synthetic standards to establish signature profiles with human tissues. Results from earlier studies established that eicosanoids derived from arachidonic acid are potent pro-inflammatory mediators (Samuelsson, 1983a; Haeggstrom and Funk, 2011) save the lipoxins, which display local anti-inflammatory and pro-resolving actions (Serhan and Petasis, 2011; Borgeson and Godson, 2012). Thus, our LC-MS-MS-based profiling approach will permit assessment of when and where individual metabolites are physiologically active and in concentrations to serve as proresolving mediators in model organisms as well as in human health and disease.
With human subjects, we found that amounts of specific SPM are substantially increased with increased availability of substrate intake. For example, NPD1/PD1 in plasma, RvE2 and RvE3 as well as D-series resolvins RvD1 and RvD2 each increase at 4 h post-PUFA administration (Colas et al., 2014). Sample storage before solid-phase extraction and LC-MS-MS injections proved critical in capturing the MS-MS to identify prostanoids and SPM. For example, at 4 weeks of sample storage, >70% of MaR1 is lost, > 50% of RvE1, and >50% of RvD1 is lost, presumably by degradation. This loss of LM-SPM in storage was also obtained with lipoxins, where >50% of LXB4 and almost 40% of LXA4 are quantitatively lost in sample storage. For a list of individual LM-SPM losses during storage of human serum samples and recoveries, see Colas et al. (2014). The chemical stability of SPM in tissue matrix is likely organ- and individual mediator-dependent. We therefore take samples for workup and LC-MS-MS profiling as soon as possible to minimize potential losses of SPM.
Several research groups have identified and measured SPM in human plasma, which increase with supplementation of n-3 PUFA; recently reviewed in Barden et al. (2016a). In a randomized trial with chronic kidney disease in 74 patients, RvD1 and upstream SPM precursors and pathway markers 18-HEPE (Figure 1) and 17-HDHA increased after 8 weeks of n-3 PUFA supplementation (Mas et al., 2016). In serum from arthritis patients, we identified RvD1, RvD3 RvD4 and other LM of interest in joint inflammation (Arnardottir et al., 2016b). RvD3 administered to arthritic mice reduced joint leukocytes, edema and clinical scores. Also, in synovial fluid of rheumatoid arthritis patients, RvD1 and RvD3 along with eicosanoids were identified via LC-MS-MS-based metabololipidomics (Norling et al., 2016). There is longstanding interest in PUFA, particularly n-3 PUFA, in pathogenesis and chronic inflammation in the joints (Norling and Perretti, 2013). Recently Barden et al. (2016b) compared knee effusions and plasma of arthritis patients taking 2.4 g/day of n-3 PUFA for 4 weeks. They identified E-series resolvins, D-series resolvins, protectins (PD1, PDx) and MaR1 in synovial fluid and plasma. Importantly, plasma SPM in the arthritic patients were found to be negatively related to erythrocyte sedimentation rate, which is an index of inflammation (Barden et al., 2016b). Also, in this study, RvE2 in synovial fluid was negatively associated with patient pain scores. These findings suggest that n-3 PUFA increase plasma and local levels of SPM that in turn reduce pain in patients, providing a mechanism for n-3 and SPM in reducing symptoms of arthritis. This is supported by a recent randomized crossover study with arthritis patients supplemented with microalgae DHA that reported increases in 14-HDHA and 17-HDHA from supplementation (Dawczynski et al., 2017).
In coronary artery disease patients, Lovaza (3.36 g daily) increased RvE2, RvD6 and the 17R aspirin-triggered (AT) forms of AT-RvD3, AT-LXB4 and AT-PD1 (Elajami et al., 2016). In these CAD patients not taking Lovaza, SPM were absent, suggesting that increased substrate can functionally impact the disease, since these SPM increased the phagocytosis of fibrin clots by human macrophages ex vivo (Elajami et al., 2016). In obese women, n-3 PUFA increase SPM, resolvins and up-regulated 15-LOX and ALX receptor (Polus et al., 2016).
SPM profiles temporally change during ICU hospitalization, and their levels in relation to eicosanoids in sepsis correlate with survival and development of acute respiratory distress syndrome (Dalli et al., 2017b). Human periodontal stem cells release SPM as well as lipoxins and prostaglandins that are functional (Cianci et al., 2016). Human tears (English et al., 2017) and, for example, breast milk also possess bioactive amounts of SPM (Figure 3B). SPM in milk stimulate resolution of inflammation in vivo in mice (Arnardottir et al., 2016a). Umbilical cord blood from mothers supplemented with n-3 PUFA showed increases in SPM biosynthetic pathway markers 17-HDHA and 14-HDHA (Fig. 1) from the maresin pathway (Mozurkewich et al., 2016).
Human skin blisters are an ideal setting to test the principles of resolution of acute inflammation in vivo and their direct relationship to LM and specifically resolvins and other SPM. Morris et al. (2010a) demonstrated the duration and severity of acute inflammation and leukocyte trafficking was associated with the activation of pro-resolution pathway and that oral administration of aspirin in vivo (low dose) increases the aspirin-triggered 15-epi-lipoxin to functional levels within blister inflammatory exudates (Morris et al., 2009). Recently, Rathod et al. (2017) identified resolvins and other SPM in skin blisters formed in response to cantharidin application. Exudates from females had higher amounts of SPM, particularly D-series resolvins, that was associated with accelerated resolution of inflammation compared to males. With E. coli-activated inflammation in human blisters, Motwani et al. (2017) obtained evidence for local SPM biosynthesis as well as SPM receptors appearing during the inflammation-resolution sequence in vivo. Direct evidence for SPM’s ability to stimulate resolution was obtained with local intrablister injections of specific SPM (LXB4, RvD1, RvE1), which accelerated resolution of PMN numbers in vivo (Motwani et al., 2017). Hence, these results provide additional evidence indicating that SPM function in vivo in humans at the concentrations in which they are produced locally during microbial challenge.
Novel SPM in Resolution of Inflammation, Pain and Tissue Regeneration
The relation between inflammation, wound response of the host and tissue regeneration is of wide interest (Eming et al., 2017). Macrophages play critical roles in wound healing and tissue regeneration. Maresins (macrophage-derived resolution mediators) produced via 14-lipoxygenation of DHA (Fig. 1B) appear well suited to engage in healing and tissue regeneration. Maresins are produced by MΦ and carry potent proresolving actions, reducing inflammation and stimulating efferocytosis of dead PMN and apoptotic cell debris (Serhan et al., 2009). Maresin 1 (MaR1) is assigned the complete stereochemistry 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid; biosynthesis from DHA is established (Serhan et al., 2012), including the assignment and bioactions of the pathway epoxide intermediate 13S,14S-epoxy-maresin (13S,14S-eMaR), which also proved to possess new potent proresolving mechanisms (Dalli et al., 2013b). Along with serving as a precursor for the enzymatic conversion to MaR1 and MaR2 (Deng et al., 2014), 13S,14S-eMaR blocks LTA4 hydrolase production of LTB4 (Dalli et al., 2013b), reduced conversion of arachidonic acid via human 12-LOX and promoted M1 MΦ conversion to the M2 phenotype.
Since the repertoire of pathways needed for tissue regeneration is likely to be highly conserved in evolution, we focus on a primordial model organisms to address the potential functions of novel lipid mediators (Serhan et al., 2012). The Platyhelminthes (e.g. Dugesia tigrina, also known as planaria) are small organisms that are capable of rapid tissue regeneration. We first questioned whether MaR1 had a role in this critical process. When planaria are surgically injured by removing their anterior portions and exposed to proresolving mediators such as MaR1 or RvE1, the rate of regeneration is enhanced (Serhan et al., 2012). MaR1-enhanced tissue regeneration is concentration dependent, and surgically injured planaria biosynthesize MaR1 from DHA. The accelerated tissue regeneration was monitored quantitatively in an unbiased fashion by introducing a tissue regeneration index that gave a linear regression coefficient of r2 = 0.836, with MaR1 in the nanomolar range.
The biological actions of MaR1 are stereospecific, since related isomers were found to be less effective in also stimulating human MF phagocytosis and apoptotic neutrophils (Serhan et al., 2012). MaR1 also reduces pain in vivo and inhibits TRPV1 currents, IC50 (nM) and TRPA1 current. In dorsal root ganglion (DRG) cell culture, pertussis toxin treatment blocked MaR1 actions, implicating a Gαi-coupled GPCR. MaR1 also dramatically reduces vincristine-initiated neuropathic pain in a cancer chemotherapy model (Serhan et al., 2012) and in temporomandibular joint pain (Park, 2015). MaR1 reduces inflammatory bowel disease (Schwanke et al., 2016), reduces fibrosis (Tang et al., 2017) and diet-induced obesity in mice (Martinez-Fernandez et al., 2017). MaR1 prevents atheroprogression in mice (Viola et al., 2016) and has direct actions on human platelets (Lannan et al., 2017) and human phagocytes (Wang et al., 2015). These results in diverse animal models confirm the potent proresolving actions of MaR1 and emphasize the importance of this separate biosynthetic pathway (Fig. 1B). MaR1 modulates T-cell responses (Fig. 2) (Chiurchiu et al., 2016) and is found in human tears (English et al., 2017).
MCTRs
The levels of potent leukocyte agonists decline during the later phase of the self-limited inflammatory response (Chiang et al., 2012), opening the possibility that other signals may be produced that regulate leukocyte responses to promote tissue repair and regeneration. Given the pivotal roles of chemical signals in infections, we investigated whether mediators within self-resolving infections could regulate tissue repair and regeneration without immunosuppression. Since maresin 1 (7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid; MaR1) displays potent pro-resolving and tissue regenerative actions, we investigated whether new undescribed chemical signals are produced during self-limited infections that regulate tissue regeneration. Along these lines, we identified a new pathway and mediators in planaria, mice and human tissues that promote repair and regeneration during infection. Identification of the first two new sulfido-conjugate (SC)-containing mediators provides the initial evidence for autacoids produced during the resolution of live infections that signal innate host responses and accelerate repair (Dalli et al., 2014).
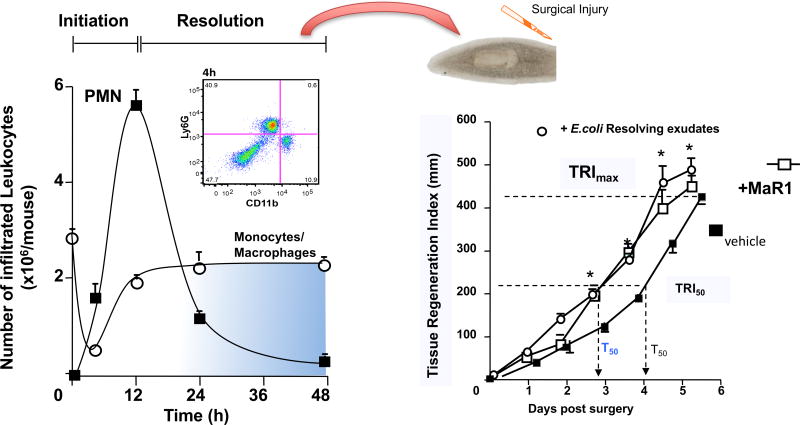
To obtain self-resolving infectious exudates in order to assess the impact of this process on tissue regeneration, we used murine Escherichia coli (E. coli) peritonitis relevant to human infections and mapped leukocyte trafficking. E. coli inoculation at 105 colony-forming units (CFU)/mouse i.p. initiates a self-limited inflammatory response that reaches maximal neutrophil infiltration at 12h and subsequently declined (Fig. 4). Monocyte/macrophage numbers increased between 4 and 24h demarking onset of the resolution phase. In these experiments, we isolated products from resolving infectious exudates (i.e. 24h) and assessed their ability, with planaria, to stimulate tissue regeneration. Since MaR1 stimulates tissue regeneration (Serhan et al., 2012) and elutes within methyl formate fractions from C18 solid-phase extractions, we sought evidence for chemical signals in distinct chromatographic fractions. In these experiments, we assessed eluates in methanol fractions for new signals that displayed tissue regenerative properties (Dalli et al., 2014).
Figure 4.
Resolvin E. coli Infectious Exudate Isolates Contain New Molecules that Promote Tissue Regeneration. To determine whether new signals were produced during self-limited infections chemical isolates were tested for their regeneration capacity (Left panel). Addition of these molecules to surgically injured planaria accelerated tissue regeneration by approximately 1 day, an action that was comparable to that displayed by the proresolving mediators MaR1.
Planaria undergo both restorative and physiological regeneration utilizing evolutionarily conserved pathways, making this an ideal system (Sanchez Alvarado, 2006) for us to identify new chemical signals involved in tissue regeneration. For these studies, planaria (Dugesia japonica) were injured on day 0 and time-dependent head regeneration monitored. To quantitate regeneration, we devised and calculated a tissue regeneration index (TRI) (Dalli et al., 2014). Following head resection or surgical removal, regeneration ensued, giving a TRImax (maximum tissue regeneration) at 6 days and T50 (the interval at which 50% regeneration, TRI50, occurred) ~4.3 days (Fig. 4, right panel). Isolates from 24h infectious-resolving exudates dose-dependently accelerated head regeneration (r2=0.91) as early as 2 days after surgery, shortening T50 to ~3.3 days. For direct comparisons, maresin 1 (Fig. 4, right panel) accelerates this regenerative process to essentially the same extent as the new materials from resolving E. coli exudates. Towards human translation, and because human milk carries nutrients and is appreciated to have products relevant to infant development and immune status (Calder et al., 2006), we also examined the tissue regenerative properties of human milk isolates obtained using the same chromatographic fractions. In these incubations of planaria with human milk isolates, we obtained clear dose-dependent acceleration of regeneration with reduced T50 from ~4.3 to ~3.5 days (Dalli et al., 2014). These findings demonstrate that both mouse resolving-exudates and human milk possess tissue regenerative properties that elute within the methanol fractions from solid-phase C18 extractions.
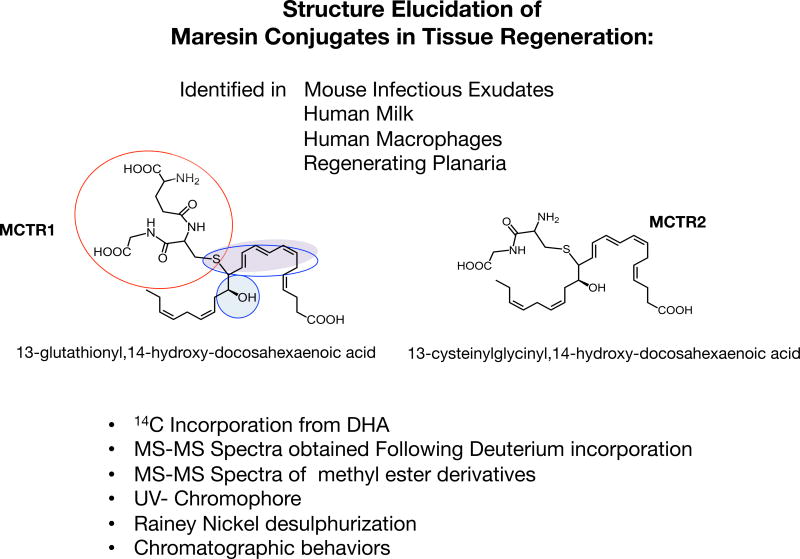
Since 14S-hydro(peroxy)-docosahexaenoic acid (14S-HpDHA) is the product of human MΦ 12-LOX biosynthetic precursor to maresins (Serhan et al., 2009), we prepared and tested 14S-HpDHA as a potential precursor of these regenerative molecules. Human MΦ incubated with 14S-HpDHA also gave these new products. Each product gave ultraviolet (UV)-chromophores with maximum absorbance at 280nm and shoulders at 270nm and 295nm in reverse phase-high pressure liquid chromatography mobile phase (Fig. 5), characteristic of a conjugated triene double bond system coupled to an auxochrome allylic to the triene such as sulphur (Samuelsson, 2012, 1983b). This was corroborated in experiments with a desulphurization reagent, Raney Nickel (Samuelsson, 2012, 1983b), that gave 14-HDHA as product. These results together with MS-MS fragmentation indicated a 13-glutathionyl,14-hydroxy- and 22-carbon backbone that originated from precursor DHA (Fig. 5) and therefore this sulfido-conjugated product I was coined MCTR1.
Figure 5.
Structure Elucidation of the Maresin Conjugates in Tissue Regeneration: Evidence for MCTR1 and MCTR2. Using several lines of evidence to interrogate the physical properties of the bioactive structures isolated from self-resolving infectious exudates, that included the incorporation of radiolabeled DHA, deuterium incorporation and methyl-ester derivatives as well as Rainey Nickel desulfurization and UV chromophores we established that the molecules carried a DHA backbone with conjugated triene double bond systems that was allylic to a peptide containing an auxochrome such as sulfur. Using these lines of evidence the structures were assigned as 13R-glutathionyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid for MCTR1, and 13R-cysteinylglycinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid for MCTR2. These mediators are evolutionary conserved and their production was established in organisms as diverse as planaria, mice and humans.
To gain further evidence for this deduced structure (Fig. 5), we assessed deuterium incorporation in MCTR1 and MCTR2 with human MΦ and d5-14S-HpDHA (Dalli et al., 2014). The d5-MCTR1 gave the expected 5-Dalton shift in the parent ion mass, from m/z 650 to m/z 655, as well as in the m/z of fragments containing carbons 21 and 22, including that resulting from a diagnostic 14–15 carbon break, which increased in mass from m/z 109 to m/z 114. The glutathione conjugate structure was corroborated by treating the bioactive products with diazomethane followed by MS-MS analysis. This was also employed to elucidate the structure of the products, i.e. 13-cysteinylglycinyl, 14-hydroxy-docosahexaenoic acid (Fig. 5). These two new structures were identified in infectious mouse exudates, human milk, human MF and planaria (Dalli et al., 2014). Their structures were deduced from chemical degradation, label tracking and mass spectrometry studies along with assessing their respective actions in diverse biological systems that suggest the molecules are highly conserved structures and function in controlling infection and tissue regeneration.
Given these structures and biological actions, the new family of macrophage-derived pro-resolving and tissue regenerative molecules was coined maresin conjugates in tissue regeneration (MCTR). Administration of MCTR1 and MCTR2 (Fig. 5), to mice with E. coli peritonitis, shortened the resolution interval (Ri) from Ri ~ 20 h to ~ 10 h, demonstrating their potent proresolving actions in inflammation and infections. With human MΦ, both MCTR stimulate efferocytosis, with MCTR2 > MCTR1 in potency with an apparent maximum of ~1 nM, and are organ protective from PMN-mediated reflow tissue injury (Dalli et al., 2014).
Using lipid mediator profiling we recently identified MCTR in human serum, lymph nodes and plasma, and investigated MCTR biosynthetic pathway in human macrophages. With human recombinant enzymes, primary cells and enantiomerically pure synthetic compounds prepared for these studies, the synthetic maresin epoxide intermediate 13S,14S-eMaR (13S,14S-epoxy- 4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid) is converted to MCTR1 (13R-glutathionyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid) by Leukotriene C4 Synthase (LTC4S) as well as Glutathione S-transferase Mu 4 (GSTM4) (Fig. 6). Of interest, human macrophages exposed to LTC4S inhibitors such as BAY-X-1005 or MK886 blocked LTC4 production and increased both resolvins and lipoxins, indicating that these inhibitors stimulate the endogenous production of proresolving mediators.
Figure 6.
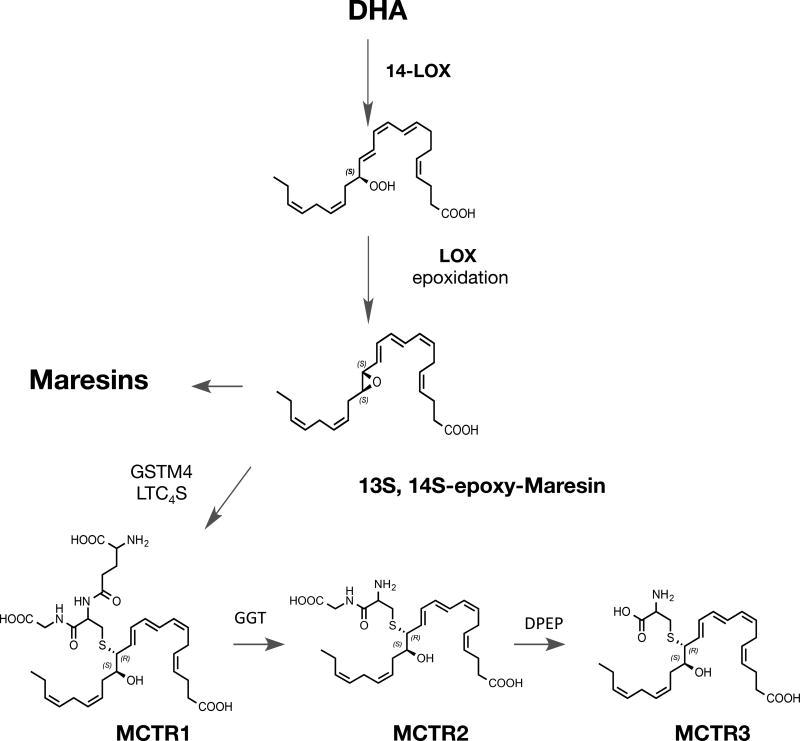
MCTR Biosynthesis and Structures. The MCTR biosynthetic pathway is initiated by lipoxygenation of DHA at carbon position 14 leading to 14S-hydro(peroxy)-4Z,7Z,10Z,12E,16Z,19Z-docosahexaenoic acid, this is converted by lipoxygenase activity to 13S,14S-epoxy −4Z,7Z,9E,11E,16Z,19Z- docosahexaenoic acid. Conversion of this allylic epoxide is to MCTR1 is catalyzed by either glutathione s-transferase mu4 (GSTM4) and by leukotriene C4 synthase (LTC4S). MCTR1 is converted by gammaglutamyl transferase (GGT) to MCTR2 that in turn is substrate for conversion by dipeptidase (DPEP) to MCTR3.
The conversion of MCTR1 to MCTR2 13R-cysteinylglycinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid is catalyzed by γ-glutamyl transferase (GGT) in human macrophages (Fig 6). Biosynthesis of MCTR3 produced by dipeptidases cleaved the cysteinyl-glycinyl bond in MCTR2 to 13R-cysteinyl, 14S-hydroxy-4Z,7Z,9E,11E,13R,14S,16Z,19Z-docosahexaenoic acid. Both GSTM4 and GGT enzymes displayed higher affinity to 13S,14S-eMaR and MCTR1 when compared to their classic substrates in the cysteinyl leukotriene metabolome (Dalli et al., 2016b). The MCTR biosynthetic pathway is established, and their roles in tissue repair and regeneration confirmed with synthetic MCTR. Among the three MCTR, MCTR3 proves to also possess potent bioactions (Fig 6).
Having identified the newest MCTR3 (Fig. 6) and established rank order potencies via matching the stereochemistries of MCTR1, MCTR2 and MCTR3 with material prepared by total organic synthesis and mediators isolated from both mouse and human systems. MCTR3 was produced from endogenous substrate by E. coli activated human macrophages and identified in sepsis patients (Dalli et al., 2016a). The three synthetic MCTR dose-dependently (1–100nM) accelerated tissue regeneration in planaria by 0.6–0.9 days. When each MCTR is administered at the onset or peak of inflammation in mice, each promoted resolution of E. coli infections. They increased bacterial phagocytosis by exudate leukocytes (~15–50%), limited neutrophil infiltration (~20–50%), promoted efferocytosis (~30%) and reduced eicosanoids. MCTR1 and MCTR2 upregulated human neutrophil and macrophage phagocytic responses where MCTR3 also proved to possess potent actions. The first synthesis of MCTRs was achieved by Rodriguez and Spur (2015). The complete stereochemistry and rank order potencies for MCTR1, MCTR2 and MCTR3 are established at this point and provide novel resolution signals in regulating responses to clear infections and signal to promote tissue regeneration (Dalli et al., 2016a).
Discovery of PCTR and RCTR
Spleens from self-limited infections, namely infectious resolving exudates in mice, human spleens (Borges da Silva et al., 2015; Mebius and Kraal, 2005) and blood from sepsis patients, each contain new mediators that also carried conjugations with glutathione (Dalli et al., 2015b). Each mediator obtained from resolving infectious exudates stimulates tissue regeneration and the removal of bacteria by human macrophages. Their structures were systematically elucidated, and their biosynthesis from DHA was determined (Figs. 7 and 8). The six new conjugated mediators stimulated phagocytosis, bacterial killing and efferocytosis of apoptotic cells and enhanced tissue regeneration in planaria as a bioassay model organism (Dalli et al., 2015b).
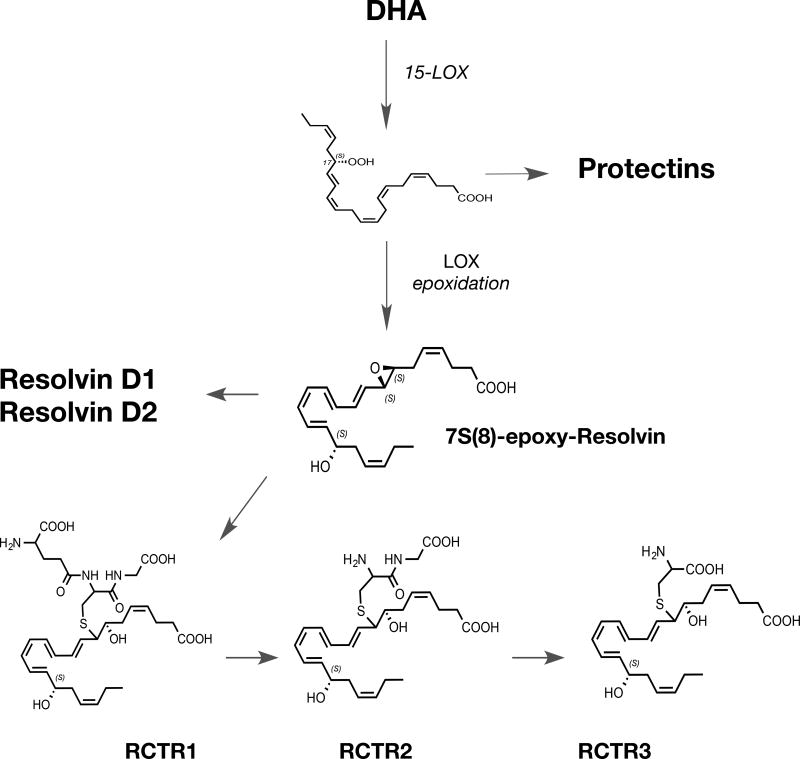
Figure 7. RCTR Biosynthesis and Structures.
15-LOX type 1 is the initiating enzyme in the RCTR pathway which catalyzes the formation of 17S-hydro(peroxy)-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid via subsequent lipoxygenase activity this substrate is converted to 7, 8-epoxy, 17S-hydroxy-4Z,9,11,13,15,19Z-docosahexaenoic acid that is precursor to RCTR1. This mediator is converted to RCTR2 that in turn produces RCTR3.
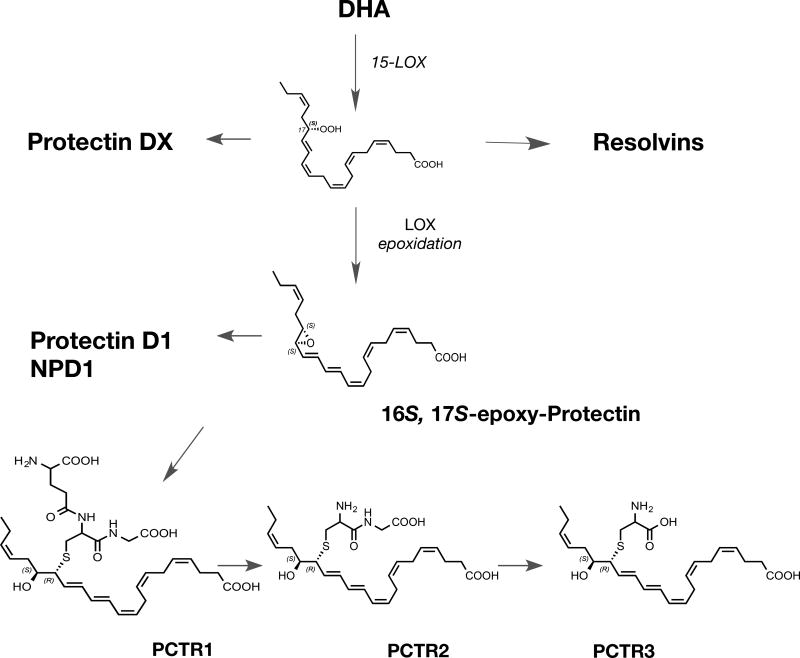
Figure 8. PCTR Biosynthesis and Structures.
Formation of 17S-hydro(peroxy)-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid from DHA by 15-LOX type 1 initiates the PCTR biosynthetic pathway. This hydroperoxide is converted to 16S,17S-epoxy-7Z,10Z,13E,14E,19Z- docosahexaenoic acid, this allylic epoxide is precursor to PCTR1, that is transformed to PCTR2 that is in turn precursor to PCTR3.
The proposed biosynthetic schemes for the RCTRs including RCTR1, RCTR2 and RCTR3 are shown in Figure 7. The PCTRs, including the new bioactive structures PCTR1, PCTR2 and PCTR3, are shown in Figure 8. The structures of the PCTRs have been rapidly confirmed by total organic synthesis (Dalli et al., 2016b; Rodriguez and Spur, 2015), and the three new structures of the RCTRs have been confirmed by total organic synthesis (Rodriguez and Spur, 2017).
The protectins and their relationship to the PCTRs and resolvins are illustrated in Figure 8. Each bioaction of the new pathway is confirmed with synthetic RCTR and PCTR (Ramon et al., 2016), and their rank orders of potency are in progress (X. de la Rosa et al., manuscript in progress). The structural elucidation of the PCTRs, since they possess a carbon-17 alcohol group and conjugated triene structure, dictated that they belong to the protectin family along with NPD1/PD1 and 10S,17S-diHDHA, a.k.a. PDX (Serhan et al., 2006; Balas and Durand, 2016), and the 17R- or aspirin-triggered protectin D1 (Serhan et al., 2011a). By a similar line of evidence, the RCTRs (Fig. 8) carried a carbon-17 position and conjugated tetraene belonging to the resolvin family. Key to their biosynthesis is the enzymatic production of an allylic epoxide that is enzymatically converted to the respective thiopeptides that are bioactive (Figs. 8 and 9). The enzymatic biosynthesis of these pivotal allylic epoxides was recently reviewed in detail in Serhan et al. (2015b)
The 16S,17S-epoxy-protectin (Fig. 8) intermediate was synthesized by total organic synthesis and enzymatically converted to PCTR1 (Ramon et al., 2016). PCTR1 is pro-resolving and is produced in greater amounts by human M2 macrophages than M1-phenotype MΦ, and in vitro is produced by M2 MΦ in the same magnitude as cysteinyl leukotrienes (Ramon et al., 2016). Vagal stimulation regulates inflammation in a process termed inflammatory reflex (Chavan et al., 2017). Vagotomy controls the local production of pro-resolving mediators such as RvD1 (Mirakaj et al., 2014). Moreover, the right vagus nerve regulates peritoneal resolution tone in mice (Dalli et al., 2017a) with production of PCTR1 (Fig. 8) via ILC3. PCTR1 restores MΦ functions and stimulates resolution of bacterial infections in mice (Dalli et al., 2017a).
Summary
In summation, results from studies on self-limited acute inflammatory response clearly demonstrated that resolution is an active biosynthetic process that connects the first response of the innate immune system to active immunity with the structural elucidation of the specialized proresolving mediators including resolvins, protectins and maresins. The precursors for these mediators prove to be n-3 essential fatty acids that have long been suspected to play critical roles in organ protection and the innate immune response to control inflammation (Calder, 2013). This structural elucidation, complete stereochemical assignments of the SPM and identification of several of their G protein-coupled receptors opens up the possibility for resolution physiology and pharmacology. It also paves the way for a deeper appreciation of the role of nutrition and the essential fatty acids in these vital processes. We identified nine new mediators during bacterial infection that stimulate tissue regeneration; their structures and biosynthesis are reviewed here. Given that their backbone structures and biosynthesis are related to resolvins, protectins and maresins, they have been named accordingly MCTR, PCTR and RCTR. Now that their structures and complete stereochemical assignments are in place, it will be possible to assess their functions and mechanisms in further molecular detail in vivo. Profiling approaches to date for the resolution metabolome clearly establish the biosynthesis and actions of SPMs in humans that evoke proresolving responses in vivo in the concentrations at which they are produced locally. Hence, these pathways and mediators illustrate new proresolving mechanisms that the host utilizes to contain as well as return from challenge and tissue injury. They could provide a basis for new approaches to treating diseases and controlling excessive inflammation as well as to tissue regeneration using active resolution pharmacology. These now permit direct assessment of personalized resolution metabolome for medicine and nutrition.
Acknowledgments
We thank Mary H. Small for expert assistance in manuscript preparations. We also thank our coauthors on the original studies reviewed here as well as our colleagues for their original contributions to this new field. JD is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant number: 107613/Z/15/Z), funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant number: 677542), the Barts Charity (Grant number: MGU0343). CNS research is supported by National Institutes of Health USA (grant numbers R01GM38765 and P01GM095467).
Abbreviations*
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- LC-MS-MS
liquid chromatography tandem mass spectrometry
- LM
lipid-derived mediators
- LOX
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- PG
prostaglandins
- MΦ
macrophage
- Maresins
macrophage mediators in resolving inflammation
- MCTR
maresin conjugate in tissue regeneration
- PCTR
protectin conjugate in tissue regeneration
- RCTR
resolvin conjugate in tissue regeneration
- PMN
polymorphonuclear leukocyte
- PD1/NPD1
protectin D1/ neuroprotectin D1
- SPM
specialized pro-resolving mediators *
- Rv, Resolvins *
bioactive omega-3 derived resolution phase interaction products
- E series Rv
resolvins from EPA
- D series Rv
resolvins from DHA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Please see English et al. (2017) for the complete stereochemical assignments and nomenclature of the bioactive SPM and their biosynthetic pathway markers.
References
- Albert BB, Derraik JG, Cameron-Smith D, Hofman PL, Tumanov S, Villas-Boas SG, Garg ML, Cutfield WS. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. 2015;5:7928. doi: 10.1038/srep07928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016a;9:757–766. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir HH, Dalli J, Norling LV, Colas RA, Perretti M, Serhan CN. Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation. J. Immunol. 2016b;197(6):2362–2368. doi: 10.4049/jimmunol.1502268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US Adults Use Dietary Supplements. JAMA Intern Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- Balas L, Durand T. Dihydroxylated E,E,Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res. 2016;61:1–18. doi: 10.1016/j.plipres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Serra MF, Diaz BL, Cordeiro RSB, Silva PMR, Lenzi HL, Bakhle YS, Serhan CN, Martins MA. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J. Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br. J. Pharmacol. 2010;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Barden AE, Mas E, Mori TA. n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr. Opin. Lipidol. 2016a;27(1):26–32. doi: 10.1097/MOL.0000000000000262. [DOI] [PubMed] [Google Scholar]
- Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids. 2016b;107:24–29. doi: 10.1016/j.plefa.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum Y. Does pioglitazone increase the production of prostacyclin (PGI2) and/or 15-epi-lipoxin with diabetes mellitus type 2? 2012 ClinicalTrials.gov, NCT01040819.
- Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdottir S, Folsgaard NV, Fink NR, Thorsen J, Pedersen AG, Waage J, Rasmussen MA, Stark KD, Olsen SF, Bonnelykke K. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016;375(26):2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound Repair Regen. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Fonseca R, Pereira RM, Cassado Ados A, Alvarez JM, D'Imperio Lima MR. Splenic Macrophage Subsets and Their Function during Blood-Borne Infections. Front Immunol. 2015;6:480. doi: 10.3389/fimmu.2015.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeson E, Godson C. Resolution of inflammation: therapeutic potential of pro-resolving lipids in type 2 diabetes mellitus and associated renal complications. Front Immunol. 2012;3:318. doi: 10.3389/fimmu.2012.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL, Jr, Goodman GE, Minasian LM, Parnes HL, Klein EA, Kristal AR. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J. Natl. Cancer Inst. 2013;105:1132–1141. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DW. Resolvyx announced positive data from trial of resolvin RX-10045 for dry eye. 2009 Eye Doc News http://eyedocnews.com/002059-resolvyx-announces-positive-data-from-trial-of-resolvin-rx-10045-for-dry-eye/
- Buechler C, Pohl R, Aslanidis C. Pro-Resolving Molecules-New Approaches to Treat Sepsis? Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, Heinrich J, Garn H, Koletzko S, Lack G, Mattelio G, Renz H, Sangild PT, Schrezenmeir J, Stulnig TM, Thymann T, Wold AE, Koletzko B. Early nutrition and immunity - progress and perspectives. Br. J. Nutr. 2006;96(4):774–790. [PubMed] [Google Scholar]
- Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay R, Raghavan S, Rao GN. Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox Biol. 2017;12:438–455. doi: 10.1016/j.redox.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity. 2017;46(6):927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015;212(8):1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017;198(2):842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery TW, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Aspects Med. 2017 doi: 10.1016/j.mam.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8(353):353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M, Miscia S, Colas RA, Dalli J, Serhan CN, Romano M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Transl Med. 2016;5(1):20–32. doi: 10.5966/sctm.2015-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone M, Cianci E, Lamolinara A, Mari VC, Nespoli A, Isopi E, Mattoscio D, Arita M, Bragonzi A, Iezzi M, Romano M, Recchiuti A. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.1036. [DOI] [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. U. S. A. 2013;110(45):18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6. W.B. Saunders Co.; Philadelphia: 1999. p. 1425. [Google Scholar]
- Dalli J, Chiang N, Serhan CN. Identification of sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E4753–4761. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 2015a;21:1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Arnardottir H, Serhan CN. Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity. 2017a;46(1):92–105. doi: 10.1016/j.immuni.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Quintana C, Barragan-Bradford D, Hurwitz S, Levy BD, Choi AM, Serhan CN, Baron RM. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations With Survival and Clinical Outcomes. Crit. Care Med. 2017b;45:58–68. doi: 10.1097/CCM.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Consalvo AP, Ray V, Di Filippo C, D'Amico M, Mehta N, Perretti M. Proresolving and Tissue-Protective Actions of Annexin A1-Based Cleavage-Resistant Peptides Are Mediated by Formyl Peptide Receptor 2/Lipoxin A4 Receptor. J. Immunol. 2013a;190:6478–6487. doi: 10.4049/jimmunol.1203000. [DOI] [PubMed] [Google Scholar]
- Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. Novel proresolving and tissue regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J. 2015b;29:2120–2136. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Sanger JM, Rodriguez AR, Chiang N, Spur BW, Serhan CN. Identification and Actions of a Novel Third Maresin Conjugate in Tissue Regeneration: MCTR3. PLoS One. 2016a;11(2):e0149319. doi: 10.1371/journal.pone.0149319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur B, Petasis NA, Chiang N, Serhan CN. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA. 2016b;113:12232–12237. doi: 10.1073/pnas.1607003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013b;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawczynski C, Dittrich M, Neumann T, Goetze K, Welzel A, Oelzner P, Volker S, Schaible AM, Troisi F, Thomas L, Pace S, Koeberle A, Werz O, Schlattmann P, Lorkowski S, Jahreis G. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2017 doi: 10.1016/j.clnu.2017.1002.1021. [DOI] [PubMed] [Google Scholar]
- De Caterina R. n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Schwartz CE, Gjorstrup P, Pflugfelder SC. Resolvin E1 (RX-10001) Reduces Corneal Epithelial Barrier Disruption and Protects Against Goblet Cell Loss in a Murine Model of Dry Eye. Cornea. 2012;31:1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016;274(1):330–353. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9(7):e102362. doi: 10.1371/journal.pone.0102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann. Rheum. Dis. 2013 doi: 10.1136/annrheumdis-2013-203338. [DOI] [PubMed] [Google Scholar]
- Easley JT, Maruyama CL, Wang CS, Baker OJ. AT-RvD1 combined with DEX is highly effective in treating TNF-alpha-mediated disruption of the salivary gland epithelium. Physiol Rep. 2016;4(19) doi: 10.14814/phy2.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. U. S. A. 2012;109(37):14983–14988. doi: 10.1073/pnas.1206641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30(8):2792–2801. doi: 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN. Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot. Essent. Fatty Acids. 2017;117:17–27. doi: 10.1016/j.plefa.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhbaatar P. Cellular Therapy for Sepsis: A New Hope? Crit. Care Med. 2016;44(12):2296–2298. doi: 10.1097/CCM.0000000000002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L, Dantes R, Magill S, Fiore A. Varying Estimates of Sepsis Mortality Using Death Certificates and Administrative Codes--United States, 1999-2014. MMWR. Morb. Mortal. Wkly. Rep. 2016;65(13):342–345. doi: 10.15585/mmwr.mm6513a2. [DOI] [PubMed] [Google Scholar]
- Feng Q, Feng F, Feng X, Li S, Wang S, Liu Z, Zhang X, Zhao Q, Wang W. Resolvin D1 reverses chronic pancreatitis-induced mechanical allodynia, phosphorylation of NMDA receptors,and cytokines expression in the thoracic spinal dorsal horn. BMC Gastroenterol. 2012;12:148. doi: 10.1186/1471-230X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda Y, Manno A, Hayashi Y, Rhodes N, Guo L, Arita M, Bamba T, Fukusaki E. Inflammation and resolution are associated with upregulation of fatty acid beta-oxidation in Zymosan-induced peritonitis. PLoS One. 2013;8(6):e66270. doi: 10.1371/journal.pone.0066270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K, Bernier J, Godbout R, Rousseau G. Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar Drugs. 2014;12(11):5396–5407. doi: 10.3390/md12115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 2011;111(10):5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs GA, Salmon JA, Henderson B, Vane JR. Pharmacokinetics of aspirin and salicylate in relation to inhibition of arachidonate cyclooxygenase and antiinflammatory activity. Proc Natl Acad Sci U S A. 1987;84(5):1417–1420. doi: 10.1073/pnas.84.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilberath JN, Carlo T, Pfeffer MA, Croze RH, Hastrup F, Levy BD. Resolution of Toll-like receptor 4-mediated acute lung injury is linked to eicosanoids and suppressor of cytokine signaling 3. FASEB J. 2011;25(6):1827–1835. doi: 10.1096/fj.10-169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada T, Ishizuka T, Aoki H, Mori M. Resolvin E1 as a novel agent for the treatment of asthma. Expert Opin Ther Targets. 2009;13(5):513–522. doi: 10.1517/14728220902865622. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Huang J, Burston JJ, Li L, Ashraf S, Mapp PI, Bennett AJ, Ravipati S, Pousinis P, Barrett DA, Scammell BE, Chapman V. Targeting the D Series Resolvin Receptor System for the Treatment of Osteoarthritis Pain. Arthritis Rheumatol. 2017;69(5):996–1008. doi: 10.1002/art.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wang C-F, Serhan CN, Strichartz G. Enduring prevention and transient reduction of post-operative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Deyama S, Shimoda K, Yoshikawa K, Ide S, Satoh M, Minami M. Rapid and sustained antidepressant effects of resolvin D1 and D2 in a chronic unpredictable stress model. Behav. Brain Res. 2017;332:233–236. doi: 10.1016/j.bbr.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012a;287(13):10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012b;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes KA, Yaffe MB. Data-driven modelling of signal-transduction networks. Nat. Rev. Mol. Cell Biol. 2006;7(11):820–828. doi: 10.1038/nrm2041. [DOI] [PubMed] [Google Scholar]
- Jaudszus A, Gruen M, Watzl B, Ness C, Roth A, Lochner A, Barz D, Gabriel H, Rothe M, Jahreis G. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. J. Lipid Res. 2013;54(4):923–935. doi: 10.1194/jlr.P031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana MR. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest. Ophthalmol. Vis. Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YY, Lee JY, Park CK. Resolvin E1 Inhibits Substance P-Induced Potentiation of TRPV1 in Primary Sensory Neurons. Mediators Inflamm. 2016;2016:5259321. doi: 10.1155/2016/5259321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Mark PJ, Keelan JA, Barden A, Mas E, Mori TA, Waddell BJ. Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J. Lipid Res. 2013;54(8):2247–2254. doi: 10.1194/jlr.M039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchegowda S, He J, Bazan HE. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88(1):27–31. doi: 10.1016/j.plefa.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int Immunopharmacol. 2012;14(4):384–391. doi: 10.1016/j.intimp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Koltsida O, Karamnov S, Pyrillou K, Vickery T, Chairakaki AD, Tamvakopoulos C, Sideras P, Serhan CN, Andreakos E. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol Med. 2013;5(5):762–775. doi: 10.1002/emmm.201201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, Neufer PD, Armstrong M, Reisdorph N, Milner JJ, Beck M, Shaikh SR. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J. Immunol. 2017;198(12):4738–4752. doi: 10.4049/jimmunol.1601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands WEM. Fish, Omega-3 and Human Health. 2. AOCS Press; Champaign, IL: 2005. [Google Scholar]
- Lannan KL, Spinelli SL, Blumberg N, Phipps RP. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J Thromb Haemost. 2017 doi: 10.1111/jth.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch. Pharm. Res. 2012;35(1):3–7. doi: 10.1007/s12272-012-0121-z. [DOI] [PubMed] [Google Scholar]
- Lee CR, Zeldin DC. Resolvin Infectious Inflammation by Targeting the Host Response. N. Engl. J. Med. 2015;373(22):2183–2185. doi: 10.1056/NEJMcibr1511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HN, Kundu JK, Cha YN, Surh YJ. Resolvin D1 stimulates efferocytosis through p50/p50-mediated suppression of tumor necrosis factor-alpha expression. J. Cell Sci. 2013;126(Pt 17):4037–4047. doi: 10.1242/jcs.131003. [DOI] [PubMed] [Google Scholar]
- Lee S, Nakahira K, Dalli J, Siempos II, Norris PC, Colas RA, Moon JS, Shinohara M, Hisata S, Howrylak JA, Suh GY, Ryter SW, Serhan CN, Choi AM. NLRP3 Inflammasome Deficiency Protects Against Microbial Sepsis via Increased Lipoxin B4 Synthesis. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M. Inflammation's stop signals. Science. 2015;347(6217):18–21. doi: 10.1126/science.347.6217.18. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J. Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, Serhan CN, Dartt DA. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013;6:1119–1130. doi: 10.1038/mi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, He J, Schwartz CE, Gjorstrup P, Bazan HEP. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. ALX-chemy: adding spice to the inflammatory soup of atherosclerosis. Cardiovasc. Res. 2015;105(1):3–5. doi: 10.1093/cvr/cvu245. [DOI] [PubMed] [Google Scholar]
- Lima-Garcia J, Dutra R, da Silva K, Motta E, Campos M, Calixto J. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, La Cava A, Wiedau-Pazos M. Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am J Neurodegener Dis. 2012;1(1):60–74. [PMC free article] [PubMed] [Google Scholar]
- Lourdudoss C, Di Giuseppe D, Wolk A, Westerlind H, Klareskog L, Alfredsson L, van Vollenhoven RF, Lampa J. Dietary Intake of Polyunsaturated Fatty Acids and Pain in spite of Inflammatory Control among Methotrexate Treated Early Rheumatoid Arthritis Patients. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CD, Dorward DA, Tait MA, Fox S, Marwick JA, Allen KC, Robb CT, Hirani N, Haslett C, Duffin R, Rossi AG. Downregulation of Mcl-1 has anti-inflammatory pro-resolution effects and enhances bacterial clearance from the lung. Mucosal Immunol. 2014;7:857–868. doi: 10.1038/mi.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T, Mangsbo SM, Scholz H, Gjorstrup P, Totterman TH, Korsgren O, Foss A. Resolvin E1 reduces proinflammatory markers in human pancreatic islets in vitro. Exp. Clin. Endocrinol. Diabetes. 2010;118(4):237–244. doi: 10.1055/s-0029-1241825. [DOI] [PubMed] [Google Scholar]
- Lundstrom SL, Yang J, Brannan JD, Haeggstrom JZ, Hammock BD, Nair P, O'Byrne P, Dahlen SE, Wheelock CE. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res. 2013;57(8):1378–1389. doi: 10.1002/mnfr.201200827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Wang J, Liu Z, Shen Z, Shi R, Liu YQ, Liu Y, Jiang M, Wu Y, Zhang Z. Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat Commun. 2016;7:12177. doi: 10.1038/ncomms12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. 2. Oxford University Press; New York: 2004. [Google Scholar]
- Martinez-Fernandez L, Gonzalez-Muniesa P, Laiglesia LM, Sainz N, Prieto-Hontoria PL, Escote X, Odriozola L, Corrales FJ, Arbones-Mainar JM, Martinez JA, Moreno-Aliaga MJ. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 2017 doi: 10.1096/fj.201600859R. [DOI] [PubMed] [Google Scholar]
- Mas E, Barden A, Burke V, Beilin LJ, Watts GF, Huang RC, Puddey IB, Irish AB, Mori TA. A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin. Nutr. 2016;35(2):331–336. doi: 10.1016/j.clnu.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Miki Y, Yamamoto K, Taketomi Y, Sato H, Shimo K, Kobayashi T, Ishikawa Y, Ishii T, Nakanishi H, Ikeda K, Taguchi R, Kabashima K, Arita M, Arai H, Lambeau G, Bollinger JM, Hara S, Gelb MH, Murakami M. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013;210(6):1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J. Exp. Med. 2014;211(6):1037–1048. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvins attenuate vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013;131:353–360. doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- Montero-Melendez T, Gobbetti T, Cooray SN, Jonassen TE, Perretti M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J. Immunol. 2015;194(7):3381–3388. doi: 10.4049/jimmunol.1402645. [DOI] [PubMed] [Google Scholar]
- Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, Gilroy DW. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. USA. 2010a;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, Gilroy DW. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A. 2010b;107(19):8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- Motwani M, George M, Flint J, Dalli J, Colas RA, de Maeyer R, Ricard-Londt A, Serhan CN, Gilroy D. Pro-resolving mediators promote resolution of acute inflammation in humans. JCI Insight, in revision. 2017 doi: 10.1172/jci.insight.94463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozurkewich EL, Greenwood M, Clinton C, Berman D, Romero V, Djuric Z, Qualls C, Gronert K. Pathway Markers for Pro-resolving Lipid Mediators in Maternal and Umbilical Cord Blood: A Secondary Analysis of the Mothers, Omega-3, and Mental Health Study. Front Pharmacol. 2016;7:274. doi: 10.3389/fphar.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Fresh approaches to anti-infective therapies. Sci. Transl. Med. 2012;4(140):140sr142. doi: 10.1126/scitranslmed.3003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Xavier RA, Newson J, Silveira VLF, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J. Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- Nebert DW. Aryl hydrocarbon receptor (AHR): "pioneer member" of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of "sensors" of foreign and endogenous signals. Prog. Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1(5):e85922. doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Perretti M. The role of omega-3 derived resolvins in arthritis. Curr Opin Pharmacol. 2013;13(3):476–481. doi: 10.1016/j.coph.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2010;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CD, Mancuso CJ, Weiss JP, Serhan CN, Guinan EC, Levy O. 17(R)-Resolvin D1 differentially regulates TLR4-mediated responses of primary human macrophages to purified LPS and live E. coli. J. Leukoc. Biol. 2011;90:459–470. doi: 10.1189/jlb.0311145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediators Inflamm. 2015;2015:275126. doi: 10.1155/2015/275126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Lü N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1 and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J. Neurosci. 2011;31:15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Cooper D, Dalli J, Norling LV. Immune resolution mechanisms in inflammatory arthritis. Nat Rev Rheumatol. 2017;13(2):87–99. doi: 10.1038/nrrheum.2016.193. [DOI] [PubMed] [Google Scholar]
- Petri MH, Laguna-Fernandez A, Arnardottir H, Wheelock CE, Perretti M, Hansson GK, Back M. Aspirin-triggered lipoxin inhibits atherosclerosis progression in ApoE-/- mice. Br. J. Pharmacol. 2017 doi: 10.1111/bph.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 2015;105(1):65–74. doi: 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai PS, Leeson S, Porter TF, Owens CD, Kim JM, Conte MS, Serhan CN, Gelman S. Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation. 2012;35(1):98–113. doi: 10.1007/s10753-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisson LM, Suhail H, Singh J, Datta I, Denic A, Labuzek K, Hoda MN, Shankar A, Kumar A, Cerghet M, Elias S, Mohney RP, Rodriguez M, Rattan R, Mangalam AK, Giri S. Untargeted Plasma Metabolomics Identifies Endogenous Metabolite with Druglike Properties in Chronic Animal Model of Multiple Sclerosis. J. Biol. Chem. 2015;290(52):30697–30712. doi: 10.1074/jbc.M115.679068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polus A, Zapala B, Razny U, Gielicz A, Kiec-Wilk B, Malczewska-Malec M, Sanak M, Childs CE, Calder PC, Dembinska-Kiec A. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim. Biophys. Acta. 2016;1861(11):1746–1755. doi: 10.1016/j.bbalip.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, Ailawadi G, Upchurch GR., Jr D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 2016;30(12):4192–4201. doi: 10.1096/fj.201600144RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Kopp MA, Brommer B, Gatzemeier N, Laginha I, Dirnagl U, Schwab JM. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain Pathol. 2011;21(6):652–660. doi: 10.1111/j.1750-3639.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6(2):e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J. Pathol. 2012;228:506–519. doi: 10.1002/path.4050. [DOI] [PubMed] [Google Scholar]
- Rajasagi NK, Bhela S, Varanasi SK, Rouse BT. Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology. J. Leukoc. Biol. 2017 doi: 10.1189/jlb.1183HI1216-1511RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Reddy PBJ, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J. Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami R, Serhan CN, Levy BD, Makrides M. Fish Oil Supplementation in Pregnancy. N. Engl. J. Med. 2016;375(26):2599–2601. doi: 10.1056/NEJMclde1614333. [DOI] [PubMed] [Google Scholar]
- Ramon S, Dalli J, Sanger JM, Winkler JW, Aursnes M, Tungen JE, Hansen TV, Serhan CN. The Protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation Am. J. Pathol. 2016;186:962–973. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 2012;189(2):1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CE. Breathing Easier with Fish Oil - A New Approach to Preventing Asthma? N. Engl. J. Med. 2016;375(26):2596–2598. doi: 10.1056/NEJMe1611723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, Van Eijl S, Gee LC, Bansal J, Pitrola K, Shaw C, D'Acquisto F, Colas RA, Marelli-Berg F, Dalli J, Ahluwalia A. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Invest. 2017;127:169–182. doi: 10.1172/JCI89429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resolvyx Pharmaceuticals Inc. Safety and efficacy study of RX-10045 on the signs and symptoms of dry eye. 2012 ClinicalTrials.gov, NCT00799552.
- Rodriguez AR, Spur BW. First total synthesis of pro-resolving and tissue regenerative maresin sulfido-conjugates. Tetraahedron Lett. 2015;56:3936–3940. [Google Scholar]
- Rodriguez AR, Spur BW. First total synthesis of pro-resolving and tissue-regenerative resolvin sulfido-conjugates. Tetrahedron Lett. 2017;58:1662–1668. [Google Scholar]
- Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 2012;189(4):1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CD, Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141:166–173. doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983a;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983b;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem. 2012;287:10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A. Planarian regeneration: its end is its beginning. Cell. 2006;124(2):241–245. doi: 10.1016/j.cell.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanke RC, Marcon R, Bento AF, Calixto JB. EPA- and DHA-derived resolvins' actions in inflammatory bowel disease. Eur. J. Pharmacol. 2016;785:156–164. doi: 10.1016/j.ejphar.2015.08.050. [DOI] [PubMed] [Google Scholar]
- Science Blog. Resolvyx announces positive data -- Phase 2 trial of resolvin RX-10045 for dry eye syndrome. 2009 http://www.scienceblog.com/
- Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem. Cell Biol. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017 doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015a;27(3):200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta. 2015b;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011a;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory -pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011b;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation-resolution. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settimio R, Clara DF, Franca F, Francesca S, Michele D. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 2012;2012:318621. doi: 10.1155/2012/318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets KG, Zhou Y, Ertel MK, Knott EJ, Regan CE, Jr, Elison JR, Gordon WC, Gjorstrup P, Bazan NG. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol. Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]
- Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78(9):658–664. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Irino Y, Shinohara M, Tsuda S, Mori T, Nagao M, Oshita T, Mori K, Hara T, Toh R, Ishida T, Hirata KI. Eicosapentaenoic Acid-Enriched High-Density Lipoproteins Exhibit Anti-Atherogenic Properties. Circ J. 2017 doi: 10.1253/circj.CJ-1217-0294. [DOI] [PubMed] [Google Scholar]
- Tang S, Gao C, Long Y, Huang W, Chen J, Fan F, Jiang C, Xu Y. Maresin 1 Mitigates High Glucose-Induced Mouse Glomerular Mesangial Cell Injury by Inhibiting Inflammation and Fibrosis. Mediators Inflamm. 2017;2017:2438247. doi: 10.1155/2017/2438247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution Therapy for the Treatment of Delayed Healing of Diabetic Wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Gomez-Galan M, Yang T, Carlstrom M, Gustavsson D, Harding RE, Lindskog M, Eriksson LI. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J. 2013;27:3564–3571. doi: 10.1096/fj.13-230276. [DOI] [PubMed] [Google Scholar]
- Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Uno H, Furukawa K, Suzuki D, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Miyazaki M. Immunonutrition suppresses acute inflammatory responses through modulation of resolvin E1 in patients undergoing major hepatobiliary resection. Surgery. 2016;160(1):228–236. doi: 10.1016/j.surg.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Vassiliou EK, Kesler OM, Tadros JH, Ganea D. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J. Immunol. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ. Res. 2016;119(9):1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- Wan M, Godson C, Guiry PJ, Agerberth B, Haeggström JZ. Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counter-regulated by lipoxin A4 and resolvin E1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- Wang CW, Colas RA, Dalli J, Arnardottir HH, Nguyen D, Hasturk H, Chiang N, Van Dyke TE, Serhan CN. Maresin 1 Biosynthesis and Proresolving Anti-infective Functions with Human-Localized Aggressive Periodontitis Leukocytes. Infect. Immun. 2015;84(3):658–665. doi: 10.1128/IAI.01131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Strichartz GR. Prevention of Chronic Post-Thoracotomy Pain in Rats By Intrathecal Resolvin D1 and D2: Effectiveness of Perioperative and Delayed Drug Delivery. J Pain. 2017;18(5):535–545. doi: 10.1016/j.jpain.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PA, Bosmann M. A historical perspective on sepsis. Am. J. Pathol. 2012;181(1):2–7. doi: 10.1016/j.ajpath.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Gronert K. The role of pro-resolving lipid mediators in ocular diseases. Mol. Aspects Med. 2017 doi: 10.1016/j.mam.2017.1003.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G. Galileo’s Gout: Science In An Age of Endarkenment. Bellevue Literary Press; New York: 2007. [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and Safety of 15(R/S)-Methyl-Lipoxin A(4) in Topical Treatment of Infantile Eczema. Br. J. Dermatol. 2013;168:172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- Xu Z-Z, Zhang L, Liu T, Park J-Y, Berta T, Yang R, Serhan CN, Ji R-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Berta T, Ji RR. Resolvin E1 Inhibits Neuropathic Pain and Spinal Cord Microglial Activation Following Peripheral Nerve Injury. J Neuroimmune Pharmacol. 2013;8:37–41. doi: 10.1007/s11481-012-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Xu Z, Yin X, Zheng F, Lin X, Pan Q, Li H. Inverse Relationship between Serum Lipoxin A4 Level and the Risk of Metabolic Syndrome in a Middle-Aged Chinese Population. PLoS One. 2015;10(11):e0142848. doi: 10.1371/journal.pone.0142848. [DOI] [PMC free article] [PubMed] [Google Scholar]