Abstract
Changes in the routine immunization schedule are common and may pose challenges to primary care clinics. We sought to assess the experiences of U.S. providers and clinic staff during the introduction of 9-valent HPV vaccine. In 2015–2016, we conducted a survey in a probability sample of 127 pediatric (40%) and family medicine (60%) clinics in three U.S. states. The 211 respondents included clinicians (63%) and staff (37%). Overall, 83% of clinics stocked 9-valent HPV vaccine, with adoption ranging from 60% among early respondents to 100% among later respondents. Almost all respondents believed that providers in their clinics would recommend the 9-valent vaccine as strongly as (66%) or more strongly than (33%) the quadrivalent vaccine. Over half (61%) had no concerns about the 9-valent vaccine, while others reported concerns about increased parental hesitancy (29%), private insurance coverage (17%), or other issues (10%). Respondents from pediatric versus family medicine clinics more often reported a concern (OR=2.06, 95% CI 1.02–4.15). Among the 169 respondents who stocked 9-valent vaccine, about half (56%, n=94) anticipated that providers in their clinics would recommend a “booster” dose of 9-valent HPV vaccine for adolescents who had completed the 3-dose series with prior versions. Among the 42 respondents who did not stock 9-valent vaccine, few (17%, n=7) believed providers would recommend adolescents delay vaccination until it was available. In conclusion, providers and staff generally had positive views of 9-valent HPV vaccine and many had no concerns. For others, responses regarding parental hesitancy, insurance coverage, and the use of booster doses suggests opportunities for enhancing future educational support.
Keywords: adolescent health, human papillomavirus vaccine, human papillomavirus infections/prevention & control, pediatric immunization schedule
INTRODUCTION
U.S. children routinely receive over a dozen vaccines before age 18, guided by the immunization schedule developed by the Advisory Committee on Immunization Practices (ACIP). To adhere to the schedule and maximize children’s protection against vaccine preventable diseases, primary care providers administer multiple doses of recommended vaccines, carefully timing administration by patients’ age. Previous research has indicated that even with the guidance of the recommended schedule providers and families may struggle find the process of keep children up-to-date challenging, particularly when immunizing older children [1]. In addition, parents often look to their pediatric primary care providers for guidance on newly introduced vaccines [2, 3]. Thus, understanding and addressing the challenges and potential concerns that providers and clinic staff identify when implementing changes to the recommended schedule may be important for providing insight into how to assist clinics during future changes.
The recent introduction of 9-valent HPV vaccine may serve as a case-study into how to support providers and staff during a change to the childhood immunization schedule. The 9-valent HPV vaccine is an update to quadrivalent HPV vaccine, which has been part of girls’ immunization schedule since 2006 [4] and boys’ schedule since 2009 [5]. The U.S. Food and Drug Administration (FDA) approved the 9-valent HPV vaccine for use in December 2014, and ACIP recommended it for routine use, in addition to the bivalent and quadrivalent versions of the vaccine, in February 2015 [6]. During the present study window, guidelines for administering the 9-valent vaccine were similar to those for the quadrivalent vaccine, with on-time vaccination requiring the receipt of three doses before age 13, although the recommendation was later updated to two doses [7, 8]. To better understand how primary care clinics experience changes to the recommended immunization schedule, we sought to assess provider and staff concerns and recommendation practices during the introduction of 9-valent HPV vaccine, using data from a multi-state sample.
METHODS
Participants and procedures
Participants were clinicians and staff from primary care clinics enrolled in a larger study. Eligible clinics were high- to medium-volume pediatric and family practice clinics in three states (Illinois, Michigan, and Washington). We defined high- to medium-volume clinics as those having at least 500 adolescent patients, ages 11–17, according to the state’s immunization information system. Of the 285 clinics randomly selected and invited to participate, 148 (or 52%) accepted a 1-hour quality improvement (QI) session delivered by state health department personnel. The goal of these sessions was to help providers understand and improve their clinics’ adolescent vaccination coverage through strategies such as the use of patient reminders.
Respondents from 127 of 148 participating clinics (86%) completed a subsequent survey. We offered 1.0 hour of free American Medical Association (AMA) Category 1 continuing medical education (CME) credit for participating in the session, including the survey. Within participating clinics, any clinician or staff member who attended the session was eligible to complete the survey, resulting in multiple respondents per clinic (mean= 1.7 respondents per clinic, range: 1 to 11 respondents). Out of 222 respondents, we excluded those with missing information on the questions pertaining to the 9-valent vaccine (n=11). Our final analytic sample consisted of the remaining 211 respondents.
We conducted our online survey from October 2015 to May 2016. Health department personnel distributed invitations to the survey using printed flyers and emails, and followed up with reminders to non-respondents by email and phone. The University of North Carolina Institutional Review Board reviewed the study protocol and determined the risk to the participants to be minimal such that informed consent was deemed unnecessary. The study was exempt from further review under the category of research for public benefit and service.
Measures
Our survey assessed specific concerns respondents had about the 9-valent HPV vaccine with one item that used the following response options: problems with private insurance coverage; problems with coverage through the Vaccines for Children (VFC) program; how to phase out current stock of 4-valent HPV vaccine; increased parental hesitancy; and no concerns.
With regard to recommendation practices, respondents indicated how strongly providers in their clinic would recommend the 9-valent HPV vaccine for 11- and 12- year-old patients in comparison to the previous versions of the vaccine; response options were more strongly, less strongly, or the same amount as previous versions. Respondents also indicated whether their clinic stocked 9-valent HPV vaccine at the time of the survey. Among clinics that did stock it, the survey assessed how often they believed providers in the clinic would recommend an additional dose as a “booster” to adolescents who had already completed the 3-dose HPV vaccine series; response options were never, rarely, sometimes, often, or always. Among clinics that did not stock the vaccine, the survey assessed how often providers would recommend that their adolescent patients delay HPV vaccination until they could receive the 9-valent vaccine; the five response options also ranged from never to always.
The survey assessed characteristics of respondents, including clinical/staff role, number of years in that role, and sex. We further categorized respondents according to role to distinguish clinicians (physician, nurse, or other vaccine provider) from non-clinical staff (office manager, front office staff, or other). We used immunization information system data to assess clinics on type of practice or clinic (private versus other), specialty (family versus pediatric), size (number of patients, ages 11–17), and HPV vaccination coverage (≥1 dose) among adolescents ages 11–12 years.
Statistical Analysis
To assess correlates of reporting a concern about 9-valent HPV vaccine, we fit generalized linear models with a logit link using Proc Genmod in SAS 9.4 (Cary, NC). These models accounted for clustering by clinic to allow for an unbiased estimate of the standard error [9]. Statistical tests were two-tailed with a critical alpha of 0.05.
RESULTS
Sample characteristics
Respondents
Almost two-thirds (63%) of the 211 respondents were clinicians, and most (94%) were female (Table 1). Similar proportions worked in private practice (53%) and other settings (47%). The majority of respondents (69%) had worked in their current role for five or more years. Respondents were located in Washington (43%), Michigan (31%) and Illinois (26%).
Table 1.
Respondent characteristics and correlates of reporting a concern about 9-valent HPV vaccine (N=211).
| N | (%) | ≥1 concern
|
||||
|---|---|---|---|---|---|---|
| n/N | (%) | OR | (95% CI) | |||
| Sex | ||||||
| Male | 13 | (6) | 4/13 | (31) | 1 | |
| Female | 198 | (94) | 79/198 | (40) | 0.67 | (0.20–2.24) |
| Rolea | ||||||
| Non-clinician | 79 | (37) | 32/79 | (39) | 1 | |
| Clinician | 132 | (63) | 51/132 | (41) | 0.92 | (0.51–1.69) |
| Years in role | ||||||
| ≥10 years | 90 | (43) | 38/90 | (42) | 1 | |
| 5–9 years | 55 | (26) | 22/55 | (40) | 0.91 | (0.46–1.81) |
| < 5 years | 66 | (31) | 23/66 | (35) | 0.73 | (0.39–1.37) |
| Practice location | ||||||
| Washington | 90 | (43) | 28/90 | (31) | 1 | |
| Illinois | 56 | (27) | 24/56 | (43) | 1.67 | (0.78–3.52) |
| Michigan | 65 | (31) | 31/65 | (48) | 2.02 | (0.87–4.69) |
| Practice typeb | ||||||
| Other | 99 | (47) | 37/99 | (37) | 1 | |
| Private | 112 | (53) | 46/112 | (41) | 1.17 | (0.69–1.97) |
| Practice specialty | ||||||
| Family medicine | 138 | (65) | 46/138 | (33) | 1 | |
| Pediatrics | 73 | (35) | 37/73 | (51) | 2.06 | (1.02–4.15)* |
Clinician includes physician, nurse, and other vaccine providers. Non-clinician includes office manager, front office staff and other roles.
Private practice includes solo, group practice or HMO. Other practice type includes Federally Qualified Health Center, hospital-based clinic, public health department, and military clinic.
p<.05
Clinics
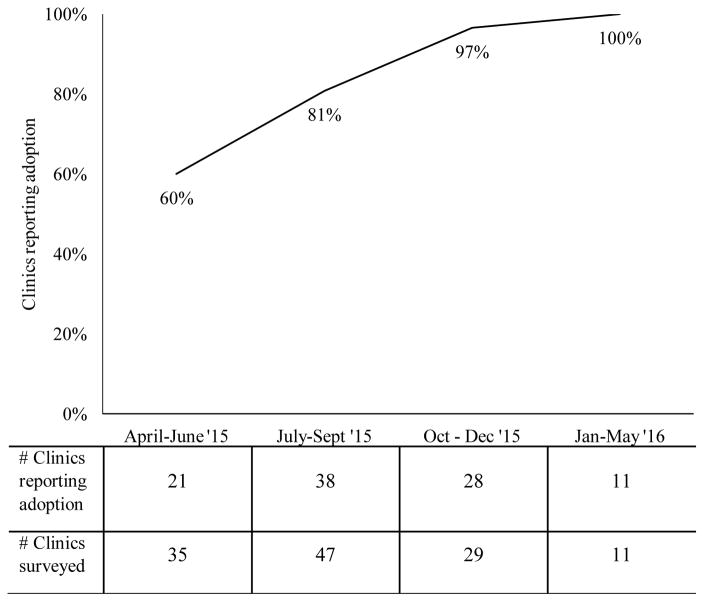
Among the 127 clinics in this study, 60% were family medicine practices, and 40% were pediatric practices. All clinics were VFC providers. Average HPV vaccination series completion among 11- and 12-year-old patients was 36% (SD 18%). At the time of the survey, 105 clinics (83%) stocked 9-valent HPV vaccine. Among clinics participating early in the study period (April to June 2015), 60% stocked the vaccine (Figure 1). Adoption increased over time, reaching 100% for clinics responding January to May 2016.
Figure 1.
Clinic adoption of 9-valent HPV vaccine
Concerns about 9-valent HPV vaccine
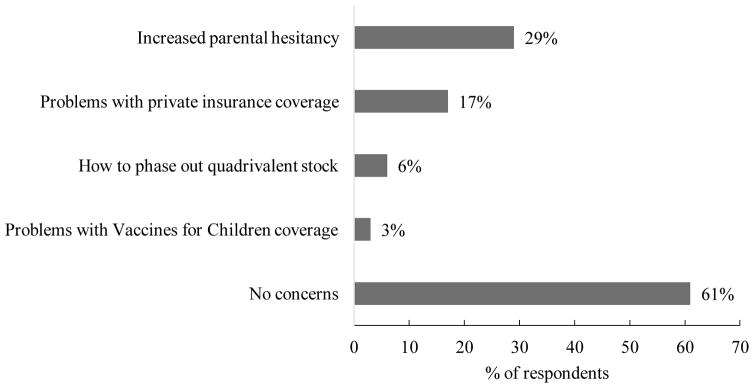
Over half (61%) of respondents reported no concerns about the 9-valent vaccine (Figure 2). However, a substantial minority reported concern about increased parental hesitancy (29%) or problems with private insurance coverage (17%). Few indicated concern about phasing out their current stock of quadrivalent HPV vaccine (6%) or VFC coverage (3%). Respondents from pediatric practices had twice the odds (OR=2.06, 95% CI 1.02, 4.15) of reporting any concern compared to those in family medicine practices (Table 1). Reporting a concern was not associated with working in private practice, respondents’ role (clinician vs. staff member), years in role, whether the clinic stocked the 9-valent vaccine, or clinics’ HPV vaccination coverage (all p>.05).
Figure 2.
Provider or staff concerns about 9-valent HPV vaccine
Recommendation practices
Almost all respondents believed that providers in their clinics would recommend the 9-valent vaccine for their 11- and 12-year-old patients as strongly as (66%) or more strongly than (33%) previous versions of the vaccine. Among the 169 respondents whose clinic stocked the vaccine, over half (n=94, 56%) indicated that providers in their clinics would recommend an additional dose of the 9-valent HPV vaccine as a “booster” for adolescents who had already completed the 3-dose series some of the time or more often. Among the 42 respondents whose clinics did not stock the vaccine, relatively few (n=7, 17%) believed that providers would sometimes or often recommend adolescents delay HPV vaccination until the 9-valent vaccine was available.
DISCUSSION
The majority of primary care providers and staff in our multi-state sample reported favorable views about the introduction of 9-valent HPV vaccine. Respondents believed that providers in their clinics would recommend the 9-valent vaccine at least as strongly as the previous versions, with about one-third reporting that providers would recommend it even more strongly. Furthermore, when asked about four different concerns they might have about the 9-valent HPV vaccine, the over half of the sample reported having no concerns. These findings are reassuring given that parents consistently identify providers’ recommendation as the most important facilitator of getting HPV vaccine [10, 11] and want their providers to exhibit high levels of confidence about the 9-valent vaccine [3].
Among providers or staff who did have concerns, increased parental hesitancy was most common. This finding is consistent with prior research that suggests providers perceive parents as being unsupportive of HPV vaccination generally [12] and uncertain about 9-valent HPV vaccine specifically [13]. Interestingly, findings from a recent focus group study suggest that many parents prefer the 9-valent vaccine over previous versions, although some did report concerns about safety and other issues [3]. Because providers may overestimate parents’ hesitancy toward HPV vaccination [14], offering tips on clinical communication might help to facilitate schedule changes. For example, providers can communicate confidence in updated vaccines by treating them like any other vaccine and by avoiding descriptions such as “new” [15, 16].
While very few providers or staff in our study had concerns regarding coverage of the 9-valent HPV vaccine by the VFC program, some expressed concern regarding private insurance coverage. The extent to which this concern may have affected providers’ recommendation of the vaccine, or parents’ decision for their child to receive it, is unclear. However, several studies have found that adolescents with private insurance are less likely to initiate the HPV vaccine series as compared to those with public insurance or eligible for the VFC program [17, 18]. Given that vaccination coverage constitutes a quality metric for private insurers [19], insurers themselves may facilitate smooth schedule changes by communicating to providers their ability to cover new and updated vaccines.
Providers and staff might have benefited from additional guidance at the time of 9-valent vaccine introduction regarding additional doses for children who had completed the series. Although ACIP does not make a recommendation with regard to additional vaccination with the 9-valent vaccine to children who completed the HPV vaccine series with the bivalent or quadrivalent vaccine [7], about half of respondents in our study who stocked the vaccine believed that providers in their clinic would recommend an additional dose of the 9-valent vaccine to those children. In a national study of parents of adolescents, this topic was also an area of confusion, with parents of previously vaccinated girls being uncertain as to whether they should seek a fourth dose [3].
Finally, we were interested to note that adoption of 9-valent HPV vaccine occurred relatively quickly. Just two months after the ACIP recommendation, over half of clinics enrolling in our study stocked the 9-valent vaccine, and by October 2015, almost all did. This rapid adoption suggests that efforts to support changes to the recommended immunization schedule must also happen quickly, with provider education campaigns ideally occurring simultaneously with ACIP recommendations. In the case of 9-valent HPV vaccine, key informational resources were available soon after the ACIP ruling, which may help to explain why providers and staff reported largely positive perceptions of the transition [20].
Our findings should be interpreted in light of several strengths and limitations. We surveyed primary care providers and staff as part of a larger study, which gave us the opportunity to explore a novel topic, adoption of the 9-valent HPV vaccine, in a probability sample of higher-volume primary care clinics in three states. However, the generalizability of our findings to providers and staff working in smaller clinics or in other states is unknown. Further research is needed to understand the concerns of smaller clinics, particularly given that vaccine storage and handling may be especially challenging in the context of a low-volume practice. Although the introduction of the 9-valent HPV vaccine was not an explicit focus of the larger study, it is also possible that some of provider and staff questions about the vaccine were addressed as part of their participation. For this reason, it is possible that our findings may underestimate providers and staff concerns.
CONCLUSIONS
Using data from a multi-state sample, we found that primary care providers and staff adopted the 9-valent HPV vaccine quickly after ACIP’s recommendation. Providers and staff expressed positive views of the 9-valent vaccine, and over half had no concerns. However, some reported concerns about increased parental hesitancy and private insurance coverage. In addition, we found variation in practice regarding the use of an additional dose of 9-valent HPV vaccine for adolescents who had already completed the HPV vaccine series. Our findings may serve as a case-study for understanding the experience of providers and staff during a change to the pediatric immunization schedule occur, and provide potential areas for educational support to ensure a smooth introduction of new vaccines in the future.
Acknowledgments
FUNDING SOURCE: Data collection was supported by a grant from the Robert Wood Johnson Foundation (71272 to NB and MB). Authors’ time was supported by grants from the National Cancer Institute (K22 CA186979 for MG; R25 CA116339 for WC).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
CONFLICT OF INTEREST: The authors declare that they have no conflicts of interest.
References
- 1.Cohen NJ, Lauderdale DS, Shete PB, Seal JB, Daum RS. Physician knowledge of catch-up regimens and contraindications for childhood immunizations. Pediatrics. 2003;111(5):925–932. doi: 10.1542/peds.111.5.925. [DOI] [PubMed] [Google Scholar]
- 2.Leask J, Kinnersley P, Jackson C, Cheater F, Bedford H, Rowles G. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatrics. 2012;12(154) doi: 10.1186/1471-2431-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontenot HB, Domush V, Zimet GD. Parental Attitudes and Beliefs Regarding the Nine-Valent Human Papillomavirus Vaccine. Journal of Adolescent Health. 2015;57(6):595–600. doi: 10.1016/j.jadohealth.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report Recommendations and Reports. 2007;56(RR-2):1–12. 13–24. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report. 2010;59(20):630–632. [PubMed] [Google Scholar]
- 6.Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 7.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2016;65(49):1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz LE, Dunne EE, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human Papillomavirus Vaccination Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report Recommendations and Reports. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 9.Desai M, Begg MD. A comparison of regression approaches for analyzing clustered data. American Journal of Public Health. 2008;98(8):1425–1429. doi: 10.2105/AJPH.2006.108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer NT, Gottlieb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, Smith JS. Longitudinal Predictors of Human Papillomavirus Vaccine Initiation Among Adolescent Girls in a High-Risk Geographic Area. Sexually Transmitted Diseases. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: Findings from the 2006–2008 National Survey of Family Growth. Vaccine. 2012;30(16):2676–2682. doi: 10.1016/j.vaccine.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PD, Brewer NT. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Preventive Medicine. 2015;77:181–185. doi: 10.1016/j.ypmed.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasting ML, Wilson S, Dixon BE, Downs SM, Kulkarni A, Zimet GD. A qualitative study of healthcare provider awareness and informational needs regarding the nine-valent HPV vaccine. Vaccine. 2016;34(11):1331–1334. doi: 10.1016/j.vaccine.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healy CM, Montesinos DP, Middleman AB. Parent and provider perspectives on immunization: Are providers overestimating parental concerns? Vaccine. 2014;32(5):579–584. doi: 10.1016/j.vaccine.2013.11.076. [DOI] [PubMed] [Google Scholar]
- 15.Farmar A-LM, Love-Osborne K, Chichester K, Breslin K, Bronkan K, Hambidge SJ. Achieving High Adolescent HPV Vaccination Coverage. Pediatrics. 2016;138(5) doi: 10.1542/peds.2015-2653. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) [Accessed July 28, 2017];You are the key to HPV cancer prevention. 2016 from https://www.cdc.gov/vaccines/ed/hpv/index.html.
- 17.Conroy K, Rosenthal SL, Zimet GD, Jin Y, Bernstein DI, Glynn S, Kahn JA. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. Journal of Womens Health. 2009;18(10):1679–1686. doi: 10.1089/jwh.2008.1329. [DOI] [PubMed] [Google Scholar]
- 18.Schluterman NH, Terplan M, Lydecker AD, Tracy JK. Human papillomavirus (HPV) vaccine uptake and completion at an urban hospital. Vaccine. 2011;29(21):3767–3772. doi: 10.1016/j.vaccine.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 19.NCQA. HEDIS. [Accessed on July 28, 2017];2016 from http://www.ncqa.org/hedis-quality-measurement/hedis-measures/hedis-2016.
- 20.Centers for Disease Control and Prevention. [Accessed on July 28, 2017];Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV vaccine. 2016 from https://www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf.