INTRODUCTION
Chronic pain is a major health problem worldwide11. There is comparably little known about the dynamic relationships between the biological mechanism(s) that contribute to the development of chronic pain, nor how to reduce its burden on everyday life.10 However, it is well established that chronic pain conditions are often associated with insufficient sleep, which in turn is a predictor of persistent pain symptoms in both general populations and those with chronic pain.1,7,25,40
Experimental sleep restriction/disruption studies in healthy adults have found associations between shorter sleep durations and increased reports of new onset spontaneous pain.13,14,36 A recent population-based study also showed that short total sleep times are associated with reports of moderate to severe pain.45 Further, experimental data have demonstrated that sleep restriction reduces pain thresholds, which supports the observed relationships between insufficient sleep and increased pain sensitivity in experimental and population-based studies.18,20,24,26,31,33,36,41 Improving sleep quantity and/or quality also reduces pain sensitivity in healthy participants32 and pain severity in chronic pain patients.37,44 These data suggest a bi-directional relationship between sleep and pain; however, the mechanistic linkage has yet to be elucidated.
Abnormalities in central pain-modulatory circuits may be one pathway through which sleep deficiency contributes to chronic pain vulnerability, as reduced pain inhibition and enhanced pain facilitation are associated with increased vulnerability to chronic pain (reviewed by38,48). Experimental sleep disruption has been shown to impair pain inhibitory function, as assessed with a cold pressor paradigm as the conditioning stimulus and pressure pain as the test stimulus.36 Insomnia disorder has been associated with lack of central pain inhibitory modulation, as assessed with a hot water stimulus as the conditioning stimulus and a heat pulse sequence as the test stimlus15 and, in clinical pain populations, short sleep duration mediates reductions in conditioned pain modulation, as assessed with cold water paradigm as the conditioning and mechanical pain applied via an algometer as the test stimulus.19 Habituation to non-threatening noxious stimuli, a process related to central inhibitory modulation,2,3 has also been observed to be reduced or absent in chronic pain disorders.8 These data suggest that insufficient sleep may play an important role in physiological processes that control vulnerability to chronic pain.
Data on the influence of sleep on pain-facilitatory circuits, as measured by heightened temporal summation (an index of central sensitization) are limited.17,47 A single night of sleep loss did not affect temporal summation in humans, but did reduce pain thresholds.34 However, in a clinical setting, temporal summation of pain increased in osteoarthritis patients in association with low sleep efficiency4. This suggests that insufficient/disrupted sleep may induce sensitization to pain, which is in part mediated by the central nociceptive system. More research is needed to better understand whether more chronic exposure to insufficient sleep reliably affects pain-facilitatory and/or pain-inhibitory circuits.
Given that sleep serves the function of maintaining bodily normal physiological processes and that dysregulation of sleep promotes pain,7 we propose that chronic exposure to insufficient sleep is one means by which central pain-modulatory circuits become less functional. We hypothesized that repeated exposure to experimentally restricted sleep with limited recovery sleep, as often occurs when sleep is reduced during the week and extended on weekends16,22,23,42,46, would be associated with compromised habituation to repeated painful stimuli and increases in pain facilitatory pathways.
METHODS
Experimental sleep model
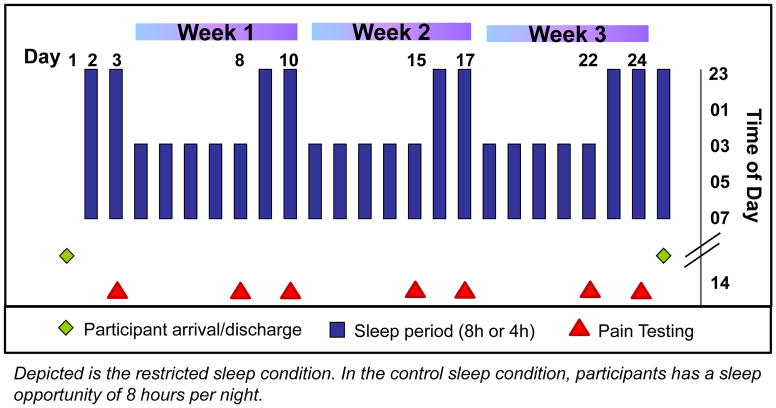
To experimentally mimic commonly reported patterns of sleep restriction on work/weekday nights and sleep recovery on non-work or weekend nights 35, we utilized an experimental model of three consecutive weeks, each beginning with five nights of sleep restricted to 4h/night (0300–0700h) followed by two nights of 8h recovery sleep (2300–0700h, Figure 1). The sleep control condition consisted of three consecutive weeks with a sleep opportunity of 8h/night. In an intra-individual balanced, randomized design, each participant underwent two 25-day in-hospital stays (restricted sleep condition and sleep control condition) separated by longer than 2 months. Each 25-day stay started with an adaptation and a baseline day, followed by three weeks of either repeated exposure to sleep restriction/recovery or control sleep, and ended with an additional night of full sleep (totaling 25 days).
Fig. 1.
Study protocol: Repeated exposure to sleep restriction-recovery patterns
Participants
The study was approved by the Institutional Review Board for the Protection of Human Subjects at the Beth Israel Deaconess Medical Center (BIDMC). Participants were recruited via internet postings and flyers in the Boston area. Seventeen healthy young women and men were studied (9 men, 8 women). The mean age of female participants was 25.1+/− 1.0 years (range 20–32y) and the mean age of male participants was 24.7+/−1.2 years (range (19–31y). Fourteen participants completed the entire study (two 25-day-in-hospital stays); three participants only completed one of the two in-hospital stays due to changes in work/family-related reasons. Thus, the women/men ratio in the sleep control sleep condition was 8/8 and 7/8 in the sleep restriction condition. Participants were screened to ensure good health, including documentation of habitual nightly sleep duration between 7–9 hours based on 10–14 days of sleep diary recordings; absence of sleep disorders based on questionnaires and polysomnography (PSG); absence of any medical or psychiatric disorders determined by questionnaires, diagnostic interviews, physician’s medical history and physical examination, and blood and urine chemistry; and absence of regular medication use (except oral contraceptives).
Study Protocol
The two 25-day in-hospital stays (restricted sleep and control sleep conditions) were conducted in the Clinical Research Center of the BIDMC. Participants were randomized to the order of experimental conditions (i.e., the first stay being sleep restriction or control) on the first day of the first 25-day hospital stay. Intensive 24h recordings were obtained on seven out of the 25 days (baseline night, every 5th day of restricted/control sleep and every 2nd night of recovery/control sleep in each of the three weeks). These intensive recording periods included PSG, blood and urine sampling, and pain testing. Ratings of spontaneous pain were assessed on computerized visual analog scales every four hours throughout waking periods. The research environment was tightly controlled, and protocols were designed to minimize potential differences between restricted sleep and control sleep in-hospital stays (full documentation in Simpson et al.).35
Measurements
Spontaneous pain was assessed every 4 hours during the waking periods of the protocol using computerized visual analogue scales (AsWin, programmed by Martin Rivers & Associates). Participants had to rate the intensity of their generalized body pain and headaches, as well as back, abdominal and muscle pain. Participants used the arrow keys to slide the cross-hatch on the scale either to the right or the left, and the rating was stored on the computer in measurement units between 0 and 100.
Ratings (five per day) were aggregated across the daytime periods (0700–2300h) of each study day for statistical analysis. This method of assessment has previously been used successfully to capture spontaneous pain experiences following single episodes of sleep restriction.13,14 In addition, the Pain Level Scale (PLS) was administered prior to each pain testing protocol on paper, in order to control for spontaneous pain experienced directly before the testing session. The PLS is a standard 0–10 scale used to rate pain in medical settings, where 0 indicates ‘no pain’ and10 indicates ‘worst pain’.
Pain testing protocol
Pain testing started at 2pm on each of the intensive recording days (baseline, every 5th and 7th day of each of the 3 weeks), and lasted about 20 minutes. The mid-day 2pm time point was selected to avoid circadian influences on morning alertness or evening sleepiness and associated influences on pain processing. Participants remained in a comfortable chair with room temperature at the individual comfort level. Potential spontaneous pretest pain was assessed prior to pain testing using the pain level scale (PLS). The following three pain testing protocols were administered:
Warmth detection threshold (WDTh) was assessed using the TSA-II NeuroSensory Analyzer (Medoc, Minneapolis, MS). A Peltier thermode, size 30 x 30 mm2 was secured on the inner palm of the hand contra-lateral to the arm used for blood drawing. From a baseline temperature of 32°C, the thermode was heated at a rate of 0.5°C/sec. Participants were requested to press a button at the first instant they detected the sensation of warmth. The stimuli were presented four times with inter-stimulus interval of 10 seconds. The mean of the responses determined the participant’s warmth detection threshold (WDTh). WDTh has been used previously to demonstrate that insufficient sleep is associated with alterations in pain processing specifically, rather than reflecting a general change in somatosensory processing.18
Heat pain threshold (HPTh) was obtained using the same method described above for WDTh. The stimuli were presented four times at a rate of 0.5°C/sec and inter-stimulus interval of 10 seconds. Heat pain thresholds have been observed to decline in models of acute sleep loss as a reflection of increased pain sensitivity.18,20,24,26,31,33,36,41 Both WDTh and HPTh were performed on the thenar eminence of the hand contra-lateral to the arm used for blood drawing.
Cold pressor test (CPT) was used to determine cold pain tolerance and temporal summation of pain. The CPT was performed on the hand that was not used for blood sampling in the general protocol to prevent interference. Participants were asked to insert their hands in a temperature-controlled water bath (Techne® water baths, Bibby Scientific US, Burlington, NJ) maintained at temperature of 2–3° C. Participants rested their hand on a plastic rack positioned in the middle of the water bath to keep the level of hand immersion constant and the forearm was insulated from coming in contact with the rim of the cold metal container. Participants were instructed to keep their hand immersed until the cold sensation became painfully unbearable, after which they were to immediately remove it from the bath. Cold pain tolerance was calculated as the time between hand immersion and removal from the water bath. The maximal immersion duration was 180 seconds. Increases in cold pain tolerance time are likely reflective of habituation to painful stimuli. However, it should be noted that habituation to pain has been largely tested using heat stimuli paradigms2,3, and validations for cold pain have not yet been conducted. To our knowledge, habituation in response to noxious stimuli following experimental sleep loss has not previously been studied. VAS scales presented on paper were used to rate pain intensity across the cold pressor test, with the instruction to draw a vertical line to indicate the intensity of the sensation on a scale of 0–100 where 0 indicated ‘no sensation’ and 100 indicated ‘very intense sensation’. VAS endpoints were anchored using ‘sensation’ endpoints rather than pain (e.g., “very intense pain”) to minimize participant expectations that a particular level of sensation will be experienced as painful (or not). Ratings were obtained prior to and for every 10 sec during the water immersion period. These ratings were used to assess progressive increases in pain perception over time as a measure of temporal summation of pain. While a single night of acute total sleep loss was not found to affect temporal summation34, to our knowledge, temporal summation of pain has not yet been characterized under conditions of chronic sleep restriction, nor has the impact of subsequent sleep recovery been assessed.
Statistics
The main analysis employed linear mixed modeling with condition (restricted sleep vs. control sleep) and study day (baseline, 5th [restricted/control sleep] and 7th [recovery/control sleep] day of each of the 3 weeks) as fixed factors, and participants and participants x day as random factors. The baseline day was used as a covariate in order to account for pre-study inter-individual differences. Accordingly, data in graphs depict estimated means from mixed model analysis. Since baseline day was used as covariate, significance of interaction as well as main condition effects were considered appropriate for follow-up post-hoc testing of single time points. In order to test whether an outcome variable showed a response decrease (habituation) or response increase (sensitization) across consecutive weeks of exposure to sleep restriction, only the 5th restriction day of each of the three weeks were used for mixed model analysis. All analyses were also conducted with a baseline general pain rating as an additional covariate, in order to control for potential interference of immediate pre-test spontaneous pain. For cold pressor-induced immersion time and temporal summation testing, time (times at which pain ratings were reported: 0, 10, 20, and 30 seconds) were entered as additional fixed factors in linear mixed modeling.
The following variables were entered as outcome variables, with the alpha value of rejection set to p≤0.05:
Subjective ratings of generalized body pain, headache pain, as well as back, abdominal, muscle and joint pains. Ratings at each site were aggregated to a single daytime mean (0700–2300) across each of the seven intensive recording days.
Warmth detection threshold (WDTh) and heat pain threshold (HPTh), averaged across the set of four assessments conducted during each of the seven intensive recording days.
-
Cold Pressor test (CPT):
Cold pain tolerance time (in seconds) of hand immersion in cold water bath;
Temporal summation of cold pain ratings of progressive increases in pain at 10s intervals while hand immersed in cold water. Both variables were assessed once during each of the seven intensive recording days. Analyses included ratings for the first 30 seconds of cold water exposure only; at this cut-point, the majority (65%) of participants were still engaged in the testing (longer time frames captured less than 50% of data points).
Graphs and text present the estimated mean ± standard error of mean (SEM) based on mixed model analyses.
RESULTS
Spontaneous pain ratings
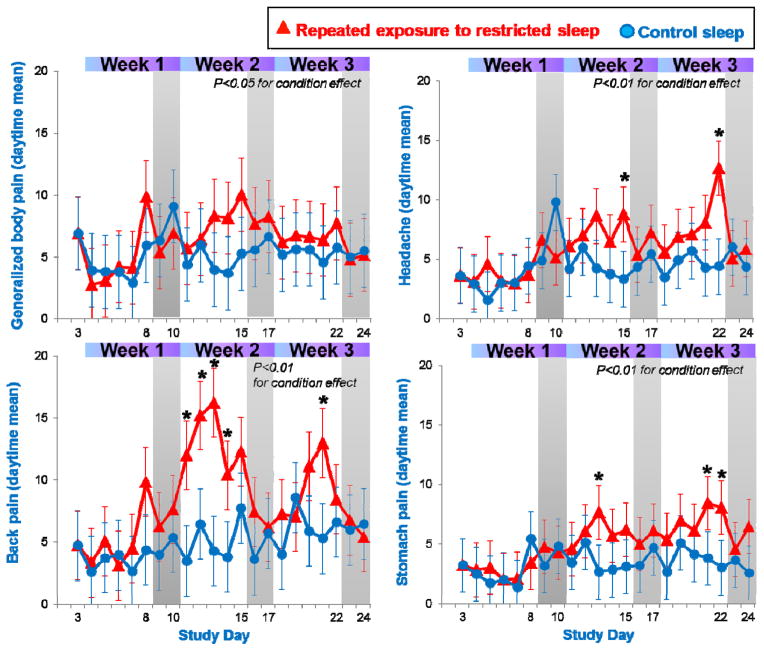
Average ratings of generalized body pain, headache, back, and abdominal pain were significantly higher in the sleep restricted compared to the control sleep condition (all p≤0.05 for condition effect, see Figure 2). Ratings of muscle and joint pain, while on average higher in the restricted sleep condition, did not differ significantly from those of the control group. Within the restricted sleep condition, ratings did not systematically increase (or decrease) across consecutive weeks of sleep restriction (all p>0.10 for comparisons for the 5th day of sleep restriction between week 1, 2, and 3).
Fig. 2.
Ratings of pain complaints during repeated exposure to sleep restriction-recovery patterns. Data were averaged across daytime hours (0700–2300), and present estimated means from mixed model analysis. *p<0.05 between conditions.
Experimental pain testing
Warmth detection thresholds (WDTh) did not show a significant difference between the restricted and control sleep condition (condition or condition by day interaction effect p>0.08).
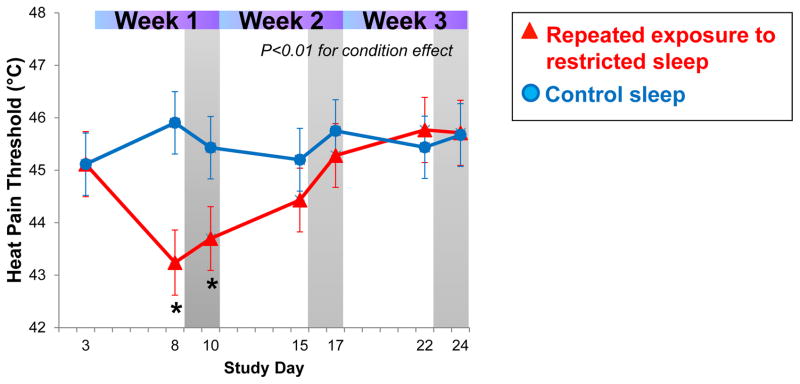
Heat pain thresholds (HPTh) showed a significant difference between the restricted and control sleep conditions (p≤0.05 for condition effect). As shown in Figure 3, HPTh decreased significantly in the first week of sleep restriction in comparison to the control sleep condition, and were still significantly lower following two nights of recovery sleep in week 1. However, during subsequent exposures to sleep restriction (week 2 and 3), heat pain thresholds gradually returned to baseline values and no longer differed significantly from the control sleep condition.
Fig. 3.
Changes in heat pain thresholds (HPTh) habituate across repeated exposure of sleep restriction and recovery.
Cold pressor test (CPT)
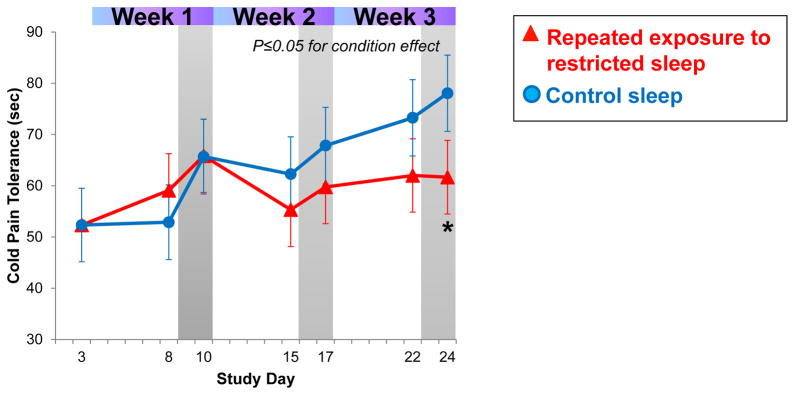
Cold pain tolerance showed a significant difference between restricted and control sleep conditions (p≤0.05 for condition effect). As shown in Figure 4, cold pain tolerance time increased in the control sleep condition by 24.0±11.4 sec from baseline to week 3, demonstrating the predicted habituation effect. In sleep restricted participants, however, cold pain tolerance increased by only 9.5±7.8 sec over the same period, indicating that comparably less habituation occurred (see Figure 4).
Fig. 4.
Cold pain tolerance (CPT) increases in the control sleep group, but shows less habituation across repeated exposure to sleep restriction. *p<0.05 between conditions.
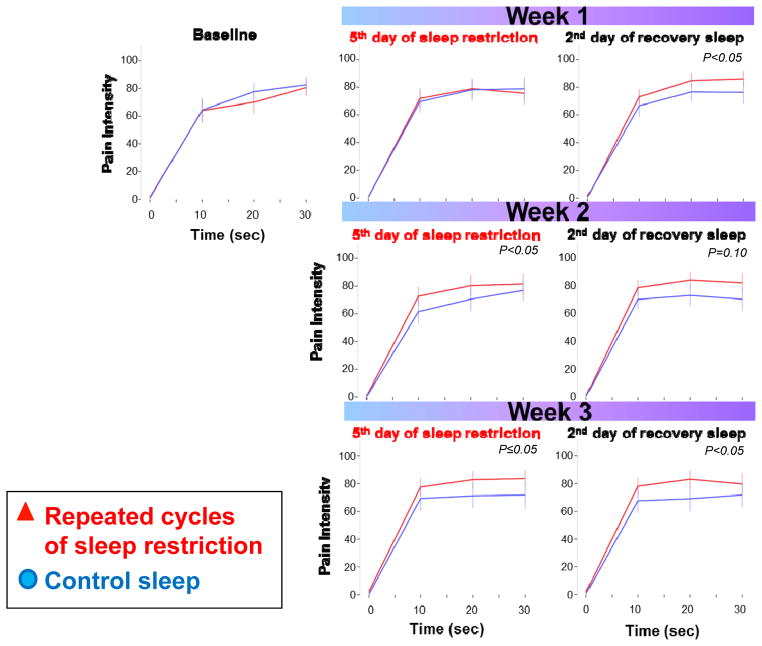
Figure 5 presents results from the temporal summation of cold pain. These analyses included ratings for the first 30 sec of cold water exposure only; at this cut-point, the majority (65%) of participants were still engaged in the testing (longer time frames captured less than 50% of data points). As can be seen in Figure 5, pain accumulated (summates) faster in the sleep restricted condition and the overall mixed model analysis indicated a significant condition and condition by time interaction effect (both p≤0.05). When analyzing testing days separately, this effect was significant during the sleep restriction testing days of week 2 and 3 (p≤0.05 for condition effect), as well as during the recovery testing days of week 1 and 3 (p≤0.05 for condition effect, indicating that summation of pain remained significantly higher across multiple weeks of sleep restriction and recovery sleep. While pain overall summated faster in the sleep restriction condition when compared to the control condition, there was no indication of a progressive increase or decrease across the repeated exposure to sleep restriction (p>0.10 between sleep restriction exposure of week 1, 2 and 3).
Fig. 5.
Temporal summation of pain during the first 30 sec of the cold pressor test. Time 0 indicates ratings directly before immersion of hand into cold water bath. P<0.05 for condition and condition by time effect in mixed model analysis. P values in graph indicate condition effect for days separately.
DISCUSSION
This study is the first to examine the domains of pain experience, sensitivity, and modulation in an experimental model of chronic insufficient sleep with limited recovery opportunity. We observed that several markers of spontaneous pain increased significantly with sleep restriction, paralleling previously reported findings 13,14,36 and that this elevation continued across the repeated weeks of sleep restriction without further amplification. In contrast, heat pain thresholds (HPTh), while significantly decreased following the first week of sleep restriction, habituated to non-significant differences between sleep restriction and control groups across the second and third weeks of sleep restriction. The acute HPTh finding from the first week of sleep restriction is in line with many studies reporting decreased heat or pressure pain thresholds after a single exposure to sleep loss;20,24,26,31 heat pain thresholds have not been studied previously under chronic conditions.
As hypothesized, tolerance to cold pain showed less habituation in the sleep restriction condition compared to the control sleep condition. Further, though the between-group difference was small, temporal summation, occurred at a significantly faster pace in the sleep restriction condition; this difference was maintained across the study period. Together, these findings demonstrate that when sleep deficiency is prolonged, the ability to habituate to evoked cold pain appears to decline. This finding differs from the subjective reporting of spontaneous pain and pain thresholds, which were not increasingly affected by the repeated exposure to restricted sleep. The physiological processes by which habituation to painful stimuli diminish under conditions of experimental sleep deficiency has not previously been studied. However, reduced habituation to painful heat stimuli has been linked to deterioration of central pain inhibition circuits, which can profoundly inhibit the experience of incoming painful stimuli.2,27,29 Thus, if the capacity to inhibit pain is reduced, habituation to pain cannot occur. This proposed link between chronic sleep loss, decreased habituation to painful stimuli and alterations in pain-inhibitory systems is supported by findings that pain-inhibitory circuits (tested directly) deteriorate in a model of experimental sleep disruption, as well as in individuals suffering from insomnia disorder.15,36
It is important to note that changes in pain habituation as assessed with the cold pressor test did not occur with the first week of sleep restriction, but rather emerged with more chronic exposure in weeks two and three. This suggests that reductions in pain habituation in acute models of experimental sleep loss are less likely to be observed, a hypothesis that is supported by previous research examining the (null) impact of one to three nights of sleep restriction on pain inhibition, a process related to pain habituation.21,36 Further, our findings suggest that changes in the habituation process with chronic sleep loss do not normalize quickly, as demonstrated by a lack of restoration to baseline with two nights of recovery sleep following the sleep restriction periods in weeks two and three. Thus, it appears that some chronicity of exposure to sleep loss is required to observe changes in ability to habituate to painful stimuli, but, once present, may require an extended recovery period to normalize.
Another potential mechanistic pathway that may underlie the observed failure to habituate is alterations in pain-facilitatory processes, i.e., temporal summation of pain. The centrally mediated process of temporal summation is frequently used as an indicator of central sensitization,47 or increased responsiveness of central pain transmission neurons located in the dorsal horn to incoming pain signals. In this study, ratings of pain intensity during immersion of the hand in cold water was used as an indicator of the summation of pain. Typically, heat or pressure pain paradigms have been used for the assessment of temporal summation; knowledge on the usefulness of cold pain paradigms is very limited. In the current study, cold pain intensity ratings increased faster in the sleep restriction-recovery condition, suggesting the presence of increased temporal summation. Using heat or pressure pain stimuli, temporal summation of pain has been shown enhanced in many chronic pain conditions, including temporomandibular joint disorder28, low back pain9, fibromyalgia39, and osteoarthritis.4 Similar to our pain habituation results, temporal summation of pain did not differ during acute sleep restriction in week 1, but differed with exposure to sleep restriction-recovery patterns in weeks 2 and 3. Of note, the presence of increased temporal summation of pain also persisted after the intermittent recovery sleep periods, similarly suggesting that more extensive recovery sleep is required to normalize alterations in this process.
In contrast to our findings that processes of habituation and sensitization to evoked cold pain were affected only with chronic exposure to insufficient sleep, the pain thresholds were observed to be altered only under conditions of acute sleep loss (week 1). Heat pain thresholds dropped by approximately 2°C in week 1 and remained lower after two nights of recovery sleep when compared to the control sleep condition, where thresholds remained stable across the protocol (Figure 3). Decreases in pain thresholds have been reported in numerous laboratory sleep loss studies of one to three nights.18,20,24,26,31,33,36,41 These results indicate that acute total sleep deprivation or restriction is associated with increased pain sensitivity. However, our findings suggest that this acute effect does not persist with more chronic exposure to insufficient sleep, as demonstrated by a gradual normalization of pain thresholds in weeks 2 and 3. Together, these findings suggest that decreases in heat pain thresholds are transient and normalize with longer exposures to restricted sleep with limited recovery periods.
Similarly, while spontaneous pain intensity ratings were mildly but significantly elevated during sleep restriction, they returned to baseline during subsequent recovery periods and overall did not show a pattern of a continued change upon repeated exposure to sleep restriction. This is similar to previous findings that elevations of spontaneous pain following experimental sleep loss normalize rapidly following a single night of recovery sleep.13,14,36 This suggests that the experience of spontaneous pain is not directly related to the observed deterioration of habituation and sensitization to cold pain under conditions of repeated exposure to sleep restriction and recovery, a conclusion that has also been supported by previous research5 and are similar to sustained elevations in physiological stress markers (e.g., cortisol, IL-6 positive monocytes) presented in an earlier paper published from this study protocol35. These findings suggest that the processes mediating spontaneous pain, pain thresholds, pain habituation, and pain summation involve different synaptic or endocrine/inflammatory mechanisms.
While this is one of the first investigations to explore mechanisms that may underlie the relationship between sleep deficiency and chronic pain, use of the cold pressor test comes with methodological limitations and may not be the optimal methodological approach to assess temporal summation of, or habituation to, pain. The cold stimulus paradigm has not been studied rigorously for sensitization of the nervous system and, therefore, validation for cold pressor test is limited at present. Interpretations of the current study findings are limited by the fact that the test likely involves not only cold nociception as the noxious trigger, but also changes in the local vasculature. This dual noxious trigger may have contributed to large observed variability of cold pain tolerance in our study, which limited the number of valid trials. Follow-up studies will need to utilize well established assessment methodologies for temporal summation, using heat and pressure stimuli.6,12,15,30 Furthermore, while we use the term ‘habituation’ to describe the phenomenon of an increase in pain tolerance across study days, the effect may be better described as sensory adaptation or fatigue. By using the term habituation, we aimed to follow terminology used in recent studies that also investigated stimuli response changes across days (rather than minutes)2,3. However, given that the noxious triggers associated with the cold pressor test are less clear, our findings need to be interpreted with caution, and follow-up studies utilizing traditional assessment approaches are warranted.
In conclusion, while pain threshold or pain sensitivity changes may be a robust marker of acute experimental sleep deficiency, we observed that abnormalities in processes related to habituation and sensitization of cold pain emerge with more chronic rather than acute forms of sleep deficiency. Given that alterations in these processes may contribute to the risk of developing chronic pain disorders,38,48 findings from this study may identify one pathway through which chronic sleep deficiency is linked to chronic pain. While exciting, generalizations that can be drawn from the current study are limited by the small sample size. There may be individual differences in vulnerability to chronic pain via altered pain processing mechanisms, or sensitivity to the effects of sleep loss,43 that are not illuminated by our study findings. Our preliminary analyses suggest that gender did not play a significant role in pain processing outcomes; however, the study was not powered to detect these differences. Nonetheless, we believe that findings from the current study contribute to our understanding of the bi-directional relationship between sleep and pain, specifically that exposure to chronic insufficient sleep may result in alterations of pain modulatory processes that in turn may increase vulnerability to chronic pain conditions. Further, our findings also suggest a potential clinical pathway through which this vulnerability may be reduced: through behavioral, or potentially pharmacological, interventions to improve sleep quality and quantity.
Acknowledgments
This work was funded by grants R01 HL 105544 from the National Heart, Lung, and Blood Institute, and grants UL1 RR02758 and M01-RR-01032 from the National Center for Research Resources to the Harvard Clinical and Translational Science Center.
Footnotes
There are no conflicts of interest to disclose.
References
- 1.Aili K, Nyman T, Svartengren M, Hillert L. Sleep as a predictive factor for the onset and resolution of multi-site pain: A 5-year prospective study. European Journal of Pain. 2015;19:341–349. doi: 10.1002/ejp.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingel U, Schoell E, Herken W, Buchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Breimhorst M, Hondrich M, Rebhorn C, May A, Birklein F. Sensory and sympathetic correlates of heat pain sensitization and habituation in men and women. European Journal of Pain. 2012;16:1281–1292. doi: 10.1002/j.1532-2149.2012.00133.x. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Care & Research. 2015;67:1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards RR, Fillingim RB. Self-reported pain sensitivity: Lack of correlation with pain threshold and tolerance. European Journal of Pain. 2007;11:594–598. doi: 10.1016/j.ejpain.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 7.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. Journal of Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flor H, Diers M, Birbaumer N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci Lett. 2004;361:147–150. doi: 10.1016/j.neulet.2003.12.064. [DOI] [PubMed] [Google Scholar]
- 9.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. Journal of Occupational Rehabilitation. 2006;16:95–108. doi: 10.1007/s10926-005-9007-y. [DOI] [PubMed] [Google Scholar]
- 10.Gereau RW, Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB, Sullivan MD, Fillingim RB. A Pain Research Agenda for the 21st Century. Journal of Pain. 2014;15:1203–1214. doi: 10.1016/j.jpain.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg DS, Mcgee SJ. Pain as a global public health priority. Bmc Public Health. 2011:11. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB. Temporal Summation of Pain as a Prospective Predictor of Clinical Pain Severity in Adults Aged 45 Years and Older With Knee Osteoarthritis: Ethnic Differences. Psychosom Med. 2014;76:302–310. doi: 10.1097/PSY.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haack M, Lee E, Cohen D, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: Potential mediator of increased spontaneous pain. Pain. 2009;145:136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. European Journal of Pain. 2012;16:522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- 17.Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 18.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, Solomon DH, Karlson EW. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis and Rheumatism. 2013;65:59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–1592. [PubMed] [Google Scholar]
- 21.Matre D, Andersen M, Knardahl S, Nilsen K. Conditioned pain modulation is not decreased after partial sleep restriction. European Journal of Pain. 2016;20:408–416. doi: 10.1002/ejp.741. [DOI] [PubMed] [Google Scholar]
- 22.Monk TH, Buysse DJ, Rose LR, Hall JA, Kupfer DJ. The sleep of healthy people - A diary study. Chronobiol Int. 2000;17:49–60. doi: 10.1081/cbi-100101031. [DOI] [PubMed] [Google Scholar]
- 23.National Sleep Foundation. Summary of Findings. Washington DC 20005: 2010. 2010 Sleep in America Poll. www.sleepfoundation.org. [Google Scholar]
- 24.Odegard SS, Omland PM, Nilsen KB, Stjern M, Gravdahl GB, Sand T. The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clinical Neurophysiology. 2015;126:1979–1987. doi: 10.1016/j.clinph.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005;39:151–159. doi: 10.1016/j.jpsychires.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 27.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raphael KG, Janal MN, Ananthan S, Cook DB, Staud R. Temporal Summation of Heat Pain in Temporomandibular Disorder Patients. Journal of Orofacial Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Rennefeld C, Wiech K, Schoell ED, Lorenz J, Bingel U. Habituation to pain: Further support for a central component. Pain. 2010;148:503–508. doi: 10.1016/j.pain.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Rhudy JL, Martin SL, Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL. Pain catastrophizing is related to temporal summation of pain but not temporal summation of the nociceptive flexion reflex. Pain. 2011;152:794–801. doi: 10.1016/j.pain.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 31.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 32.Roehrs TA, Harris E, Randall S, Roth T. Pain Sensitivity and Recovery From Mild Chronic Sleep Loss. Sleep. 2012;35:1667–1672. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuh-Hofer S, Wodarski R, Pfau DB, Caspani O, Magerl W, Kennedy JD, Treede RD. One night of total sleep deprivation promotes a state of generalized hyperalgesia: A surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154:1613–1621. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 34.Schuh-Hofer S, Wodarski R, Pfau DB, Caspani O, Magerl W, Kennedy JD, Treede RD. One night of total sleep deprivation promotes a state of generalized hyperalgesia: A surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154:1613–1621. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 35.Simpson NS, Diolombi M, Scott-Sutherland J, Yang H, Bhatt V, Gautam S, Mullington J, Haack M. Repeating patterns of sleep restriction and recovery: Do we get used to it? Brain Behav Immun. 2016;58:142–151. doi: 10.1016/j.bbi.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 37.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, McInrue E, Han D, Leoutsakis J, Haythornthwaite JA. Cognitive-behavior therapy for insomnia in knee osteoarthritis: A double-blind, randomized, active placebo controlled clinical trial. Arthritis Rheumatol. 2015 doi: 10.1002/art.39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Review of Neurotherapeutics. 2012;12:577–585. doi: 10.1586/ern.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 40.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 41.Tiede W, Magerl W, Baumgaertner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Tsui YY, Wing YK. A Study on the Sleep Patterns and Problems of University Business Students in Hong Kong. Journal of American College Health. 2009;58:167–176. doi: 10.1080/07448480903221418. [DOI] [PubMed] [Google Scholar]
- 43.Van Dongen HPA, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: Importance and techniques. Aviation Space and Environmental Medicine. 2004;75:A147–A154. [PubMed] [Google Scholar]
- 44.Vitiello MV, McCurry SM, Shortreed SM, Baker LD, Rybarczyk BD, Keefe FJ, Von Koff M. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155:1547–1554. doi: 10.1016/j.pain.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weingarten JA, Dubrovsky B, Basner RC, Redline S, George L, Lederer DJ. Polysomnographic Measurement of Sleep Duration and Bodily Pain Perception in the Sleep Heart Health Study. Sleep. 2016;39:1583–1589. doi: 10.5665/sleep.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing YK, Li SX, Li AM, Zhang JH, Kong APS. The Effect of Weekend and Holiday Sleep Compensation on Childhood Overweight and Obesity. Pediatrics. 2009;124:E994–E1000. doi: 10.1542/peds.2008-3602. [DOI] [PubMed] [Google Scholar]
- 47.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Current Opinion in Anesthesiology. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]