Abstract
We introduce a high-resolution adult foraging assay (AFA) that relates pre- and post-ingestive walking behavior to individual instances of food consumption. We explore the utility of the AFA by taking advantage of established rover and sitter strains known to differ in a number of feeding-related traits. The AFA allows us to effectively distinguish locomotor behavior in Fed and Food-Deprived (FD) rover and sitter foragers. We found that rovers exhibit more exploratory behavior into the center of an arena containing sucrose drops compared to sitters who hug the edges of the arena and exhibit thigmotaxic behavior. Rovers also discover and ingest more sucrose drops than sitters. Sitters become more exploratory with increasing durations of food deprivation and the number of ingestion events also increases progressively with prolonged fasting for both strains. AFA results are matched by strain differences in sucrose responsiveness, starvation resistance, and lipid levels, suggesting that under the same feeding condition, rovers are more motivated to forage than sitters. These findings demonstrate the AFA’s ability to effectively discriminate movement and food ingestion patterns of different strains and feeding treatments.
Keywords: Drosophila melanogaster, adult foraging assay, food deprivation, food search, ingestion, thigmotaxis
Graphical abstract
Introduction
Animals meet their daily energetic demands by searching for and consuming food (Stephens et al, 2007). Energy needs are more easily satisfied when food is abundant, and negative perturbations in metabolic set points stimulate increased locomotion and food search (Corrales-Carvajal et al., 2016; Dethier, 1976). In the fruit fly (Drosophila melanogaster) foraging behavior combines the detection of olfactory and gustatory cues through contact chemo-sensation (Dahanukar et al., 2005; Dethier, 1976). Gustatory receptor neurons, located in tarsi and labella, allow a fly to detect attractive nutrients and aversive toxins (e.g. by-products of decomposition); this information is used to make decisions on whether to ingest a specific food item (Itskov and Ribeiro, 2013; Ling et al., 2014). In the wild, flies face heterogeneity in both the quantity and quality of nutrients as they encounter fallen fruit in orchards (Reaume and Sokolowski, 2006). Due to the complexity of natural habitats, Drosophila researchers use controlled laboratory environments to characterize foraging behavior. Food consumption, or its likelihood, is quantified using a variety of techniques (Deshpande et al., 2014; Itskov and Ribeiro, 2013) such as measuring the volume ingested from a capillary tube over time in the Capillary Feeder (CAFE) assay (Ja et al., 2007) or by counting the number of proboscis extensions when fly tarsi are stimulated by food as in the Proboscis Extension Response (PER) assay (Scheiner et al. 2004; Shiraiwa and Carlson, 2007). Alternatively, post-hoc analyses measure the quantity of coloured, fluorescent, or radiolabeled food ingested (e.g. Allen et al., 2017; Carvalho et al., 2005; Tanimura et al., 1982; Thompson et al., 1991). Further approaches to measuring ingestion include the flyPAD (fly proboscis and activity detector) and FLIC (fly liquid food interaction counter), which rely on changes in capacitance or resistance across sensors to measure the physical interaction of an individual fly with food (Itskov et al., 2014); Ro et al., 2014). Automated systems for measuring actual consumption with high resolution, the Expresso and ARC (Activity Recording CAFE) have also been described (Yapici et al., 2016, Murphy et al., 2016). While these assays provide excellent measurement of food intake, they do not provide any information about how the animals find food in a large open space.
Food search is a more complex behavior than simply food intake and its study in the laboratory requires artificial foraging environments (Bell et al., 1985; Corrales-Carvajal et al., 2016; Pereira and Sokolowski, 1993; Tanimura et al., 1982; Toshima et al., 2014). The characterization of walking behavior during foraging requires precise and reliable tracking of a fly’s movement. Measures such as distance traveled and speed in addition to food intake allow us to examine specific pre- and post-ingestion exploratory patterns that may influence successful food search. It is important to distinguish pre- and post-ingestion exploratory behaviors since previous research has shown that they are influenced by metabolism and differ from each other (Bell et al., 1985; Nagle and Bell, 1987; Burns et al., 2012). When flies find a food patch they perform either local search or ranging behaviour (Bell et al., 1985, Kim and Dickinson, 2017). In local search, flies remain close to the food source and often circle around it (Kim and Dickinson, 2017). In ranging, flies move further from the food source and their movement patterns are in straighter paths. Hungry flies perform more intensive search behavior than well-fed flies (Bell, 1990). Here, we introduce a novel cost-effective design for an adult foraging assay (AFA) that permits quantification of pre- and post-ingestive behavior as well as the number of times a fly feeds on a food source. The AFA is flexible with respect to the configuration of drops of sucrose or other food sources and is designed to minimize handling stress by allowing the fly to enter the chamber on its own accord. This entry method eliminates the potential confounds of handling effects that arise from aspirating or tapping a fly into a test arena (Barron, 1999; Burns et al., 2012; Trannoy et al., 2015). The AFA arena is surrounded with a water moat to prevent flies from walking onto the arena wall and lid. This obviates the need for a slippery fluon coating of arena surfaces (Dierick and Greenspan, 2006), a method that may lead to excessive grooming in test subjects. We assess the ability of this assay to characterize variation in foraging behavior by using rover (forR) and sitter (fors) strains of the foraging (for) gene of D. melanogaster (Burns et al., 2012; de Belle et al., 1989; Kaun et al., 2007; Osborne et al., 1997; Sokolowski, 1980). Rovers and sitters have been shown to differ in feeding behavior both as larva (Sokolowski, 1980) and adult flies (Pereira and Sokolowski, 1993; Kent et al. 2009; Burns et al., 2012). Our results show that the AFA provides an enhanced method for the phenotypic characterization of adult rover and sitter foraging behavior and their plastic responses to food deprivation, and that these differences correlate with several metabolic traits.
Materials and Methods
Fly strains and handling
The Rover (forR) and sitter (fors) strains used in these experiments have isogenized forR 2nd chromosomes, originally described and called B15 in Bauer and Sokolowski (1985), or fors, 2nd chromosomes, originally described and called E2E3 in Sokolowski (1980); and share isogenized X and 3rd chromosomes from the rover B15 strain (25). Rover and sitter strains were reisogenized in 2013 as described in Allen et al. (2017), and share the same newly isogenized X and 3rd chromosome. Flies were maintained at 25 °C, in a 12:12-h light/dark cycle at 60% relative humidity with lights on at 0800h. Flies were reared on a standard yeast-sugar-agar medium (Anreiter et al., 2016). Virgin female flies were collected immediately after eclosion and placed into vials of n=20 containing 10 ml of standard food. For proof of principle of the AFA, we chose to test virgin females to avoid confounding effects of oviposition site selection. Approximately 24 hours after collection, flies had their wings clipped under brief anesthesia with CO2. We found that wing clipping did not abolish strain differences in food-search behavior, but prevented flies from jumping onto the arena lid. Un-clipped Fed flies would often jump onto the arena lid, which prevented consistent filming and resulted in too many mistrials. Flies were transferred to fresh food vials each day. Fed and FD (24h and 48h) treatment groups were prepared by placing n=8–10 virgin female flies (age 5–8 days old) in either a vial with 10 ml of standard food, or a vial with 10 ml of 1% (w/v) agar that contained a water-moistened cotton ball for 24 or 48h prior to the assay. In this way, flies were deprived of food but not water prior to the experiments. Flies were moved between vials, and from vials into the loading arm of the testing apparatus, using a mouth aspirator.
AFA
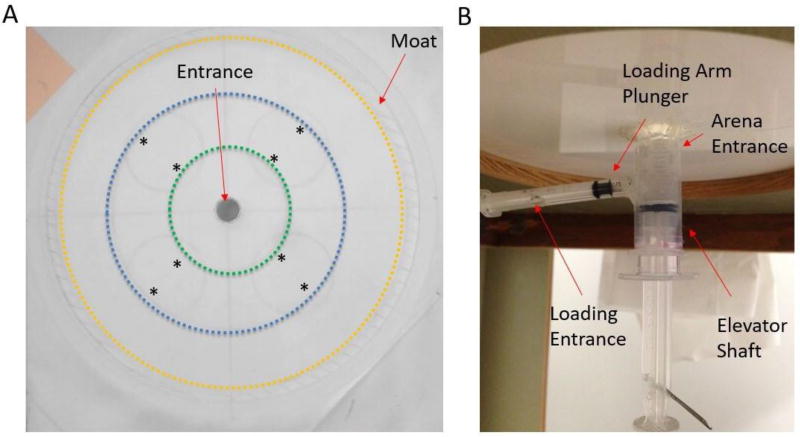
The bottom of the circular AFA arena (Fig. 1A) consists of 7mm thick Plexiglas, 190mm in diameter, including a 2mm deep well edged around the outer perimeter, resulting in an inner diameter of 180mm. The center of the arena bottom has an entrance hole 15mm in diameter. A 10mm high and 5mm thick wall surrounds the outside of the arena. The lid of the arena is removable to facilitate removal of flies and cleaning, and consist of 5mm thick Plexiglas, with a 1mm deep, 5mm wide depression edged around the outer perimeter that fits onto the arena wall to prevent displacement of the lid. The Plexiglas bottom of the arena is etched for improved traction, and to reduce reflections on the camera. The arena is placed on an elevated platform that allows for the positioning of the fly loading arm below the arena. The platform is uniformly white, and surrounded by a 10cm high white cardboard wall used to eliminate spatial cues and to prevent flies from detecting movement outside of the arena. The camera is positioned above the center of the arena. The loading arm of the assay (Fig. 1B) consists of a 1mL syringe attached horizontally to a 10mL vertically positioned syringe; the 1mL syringe acts as a holding arm and its end leads directly into a hole in the wall of the 10mL syringe. Flies are introduced to the holding arm via a hole cut into its distal end, and allowed to walk to the 1/10mL syringe interface. A 5mL syringe is placed freely inside the 10mL vertical syringe. The 10mL syringe acts as a movable collar around the 5mL syringe that can be spun from a ‘closed’ to ‘open’ position. When ‘closed’, the opening at the proximal end of the 10mL syringe faces the solid exterior of the 5mL syringe. When ‘open’, the proximal end of the 10mL syringe coincides with the opening in the 5-mL syringe wall. Flies move freely from the holding arm into the 5mL syringe ‘elevator shaft’, and enter the arena by ascending the shaft. The ‘floor’ (i.e.: syringe plunger) of the elevator shaft, covered with white paper, is then pushed upwards to complete a flat surface for the arena floor. For the purposes of quantifying fly walking behavior, the arena is divided into an inner zone, middle and outer zone. The middle zone contained blue colored sucrose drops (described below) that are visible to both the researcher and the camera. In the present study, we positioned four food patches consisting of paired 0.2µL sucrose drops (described below), separated by 1.5cm within the middle radial zone of the arena, but any configuration of sucrose drops or any food source (e.g., yeast drops or food with different amino acid compositions) could be used in the AFA.
Figure 1.
The Adult Foraging Assay (AFA) for examining locomotion and feeding in adult flies. (A) The AFA testing arena. The entrance is shown in the open position at the center of the arena, and the space for the moat is noted at the edge. The arena is 90mm in radius from the center to the inner edge of the moat. * Denotes the location of the sucrose drops. The dashed circumference lines mark the inner (green), middle (blue) and outer (yellow) zones. The movable opening in the floor attached to the loading arm is shown open in this figure but, once closed, the entire surface of the arena is uniformly white, except for the sucrose drops that were dyed blue to increase visibility. (B) Fly loading arm underneath the arena. The loading arm consists of a 1mL horizontal syringe, with a hole large enough for a single fly to enter, leading into a 5mL vertical syringe, placed within a 10mL vertical syringe. The front ends of both syringes were cut, with the end of the horizontal 1mL syringe leading into a hole at the bottom of the vertical 5mL and 10mL syringes, and the open end of the 5mL syringe leading into the arena. Moving the outer 10mL syringe results of displacement of the holes in the two vertical syringes, closing the entrance into the shaft and preventing the fly from moving backwards. The loading arm is shown in the closed position, when open the plunger is pulled back past the loading entrance.
Arena preparation and data collection
Before each test, the surface of the entire foraging arena was wiped down with a water-moistened Kim-wipe. Water was added to the depression in the outer edge of the AFA arena dish to create an impassable moat. Eight 0.2µL drops of 10% sucrose solution (dyed blue with 0.1% erioglaucine) were placed using a pipette on marked food patches. Individual flies were gently aspirated from a treatment vial and were allowed to walk from the aspirator tip into the loading arm of the assay. The limited diameter of the loading arm prompted flies to walk forward, towards the elevator shaft, and remain at the closed entrance to the elevator shaft until the loading arm was rotated, allowing the fly to enter the elevator shaft. All flies were given a 2min acclimatization period before the loading arm was rotated to open position. Video recording was started as soon as the fly entered the arena though the elevator shaft. Because adult flies exhibit strong phototactic behavior, the positioning of the elevator shaft in the darker area below the arena prompted flies to climb the elevator shaft towards the lighter arena entrance. The fly’s locomotion was recorded using a Microsoft LifeCam Studio webcam positioned 0.3m above the arena, which allowed the live tracking of a fly’s position using a custom tracker developed in the public-domain JavaGrinders Framework (available for free download at http://iEthology.com/). The position of the fly was tracked for 10min as a dark object (the fly) relative to a reference frame of an unoccupied arena. At a rate of 10 fps, the tracker logged the fly’s time-stamped (ms) coordinate data to a file for post hoc analyses of movement patterns and space utilization. Python 2.7 analysis scripts were used to extract the following parameters: location, distance traveled, speed, and time spent in defined arena regions (https://github.com/njc9295/AFA) for an analysis of strain and treatment differences in exploratory and local search behavior. A detection and movement threshold was used to eliminate false positives from the analysis. Video files of each test were collected using screen capture software (https://itunes.apple.com/us/app/capturer/id652792633?mt=12 ‘capturer’ software). It is also possible to create avi videos at specific frame rates from within the tracker without the need for any additional software. Tests were conducted between 1300h and 1700h to control for circadian influences on foraging behavior; all strain and treatment conditions were tested in a randomized order on each day. Tests were aborted if a fly hopped onto the arena lid; the frequency of which did not differ between strains or treatments. The software detected the location of each dyed sucrose drop during the analysis. After completion of the AFA assay, each of the 8 dyed sucrose drops in the assay were examined visually to determine whether they were partially or fully ingested. Fully ingested drops could be easily scored by eye, due to the absence of any blue solution. Partially ingested drops were confirmed by examining every test fly’s proboscis for blue dye staining after the test. Visual inspection of the fly’s proboscis for blue staining allowed us to confirm that the fly ingested some of the droplet, and did not remain motionless over the droplet without feeding.
Starvation resistance assay (SRA)
Starvation vials were made using standard fly vials (Diamed, Cat #GEN32-120) containing 10mL of 1% agar and a cotton ball soaked with water. Virgin female flies were reared on standard food at 25°C in a 12:12h light/dark cycle. Groups of 10 flies (n=10; 7±1 day old) were aspirated into each vial and the number of dead flies was counted every 6–8h. Starvation resistance was performed under rearing temperature, light and humidity conditions.
Proboscis extension response (PER) assay
PER assays were modified from Scheiner et al (2004) and Shiraiwa and Carlson (2007). Virgin female flies (7±1 day old) were aspirated into 100µL pipette tips so that only the head and one foreleg extended from the opening. Foreleg tarsi were stimulated by contact (<1s) with Kim wipe tissue ‘threads’ soaked with either water or various sucrose solutions (0.1%, 0.3%, 1%, 3%, 10%, or 30%, all w/v). Sucrose treatments were presented consecutively in randomized order to minimize experimental bias by the sequence of applied stimuli, and water was presented before and after each sucrose stimulus. A positive result was recorded for every full extension of the proboscis. Flies that responded positively to water were eliminated from analysis, as they were no longer responding exclusively to feeding cues. The number of positive responses for each fly was summed to yield an individual sucrose response (SR) score. The mean SR score was then calculated for each strain-treatment group. Flies were scored separately to yield an individual and strain-averaged sucrose response (SR) score. Flies were treated as described above for Fed, 24h and 48h FD groups. All experiments were conducted between 1300h and 1700h.
Triglyceride quantification
To obtain a measure of lipid stores, total triacylglyceride (TAG) content was quantified as described in Allen et al. (2017). Feeding treatments were conducted as described above. Virgin females (n=8; 7±1 day old) were homogenized in 200µL of 1×PBT with 0.5% TritonX (0.5% PBT-X) and the solution topped up to 1000µL, vortexed and heat inactivated at 70°C for 5min. Samples were centrifuged at 5000rpm at 4°C for 1min, the supernatant was removed and centrifuged again at 13000rpm for 3min. Supernatant was removed and stored at −20°C for later use, or immediately pipetted into a Corning Falcon 96-well cell culture plate (VWR 351172): 50µL of sample were added to each well, and 3 technical replicates were prepared for each sample. A blank reading of the plate was made at 562nm using a BioTek Synergy HT spectrophotometer and Gen5 analytical software. Infinity TAG reagent (Thermo Scientific 796704) was preheated to 37°C and 200µL were added to each well. The plate was incubated at 37°C for 15min and read at 562nm. Total TAG was determined using a standard curve made using a TAG standard (Trace DMA TR2291-030).
Protein was quantified using the Pierce BCA protein assay (Thermo Scientific 23225). 50µL of supernatant prepared as per the TAG assay were loaded in triplicate into a Corning Falcon 96-well cell culture plate (VWR 351172). The plate was read at 562nm for a blank reading. 150µL of BCA solution were added to each well. The plate was incubated at 37°C for 3min and read at 562nm. The blank reading was subtracted from the final reading and total protein content was determined using a standard curve made from a protein standard (BCA kit). TAG levels were standardized over protein levels for each sample.
Statistics
Effects of strain (rover or sitter) and FD treatment (Fed, 24FD and 48FD) were analyzed with a two-way ANOVA followed by a Tukey's post hoc test. AFA post-ingestive behavior data were analyzed with the sign test, with an adjusted significance threshold to correct for multiple comparisons. All statistical analysis was performed in GraphPad Prism version 7.00 for Mac OS X, GraphPad Software, La Jolla California USA.
Results
Locomotion in the AFA differs between strain and treatment groups
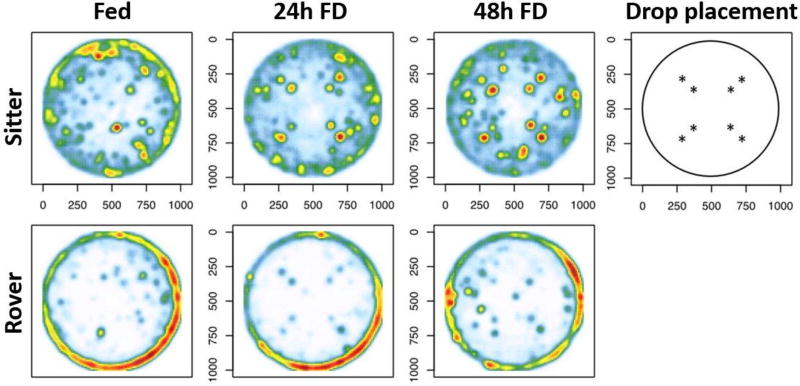
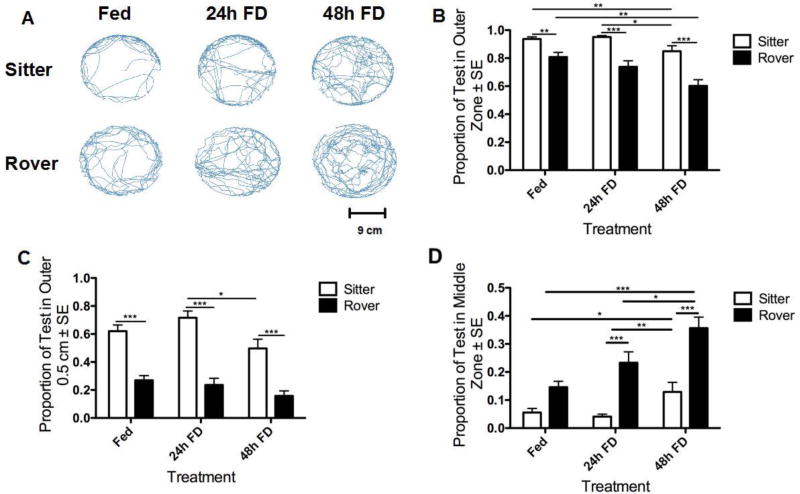
We tested the effect of Fed and Food Deprived (24h FD and 48h FD) treatments on individual exploratory behavior of 5–8 day old virgin female rovers and sitters. Compound heat maps for treatment combinations indicated the presence of distinct overall patterns in arena utilization (Fig. 2). While sitters were much more likely to remain on the perimeter, members of both strains were increasingly likely to venture into the arena's center under food deprivation. Representative examples of locomotion patterns for each strain and treatment group are shown in Fig. 3A. Analysis of these patterns revealed a significant effect of strain and FD treatment on the number of flies found in the outer radial zone of the arena (Two-way ANOVA, FD: F(2,116) = 10.61, p < 0.001; Strain: F(1,116) = 26.40, p < 0.01; Interaction: F(2,116) = 1.66, p = 0.2; Fig. 3B). In all feeding treatments, rovers spent less time in the outer radial zone than sitters, and were increasingly likely to leave the outer zone and explore the middle and inner zones as FD time increased (Fig. 3B). Sitters showed a significant increase in exploratory behavior only in the 48h FD treatment group (Fig. 3B). Since the preference of a fly for the outer zone may be related to thigmotaxis, an attraction to the edge of the arena, we also investigated fly preferences for the most outer edge (outer 0.5 cm) of the arena. Both strain and FD significantly affected the amount of time spent in the outer 0.5 cm, with strain accounting for 48% and feeding treatment accounting for 5.1% of the total variation (Fig. 3C: Two-way ANOVA, FD: F(2,116) = 5.88 p < 0.01; Strain: F(1,116) = 110.30 p < 0.001; Interaction: F(2,116) = 1.39, p = 0.3). Sitters spent significantly more time in the outer 0.5 cm than rovers in all feeding treatments. The FD treatments did not significantly affect rover preference for the outer 0.5 cm (Fig. 3B). These results demonstrate a strong effect of both strain and treatment on exploratory behavior, and identify a strong strain effect on thigmotaxis. We also quantified the time each strain spent in the middle zone, under each feeding condition because all the sucrose drops were placed in the middle zone of the arena, (Fig. 3D). We found a significant effect of strain, FD treatment and their interaction on the number of flies found in the middle zone of the arena (Two-way ANOVA, FD: F(2,116) = 12.58, p < 0.001; Strain: F(1,116) = 26.18, p < 0.001; Interaction: F(2,116) = 3.02, p = 0.05). 24h and 48h FD rovers spent significantly more time in the middle zone than did 24h and 48h FD sitters. Rovers significantly increased the time spent in the middle zone with each increase in FD time, while sitters only increased the time spent in the middle zone after 48h FD. Together these results show that exploratory behavior, measured as time spent at the edge versus time spent in the middle of the arena, increases with FD, and rovers are generally more exploratory than sitters. FD affects sitter and rover behavior differently, with sitters needing longer periods of FD to show a behavioral response.
Figure 2.
Spatial visualization of arena use by flies in a two strain (rover vs. sitter) by three starvation treatments (Fed, 24h FD, 48h FD) factorial design. Each panel depicts a compound spatial density function, derived from data merged across multiple animals within each combination of strain and starvation status. Binned frequencies were used to calculate a 2D probability function via R:MASS's kernel density estimator (kde2d), with z-axis plotted on a progressive color gradient (white -> blue -> green -> yellow -> red). Sitters exhibit thigmotaxic preference for the arena's edges, while rovers do not. With longer periods of starvation, space utilization increasingly transfers to the sites of eight sucrose drops in the interior of the arena. The location of sucrose drops is marked by * in the top right panel. n=19 sitters Fed; n=22 rovers Fed; n=22 sitters 24h FD; n=20 rovers 24h FD; n=16 sitters 48h FD; n=21 rovers 48h FD.
Figure 3.
Locomotor behavior of adult female virgin Fed, 24h FD and 48h FD rovers and sitters (A) Representative tracings of walking patterns of adult virgin female Fed, 24, and 48 hours food deprived (FD) rovers and sitters during a 10 min test in the AFA. Scale bar represents 9 cm. (B) Sitters spent significantly more time in the outer zone than rovers; this trend decreased in both strains with 24h and 48h FD. (C) Rovers spent significantly less time in the outer 0.5 cm than sitters. FD affected the amount of time sitters, but not rovers, spent in the outer 0.5 cm. (D) Rovers spend significantly more time in the middle area of the arena than sitters. n=19 sitters Fed; n=22 rovers Fed; n=22 sitters 24h FD; n=20 rovers 24h FD; n=16 sitters 48h FD; n=21 rovers 48h FD. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
The average total distance covered during the test did not significantly differ between flies in the strain and FD treatment groups (Fig. 4A). Walking speed over the course of a test did not differ between FD treatments in sitters, while rovers showed a significant increase in walking speed between Fed and 48h FD treatments (Fig. 4B: Two-way ANOVA, FD: F(2,116) = 4.21, p < 0.05; Strain: F(1,116) = 2.65, p = 0.1; Interaction: F(2,116) = 0.9, p = 0.4). In the outer zone of the arena, rovers showed a significant increase in walking speed with progressive FD treatment; this effect was absent in sitters (Fig. 4C: Two-way ANOVA, FD: F(2,116) = 6.44, p < 0.01; Strain: F(1,116) = 7.76, p < 0.01; Interaction: F(2,116) = 1.58, p = 0.2).
Figure 4.
Total distance and average speed do not differ between strains. (A) Neither strain nor FD treatment had a significant effect on the total distance travelled during a t(B) The mean speed of rovers was significantly increased by FD. (C) In rovers, but not sitters, FD significantly increased walking speed in the outer zone. FD produced significant inter-strain differences in walking. n=19 sitters Fed; n=22 rovers Fed; n=22 sitters 24h FD; n=20 rovers 24h FD; n=16 sitters 48h FD; n=21 rovers 48h FD. * = p < 0.05, ** = p < 0.01.
Ingestion varies significantly between strain and treatment groups
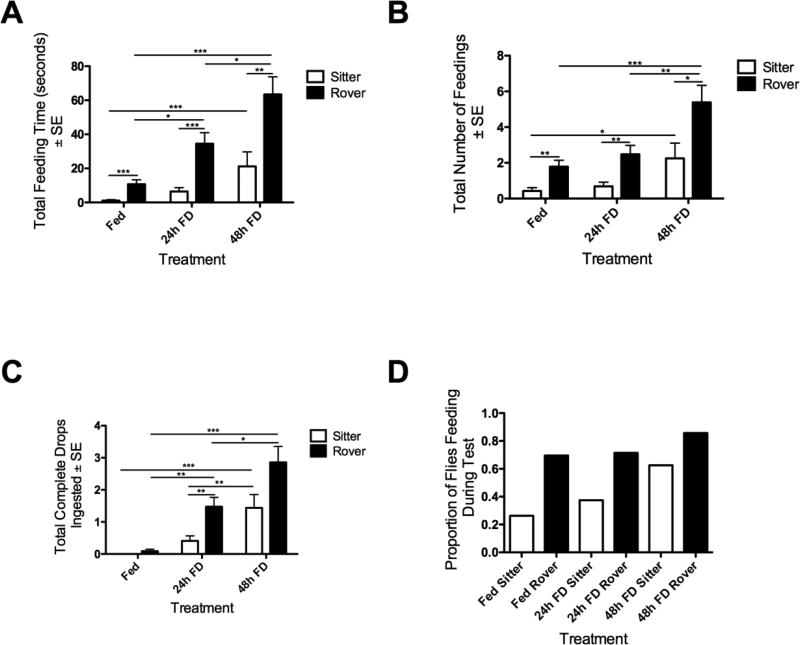
We also assessed the ability of the AFA to measure success at food search and how it is related to movement patterns. In our analysis, an ingestion event was defined as any amount of time that a foraging fly remained stationary over top of a sucrose drop: a drop did not have to be completely consumed during a single ingestion event. We found a significant effect on the average total time of ingestion events by strain and treatment. Ingestion time increased significantly with increasing FD treatment: rovers consistently showed greater average feeding time (Fig. 5A: Two-way ANOVA, FD: F(2,116) = 17.58, p < 0.001; Strain: F(1,116) = 29.02, p < 0.001; Interaction: F(2,116) = 3.56, p < 0.05). Within a treatment group, a greater proportion of rovers found and ingested food; within strains, the number of ingestion events increased progressively with increasing FD treatment (Fig. 5B: Two-way ANOVA, FD: F(2,116) = 12.14, p < 0.001; Strain: F(1,116) = 20.04, p < 0.001; Interaction: F(2,116) = 1.23, p = 0.3), as well as in the average number of drops that were completely ingested (Two-way ANOVA, FD: F(2,116) = 25.60, p < 0.001; Strain: F(1,116) = 13.17, p < 0.001; Interaction: F(2,116) = 2.83, p = 0.06) (Fig. 5C). Overall, the proportion of flies feeding in the AFA increased with FD (Fig. 5D). Interestingly, Fed rovers differed significantly from both 24h and 48h FD rovers in feeding time, the number of feedings, and the number of complete drops ingested. In contrast, Fed sitters only differed significantly from 48h FD sitters. This suggests that sitters are more resistant to food-deprivation, needing longer periods of food deprivation to show changes in food ingestion.
Figure 5.
Ingestion behavior of adult female virgin Fed, 24h FD and 48h FD rovers and sitters. (A) FD significantly increases average duration of ingestion for both strains, with rovers feeding significantly longer than sitters. (B) Rovers ingested sucrose drops significantly more times than sitters; the number of ingestion events increased with FD duration in both strains. (C) Rovers consumed significantly more complete drops than sitters; FD increased the tendency of flies to completely ingest drops in both strains. (D) Overall, more rovers than sitters fed in the AFA and the proportion of flies feeding tended to increase with FD. n=19 sitters Fed; n=22 rovers Fed; n=22 sitters 24h FD; n=20 rovers 24h FD; n=16 sitters 48h FD; n=21 rovers 48h FD.. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
Proboscis extension responses (PER) vary significantly between strains and treatments and parallel results in the AFA
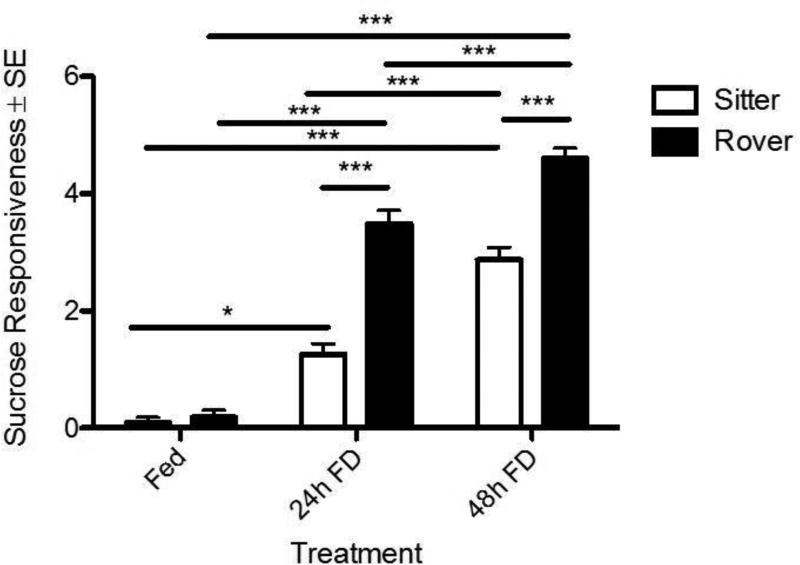
In flies, proboscis extension response (PER) provides a quantifiable response to appetitive gustatory stimuli (i.e. sugar). In order to further investigate strain differences, we conducted PER assays to measure sucrose response (SR) in Fed, 24h FD and 48h FD adult females (Scheiner et al., 2004; Shiraiwa and Carlson, 2007). Although there were no differences between strains in the Fed treatment, robust rover-sitter differences exist in both the 24 and 48h FD treatment groups (Fig. 6; Two-way ANOVA, FD: F(2,176) = 86.54, p < 0.001; Strain: F(1,176) = 42.31, p < 0.001; Interaction: F(2,176)= 7.21, p < 0.001). Rovers showed greater SR than sitters in both FD treatments, and significant intra-strain increases in SR were observed in response to increasing FD. These data suggest that rovers were more responsive to the sucrose following FD treatment, and that this responsiveness increased with increasing duration of FD. Thus, the SR data from the PER assay reflect those found for the sucrose drop ingestion in the AFA assay and show that a 24h FD rover behaves like a 48h FD sitter fly. These results are consistent with those found by Scheiner et al. 2004.
Figure 6.
Proboscis extension responses (PER) vary significantly between strains and treatments. Fed adult virgin female rovers and sitters do not differ in sucrose responsiveness (SR). Both strains increase their sucrose responsiveness with increased food deprivation. Rovers show significantly greater sucrose responsive scores than sitters after 24h and 48h FD. n=11 sitters Fed; n=11 rovers Fed; n=40 sitters 24h FD; n=40 rovers 24h FD; n=40 sitters 48h FD; n=39 rovers 48h FD. * = p < 0.05, *** = p < 0.001.
Post-ingestion locomotion patterns show strain-treatment effects
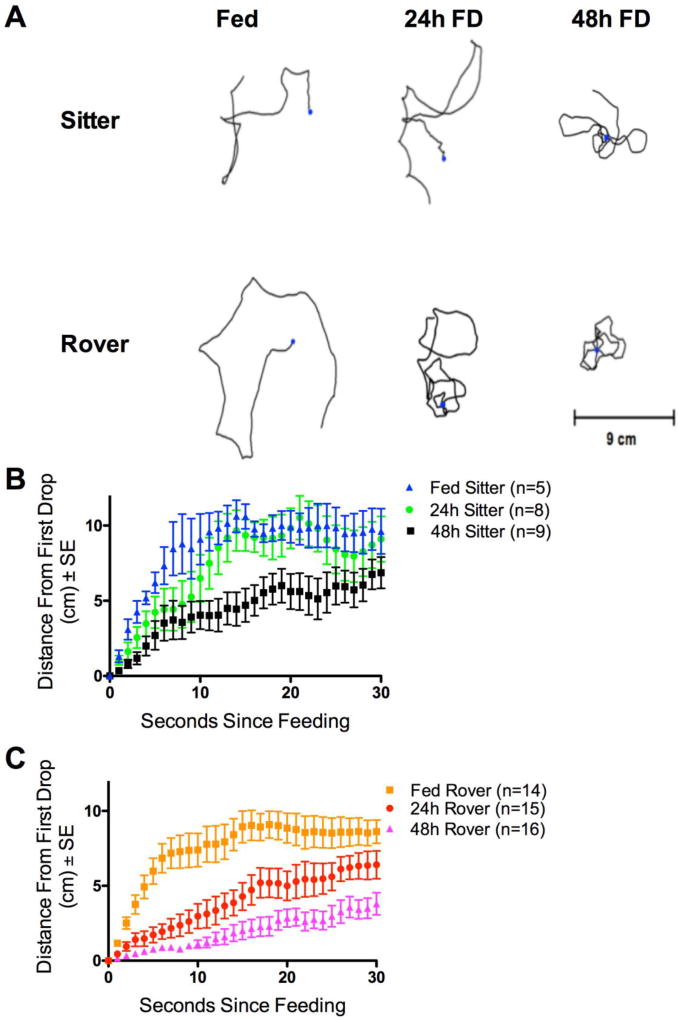
We used the AFA and Tracker to quantify the distance moved from the drop during the 30sec after ingestion following Pereira and Sokolowski (1993). Representative examples of these patterns for each strain and treatment group show that the distance from the drop changes with FD. Tracks show more ranging in Fed treatment groups and increasing local search behavior in 24h and 48h FD treatment groups (Fig. 7A). The distance traveled from a food site following the first ingestion reflects these patterns with 48h FD sitters remaining closer to the sucrose drop than Fed and 24h FD sitters (Fig. 7B), and with the distance that rovers move from the sucrose drop decreasing progressively as FD treatment increases (Fig. 7C) (Sign Test: P<0.001). The number of test flies in which post-ingestion behavior could be analyzed was smaller in sitters than in rovers, due to the smaller number of ingestion events in sitters. Out of the flies that Fed, only 2/5 Fed sitters, 5/8 of 24h FD sitters, and 7/9 of 48h FD sitters had a second feeding event during the test. Among rovers, 10/14 of Fed, 13/15 of 24h FD, and 15/16 of 48 FD rovers had a second feeding event. These small sample sizes limit statistical analyses for post-ingestion movement after the second feeding event, but show that the rover-sitter behavior trends are maintained after the first feeding. Overall, the post-ingestion movement patterns show that flies remain closer to the food drop post-feeding with longer periods of food deprivation, and in any FD treatment, rovers show more intense local search than sitters. This suggests that in any given FD treatment rovers are “hungrier” than sitters, and thus more motivated to search for more food after they found a sucrose drop.
Figure 7.
Post-ingestion walking behavior following the first feeding event in Fed, 24h FD and 48h FD adult female virgin rovers and sitters. (A) Representative plots for the movement of rover and sitter individuals for 30 seconds immediately after the end of an ingestion event. (B) Fed and 24h FD sitters display similar trends, and 48h FD decreases the tendency of sitters to walk away from a sucrose drop. (C) FD had the effect of progressively decreasing the tendency of rovers to leave the site of a sucrose drop. Both 24 and 48 hours of FD altered the movement of rovers compared to Fed state. Scale bars all represent 5 cm. n=5 sitters Fed; n=14 rovers Fed; n=8 sitters 24h FD; n=15 rovers 24h FD; n=9 sitters 48h FD; n=16 rovers 48h FD.
Differences in starvation resistance and lipid levels predict behavior in the AFA
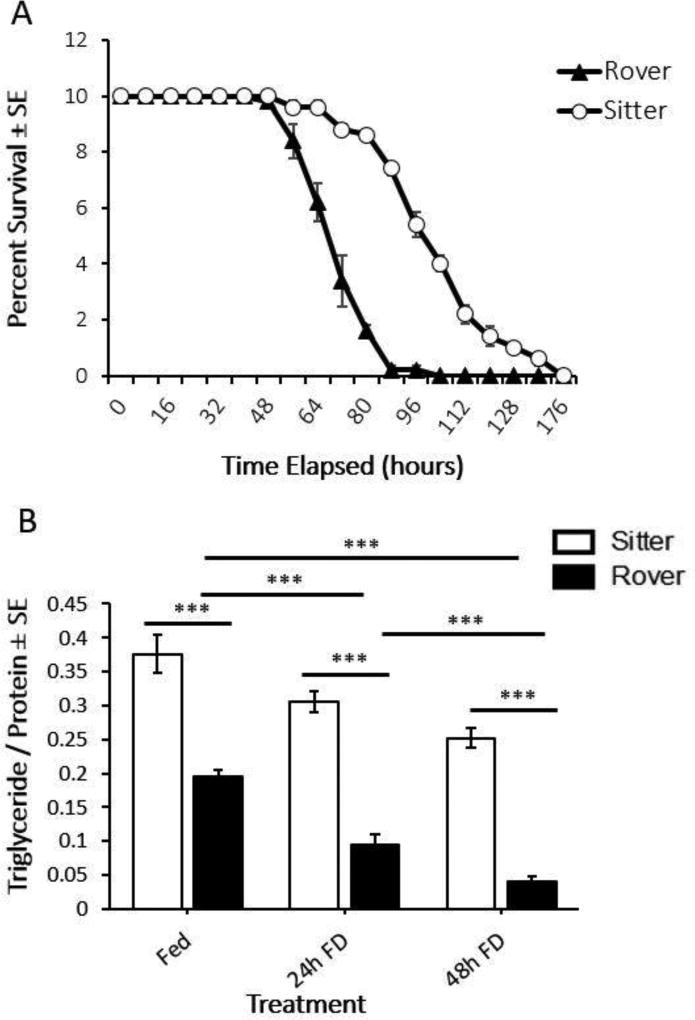
The results of the starvation resistance assay demonstrate that rovers die of starvation faster than sitters (Fig. 8A; log-rank (Mantel–Cox) test: P < 0.001). In addition, adult virgin female rovers fed ad libitum have lower whole body lipid content than sitters, and these inter-strain differences persist across 24h and 48h FD treatments (Fig. 8B). This lower starvation resistance in rovers is predicted from their lower lipid levels relative to sitters. Sitters had higher TAG levels, greater starvation resistance, and lower SR, suggesting sitters are more resistant to metabolic stress than rovers, potentially leading to less motivation to forage than rovers. Accordingly, we found that sitters showed relatively low exploratory behavior, food finding success, and local search intensity in the AFA and longer periods of food deprivation were needed to generate rover-like behavior in sitter flies in the AFA.
Figure 8.
Starvation resistance and lipid metabolism in Fed, 24h FD and 48h FD adult female virgin rovers and sitters. (A) Rovers starve to death faster than sitters. The average percent survival of in five starvation resistance assays with n=10 virgin female flies of each strain per trial for a total of 5 trials. (B) Adult virgin female sitters have a significantly higher homeostatic set point for lipid metabolism than rovers; this trend persists as lipid levels decrease with starvation. 4 flies/sample with the following sample sizes: n=10 sitters Fed; n=8 rovers Fed; n=10 sitters 24h FD; n=7 rovers 24h FD; n=10 sitters 48h FD; n=9 rovers 48h FD. *** = p < 0.001
Discussion
Methodological advantages of the AFA
The AFA is a simple setup that does not require the purchase of expensive equipment and can be constructed easily and cost effectively from readily available materials. Depending on the specific research question, any food source in any configuration of drops can be used. The arena provides the resolution necessary to record videos that allow simultaneously tracking of multiple flies. In this study, we intended to remove any confounding social interaction effects when assaying strain-specific food search behaviors, but the assay could also be used to test for the effect of social interactions on food-search behavior. Social interactions have been shown to play a role in feeding behavior (Lihoreau et al, 2016). The software used for tracking and analysis of fly movement is open source and can be modified to fit specific needs. Rover and sitter behavioral responses to food deprivation demonstrate that strain and treatment-specific movement patterns can be easily distinguished in the AFA. The acclimatization chamber and loading arm underneath the arena reduces the stress on the fly during its introduction into the AFA test arena because the fly walks into the arena without being blown or tapped. Flies engaged in exploratory behavior in the AFA as they entered the arena. Lastly, our effective use of a water moat instead of the traditionally slippery chemicals avoided any confounding effects of chemical sensing on feeding behavior and prevented flies from climbing on the sides and lid of the arena.
Effectiveness of the AFA in discriminating strain-specific movement patterns
Work using mutant and transgenic analyses has shown that differences in feeding-related traits arise from variation in foraging (Scheiner et al, 2004; Belay et al, 2007; Burns et al, 2012; Anreiter et al, in press). Here we used the rover and sitter strains to show the efficacy of the AFA. We found that rovers and sitters differ in a number of adult foraging traits, but not in general locomotion parameters. Rovers and sitters did not differ in total walking distance or speed. However, flies of both strains increased their walking speed in response to FD, consistent with what has been reported about D. melanogaster in the literature (Bell, 1985; Corrales-Carvajal et al., 2016). We also observed differences in thigmotaxis with rovers more exploratory than sitters (see also Burns et al. 2012). Thigmotaxis decreased in FD rovers compared to Fed rovers. This resulted in increased walking through the inner and middle zones of the arena, a potentially risky exploratory behavior. However, under FD conditions, rovers found more sucrose drops, spent more time ingesting a drop, and ingested more complete drops than sitters. The greater thigmotaxis of sitters and exploratory behavior of rovers in the AFA is reminiscent of previously described strain differences in thigmotaxis and exploratory behaviors in an open field assay in Drosophila (Burns et al., 2012) and rodents (Wilson et al. 1976; Crusio 2001). Ingesting a sugar drop triggers a distinctive local search pattern in flies, where the fly stays close to the sugar drop, circles and repeatedly returns to the spot where it found food (Murata et al, 2017; Kim and Dickinson, 2017). After ingesting a sucrose drop, FD rovers moved shorter distances from the drop than FD sitters. This contradicts previous reports using a different assay (Nagle and Bell, 1987; Pereira and Sokolowski, 1993), where rovers walked farther than sitters after consuming a sucrose drop suggesting that they show more ranging behavior. However, the assays used by Nagle and Bell (1987) and Pereira and Sokolowski (1993) had several limitations. Firstly, the only measure obtained was the maximum distance walked by the fly; secondly, data were collected only from flies that consumed the entire 0.2-µL sucrose drop; and thirdly, flies did not perform search behavior to find the drop because they were placed directly onto the sucrose drop. As a result, their assay discounts the influence of pre-ingestion food search behavior on feeding and post-ingestive behavior. In contrast, the AFA allowed the fly to freely walk within the arena, search and find a drop and consume it. This suggests that the means by which post-ingestion local search behavior is assayed is highly sensitive to how a fly finds and ingests food prior to the post-ingestion assay of local search. In support of previous studies, we did find an increase in local search intensity in both rovers and sitters with increasing FD treatments (Bell et al., 1987; Corrales-Carvajal et al., 2016). Indeed, a hungry fly is more likely to exhibit local search behavior following ingestion of food compared to flies fed ad libitum that show more ranging behavior following an ingestion event (Bell et al., 1985; Corrales-Carvajal et al., 2016).
Rover-sitter foraging patters and other metabolic measures
We showed that rovers have higher sucrose responsiveness (SR), and lower starvation resistance and triglyceride levels than sitters. Our findings support previous studies that show that in the absence of food, adult rovers starve to death faster than sitters (Donlea et al. 2012) and that rovers are more likely to ingest sucrose following a 24h or 48h FD treatment than sitters (Scheiner et al. 2004). We have previously shown that sitter larvae have higher triglyceride levels, eat more, and move less than rovers (Allen et al, 2017), and that the foraging gene directly regulates these phenotypes in larvae (Allen et al, 2017). The rover and sitter adult TAG differences reported in the present study might have origins in larval TAG differences as a carryover from larval development or alternatively foraging may regulate TAG during the adult stage irrespective of its role in larvae. Kent et al (2009) compared the heads of 24h old FD mutant sitter and rover heads and found higher lipid levels in mutant sitter heads. Even though these results parallel the results of the present study, it should be noted that Kent et al. (2009) is not directly comparable because they used different strains, mated female flies, fly heads, a richer rearing medium and different TAG measurements. Overall our differences in rover and sitter behavior in the AFA are interpretable from the perspective of metabolically-related phenotypes, which together suggest that rovers are more motivated to forage than their sitter counterparts. Sitters that undergo FD treatments become more rover-like, with 24h FD rovers showing similar patterns to 48h FD sitters. That sitters become more rover-like with increasing FD suggests that rover-sitter differences in any Fed or FD state are a product of greater starvation sensitivity in rovers. This is in line with theory from the behavioral-ecology literature that shows that the cost associated with increased risk taking must be outweighed by the need for finding food (Herberholz and Marquart, 2012; Lima and Dill, 1990). The clear continuity in characteristics demonstrates the high resolving capacity of the AFA to distinguish between behavioral patterns that are influenced by metabolism.
Highlights.
We present a new Drosophila adult foraging assay (AFA).
The AFA is easy, cost effective, flexible, and uses open source tracker software.
The AFA is effective at teasing apart strain differences in foraging behavior.
We use the rover and sitter strains to measure movement and feeding patters.
Acknowledgments
This research was supported by a National Sciences and Engineering Research Council (NSERC) discovery grant to MBS. IA was supported by an Ontario Graduate Scholarship and NLJC by an NSERC USRA studentship. We thank Fred Mery for early discussions on the design of the AFA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AM, Anreiter I, Neville MC, Sokolowski MB. Feeding-related traits are affected by dosage of the foraging gene in Drosophila melanogaster. Genetics. 2017;205:761–773. doi: 10.1534/genetics.116.197939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anreiter I, Vasquez OE, Allen AM, Sokolowski MB. Foraging path-length protocol for Drosophila melanogaster larvae. J. Vis. Exp. 2016;110:e53980. doi: 10.3791/53980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anreiter I, Kramer J, Sokolowski MB. Epigenetic mechanisms modulate differences in Drosophila foraging behavior. PNAS. doi: 10.1073/pnas.1710770114. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AB. Anaesthetizing Drosophila for behavioral studies. J. Insect. Physiol. 2000;46:439–442. doi: 10.1016/s0022-1910(99)00129-8. [DOI] [PubMed] [Google Scholar]
- Bauer SJ, Sokolowski MB. A genetic analysis of path length and pupation height in a natural population of Drosophila melanogaster. Can J Genet Cytol. 1985;27:334–340. [Google Scholar]
- Belay AT, Scheiner R, So AK-C, Douglas SJ, Chakaborty-Chatterjee M, Levine JD, Sokolowski MB. The foraging gene of Drosophila melanogaster: spatial-expression analysis and sucrose responsiveness. Journal of Comparative Neurology. 2007;504:570–582. doi: 10.1002/cne.21466. [DOI] [PubMed] [Google Scholar]
- Bell WJ. Chapman and Hall Animal behavior series. Springer; 1990. z ecology of finding resources. [Google Scholar]
- Bell WJ, Cathy T, Roggero RJ, Kipp LR, Tobin TR. Sucrose-stimulated searching behavior of Drosophila melanogaster in a uniform habitat: modulation by period of deprivation. Anim. Behav. 1985;33:436–448. [Google Scholar]
- Burns JG, Svetec N, Rowe L, Mery F, Dolan MJ, Boyce WT, Sokolowski MB. Gene–environment interplay in Drosophila melanogaster: Chronic food deprivation in early life affects adult exploratory and fitness traits. PNAS. 2012;109:17239–17244. doi: 10.1073/pnas.1121265109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nature Methods. 2005;11:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales-Carvajal VM, Faisal AA, Ribeiro C. Internal states drive nutrient homeostasis by modulating exploration-exploitation trade-off. Elife. 2016;5:e19920. doi: 10.7554/eLife.19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE. Genetic dissection of mouse exploratory behavior. Behav. Brain. Res. 2001;125:127–132. doi: 10.1016/s0166-4328(01)00280-7. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr. Opin. Neurobiol. 2005;15:423–30. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat. Genetics. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, Ja WW. Quantifying Drosophila food intake: comparative analysis of current methodology. Nature Methods. 2014;11:535–40. doi: 10.1038/nmeth.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding. Harvard UPress; Cambridge, MA: 1976. [Google Scholar]
- Donelson NC, Kim EZ, Slawson JB, Vecsey CG, Huber R, Griffith LC. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “Tracker” program. Plos One. 2012;7:e37250. doi: 10.1371/journal.pone.0037250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J, Leahy A, Thimgan MS, Suzuki Y, Hughson BN, Sokolowski MB, Shaw PJ. foraging alters resilience/vulnerability to sleep disruption and starvation in Drosophila. Proc. Natl. Acad. Sci. 2012;109:2613–2618. doi: 10.1073/pnas.1112623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberholz J, Marquart GD. Decision making and behavioral choice during predator avoidance. Front. Neurosci. 2012;6:125. doi: 10.3389/fnins.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front. Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov PM, Moreira J-M, Vinnik E, Lopes G, Safarik S, Dickinson MH, Ribeiro C. Automated monitoring and quantitative analysis of feeding behavior in Drosophila. Nat. Commun. 2014;5:4560. doi: 10.1038/ncomms5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, del la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Riedl CAL, Chakaborty-Chatterjee M, Belay AT, Douglas SJ, Gibbs AG, Sokolowski MB. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J. Exp. Biol. 2007;210:3547–3558. doi: 10.1242/jeb.006924. [DOI] [PubMed] [Google Scholar]
- Kent CF, Daskalchuk T, Cook L, Sokolowski MB, Greenspan RJ. The Drosophila foraging gene mediates adult plasticity and gene–environment interactions in behavior, metabolites, and gene expression in response to food deprivation. Plos Genet. 2009;5:e1000609. doi: 10.1371/journal.pgen.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Dickinson MH. Idiothetic Path Integration in the Fruit Fly Drosophila melanogaster. Curr Biol. 2017 doi: 10.1016/j.cub.2017.06.026. pii: S0960-9822(17)30728-5. [DOI] [PubMed] [Google Scholar]
- Lihoreau M, Clarke IM, Buhl J, Sumpter DJT, Simpson SJ. Collective selection of food patches in Drosophila. Journal of Experimental Biology. 2016;219:668–675. doi: 10.1242/jeb.127431. [DOI] [PubMed] [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool. 1990;68(4):619–640. [Google Scholar]
- Ling F, Dahanukar A, Weiss LA, Kwon JY, Carlson JR. The molecular and cellular basis of taste coding in the legs of Drosophila. J. Neurosci. 2014;34:7148–7164. doi: 10.1523/JNEUROSCI.0649-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S, Brockmann A, Tanimura T. Pharyngeal stimulation with sugar triggers local searching behavior in Drosophila. J. Exp. Biol. 2017 doi: 10.1242/jeb.161646. Advance Online Article. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Deshpande SA, Yurgel ME, Quinn JP, Weissbach JL, Keene AC, Dawson-Scully K, Huber R, Tomchik SM, Ja WW. Postprandial sleep mechanics in Drosophila. Elife. 2016;5:e19334. doi: 10.7554/eLife.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle KJ, Bell WJ. Genetic control of the search tactic of Drosophila melanogaster: an ethometric analysis of rover/sitter traits in adult flies. Behav. Genet. 1987;17(4):385–408. doi: 10.1007/BF01068138. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:843–846. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Pereira HS, Sokolowski MB. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proc. Natl. Acad. Sci. 1993;90:5044–6. doi: 10.1073/pnas.90.11.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume CJ, Sokolowski MB. Natural history and ecology of Drosophila melanogaster. Curr. Biol. 2006;16:R623–8. doi: 10.1016/j.cub.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Ro J, Harvanek ZM, Pletcher SD. FLIC: High-Throughput, Continuous Analysis of Feeding Behaviors in Drosophila. PLoS One. 2014:e101107. doi: 10.1371/journal.pone.0101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R, Sokolowski MB, Erber J. Learn. Mem. Vol. 11. Cold Spring Harb. N. Y.: 2004. Activity of cGMP-dependent protein kinase (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster; pp. 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J. Vis. Exp. 2007;3:193. doi: 10.3791/193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski MB. Foraging strategies of Drosophila melanogaster: a chromosomal analysis. Behav. Genet. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Stephens DW, Brown JS, Ydenberg RC. Foraging: Behavior and Ecology. University of Chicago Press; Chicago, Illinois, USA: 2007. [Google Scholar]
- Tanimura T, Isono K, Takamura T, Shimada I. Genetic dimorphism in the taste sensitivity to trehalose in Drosophila melanogaster. J. Comp. Physiol. A. 1982;147:433–437. [Google Scholar]
- Thompson ED, Reeder BA, Bruce RD. Characterization of a method for quantitating food consumption for mutation assays in Drosophila. Environ. Mol. Mutagen. 1991;18:14–21. doi: 10.1002/em.2850180104. [DOI] [PubMed] [Google Scholar]
- Toshima N, Hara C, Scholz CJ, Tanimura T. Genetic variation in food choice behavior of amino acid-deprived Drosophila. J. Insect. Physiol. 2014;69:89–94. doi: 10.1016/j.jinsphys.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Trannoy S, Chowdhury B, Kravitz EA. Handling alters aggression and “loser” effect formation in Drosophila melanogaster. Learn. Mem. 2015;22:64–8. doi: 10.1101/lm.036418.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Vacek T, Lanier DL, Dewsbury DA. Open-field behavior in muroid rodents. Behav. Biol. 1976;17:495–506. doi: 10.1016/s0091-6773(76)90901-9. [DOI] [PubMed] [Google Scholar]
- Yapici N, Cohn R, Schusterreiter C, Ruta V, Vosshall LB. A taste circuit that regulates ingestion by integrating food and hunger signals. Cell. 2016;165:715–29. doi: 10.1016/j.cell.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]