Abstract
Magnetic resonance elastography (MRE) is a quantitative imaging technique for noninvasively characterizing tissue mechanical properties, and has recently emerged as a valuable tool for neuroimaging. The measured mechanical properties reflect the microstructural composition and organization of neural tissue, and have shown significant effects in many neurological conditions and normal, healthy aging, and evidence has emerged supporting novel relationships between mechanical structure and cognitive function. The sensitivity of MRE to brain structure, function, and health make it an ideal technique for studying the developing brain; however, brain MRE studies on children and adolescents have only just begun. In this article, we review brain MRE and its findings, discuss its potential role in developmental neuroimaging, and provide suggestions for researchers interested in adopting this technique.
Keywords: Elastography, Development, Brain, Stiffness, Viscoelasticity
1. Introduction
The advent of novel neuroimaging techniques, including magnetic resonance elastography (MRE (Muthupillai et al., 1995)), provides researchers with a powerful tool to noninvasively investigate the mechanical properties of the human brain. MRE probes tissue stiffness and viscoelasticity, providing measures of the integrity of the underlying neural microstructure, and is a sensitive measure of brain tissue structure (Sack et al., 2013). Over the past several years, MRE has begun to receive attention in clinical neuroimaging research as well as in the study of normal aging (Hiscox et al., 2016). However, until recently, MRE methods lacked sufficient resolution necessary to accurately examine specific neuroanatomical structures in the brain, and thus could not contribute to examination of specific structure–function relationships. Researchers have recently developed new MRE techniques that allowed us, for the first time, to collect in vivo measurement of local variations in mechanical properties of specific subregions of the human brain, including subcortical regions (Johnson et al., 2016a). Using these new methods, studies have begun to emerge linking mechanical properties of the brain with functional performance (Schwarb et al., 2016), and many labs have shown strong developmental changes in tissue viscoelasticity, albeit all focused on the aging brain and cognitive decline (Arani et al., 2015, Sack et al., 2011). Despite these exciting advances in the field, MRE has not yet received attention in young developmental populations, although several groups, including our own, have begun to collect MRE data in children and adolescents. In the current manuscript, we will describe the benefits of MRE for examining developmental changes in brain tissue and offer readers important methodological considerations. Few universities currently have equipment for acquiring MRE. The lead author has developed protocols for collecting brain MRE data in developmental, aging, and clinical populations, and we will provide logistical considerations for setting up and acquiring MRE data.
2. Magnetic resonance elastography of the brain
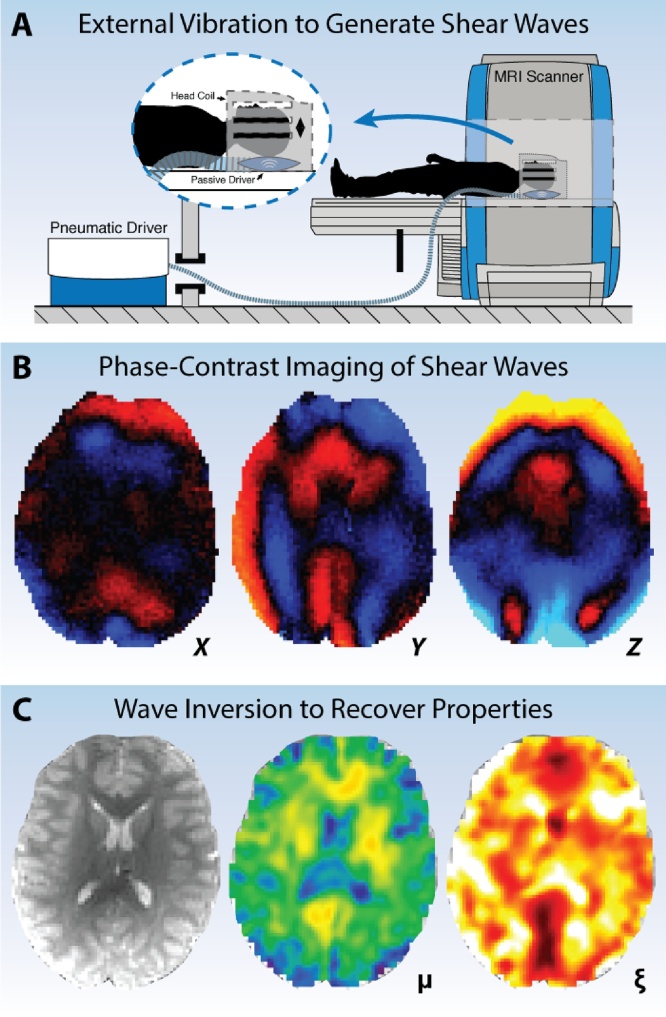
MRE is a technique widely applied to many organs in the human body (Manduca et al., 2001, Mariappan et al., 2010, Glaser et al., 2012), particularly the liver, where MRE is one of the most accurate and robust methods for characterizing chronic liver disease (Venkatesh et al., 2013, Yin et al., 2016). Each MRE exam, regardless of tissue, requires the same basic experimental elements (Fig. 1): the tissue is vibrated by an external mechanical actuator; the resulting deformations are encoded through MRI images using customized phase-contrast pulse sequences; and these deformations are “inverted” to recover the underlying mechanical properties of tissue. Brain MRE utilizes the same basic elements, but the complex tissue architecture of the brain and its protection from deformation via the skull require more advanced experimental techniques (Hiscox et al., 2016). These include actuators that vibrate the entire head to induce shear deformation; MRE pulse sequences capable of capturing full vector wave fields at high spatial resolution; and inversion algorithms that account for significant material heterogeneity.
Fig. 1.
Overview of MRE data acquisition and processing: (A) An external mechanical actuator is used to lightly vibrate the head and generate shear deformations in brain tissue; (B) specialized phase-contrast MRI sequences image the resulting deformation through synchronization with applied vibration; and (C) an inversion algorithm is used to calculate the mechanical property distribution from imaged wave field.
The first brain MRE studies were published nearly a decade ago (Kruse et al., 2008, Sack et al., 2008, Green et al., 2008) and sought to provide baseline values for the global stiffness of the healthy adult human brain. Despite initial studies showing a lack of agreement between reported properties, recent years have found highly reliable property measures and a convergence on a common set of properties in the normal brain, even across different experimental methodologies and reported viscoelastic measures. In general, MRE recovers the complex shear modulus, G = G′ + iG′′, where G′ is the storage modulus reflecting elasticity, and G′′ is the loss modulus reflecting viscosity. The shear stiffness, , is a commonly reported composite parameter (Manduca et al., 2001), and in the range of 50–60 Hz vibration frequency the stiffness of the cerebrum is approximately 3 kPa (Hiscox et al., 2016). The viscous behavior of tissue is often reported as the dimensionless damping ratio, ξ = G′′/2G′ (McGarry and Van Houten, 2008), or phase angle, (Sack et al., 2013), though G′′ is a more challenging measure to recover (McGarry et al., 2012, Dittmann et al., 2016) and thus there is no current consensus value for brain tissue viscosity measured with MRE.
Recent developments in MRE technology have focused on achieving reliable property measures localized to specific regions of interest. Murphy et al. developed a pipeline to robustly characterize the stiffness of cerebral lobes and the cerebellum (excluding cortical gray matter) with measurement variation on the order of 2% (Murphy et al., 2013). They found differences in stiffness between several lobes, and between the cerebrum and the cerebellum (2.99 kPa vs. 2.38 kPa), which echoes a previous finding of the cerebellum being softer (Zhang et al., 2011). Johnson et al. reported reliable measurements of the corpus callosum and corona radiata white matter tracts (variation 5–8%), and found different elastic and viscous properties reflective of expected microstructure (Johnson et al., 2013a). Other reports have characterized white matter tracts (Guo et al., 2013, Anderson et al., 2016, Fehlner et al., 2015), including the corticospinal tracts using an anisotropic inversion algorithm (Romano et al., 2012).
Using a higher resolution imaging sequence (Johnson et al., 2014) and inversion algorithm designed to improve accuracy of local properties in small regions (McGarry et al., 2013), Johnson et al. recently reported the first reliable MRE measurements in subcortical gray matter structures (variation 3–7%), including the hippocampus and thalamus. This approach allowed for the first observation of a structure-function relationship using MRE (see section below) (Schwarb et al., 2016). This finding highlights the continued push for novel technologies capable of improving accuracy and reliability of viscoelastic measures of specific neuroanatomical structures differentially affected by disease, determine aspects of behavior and cognitive function, or exhibit unique trajectories during development. Despite early reports of differences between white matter and gray matter (primarily cortical gray matter) (Kruse et al., 2008, Green et al., 2008, Zhang et al., 2011, Johnson et al., 2013a), the thin structure of the cortex and its proximity to cerebrospinal fluid likely contaminate these measures. There have been efforts towards examining cortical gray matter (McGarry et al., 2013, Braun et al., 2014), though this remains an open area of investigation.
2.1. MRE studies of neurodegeneration
The overwhelming majority of brain MRE studies have considered the adult brain, and specifically have sought to identify signatures related to neurodegeneration through neural tissue mechanics. These studies have consistently found a softening of brain tissue (i.e. loss of viscoelasticity) in many neurological conditions, including Alzheimer’s disease (Murphy et al., 2011, Murphy et al., 2016), multiple sclerosis (Wuerfel et al., 2010, Streitberger et al., 2012, Fehlner et al., 2016), Parkinson’s disease (Lipp et al., 2013), and amyotrophic lateral sclerosis (Romano et al., 2014).
Three major takeaways from these MRE studies of neurodegeneration are (1) the relatively large differences between healthy and diseased brains; (2) the progressive nature of the softening effect with disease stage; and (3) the region-specific patterns of softening. Wuerfel, Streitberger et al. found that the brain is 12.7% softer than healthy in relapsing-remitting multiple sclerosis (Wuerfel et al., 2010) and 20.5% softer in progressive multiple sclerosis (accompanied with a 6.1% reduction in viscosity) (Streitberger et al., 2012). In a study of Alzheimer’s disease, Murphy et al. found a global brain softening of 7.2% compared to cognitively normal healthy controls (Murphy et al., 2011). In follow-up studies, Murphy et al. demonstrated that this softening is primarily in the frontal, temporal, and parietal lobes (Murphy et al., 2016), and Huston et al. showed that this pattern is different in frontotemporal dementia, which shows softening in only the frontal and temporal lobes (Huston et al., 2016).
MRE studies reporting significant effects in clinical populations speak to the sensitivity of tissue mechanics to microstructural health, and suggests a role for characterizing the healthy brain. Several groups have reported brain MRE measures in aging and found softening on the order of 0.3-1.0% per year (Arani et al., 2015, Sack et al., 2011, Sack et al., 2009), along with regional variability in softening rate and evidence of sexual dimorphism. This softening reflects the natural degradation of brain tissue in aging through similar processes accompanying the neurological conditions described above. This strong age-related change is equal to or greater than changes in other structural imaging measures, such as volume of gray matter regions (Kennedy et al., 2009) and diffusivity metrics from diffusion tensor imaging (DTI) (Davis et al., 2009, Lebel et al., 2012). The potentially greater sensitivity of MRE measures are likely due to the more direct relationship with microstructure than volumetrics, and thus tissue pathophysiology, and the reflection in mechanics of both axonal and extra-axonal contributions to structural integrity, unlike DTI. However, further research is needed to determine the relationships between MRE and other structural neuroimaging metrics, both in aging populations and developing populations.
2.2. Microstructural basis of mechanical properties
The mechanical properties of the brain measured with MRE reflect the behavior of the tissue as a microscale composite, and through that are sensitive to the microstructural health of tissue. In particular, elastic tissue properties are generally reflective of contributions from individual tissue components, e.g. axons, and their mechanical properties, while viscous properties arise from geometric organization of components and their network complexity (Sack et al., 2013, Posnansky et al., 2012, Guo et al., 2012). However, directly relating aspects of microstructure to observed properties is a challenging problem from both modeling and experimental mechanical testing perspectives. Much of what is known about the relationship between MRE measures and microstructure come from in vivo measurements on animal models of disease that are then compared with ex vivo histology of tissue slices.
In a study of cuprizone-induced toxic demyelination in mice, Schregel et al. observed relative decreases in both stiffness and phase angle over time that was partially reversible upon stopping the cuprizone diet (Schregel et al., 2012). By comparing the MRE results with histological samples, they attributed the viscoelasticity alterations to myelination and extracellular matrix remodeling, and particularly highlight that phase angle decrease may be specific to demyelination. In studies of mice with experimental autoimmune encephalomyelitis (EAE), a neuroinflammatory model, Riek, Millward et al. found reduced brain stiffness that correlated with indices of inflammation, but not reduced phase angle (Riek et al., 2012, Millward et al., 2015). This finding was also echoed in a murine stroke model (Freimann et al., 2013). Contrasting these findings with DTI studies of cuprizone mice, which are able to detect demyelination but are confounded by inflammation (Song et al., 2005; Sun et al., 2006; Wang et al., 2011), the results from initial MRE studies suggest that the mechanical contrast may be specific to both axonal and extra-axonal elements of brain tissue microstructure.
2.3. Evidence of structure-function relationships
Based on the relationship between tissue mechanics and microstructure, the large softening effects in neurological conditions associated with cognitive impairments, and the age-related decline in tissue viscoelasticity, we expect these properties to exhibit a relationship with cognitive function and behavior. Schwarb et al. recently used MRE to measure hippocampal viscoelasticity in healthy young adult males (age 18–33) and found a strong correlation with memory function (Schwarb et al., 2016). Specifically, hippocampal damping ratio correlated significantly with performance on a spatial reconstruction task of relational memory (Watson et al., 2013; Monti et al., 2015). This was the first observation of any relationship between MRE measures and cognition. Importantly, this relationship was stronger than the relationship between memory and hippocampal volume, thus reinforcing the idea that tissue viscoelasticity is a more direct and more sensitive measure of structural integrity.
Recent work has replicated this finding in a larger group of both young men and young women (Schwarb et al., 2017). In addition to replicating the viscoelasticity relationship, and lack of volume relationship, this study also found that hippocampal viscoelasticity mediates the relationship between aerobic fitness and memory performance. This is an exciting development, as fitness and exercise are known to improve brain structure and function in older adults and children (Hillman et al., 2008, Stillman et al., 2016), and MRE may provide a sensitive window into the mechanisms of this relationship. This finding was buttressed by a study of an exercise intervention for adults with multiple sclerosis, who exhibited improvements in memory and corresponding changes in hippocampal viscoelasticity, both an increase in stiffness and decrease in damping ratio, with training (Sandroff et al., 2017). Ultimately, this relationship with fitness and exercise points to MRE measures reflecting elements of neurogenesis, which exercise is known to promote (van Praag, 2008), and which has been observed with MRE in mice, albeit only as a transient effect (Klein et al., 2014, Hain et al., 2016). Further research aimed at examining and reconciling these exciting findings is needed.
3. Magnetic resonance elastography in developmental populations
Development involves constantly changing dynamical systems (Novak and Pelaez, 2004). Gottlieb defined this process as being “characterized by an increase of complexity or organization—that is, the emergence of new structural and functional properties and competencies—at all levels of analysis (molecular, subcellular, cellular, organismic) as a consequence of horizontal and vertical coactions among its parts, including organism-environment coactions” (Gottlieb, 1997). Contemporary theories stress that relative plasticity exists across the life span, although the magnitude of this plasticity may vary across ontogeny (Lerner, 1984). While developmental methods have been applied extremely successfully using longitudinal methods in large samples to examine linear and nonlinear growth trajectories across large age spans, this work has almost exclusively focused on behavioral measures, such as the National Longitudinal Study of Adolescent to Adult Health (Add Health, www.cpc.unc.edu/projects/addhealth) and the NICHD Study of Early Child Care and Youth Development (SECCYD, www.nichd.nih.gov/research/supported/Pages/seccyd.aspx). Large, longitudinal developmental studies that incorporate neuroimaging measures are just beginning to emerge, such as The Adolescent Brain and Cognitive Development Study (ABCD, www.abcdstudy.org).
Over the past two decades, there has been an explosion of research examining the developing human brain. Contrary to early beliefs that the brain undergoes most development prior to the adolescent years, we now know that brain development is protracted, extending into the third decade of life. Growing access to large-scale, longitudinal neuroimaging datasets have significantly increased our understanding of structural brain development (Mills and Tamnes, 2014). For instance, cortical gray matter volume tends to be greatest in childhood and decreases throughout adolescence, whereas white matter volume increases across childhood and adolescence and into early adulthood (Mills and Tamnes, 2014, Lebel and Beaulieu, 2011). Studies examining different cortical and subcortical brain regions find significant heterogeneity in developmental timing, with parietal and occipital gray matter volume showing a linear decline from childhood through adolescence, the caudate, putamen, and nucleus accumbens decreasing in volume, and the amygdala and hippocampus showing little change or even increasing in volume during this period (Mills and Tamnes, 2014). Moreover, striatal and thalamic subcortical regions show protracted volumetric maturation peaking after cortical volume has already begun to decline, with males and females showing different patterns of brain development in both absolute volume and timing (Raznahan et al., 2014). White matter development is also regionally specific, with diffusion imaging studies showing that fronto-temporal connections develop more slowly than other tracts, with the cingulum showing particularly protracted development (Lebel et al., 2012). This work has revolutionized our understanding of the human brain, and highlighted the continued development of cortical and subcortical brain structures well into the second and third decade of life (Giedd and Rapoport, 2010). Although being roughly equal in size, the brains of children, adolescents, and adults differ in complex ways, ranging from structural to functional to network-level differences (Somerville, 2016). Indeed, the developing nervous system is qualitatively different from the adult nervous system, and many of the neural changes that occur in the first two decades of life set the stage for later neurocognitive functions (Deary et al., 2006, Kochunov et al., 2012) and neuropsychiatric disorders (Gogtay et al., 2011, Paus et al., 2008). Thus, understanding normative ontogeny of neural development will be key for understanding when and how development goes awry (i.e. psychopathology and disease) and how decline occurs. Although significant advances have been made in the field of developmental neuroscience, many questions regarding healthy brain development remain.
The trajectory of brain development occurs in many stages, with different brain regions showing unique courses of ontogeny, and such development occurs differently across structural, functional, and network level indices. When examining different brain indices, or different brain regions, developmental maturity (i.e. when neural measures reach an asymptote) occurs at different ages. For instance, resting state functional connectivity of the whole-brain asymptotes in the mid 20s, cortical thickness values asymptote in the early 20 s (parietal cortex) or mid 20 s (frontal cortex), and fractional anisotropy asymptotes in the late 20 s (parietal cortex) or even mid 30 s (frontal cortex; reviewed in (Somerville, 2016)). These findings highlight that different neural indices reveal maturity of different brain regions at very different ages, underscoring the need for multimodal techniques in understanding brain maturation.
Although significant work has begun to unpack the developing brain via gray and white matter changes as well as functional network level changes, no published work has used MRE on children, a population of particular interest because of rapid brain development, or adolescence, a population that shows rates of behavioral and neural change that parallel early postnatal development (Spear, 2007, Spear, 2000). Nonetheless, studies have emerged revealing significant changes in mechanical properties with microstructural health in aging populations (Arani et al., 2015, Sack et al., 2011), underscoring the usefulness of MRE for examining neurodevelopmental processes. Therefore, the time is ripe to extend MRE methods into developmental neuroscience research in light of evidence indicating rapid brain growth and heightened plasticity to environmental contexts during childhood and adolescence. Longitudinal research of healthy brain development should combine high quality multi-sequence, multi-modal MR (structural MRI, magnetic resonance spectroscopy (MRS), DTI, and MRE) measures to carefully unpack developmental trajectories in brain growth and decline. Ultimately, MRE can extend and complement our understanding of human brain development and provide a sensitive measure to studying how the brain matures.
4. Adding MRE to developmental neuroimaging protocols
For researchers interested in adding brain MRE to developmental neuroimaging protocols, we would like to stress the importance of several experimental considerations: (1) simplicity of mechanical actuation system; and (2) choice of imaging and inversion scheme. While all MRE protocol decisions involve similar factors, speed and robustness for imaging a potentially challenging population are critically important.
4.1. Mechanical actuation systems
There are several different categories of mechanical actuators used in brain MRE experiments, however the Resoundant pneumatic driver system (resoundant.com, Resoundant, Inc., Rochester, MN) has become one of the most popular in recent years (Arani et al., 2015, Murphy et al., 2013, Murphy et al., 2011, Murphy et al., 2016). The Resoundant system includes an active driver in the MRI control room coupled with a passive driver that delivers vibrations to the body part of interest via pneumatic hose. For brain MRE, this passive driver is a “pillow” that the head rests on, and results in a subtle up-down motion when active. This pillow replaces the typical pad used in the MRI head coil, is simple to set up by researchers and technicians, and is comfortable enough to be rested on throughout the entire protocol lasting 1–2 h, based on our experience with adolescents. Further, the pillow driver does not interfere with visual stimulus presentation with projection or goggle systems, thus allowing for MRE to be added to protocols with fMRI scans without the need to change equipment during a scan session. The Resoundant system with passive drivers suitable for the abdomen is commercially available through all major MRI vendors, and the pillow driver for the head is expected to be available soon. Other types of mechanical actuation systems are in use for brain MRE that may provide advantages over the Resoundant in terms of quality of applied motion, though definitive comparison studies are lacking, and actuators designed specifically for pediatric populations should be an avenue for future development in the community.
4.2. Imaging and inversion schemes
Choosing imaging and inversion schemes are critical as they determine the reliability of local and regional mechanical property measures. The single-shot echo-planar imaging (EPI) sequence is the most common for brain MRE (Murphy et al., 2013; Zhang et al., 2011; Guo et al., 2013) as it is fast, robust, and flexible, though limited in spatial resolution due to artifacts arising from magnetic field inhomogeneity. Coupled with a brain-specific, direct inversion algorithm, an EPI sequence with 3 mm isotropic resolution is capable of providing reliable estimates of the global brain properties, lobes of the cerebrum, and the cerebellum (Murphy et al., 2013). This approach has been used to find regional softening effects in specific lobes in aging and dementia (Arani et al., 2015, Murphy et al., 2016, Huston et al., 2016). An EPI-based brain MRE imaging and inversion package is expected to be available soon on all major MRI platforms.
An alternative to the single-shot EPI-based MRE sequences are the multishot spiral MRE sequences (Johnson et al., 2014; Johnson et al., 2013b; Johnson et al., 2016b) that overcome the distortion limitation by shortening data readout, and thus allowing greater spatial resolutions. Our team has used these sequences in conjunction with the nonlinear inversion algorithm (NLI) (McGarry et al., 2012; McGarry et al., 2013; Van Houten et al., 2011; Tan et al., 2017) as we seek to improve the ability of MRE to mechanically map the human brain, and have reported on the ability to obtain reliable local measures of neuroanatomical structures, including white matter tracts (Johnson et al., 2013a, Anderson et al., 2016) and subcortical gray matter structures (Johnson et al., 2016a). NLI is a finite-element based algorithm that has the potential for generating high-resolution mechanical property maps through its formulation of the nonhomogeneous Navier’s equation that avoids the local homogeneity assumption central to most other inversion approaches. However, the regularization needed to stabilize the results in the presence of noise and model-data mismatch currently keeps the results from achieving a spatial resolution equal to the nominal imaging resolution. The resulting property map resolution from NLI has been estimated as approximately half that of the imaging resolution (McGarry, 2013), highlighting the importance of acquiring high-resolution MRE data. Another approach that seeks to improve the spatial resolution of MRE property maps is the multifrequency dual elasto visco (MDEV) inversion (Hirsch et al., 2013), which uses wave fields at multiple frequencies to overcome the limitations of algebraically inverting displacement data with a single spatial frequency, specifically the instabilities at boundaries due to the local homogeneity assumption. This approach generates property maps with sharp features and has shown success in characterizing several subcortical structures (Guo et al., 2013; Lipp et al., 2013; Hetzer et al., 2017), however a thorough examination of the reliability and robustness of these features has yet to be reported.
The imaging sequences and inversion algorithms described above are continuously under development, and are currently available only through collaborations or research agreements with developers. We strongly encourage researchers interested in adopting such techniques to contact developers directly for assistance.
4.3. Specific considerations for developmental populations
The biggest challenge associated with adopting MRE in a developmental neuroimaging protocol is the acquisition time, which is generally between 5 and 10 min, and may be too long for children who could move during the scan and corrupt data. MRE is a motion-sensitive technique, and unwanted subject motion can cause phase errors that appear as inconsistent phase between volumes (e.g. the common inter-slice phase discontinuity artifact (Murphy et al., 2012)) and signal loss in multishot sequences (Johnson et al., 2013b). While strategies for correcting the effects of small motions exist (Johnson et al., 2014, Johnson et al., 2013b, Fehlner et al., 2017), large motions that might be expected towards the end of a long scan as a child becomes restless are likely to appear as corrupted and unusable images, such as those encountered in DTI (Gallichan et al., 2009).
The need to keep scans short challenges the ability to acquire high-resolution MRE images and thus the ability to extract reliable measures from specific brain structures. One approach to mitigate this is to adopt a data sampling acceleration strategy, such as SLIM, which can reduce scan times by up to 3 x (Klatt et al., 2015, Klatt et al., 2013). In general, we recommend measuring data quality by monitoring subject motion and calculating the octahedral shear strain-based signal-to-noise ratio (OSS-SNR) (McGarry et al., 2011) to determine whether an acquisition is successful. Additionally, it is recommended to investigate repeatability, especially when using a new protocol, as this is the most common way to characterize measurement reliability (Johnson et al., 2016a, Murphy et al., 2013, Johnson et al., 2013a).
5. Conclusions
Despite the relative novelty of MRE in the neuroimaging toolbox, the observations of brain viscoelasticity changes in neurological disease and normal aging highlight the potential of mechanical properties as a valuable markers of neural tissue structure, function, and health. The inherent sensitivity of MRE measures to microstructural composition and organization make it an attractive technique to apply to the study of the developing brain. We encourage neurodevelopmental researchers to consider adopting MRE in imaging protocols as mechanical properties could provide new insights to microstructural aspect of brain maturation, and contributions to behavior and cognitive function through childhood, adolescence, and into adulthood.
Conflict of Interest
None.
Acknowledgments
CLJ receives support from the University of Delaware Research Foundation, from the Delaware INBRE program with a grant from the National Institutes of General Medical Sciences (P20-GM103446) from the National Institutes of Health and the State of Delaware, and from Delaware CTR ACCEL Program with a grant from the National Institutes of General Medical Sciences (U54-GM104941) from the National Institutes of Health. EHT receives support from the Department of Psychology and Neuroscience at UNC-CH, with a grant from the National Science Foundation (SES-1459719) and National Institutes of Health (R01-DA039923).
Contributor Information
Curtis L. Johnson, Email: clj@udel.edu.
Eva H. Telzer, Email: ehtelzer@unc.edu.
References
- Anderson A.T., Van Houten E.E.W., McGarry M.D.J., Paulsen K.D., Holtrop J.L., Sutton B.P., Georgiadis J.G., Johnson C.L. Observation of direction-Dependent mechanical properties in the human brain with multi-Excitation MR elastography. J. Mech. Behav. Biomed. Mater. 2016;59:538–546. doi: 10.1016/j.jmbbm.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arani A., Murphy M.C., Glaser K.J., Manduca A., Lake D.S., Kruse S.A., Jack C.R., Ehman R.L., Huston J. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage. 2015;111:59–64. doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Guo J., Lutzkendorf R., Papazoglou S., Hirsch S., Sack I., Bernarding J. High-resolution mechanical imaging of the human brain by three-dimensional multifrequency magnetic resonance elastography at 7T. Neuroimage. 2014;90:308–314. doi: 10.1016/j.neuroimage.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Davis S.W., Dennis N.A., Buchler N.G., White L.E., Madden D.J., Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I.J., Bastin M.E., Pattie A., Clayden J.D., Whalley L.J., Starr J.M., Wardlaw J.M. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Dittmann F., Hirsch S., Tzschätzsch H., Guo J., Braun J., Sack I. In vivo wideband multifrequency MR elastography of the human brain and liver. Magn. Reson. Med. 2016;76:1116–1126. doi: 10.1002/mrm.26006. [DOI] [PubMed] [Google Scholar]
- Fehlner A., Papazoglou S., McGarry M.D.J., Paulsen K.D., Guo J., Streitberger K.-J., Hirsch S., Braun J., Sack I. Cerebral multifrequency MR elastography by remote excitation of intracranial shear waves. NMR Biomed. 2015;28:1426–1432. doi: 10.1002/nbm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlner A., Behrens J.R., Streitberger K.-J., Papazoglou S., Braun J., Bellmann-Strobl J., Ruprecht K., Paul F., Wuerfel J., Sack I. Higher-resolution MR elastography reveals early mechanical signatures of neuroinflammation in patients with clinically isolated syndrome. J. Magn. Reson. Imaging. 2016;44:51–58. doi: 10.1002/jmri.25129. [DOI] [PubMed] [Google Scholar]
- Fehlner A., Hirsch S., Weygandt M., Christophel T., Barnhill E., Kadobianskyi M., Braun J., Bernarding J., Lutzkendorf R., Sack I., Hetzer S. Increasing the spatial resolution and sensitivity of magnetic resonance elastography by correcting for subject motion and susceptibility-induced image distortions. J. Magn. Reson. Imaging. 2017;46:134–141. doi: 10.1002/jmri.25516. [DOI] [PubMed] [Google Scholar]
- Freimann F.B., Müller S., Streitberger K.-J., Guo J., Rot S., Ghori A., Vajkoczy P., Reiter R., Sack I., Braun J. MR elastography in a murine stroke model reveals correlation of macroscopic viscoelastic properties of the brain with neuronal density. NMR Biomed. 2013;26:1534–1539. doi: 10.1002/nbm.2987. [DOI] [PubMed] [Google Scholar]
- Gallichan D., Scholz J., Bartsch A., Behrens T.E.J., Robson M.D., Miller K.L. Addressing a systematic vibration artifact in diffusion-weighted MRI. Hum. Brain Mapp. 2009;31:193–202. doi: 10.1002/hbm.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser K.J., Manduca A., Ehman R.L. Review of MR elastography applications and recent developments. J. Magn. Reson. Imaging. 2012;36:757–774. doi: 10.1002/jmri.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Vyas N.S., Testa R., Wood S.J., Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr. Bull. 2011;37:504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Erlbaum; Mahwah, NJ: 1997. Synthesizing Nature-Nurture. Prenatal Roots of Instinctive Behavior. [Google Scholar]
- Green M.A., Bilston L.E., Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21:755–764. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- Guo J., Posnansky O., Hirsch S., Scheel M., Taupitz M., Braun J., Sack I. Fractal network dimension and viscoelastic powerlaw behavior: II: an experimental study of structure-Mimicking phantoms by magnetic resonance elastography. Phys. Med. Biol. 2012;57:4041–4053. doi: 10.1088/0031-9155/57/12/4041. [DOI] [PubMed] [Google Scholar]
- Guo J., Hirsch S., Fehlner A., Papazoglou S., Scheel M., Braun J., Sack I. Towards an elastographic atlas of brain anatomy. PLoS One. 2013;8:e71807. doi: 10.1371/journal.pone.0071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain E.G., Klein C., Munder T., Braun J., Riek K., Mueller S., Sack I., Steiner B. Dopaminergic neurodegeneration in the mouse is associated with decrease of viscoelasticity of substantia nigra tissue. PLoS One. 2016;11:e0161179. doi: 10.1371/journal.pone.0161179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer S., Birr P., Fehlner A., Hirsch S., Dittmann F., Barnhill E., Braun J., Sack I. Perfusion alters stiffness of deep gray matter. J. Cereb. Blood Flow Metab. 2017 doi: 10.1177/0271678X17691530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hirsch S., Guo J., Reiter R., Papazoglou S., Kroencke T., Braun J., Sack I. MR elastography of the liver and the spleen using a piezoelectric driver, single-shot wave-field acquisition, and multifrequency dual parameter reconstruction. Magn. Reson. Med. 2013;71:267–277. doi: 10.1002/mrm.24674. [DOI] [PubMed] [Google Scholar]
- Hiscox L.V., Johnson C.L., Barnhill E., McGarry M.D.J., Huston J., van Beek E.J.R., Starr J.M., Roberts N. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys. Med. Biol. 2016;61:R401–R437. doi: 10.1088/0031-9155/61/24/R401. [DOI] [PubMed] [Google Scholar]
- Huston J., Murphy M.C., Boeve B.F., Fattahi N., Arani A., Glaser K.J., Manduca A., Jones D.T., Ehman R.L. Magnetic resonance elastography of frontotemporal dementia. J. Magn. Reson. Imaging. 2016;43:474–478. doi: 10.1002/jmri.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., McGarry M.D.J., Gharibans A.A., Weaver J.B., Paulsen K.D., Wang H., Olivero W.C., Sutton B.P., Georgiadis J.G. Local mechanical properties of white matter structures in the human brain. Neuroimage. 2013;79:145–152. doi: 10.1016/j.neuroimage.2013.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., McGarry M.D.J., Van Houten E.E.W., Weaver J.B., Paulsen K.D., Sutton B.P., Georgiadis J.G. Magnetic resonance elastography of the brain using multishot spiral readouts with self-navigated motion correction. Magn. Reson. Med. 2013;70:404–412. doi: 10.1002/mrm.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., Holtrop J.L., McGarry M.D.J., Weaver J.B., Paulsen K.D., Georgiadis J.G., Sutton B.P. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn. Reson. Med. 2014;71:477–485. doi: 10.1002/mrm.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., Schwarb H., McGarry M.D.J., Anderson A.T., Huesmann G.R., Sutton B.P., Cohen N.J. Viscoelasticity of subcortical gray matter structures. Hum. Brain Mapp. 2016;37:4221–4233. doi: 10.1002/hbm.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.L., Holtrop J.L., Anderson A.T., Sutton B.P. Brain MR elastography with multiband excitation and nonlinear motion-induced phase error correction. Proceedings of the 24th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Singapore; 2016. p. 1951. [Google Scholar]
- Kennedy K.M., Erickson K.I., Rodrigue K.M., Voss M.W., Colcombe S.J., Kramer A.F., Acker J.D., Raz N. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol. Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt D., Yasar T.K., Royston T.J., Magin R.L. Sample interval modulation for the simultaneous acquisition of displacement vector data in magnetic resonance elastography: theory and application. Phys. Med. Biol. 2013;58:8663–8675. doi: 10.1088/0031-9155/58/24/8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt D., Johnson C.L., Magin R.L. Simultaneous, multidirectional acquisition of displacement fields in magnetic resonance elastography of the in vivo human brain. J. Magn. Reson. Imaging. 2015;42:297–304. doi: 10.1002/jmri.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Hain E.G., Braun J., Riek K., Mueller S., Steiner B., Sack I. Enhanced adult neurogenesis increases brain stiffness: in vivo magnetic resonance elastography in a mouse model of dopamine depletion. PLoS One. 2014;9:e92582. doi: 10.1371/journal.pone.0092582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P., Williamson D.E., Lancaster J., Fox P., Cornell J., Blangero J., Glahn D.C. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol. Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse S.A., Rose G.H., Glaser K.J., Manduca A., Felmlee J.P., Jack C.R., Ehman R.L. Magnetic resonance elastography of the brain. Neuroimage. 2008;39:231–237. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lerner R.M. Cambridge University Press; New York: 1984. On the Nature of Human Plasticity. [Google Scholar]
- Lipp A., Trbojevic R., Paul F., Fehlner A., Hirsch S., Scheel M., Noack C., Braun J., Sack I. Cerebral magnetic resonance elastography in supranuclear palsy and idiopathic Parkinson's disease. NeuroImage Clin. 2013;3:381–387. doi: 10.1016/j.nicl.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A., Oliphant T.E., Dresner M.A., Mahowald J.L., Kruse S.A., Amromin E., Felmlee J.P., Greenleaf J.F., Ehman R.L. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med. Image Anal. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Mariappan Y.K., Glaser K.J., Ehman R.L. Magnetic resonance elastography: a review. Clin. Anat. 2010;23:497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.D.J., Van Houten E.E.W. Use of a rayleigh damping model in elastography. Med. Biol. Eng. Comput. 2008;46:759–766. doi: 10.1007/s11517-008-0356-5. [DOI] [PubMed] [Google Scholar]
- McGarry M.D.J., Van Houten E.E.W., Perriñez P.R., Pattison A.J., Weaver J.B., Paulsen K.D. An octahedral shear strain-Based measure of SNR for 3D MR elastography. Phys. Med. Biol. 2011;56:N153–N164. doi: 10.1088/0031-9155/56/13/N02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.D.J., Van Houten E.E.W., Johnson C.L., Georgiadis J.G., Sutton B.P., Weaver J.B., Paulsen K.D. Multiresolution MR elastography using nonlinear inversion. Med. Phys. 2012;39:6388–6396. doi: 10.1118/1.4754649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.D.J., Johnson C.L., Sutton B.P., Van Houten E.E.W., Georgiadis J.G., Weaver J.B., Paulsen K.D. Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE Trans. Med. Imaging. 2013;32:1901–1909. doi: 10.1109/TMI.2013.2268978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.D.J. Dartmouth College; 2013. Improvement and Evaluation of Nonlinear Inversion MR Elastography. [Google Scholar]
- Mills K.L., Tamnes C.K. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cogn. Neurosci. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward J.M., Guo J., Berndt D., Braun J., Sack I., Infante-Duarte C. Tissue structure and inflammatory processes shape viscoelastic properties of the mouse brain. NMR Biomed. 2015;28:831–839. doi: 10.1002/nbm.3319. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Cooke G.E., Watson P.D., Voss M.W., Kramer A.F., Cohen N.J. Relating hippocampus to relational memory processing across domains and delays. J. Cogn. Neurosci. 2015;27:234–245. doi: 10.1162/jocn_a_00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.C., Huston J., Jack C.R., Glaser K.J., Manduca A., Felmlee J.P., Ehman R.L. Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography. J. Magn. Reson. Imaging. 2011;34:494–498. doi: 10.1002/jmri.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.C., Huston J., Glaser K.J., Manduca A., Felmlee J.P., Ehman R.L. Phase correction for interslice discontinuities in multislice EPI MR elastography. Proceedings of the 20th Annual Meeting of ISMRM; Melbourne, Australia; 2012. p. 3426. [Google Scholar]
- Murphy M.C., Huston J., Jack C.R., Glaser K.J., Senjem M.L., Chen J., Manduca A., Felmlee J.P., Ehman R.L. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One. 2013;8:e81668. doi: 10.1371/journal.pone.0081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.C., Jones D.T., Jack C.R., Glaser K.J., Senjem M.L., Manduca A., Felmlee J.P., Carter R.E., Ehman R.L., Huston J. Regional brain stiffness changes across the Alzheimer's disease spectrum. NeuroImage Clin. 2016;10:283–290. doi: 10.1016/j.nicl.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthupillai R., Lomas D.J., Rossman P.J., Greenleaf J.F., Manduca A., Ehman R.L. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- Novak G., Pelaez M. Sage; 2004. Child and Adolescent Development: A Behavioral Systems Approach. [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnansky O., Guo J., Hirsch S., Papazoglou S., Braun J., Sack I. Fractal network dimension and viscoelastic powerlaw behavior: I: a modeling approach based on a coarse-graining procedure combined with shear oscillatory rheometry. Phys. Med. Biol. 2012;57:4023–4040. doi: 10.1088/0031-9155/57/12/4023. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P.W., Lerch J.P., Clasen L.S., Greenstein D., Berman R., Pipitone J., Chakravarty M.M., Giedd J.N. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1592–1597. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek K., Millward J.M., Hamann I., Mueller S., Pfueller C.F., Paul F., Braun J., Infante-Duarte C., Sack I. Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis. NeuroImage Clin. 2012;1:81–90. doi: 10.1016/j.nicl.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A.J., Scheel M., Hirsch S., Braun J., Sack I. In vivo waveguide elastography of white matter tracts in the human brain. Magn. Reson. Med. 2012;68:1410–1422. doi: 10.1002/mrm.24141. [DOI] [PubMed] [Google Scholar]
- Romano A.J., Guo J., Prokscha T., Meyer T., Hirsch S., Braun J., Sack I., Scheel M. In vivo waveguide elastography: effects of neurodegeneration in patients with amyotrophic lateral sclerosis. Magn. Reson. Med. 2014;72:1755–1761. doi: 10.1002/mrm.25067. [DOI] [PubMed] [Google Scholar]
- Sack I., Beierbach B., Hamhaber U., Klatt D., Braun J. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed. 2008;21:265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- Sack I., Beierbach B., Wuerfel J., Klatt D., Hamhaber U., Papazoglou S., Martus P., Braun J. The impact of aging and gender on brain viscoelasticity. Neuroimage. 2009;46:652–657. doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Sack I., Streitberger K.-J., Krefting D., Paul F., Braun J. The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PLoS One. 2011;6:e23451. doi: 10.1371/journal.pone.0023451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack I., Jöhrens K., Wuerfel J., Braun J. Structure-Sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter. 2013;9:5672–5680. [Google Scholar]
- Sandroff B.M., Johnson C.L., Motl R.W. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology. 2017;59:61–67. doi: 10.1007/s00234-016-1767-x. [DOI] [PubMed] [Google Scholar]
- Schregel K., Wuerfel E., Garteiser P., Gemeinhardt I., Prozorovski T., Aktas O., Merz H., Petersen D., Wuerfel J., Sinkus R. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6650–6655. doi: 10.1073/pnas.1200151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H., Johnson C.L., McGarry M.D.J., Cohen N.J. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage. 2016;132:534–541. doi: 10.1016/j.neuroimage.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarb H., Johnson C.L., Daugherty A.M., Hillman C.H., Kramer A.F., Cohen N.J., Barbey A.K. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. Neuroimage. 2017;153:179–188. doi: 10.1016/j.neuroimage.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. Searching for signatures of brain maturity: what are we searching for? Neuron. 2016;92:1164–1167. doi: 10.1016/j.neuron.2016.10.059. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Yoshino J., Le T.Q., Lin S.-J., Sun S.-W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Neurobehavioral changes in adolescence. Curr. Direct. Psychol. Sci. 2000;9:111–114. [Google Scholar]
- Spear L.P. The psychobiology of adolescence. In: Kline K.K., editor. Authoritative Communities: The Scientific Case for Nurturing the Whole Child. 2007. pp. 263–280. [Google Scholar]
- Stillman C.M., Cohen J., Lehman M.E., Erickson K.I. Mediators of physical activity on neurocognitive function: a review at multiple levels of analysis. Front. Hum. Neurosci. 2016;10:626. doi: 10.3389/fnhum.2016.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitberger K.-J., Sack I., Krefting D., Pfüller C., Braun J., Paul F., Wuerfel J. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS One. 2012;7:e29888. doi: 10.1371/journal.pone.0029888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.-W., Liang H.-F., Trinkaus K., Cross A.H., Armstrong R.C., Song S.-K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tan L., McGarry M.D.J., Van Houten E.E.W., Ji M., Solamen L., Weaver J.B., Paulsen K.D. Gradient-based optimization for poroelastic and viscoelastic MR elastography. IEEE Trans. Med. Imaging. 2017;36:236–250. doi: 10.1109/TMI.2016.2604568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromol. Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- Van Houten E.E.W., Viviers D.V., McGarry M.D.J., Perriñez P.R., Perreard I.M., Weaver J.B., Paulsen K.D. Subzone based magnetic resonance elastography using a rayleigh damped material model. Med. Phys. 2011;38:1993–2004. doi: 10.1118/1.3557469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S.K., Yin M., Ehman R.L. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J. Magn. Reson. Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Haldar J.P., Yeh F.C., Xie M., Sun P., Tu T.W., Trinkaus K., Klein R.S., Cross A.H., Song S.-K. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P.D., Voss J.L., Warren D.E., Tranel D., Cohen N.J. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23:570–580. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerfel J., Paul F., Beierbach B., Hamhaber U., Klatt D., Papazoglou S., Zipp F., Martus P., Braun J., Sack I. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage. 2010;49:2520–2525. doi: 10.1016/j.neuroimage.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Yin M., Glaser K.J., Talwalkar J.A., Chen J., Manduca A., Ehman R.L. Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations. Radiology. 2016;278:114–124. doi: 10.1148/radiol.2015142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Green M.A., Sinkus R., Bilston L.E. Viscoelastic properties of human cerebellum using magnetic resonance elastography. J. Biomech. 2011;44:1909–1913. doi: 10.1016/j.jbiomech.2011.04.034. [DOI] [PubMed] [Google Scholar]