Abstract
There is renewed interest in leveraging the thermogenic capacity of brown adipose tissue (BAT) and browning of white adipose tissue (WAT) to improve energy balance and prevent obesity. In addition to these effects on energy expenditure, both BAT and WAT secrete large numbers of hormones and cytokines that play important roles in maintaining metabolic health. Both BAT and WAT are densely innervated by the sympathetic nervous system (SNS) and this innervation is crucial for BAT thermogenesis and WAT browning, making it a potentially interesting target for manipulating energy balance and treatment of obesity and metabolic disease. Peripheral neuromodulation in the form of electrical manipulation of the SNS and parasympathetic nervous system (PSNS) has been used for the management of pain and many other conditions, but progress is hampered by lack of detailed knowledge of function-specific neurons and nerves innervating particular organs and tissues. Therefore, the goal of the National Institutes of Health (NIH) Common Fund project “Stimulating Peripheral Activity to Relieve Conditions (SPARC)” is to comprehensively map both anatomical and neurochemical aspects of the peripheral nervous system in animal model systems to ultimately guide optimal neuromodulation strategies in humans. Compared to electrical manipulation, neuron-specific opto- and chemogenetic manipulation, now being extensively used to decode the function of brain circuits, will further increase the functional specificity of peripheral neuromodulation.
Keywords: sympathetic innervation, adipose tissue, gene therapy, energy expenditure, neuromodulation
1. Introduction
Despite improvements in understanding the regulation of energy balance, there are still only a few efficient treatment options for obesity. Bariatric surgery, although highly effective, is only accessible to few, and pharmacotherapy has not been as effective as hoped for. It is increasingly realized that mono-therapies may not be optimal for a complex disease such as obesity and its comorbidities. Given that energy balance ultimately depends on both intake and expenditure, therapies that impinge on both components are more likely to be successful. Limiting energy intake alone through dieting has not been successful for long-term weight loss because of strong adaptive counter-regulatory biological responses [1, 2]. Increasing energy expenditure through exercise has also been questioned as an effective weight loss therapy, partly because of upregulated food intake and lack of long-term compliance [3, 4].
Another strategy to increase energy expenditure has only recently been revived. Although the initial excitement about the possibility of burning excess energy by diet-induced activation of BAT [5] lost its momentum for many years, it was recently revived when significant amounts of BAT were detected in humans [6–10]. In addition, new research has shown that WAT has the potential to increase uncoupling protein 1 (UCP1) expression and start behaving more like BAT (called beiging or browning), not just storing fat but burning energy [11, 12]. Therefore, intense research is underway with the goal to leverage the energy burning capacities of both BAT and WAT in the fight against obesity. Ultimately combined with dieting and exercise, this strategy could be very powerful in resetting energy balance at a lower body weight.
Here, we discuss the possibilities of stimulating adipose tissue thermogenesis through targeted manipulation of its sympathetic innervation. Peripheral neuromodulation is the focus of a newly launched NIH Common Fund Initiative SPARC (https://commonfund.nih.gov/sparc/index), which includes neuromodulation of the sympathetic innervation of adipose tissue. The anatomical and chemical organization of adipose tissue innervation has been comprehensively studied by the pioneering work of Timothy Bartness and his colleagues [13] and will be summarized only briefly in the first part of this review. Similarly, the diverse functions of adipose tissue have been discussed in excellent recent reviews [14, 15] and will only be summarized here. The main purpose of this review is to discuss different strategies for manipulating specific functions of adipose tissue with a focus on genetics-driven technologies.
2. Innervation of adipose tissue
The first studies on the sympathetic innervation of adipose tissue involved histological staining for nerves and later more specific staining for the sympathetic neurotransmitter noradrenaline (NA) [16], supported by biochemical assays to demonstrate increased lipolysis after adrenergic WAT stimulation [17]. More recently, Timothy Bartness and colleagues spearheaded the study of neuronal circuits involved in sympathetic adipose tissue innervation and made a major contribution to science by linking the brain to BAT and WAT bi-directionally. Using retrograde trans-synaptic neuronal tracer, pseudorabies virus (PRV), they were the first to define pre- and post-ganglionic sympathetic innervation of adipose tissue in the Siberian Hamster and rats [18, 19]. PRV neurotropic capacity which enables it to jump across synapses allows tracking connectivity in adipose tissue sympathetic outflow pathway and its central neural command circuits [20]. Both neuroanatomical and functional studies using sympathetic nerve denervation led to extensive discoveries on adipocyte innervation and biology.
2.1. Sympathetic BAT innervation
In rodents, interscapular BAT is densely innervated by tyrosine hydroxylase (TH) and Neuropeptide Y (NPY) -positive nerve fibers, with fibers expressing both markers selectively innervating the microvasculature and fibers expressing only TH innervating brown adipocytes [21]. Fibers expressing only TH are in a position to release NA and stimulate β-adrenergic receptors (β-ARs) expressed by brown adipocytes, triggering a cascade of intracellular changes via the cyclic AMP-protein kinase A signaling pathway, including upregulation of UCP1 gene, increased lipolysis and glucose uptake, which results in an increased BAT thermogenic capacity and substrate availability [22]. Retrograde tracing identified postganglionic sympathetic neurons expressing the same markers bilaterally in ganglia of the sympathetic chain, particularly stellate ganglia [23] (Fig. 1). Clearly, additional postganglionic neurons are also located in more caudal sympathetic chain ganglia (T2–T6), but there is considerable uncertainty whether they extend even more caudal ([23] in rat, [24] in hamster, and our own unpublished data). The corresponding preganglionic neurons innervating BAT have been identified in the intermediolateral column of the spinal cord extending from the T1– T7 region and higher order neurons are located in various brain regions including the raphe pallidus and dorsomedial hypothalamus [19, 23, 25]. The functional necessity of the SNS for intact BAT function is demonstrated by surgical and chemical denervation methods, which block or greatly reduce most cold-induced BAT adaptations like UCP1 induction, increased blood flow, higher mitochondrial density, enhanced glucose uptake and activation of thyroid-activating enzymes [26].
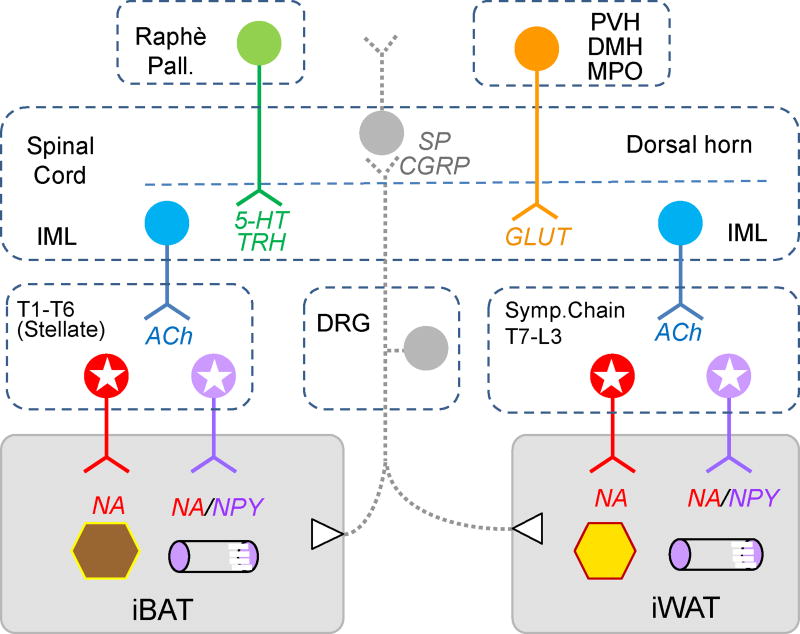
Fig. 1.
Schematic diagram showing sympathetic and afferent innervation of interscapular brown fat (iBAT) and inguinal white fat (iWAT). Postganglionic sympathetic neurons are located in the upper (stellate ganglia, T1/T2 to about T6) for iBAT, and in the lower (T7 to L3) sympathetic chain ganglia for iWAT. Postganglionic neurons innervating brown and white adipocytes express noradrenaline (NA, red), while neurons innervating the vasculature express NA and neuropeptide Y (NA/NPY, purple). Preganglionic neurons for both fat pads are located in the intermediolateral column (IML) of the spinal cord and express acetylcholine (Ach, blue). Both fat pads are also innervated by afferents with cell bodies located in the dorsal root ganglia (DRG), expressing substance P (SP) and calcitonin gene-related peptide (SP/CGRP, gray). Higher order sympathetic outflow is mainly originating in the nucleus raphè pallidus with 5-hydroxytryptamine (5-HT) and thyrotropin-releasing hormone (TRH) and hypothalamic nuclei including the paraventricular nucleus (PVH), dorsomedial nucleus (DMH), and medial preoptic nucleus (MPO), with glutamate as major transmitter. For simplicity, innervations of epididymal white fat (eWAT) and mesenteric white fat (mWAT) are not shown. For more information on eWAT and mWAT innervation, please refer to [27, 28].
2.2. Sympathetic WAT innervation
The thoracic ganglion 13 (T13) and the lumbar ganglia 1–2 (L1, L2) in the sympathetic chain provide the main sympathetic innervation of subcutaneous WAT, with minor contributions from more rostral sympathetic chain ganglia [27] (Fig. 1). Innervation of epididymal (eWAT) and abdominal mesenteric WAT (mWAT) has also been shown to originate in lower thoracic and lumbar sympathetic chain ganglia, with a certain degree of topographic segregation and overlap [28–30]. This topographic segregation may be underlying some of the differential effects of sympathetic stimulation, with eWAT showing stronger NA turnover rates than inguinal WAT (iWAT) [28]. A similar topographical segregation has been noted in the preganglionic neurons located in the intermediolateral column of the spinal cord that innervate different WAT depots [27].
In summary, we have a good general idea of where the sympathetic pre- and postganglionic, as well as higher order neurons innervating BAT and WAT are located. However, more comprehensive maps are necessary for successful neuromodulatory approaches. For example, besides neuronal cell bodies, projection pathways in connecting nerves need to be identified for successful and selective electrical stimulation or inhibition. Furthermore, existing maps are a synthesis from at least 3 different species, hamster, rat, and mouse. It will be particularly important to generate comprehensive maps of sympathetic innervation in the mouse, as this species is the prime experimental model applying genetics-driven neuromodulation approaches. It is hoped that understanding adipose tissue innervation in the mouse and other model organisms will ultimately lead to better neuromodulation strategies in humans.
2.3. Sensory innervation of adipose tissue through dorsal root ganglia
The Bartness laboratory also spearheaded investigations to show the existence of sensory nerves in both BAT and WAT. The cell bodies of these sensory nerves are found in dorsal root ganglia and receive their sensory input from the adipose tissue and further propagate their signal to the central nervous system (CNS) [26, 31, 32]. Recent work further supports crosstalk between WAT and BAT. Anatomical studies found that WAT and BAT share discrete sympathetic outflows from the CNS [30], and sensory circuits of WAT and sympathetic circuits of BAT (and vice versa) also have significant overlap in the CNS [24]. These data provide evidence for feedback loops which permit crosstalk within the SNS control of WAT and BAT, and also for sensory information from one depot to influence sympathetic outflow to the alternate depot. Functional studies confirmed that the absence of SNS input to BAT is compensated by increasing sympathetic outflow to WAT in response to cold challenges [30]. Additional research demonstrated that lipolysis in WAT stimulates afferent sensory nerves and is sufficient to induce thermogenesis in BAT [33], confirming that WAT sensory activation can influence SNS outflow to BAT. Thus, these sensory nerves may sense products of lipolysis or consequences of sympathetically activated WAT. The nature of signals for adipose tissue sensory neurons remains unknown but it is predicted that the sensory information from adipose tissue could play an important role to influence adipocyte and depot size, inflammatory and metabolic responses [32].
3. Adipose tissue functions
3.1. BAT functions
The major function of BAT is to generate heat by burning energy and maintain body temperature [34]. Whether BAT thermogenesis also functions to get rid of excess energy has been a matter of intense debate over the last 40 years [5]. When BAT is activated, high amounts of lipids and glucose are combusted in this mitochondria-rich tissue. This fatty acidinduced thermogenesis depends on UCP1 [34], which is widely used as a marker for BAT. Following the influential paper from Rothwell and Stock on diet-induced thermogenesis [5], the initial excitement for BAT function to combat human obesity was soon dampened by the fact that only human newborns showed significant amounts of BAT while there was little support for significant amount of BAT in adult humans. However, over the last decade, metabolic imaging based on the use of an isotopic glucose analogue has provided conclusive evidence that functional BAT exists in adult humans [35], which has driven a renewed interest for the role of BAT in energy balance regulation in relation with obesity.
Recent studies have confirmed an additional and important secretory role of BAT (see [14] for a recent review). Briefly, so-called batokines can act locally in an autocrine and/or paracrine fashion to either enhance (bone morphogenetic proteins, fibroblast growth factors, prostaglandins and lipocalin D synthase, interleukin 6 and chemerin) or inhibit (soluble form of the LDL receptor sLR11, endocannabinoids and endothelin-1) thermogenesis and modulate other BAT functions [14, 36]. In addition to these autocrine and/or paracrine factors, BAT also interacts with endocrine systems where a sympathetic NA release induces type-2-iodothyronine-deiodinase (DIO2) activity to convert the thyroid prohormone thyroxine (T4) into its biologically active form triiodothyronine (T3), a major regulator of metabolism. DIO2 is present in brown adipocytes, but not white adipocytes [37], and BAT can be an important contributor of circulating T3 apart from the thyroid gland [38]. Importantly, T3 also contributes to BAT-induced adaptive thermogenesis by promoting UCP1 expression and other components of the thermogenic BAT machinery [39].
The role of cytokines in the regulation of BAT has only begun to be explored. They seem to be key functional regulators of insulin sensitivity and thermogenic adaptation to cold stress in healthy BAT [40]. Increased expression of some pro-inflammatory cytokines have been reported in BAT under obesogenic conditions in rodents, potentially to counteract the increase in thermogenesis, and BAT-derived anti-inflammatory cytokines have also been associated with heat production [14].
The strong correlation between increased BAT activity and protection against obesity, hyperglycemia and hyperlipidemia [41] suggests that manipulating BAT activity and mass would bring substantial clinical benefits and could lead to the development of new therapeutic tools to combat obesity and metabolic disorders.
3.2. WAT functions
Besides its fat storage capacity, WAT is now recognized as a metabolically dynamic endocrine organ that is capable of synthesizing hormones like leptin and adiponectin, and several cytokines that regulate metabolic and other diverse biological functions [42, 43]. For instance, leptin can induce UCP1 expression in BAT and WAT in an SNS-dependent manner [44, 45], in addition to its well-known anorexigenic function. Adiponectin, also mainly secreted by WAT, is regulated by long-term metabolic changes [46], and has impressive health-promoting characteristics [43, 47].
WAT can secrete other factors that increase insulin resistance (retinol binding protein-4, resistin, and visfatin) or improve insulin sensitivity, glucose uptake or storage of triglycerides (apelin, acylation stimulating protein) [43, 48]. The onset of obesity-associated insulin resistance is thought to be due to an altered production of adipokines and an increase in WAT pro-inflammatory cytokines [49]. This can partly be explained by an increased number of white adipocytes, but mainly by a greater number of infiltrating macrophages associated with the expansion of WAT in obesity [50]. The strong association between adipose tissue mass and the secretion of pro-inflammatory adipokines has led to the idea that inhibiting pro-inflammatory adipokines is a promising strategy for the treatment of obesity-related diseases.
Small non-coding RNA molecules, especially microRNAs (miRNAs) are also relevant for adipose tissue functions such as adipogenesis, differentiation, proliferation, and browning of WAT [51, 52]. Many studies have found that dysregulation of various miRNAs can have profound effects on BAT/WAT physiology including energy expenditure, insulin sensitivity, and lipolysis [51] (for a comprehensive review of individual miRNA function in adipose tissue, see [53]). These effects are mediated at different levels within adipocytes, influencing intracellular signaling (e.g. growth factor signaling), production and release of adipokines, (e.g. serum adiponectin levels) and crosstalk between adipocytes and immune cells [53]. miRNAs were shown to be secreted by exosomes into the circulation and thus are capable of acting in distant tissues [54]. Therapeutic use of miRNAs is thus a promising strategy and several miRNA constructs are already in development for the treatment of obesity and related conditions [52, 53].
The new view of BAT and WAT as endocrine organs has not been well integrated in our current understanding of adipose tissue innervation and central regulation of adipose tissue function. Much work remains to determine the role of the SNS in these diverse functions of adipose tissue, and neuromodulation will be an essential strategy to reach this goal. Undoubtedly, modulation of higher order sympathetic neurons in the brain may result in beneficial health effects, particularly if we view central “command neurons” as orchestrators of a pattern of sympathetic (and even parasympathetic) outflow to achieve a particular beneficial effect. However, with such potential advantages also come disadvantages, such as difficulty to selectively manipulate and rule out unwanted side effects. For the time being, peripheral neuromodulation seems to be more feasible because it is likely to have more selective functional effects and is easier to implement.
4. Peripheral neuromodulation of adipose tissue
One of the goals of the SPARC program is to generate comprehensive and detailed anatomical and functional maps of the peripheral nervous system, which can then be used for the design of neuromodulation strategies. For example, recently developed tools for targeted neuromodulation through genetically engineered channels and receptors, now widely used in the CNS of animal models, represent promising opportunities for peripheral neuromodulation in humans. We will therefore focus our discussion on the potential and limitations of old and new methods of peripheral neuromodulation.
4.1. Pharmacological manipulation
Getting rid of excess fat by increasing metabolic rate is not a new idea. For example, the chemical uncoupler dinitrophenol, the nonselective sympathomimetic ephedrine, and the serotonin-noradrenaline reuptake inhibitor sibutramine had been used for weight loss therapy, but their often severe and sometimes fatal side effects due to nonspecific functions caused them to be banned or highly regulated in many countries [55]. Other β-AR agonists can similarly increase energy expenditure and promote weight loss but share the same problem of unwanted side effects with the scope and severity of such side effects depending on the type of targeted receptors [56]. These pharmacological agents are technically not neuromodulators because they mostly act on cell surface receptors or intracellular components of end organs. However, β3-AR is a direct target of sympathetic BAT activation and the β3-AR agonist mirabegron, approved for treatment of overactive bladder in humans, effectively induces energy expenditure in humans without significant side effects, at least during the short-term test period [57]. This study also reassured that enhancing the sympathetic output to adipose tissue can be a feasible option to treat obesity and diabetes
4.2. Electrical manipulation
Electrical stimulation of the central and peripheral nervous system has been applied therapeutically for centuries. Several devices aiming to electrically modulate regions of the brain have been approved by the US Food and Drug Administration, including deep brain stimulation [58] and electroconvulsive therapy [59], both used clinically to treat depression and other psychiatric disorders. Electrical stimulation at the level of peripheral nerves is also currently used in patients [60]. Peripheral nerve and spinal cord stimulation are both efficient therapies to treat chronic pain via the implantation of a permanent stimulator under the skin [61]. Transcutaneous electrical nerve stimulation is also used for pain therapy as a noninvasive alternative [62].
Neuromodulation specifically targeting the vagus nerve has been used to treat a variety of conditions, including epilepsy, depression, migraines, Alzheimer and Parkinson diseases as well as inflammatory bowel disease, irritable bowel syndrome, rheumatoid arthritis, psoriasis [63, 64]. Sacral nerve stimulation is successfully used in patients to treat incontinence and constipation [65]. The drawbacks of implanted electrical stimulation systems are that they require invasive surgeries to ensure the proper placement and maintenance of a stimulator [66]. Furthermore, as is the case with most metal implants, neuro-stimulators usually prevent the use of magnetic resonance imaging. In general, electrical stimulation of peripheral nerves lacks specificity because they are typically composed of nerve fibers with many different functions. For example, the vagus nerve contains both sensory and motor fibers with a host of different functions [63]. More comprehensive and detailed maps will greatly improve the development of electro-neuromodulation therapies.
4.3. Genetics-based manipulation
Gene therapy has gained momentum as a method to treat human disease. The molecular tools for gene delivery have evolved and recombinant vectors based upon adeno-associated virus (AAV) have made significant progress toward clinical application [67, 68]. In fact, AAV-based gene therapy shows promising results in currently ongoing human clinical trials in the treatment of Parkinson disease [69, 70], choroideremia [71] and cardiovascular disease [72]. Thanks to a better biological understanding of these advanced tools, we are witnessing an unprecedented expansion of possibilities in genetics-based therapies. We will discuss some noteworthy breakthroughs and how they can be applied to stimulate adipose tissue.
4.3.1. Optogenetic manipulation
Neuromodulation mediated by optogenetics represents a novel gene therapy strategy that uses light as a stimulus to activate photosensitive channel proteins in order to affect neural activity. It has been widely used in the CNS of rodents since its development about a decade ago [73] and more recently applied to modulate various neural circuits in the periphery in rodents. For example, optogenetics was used to activate or inhibit motor neurons and muscle activity using an implantable light delivery system in freely moving animals [74, 75]. Application of optogenetics in the peripheral nervous system has also been successfully used to inhibit pain [76] and return vision to blind mice [77]. In a non-viral genetic approach, optogenetics was previously used to specifically activate sympathetic TH-fibers innervating iWAT [78]. This resulted in increased lipolysis (NA release, phosphorylation of hormone-sensitive lipase) and was sufficient to decrease the adipose tissue size.
The animal studies described above give a glimpse at future possibilities for human therapies, but there are several hurdles to be overcome for clinical applications of optogenetics, such as target specificity, safety, long-term effectiveness, and optimal light delivery [79]. In mice, a cell type- or tissue-specific opsin protein expression is achieved mainly by the transgenic Cre-LoxP system, but this is not applicable to humans or most other species. Within the CNS, this limitation has been overcome, e.g. in rat studies, by injecting viral constructs with gene expression under the control of a cell- or tissue-specific promoter [80–82].
While virally-driven genetic approaches are constantly developed and improved at a fast pace and with a greater precision, viral tools tailored for the peripheral nervous system have surprisingly lagged behind. One hindering factor is that autonomic ganglia (e.g. paravertebral sympathetic chain or prevertebral ganglia like the celiac and mesenteric ganglia) are difficult to target and typically consist of a mix of neurons that serve distinct peripheral organs. This can be circumvented by a direct delivery of virus into a peripheral end organ, as has been done in the muscle to express organ-specific opsin genes [75]. In this study, the muscle-injected virus retrogradely transduced motor neurons in the sacral spinal cord and their innervation of the muscle was stimulated optogenetically.
However, the direct delivery of virus into an end organ does not completely solve the problem of specificity because peripheral organs are normally innervated by multiple neuronal cell types. For example, adipose tissue is innervated by both sympathetic and sensory neurons and injecting AAV-ChR2 into adipose tissue may transduce both cell types. This issue can be resolved by driving ChR2 expression with a promoter/enhancer that is selectively active in sympathetic neurons, not in sensory neurons, or by engineering AAV capsid proteins to grant a specific tropism toward sympathetic nerve endings. Both strategies require detailed molecular understanding of neurons innervating adipose tissues, which is one of the main goals of the SPARC program.
Even after all molecular and genetic strategies have been figured out, many technical issues still have to be carefully assessed such as the most effective and safe virus titer, the most ideal frequency of viral injection, and the most efficient light delivery method. Recent advancement in optimal viral gene expression and wireless optical devices are expected to push forward optogenetics as a therapeutic option [83, 84]. Finally, non-human primate models for optogenetics-based neuromodulation would be an important step toward accelerating its application in humans [85].
4.3.2. Chemogenetic manipulation
Chemogenetic neuromodulation uses a small molecule ligand to activate synthetic G protein-coupled receptors, designer receptors exclusively activated by designer drugs (DREADDs), with various downstream consequences on neuronal excitability [86]. DREADDs were originally created by directed mutagenesis of human M3 muscarinic receptor (hM3) to achieve selective activation by the inactive clozapine analog clozapine-N-oxide but insensitivity by the endogenous ligand acetylcholine [87]. Chemogenetics has been applied in the periphery in one recent study in primary afferent nociceptors for sustained inhibition of pain [88]. The main advantage of chemogenetics over optogenetics is that it does not require an optical device, which may involve invasive surgeries, frequent battery changes, and high cost. Another advantage is that different flavors of DREADDs can modulate a variety of internal signaling that does not directly affect neuronal activity but rather adjust neuronal plasticity. This type of modulation can potentially be useful when a subtle change is more desirable than a blunt stimulation or inhibition of neurons. However, the use of a chemical ligand for receptor stimulation lacks temporal control of neuronal activity as opposed to optogenetics. The choice between optogenetics and chemogenetics would depend on whether the target organ and symptoms require an instant on-off control (optogenetics) or long-lasting effects (chemogenetics).
4.3.3. Radio- and magneto-genetic manipulation
Recently a new genetic way of controlling neural activity or gene expression was introduced, which has distinctive advantages over optogenetics and chemogenetics [89–91]. In this approach, an engineered heat- and mechano-sensing transient receptor potential vanilloid 1 (TRPV1) is co-expressed with iron-storing ferritin protein that is tethered to TRPV1. When low radio frequencies or magnetic fields are applied to cells expressing both TRPV1 and ferritin, TRPV1 opens up to allow Ca2+ entry into the cell for neuronal activation or transgene expression. By using deep-penetrating radio waves or magnets for channel activation, the invasive optical cable implantation of optogenetics can be avoided. Furthermore, the instant on-off capability and no requirement of chemical injection are advantages over chemogenetics. Drawbacks of this approach would be a relatively big expression cassette that may be too big to be packaged into AAV for safer and longer expression, and a potential problem with using radio waves or magnets in people with metal implants.
4.3.4. Other potential gene therapy strategies
In addition to delivering ChR2- or DREADD-expressing viral vectors into the fat-innervating sympathetic neurons, AAV-based gene therapy can be applied to enhance the sympathetic output to adipose tissue or to amplify β-AR signaling in adipocytes. The most direct and perhaps most effective way would be delivering UCP1 gene into iWAT. However, it requires further investigation whether simply over-expressing UCP1 in iWAT without inducing browning would be an effective long-term solution. Other potential targets for gene therapy could be genes involved in browning of white adipocytes, β-AR signaling, or synthesis and release of NA. Secreted molecules from other tissues that are known to stimulate BAT or promote WAT browning would also be worth consideration. With better understanding of adipose tissue browning and underlying neural circuits, we will have a greater repertoire at our disposal.
AAV-based gene expression levels may be hard to control and overexpression can potentially cause unexpected side effects while insufficient expression can render the therapy ineffective. Also, AAV-introduced genes may have a limited expression time, which would be problematic for human therapy where long-term cures would require efficient expression over decades.
In rare cases of monogenic obesity syndromes with a known genetic variance (e.g. leptin receptor deficiency), a recent innovation of gene editing, CRISPR/Cas9 may be applied to correct genetic errors (for detailed information about CRISPR/Cas9, see reference [92]). This method may even be used to introduce a genetic variance that is known to enhance BAT function or stimulate WAT browning to individuals with an increased risk of obesity as a preventive measure. By editing the endogenous genome permanently, CRISPR/Cas9 can overcome the longevity problem of the exogenous gene expression by viruses. In animal models, successful applications of the technology to congenital genetic diseases have already been reported [93, 94]. The most important task for human application of CRISPR/Cas9 would be minimizing off-target effects of guide RNAs [95, 96].
5. Conclusions
The re-discovery of significant depots of brown fat in humans and the potential for transforming white into brown-like fat has generated considerable interest in leveraging adipose tissue thermogenesis for treatment and prevention of obesity and metabolic diseases. Given the crucial role of sympathetic innervation in adipose tissue thermogenesis, it has become an important target for neuromodulation therapies. Although exciting recent progress has been made on characterizing cross-talk between sensory and sympathetic innervation of BAT and WAT, much work remains to anatomically and molecularly identify function-specific pre- and postganglionic neurons and making them accessible for peripheral neuromodulation. Genetics-based viral tools for whole-body visualization and mapping of peripheral sympathetic neuronal circuits innervating brown and white fat in mice are currently being developed. Genetics-based tools and techniques will also be important for transfection of specific pools of chemically identified pre- and postganglionic neurons selectively innervating BAT and WAT. This will open up the possibility for opto- and chemogenetic modulation to selectively modulate BAT thermogenesis, browning of white fat, and other functions of adipose tissue. Ultimately, novel gene therapy approaches should make sympathetic innervation of adipose tissue also accessible for neuromodulation in humans.
Highlights.
Neuromodulation of adipose tissue can be a new therapeutic strategy for obesity.
Genetics-based neuromodulation can reduce side effects with improved specificity.
AAV-based gene therapy is promising for genetics-based neuromodulation.
Acknowledgments
Funding: This work was supported by AHA053298N, DK020572-30, P20RR02195, 2P30DK072476-06, R01DK092587 (HM), R01DK047348 (HRB), 2P20GM103528 (SY), T32DK064584 (EQC), and 1OT2OD023864-01 (HM, HRB, SY). This work utilized the facilities of the Cell Biology and Bioimaging Core, supported in part by COBRE (NIH P20RR021945) and CNRU (NIH 1P30DK072476) center grants from the National Institutes of Health. Partial support was provided through the Animal Phenotyping Core supported through NIDDK NORC Center Grant 2P30DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ at the Pennington Biomedical Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflict of interest.
References
- 1.Polidori D, Sanghvi A, Seeley RJ, Hall KD. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity (Silver Spring) 2016;24(11):2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Hall KD. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring) 2016;24(8):1612–9. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–7. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, Heymsfield SB. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13(10):835–47. doi: 10.1111/j.1467-789X.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281(5726):31–5. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 6.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American journal of physiology. Endocrinology and metabolism. 2007;293(2):E444–52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 7.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Teule GJ. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360(15):1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Nuutila P. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 11.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 12.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 13.Bartness TJ, Ryu V. Neural control of white, beige and brown adipocytes. Int J Obes Suppl. 2015;5(Suppl 1):S35–9. doi: 10.1038/ijosup.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13(1):26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 15.Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22(4):546–59. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirsen C. Adrenergic Innervation of Adipose Tissue Examined by Fluorescence Microscopy. Nature. 1964;202:913. doi: 10.1038/202913a0. [DOI] [PubMed] [Google Scholar]
- 17.Paoletti R, Smith RL, Maickel RP, Brodie BB. Identification and physiological role of norepinephrine in adipose tissue. Biochemical and Biophysical Research Communications. 1961;5(6):424–429. [Google Scholar]
- 18.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275(1 Pt 2):R291–9. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 19.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6 Pt 2):R1569–78. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 20.Strack AM, Loewy AD. Pseudorabies virus: a highly specific transneuronal cell body marker in the sympathetic nervous system. J Neurosci. 1990;10(7):2139–47. doi: 10.1523/JNEUROSCI.10-07-02139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grkovic I, Anderson CR. Calbindin D28K-immunoreactivity identifies distinct subpopulations of sympathetic pre- and postganglionic neurons in the rat. J Comp Neurol. 1997;386(2):245–59. [PubMed] [Google Scholar]
- 22.Collins S, Yehuda-Shnaidman E, Wang H. Positive and negative control of Ucp1 gene transcription and the role of beta-adrenergic signaling networks. Int J Obes (Lond) 2010;34(Suppl 1):S28–33. doi: 10.1038/ijo.2010.180. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110(3):515–26. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 24.Ryu V, Watts AG, Xue B, Bartness TJ. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2017;312(3):R324–R337. doi: 10.1152/ajpregu.00456.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460(3):303–26. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 26.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(Suppl 1):S36–42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2014;306(6):R375–86. doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol. 1995;268(3 Pt 2):R744–51. doi: 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- 29.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306(12):R886–900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen NL, Barr CL, Ryu V, Cao Q, Xue B, Bartness TJ. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R132–R145. doi: 10.1152/ajpregu.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R501–11. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318(1–2):34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab. 2016;5(8):626–34. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 35.Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR, Ho KK. High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab. 2011;96(8):2450–5. doi: 10.1210/jc.2011-0487. [DOI] [PubMed] [Google Scholar]
- 36.Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15(4):277–87. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 37.Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 1983;305(5936):712–3. doi: 10.1038/305712a0. [DOI] [PubMed] [Google Scholar]
- 38.Silva JE, Larsen PR. Potential of brown adipose tissue type II thyroxine 5'-deiodinase as a local and systemic source of triiodothyronine in rats. J Clin Invest. 1985;76(6):2296–305. doi: 10.1172/JCI112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianco AC, Silva JE. Optimal response of key enzymes and uncoupling protein to cold in BAT depends on local T3 generation. Am J Physiol. 1987;253(3 Pt 1):E255–63. doi: 10.1152/ajpendo.1987.253.3.E255. [DOI] [PubMed] [Google Scholar]
- 40.Ortega MT, Xie L, Mora S, Chapes SK. Evaluation of macrophage plasticity in brown and white adipose tissue. Cell Immunol. 2011;271(1):124–33. doi: 10.1016/j.cellimm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology. 1996;137(1):21–9. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- 42.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60(3):329–39. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 43.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9(2):191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter R, Gettys TW. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140(10):4772–8. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 45.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140(1):292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 46.Palanivel R, Fang X, Park M, Eguchi M, Pallan S, De Girolamo S, Sweeney G. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75(1):148–57. doi: 10.1016/j.cardiores.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 48.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56(3):564–73. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 51.Price NL, Fernandez-Hernando C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim Biophys Acta. 2016;1861(12 Pt B):2104–2110. doi: 10.1016/j.bbalip.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Pan R, Pfeifer A. Regulation of brown and beige fat by microRNAs. Pharmacol Ther. 2017;170:1–7. doi: 10.1016/j.pharmthera.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Brandao BB, Guerra BA, Mori MA. Shortcuts to a functional adipose tissue: The role of small non-coding RNAs. Redox Biol. 2017;12:82–102. doi: 10.1016/j.redox.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whittle AJ, Lopez M, Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med. 2011;17(8):405–11. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Clapham JC, Arch JR. Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab. 2007;9(3):259–75. doi: 10.1111/j.1463-1326.2006.00608.x. [DOI] [PubMed] [Google Scholar]
- 57.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, Kolodny GM. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–8. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159–74. doi: 10.1097/YCT.0b013e3181e48165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakravarthy K, Nava A, Christo PJ, Williams K. Review of Recent Advances in Peripheral Nerve Stimulation (PNS) Curr Pain Headache Rep. 2016;20(11):60. doi: 10.1007/s11916-016-0590-8. [DOI] [PubMed] [Google Scholar]
- 61.Raphael JH, Raheem TA, Southall JL, Bennett A, Ashford RL, Williams S. Randomized double-blind sham-controlled crossover study of short-term effect of percutaneous electrical nerve stimulation in neuropathic pain. Pain Med. 2011;12(10):1515–22. doi: 10.1111/j.1526-4637.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- 62.Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4(3):197–209. doi: 10.2217/pmt.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29(3):493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25(3):208–21. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- 65.Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;(8):CD004464. doi: 10.1002/14651858.CD004464.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeon YH. Spinal cord stimulation in pain management: a review. Korean J Pain. 2012;25(3):143–50. doi: 10.3344/kjp.2012.25.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583–93. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wirth T, Parker N, Yla-Herttuala S. History of gene therapy. Gene. 2013;525(2):162–9. doi: 10.1016/j.gene.2013.03.137. [DOI] [PubMed] [Google Scholar]
- 69.Feigin A, Kaplitt MG, Tang C, Lin T, Mattis P, Dhawan V, Eidelberg D. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104(49):19559–64. doi: 10.1073/pnas.0706006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 71.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammoudi N, Ishikawa K, Hajjar RJ. Adeno-associated virus-mediated gene therapy in cardiovascular disease. Curr Opin Cardiol. 2015;30(3):228–34. doi: 10.1097/HCO.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18(9):1213–25. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liske H, Towne C, Anikeeva P, Zhao S, Feng G, Deisseroth K, Delp S. Optical inhibition of motor nerve and muscle activity in vivo. Muscle Nerve. 2013;47(6):916–21. doi: 10.1002/mus.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One. 2013;8(8):e72691. doi: 10.1371/journal.pone.0072691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol. 2014;32(3):274–8. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, Huberman AD. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci. 2016;19(8):1073–84. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Domingos AI. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163(1):84–94. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji ZG, Ishizuka T, Yawo H. Channelrhodopsins-Their potential in gene therapy for neurological disorders. Neurosci Res. 2013;75(1):6–12. doi: 10.1016/j.neures.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Brown AJ, Fisher DA, Kouranova E, McCoy A, Forbes K, Wu Y, Cui X. Whole-rat conditional gene knockout via genome editing. Nat Methods. 2013;10(7):638–40. doi: 10.1038/nmeth.2516. [DOI] [PubMed] [Google Scholar]
- 81.Schonig K, Weber T, Frommig A, Wendler L, Pesold B, Djandji D, Bartsch D. Conditional gene expression systems in the transgenic rat brain. BMC Biol. 2012;10:77. doi: 10.1186/1741-7007-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H, Huang C, Yang M, Landel CP, Xia PY, Liu YJ, Xia XG. Developing tTA transgenic rats for inducible and reversible gene expression. Int J Biol Sci. 2009;5(2):171–81. doi: 10.7150/ijbs.5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park SI, Brenner DS, Shin G, Morgan CD, Copits BA, Chung HU, Rogers JA. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol. 2015;33(12):1280–1286. doi: 10.1038/nbt.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Poon AS. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods. 2015;12(10):969–74. doi: 10.1038/nmeth.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14(3):387–97. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- 87.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(12):5163–8. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iyer SM, Vesuna S, Ramakrishnan C, Huynh K, Young S, Berndt A, Delp SL. Optogenetic and chemogenetic strategies for sustained inhibition of pain. Sci Rep. 2016;6:30570. doi: 10.1038/srep30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science. 2012;336(6081):604–8. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Friedman JM. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531(7596):647–50. doi: 10.1038/nature17183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stanley SA, Sauer J, Kane RS, Dordick JS, Friedman JM. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat Med. 2015;21(1):92–8. doi: 10.1038/nm.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang F, Doudna JA. CRISPR-Cas9 Structures and Mechanisms. Annu Rev Biophys. 2017;46:505–529. doi: 10.1146/annurev-biophys-062215-010822. [DOI] [PubMed] [Google Scholar]
- 93.Ruan GX, Barry E, Yu D, Lukason M, Cheng SH, Scaria A. CRISPR/Cas9-Mediated Genome Editing as a Therapeutic Approach for Leber Congenital Amaurosis 10. Mol Ther. 2017;25(2):331–341. doi: 10.1016/j.ymthe.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bengtsson NE, Hall JK, Odom GL, Phelps MP, Andrus CR, Hawkins RD, Chamberlain JS. Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun. 2017;8:14454. doi: 10.1038/ncomms14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Root DE. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34(2):184–91. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–6. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]