Abstract
Objective
Although patients with schizophrenia exhibit impaired suppression of the P50 event-related brain potential (ERP) to the second of two identical auditory stimuli during a paired-stimulus paradigm, uncertainty remains over whether this deficit in inhibitory gating of auditory sensory processes has relevance for patients’ clinical symptoms or cognitive performance. We examined associations between P50 suppression deficits and several core features of schizophrenia to address this gap.
Method
P50 was recorded from 52 patients with schizophrenia and 41 healthy individuals during a standard auditory paired-stimulus task. The Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS) were used to assess clinical symptoms, and the MATRICS Cognitive Consensus Battery (MCCB) measured cognitive performance in a subsample of 39 patients. Correlation and regression analyses were used to examine P50 suppression in relation to clinical symptom and cognitive performance measures.
Results
Patients with schizophrenia demonstrated a deficit in P50 suppression when compared to healthy participants, replicating prior research. Within the patient sample, impaired P50 suppression covaried reliably with greater difficulties in attention, poorer working memory, and reduced processing speed.
Conclusions
Impaired suppression of auditory stimuli is associated with core pathological features of schizophrenia, increasing confidence that P50 inhibitory processing can inform the development of interventions that target cognitive impairments in this chronic and debilitating mental illness.
Understanding the biological processes that accompany debilitating clinical symptoms holds great potential for identifying and ameliorating chronic mental illness, and exploring biological aspects of basic cognitive systems is a priority within the NIMH Research Domain Criteria (RDoC) initiative (1). Consistent with these views, individuals diagnosed with schizophrenia reliably demonstrate dysfunction in inhibitory gating of auditory sensory processes. Specifically, using an S2/S1 ratio score to assess the degree of suppression of the P50 event-related potential (ERP) to the second of paired auditory stimuli (S1–S2), patients with schizophrenia consistently exhibit higher P50 suppression scores than healthy individuals (e.g., see 2).
Disruption of the inhibitory mechanism activated by S1 is postulated to reflect a fundamental neural deficit in schizophrenia that contributes to attentional difficulties associated with the illness (3). Although P50 suppression deficits in schizophrenia appear to be robust, evidence relating this impairment to overt clinical symptoms and cognitive disturbances is mixed. Some studies demonstrate clear associations between P50 abnormalities and clinical symptom ratings (4–8) while others do not (9–12). In the domain of cognitive performance, P50 is associated with working memory and attention-related processes in schizophrenia (5, 13, 14), though again, contradictory findings cast doubt on these effects (15, 16, for a review, see 17). Although evidence linking P50 to symptoms and cognition may be spurious, the null findings could reflect underpowered studies or dichotomized scores on key variables, thereby reducing statistical power and underestimating effect estimates. Reliance on heterogeneous clinical composite measures that include domains presumed to be minimally related to attention (e.g., affective flattening, anhedonia) may also obscure important relationships between P50 suppression and specific symptoms (9, 11).
In view of the apparent promise of P50 inhibitory processes as a candidate mechanism for further biological elaboration on the one hand, and the ambiguous findings relating P50 to specific behavioral and cognitive components of the disorder on the other, the present study enrolled relatively large samples of clinically stable schizophrenia outpatients and demographically-matched healthy comparison subjects to evaluate disturbances associated with compromised P50 processing. More critically, we tested whether higher P50 ratio scores covary with clinical observations of attentional difficulties in patients with schizophrenia and whether greater P50 suppression deficits accompany more pronounced impairments on performance-based measures of working memory, speed of processing, and attention. This second prediction was addressed using the MATRICS Consensus Cognitive Battery (MCCB; 18), a comprehensive and well-validated assessment battery specifically devised to support the development of interventions for prevalent cognitive difficulties in schizophrenia.
Method
Participants
Participants were 54 outpatients with schizophrenia and 45 healthy individuals who were screened using the Structured Clinical Interview for DSM-IV Disorders (SCID). Patients met DSM-IV diagnostic criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder, depressed type, were clinically stable, and were receiving antipsychotic medication at the time of participation. Healthy comparison participants had no history of a major psychiatric disorder according to the SCID assessment or a family history of a psychotic disorder. Participants with a premorbid IQ less than 70, evidence of a known neurological disorder or significant head injury, and substance abuse (in the past month) or dependence (in the past 6 months) were excluded. To avoid anticholinergic effects on dependent variables, antiparkinsonian medications were discontinued in 9 patients 24–48 hours prior to electroencephalography (EEG) recording. Participants refrained from cigarette smoking during the hour prior to data acquisition given prior evidence of a brief, transient effect of nicotine on P50 suppression in schizophrenia (19). The study was approved by the institutional review board of the University of California, Los Angeles, and participants provided written informed consent.
Data from 2 patients and 4 comparison participants were excluded because their data did not meet P50 inclusionary criteria (see below). Of the remaining 52 patients, 45 were prescribed risperidone, whereas 7 were stabilized with olanzapine, ziprasidone, fluphenazine, haloperidol, clozapine, or aripiprazole. There were no significant differences on any dependent variables as a function of medication type; unless noted, analyses are reported for all 52 patients.
Clinical Assessment
Patients’ symptoms were assessed at the UCLA Aftercare Research Program using the clinician-rated Scale for the Assessment of Positive Symptoms (SAPS; 20) and Scale for the Assessment of Negative Symptoms (SANS; 21). The positive symptoms summary score was the sum of the hallucinations, delusions, bizarre behavior, and positive formal thought disorder global subscale scores. The negative symptoms summary score was the sum of the affective flattening, alogia, avolition/apathy, anhedonia/asociality, and inattention global subscale scores.
Cognitive Assessment
The MCCB was administered to assess cognitive function in a subsample of 39 patients across 7 domains: attention, working memory, speed of processing, verbal learning, visual learning, reasoning and problem solving, and social cognition. Performance in each MCCB domain was converted to age- and gender-corrected T-scores, using the MCCB scoring program which also provided a composite T-score (18).
Psychophysiological Recording Methods
EEG recordings were obtained using a SynAmps amplifier system (Neuroscan, Charlotte, NC) with a cap containing 124 Ag/AgCl sintered electrodes, with an equidistant layout. Electrooculogram (EOG) was recorded from electrodes placed above and below the right eye and near the outer canthi of the eyes. Electrode sites were referenced to the left earlobe during data collection and re-referenced offline to averaged earlobes. All impedances were below 10 kI. The EEG was amplified 2,500 times and EOG signals 500 times, respectively. Signals were acquired at a sampling rate of 2000 Hz, with filters from 0.5 to 200 Hz.
Epochs were extracted from 200 ms before stimulus onset to 1,000 ms following stimulus presentation, with the first 200 ms used for baseline correction, and EEG trials were digitally filtered with a bandpass of 10 to 50 Hz for measuring the P50 ERP component. Blind source separation by independent component analysis (ICA) was conducted using MATLAB with the open-source toolbox EEGLAB, and eye movements, blink artifact, and electrocardiographic activity were removed. Epochs containing artifacts (voltages exceeding ±100µV) were rejected. The number of trials retained for patients (M = 78.73, SD = 4.62) and healthy participants (M = 79.88, SD = 0.33) did not differ (p > 0.05). EEG trials were averaged across all trials for each participant, and P50 was identified as the maximum positivity between 40 and 80 ms after stimulus onset at the Cz site and was measured relative to the preceding N40. P30 amplitude and latency were identified based on the most positive peak between 20 and 40 ms after stimulus onset, and the maximum negativity between P30 and P50 was identified as N40. Two raters, blind to participant group, independently verified scoring of each ERP. As noted above, 6 participants were excluded from analyses as their P50 amplitudes to S1 did not exceed 0.5 µV. One P50 ratio value was truncated to 2.00 to prevent extreme scores from having a disproportionate effect on the results (e.g., see 22).
Procedure
Participants completed an audiometric screening, consisting of sound intensities presented in 5-dB increments at frequencies ranging from 500 to 8000 Hz, to ensure all participants detected sounds at each frequency above 30 dB SPL with each ear. P50 recordings were collected during presentation of 80 trials of paired auditory stimuli that were each 3 ms in duration and 80 dB SPL, with a 500 ms interstimulus interval, and a variable intertrial interval of 9–11 s between pairs of stimuli. Participants were instructed to sit comfortably in a sound-attenuated room while auditory stimuli were presented through foam-insert earphones. Clinical ratings were completed during a separate visit by trained clinicians who evaluated symptoms over the past 3 months, including the day of EEG data collection. For the patient subsample completing the MCCB, the assessment was conducted within one month of EEG collection as part of a separate study (23).
Statistical Analysis
Analyses of variance (ANOVA) and chi-square tests were used to compare schizophrenia and healthy comparison groups on demographic characteristics. A repeated-measures ANOVA involving group (patient vs. healthy comparison) and P50 amplitude (S1 vs. S2) was used to provide a difference measure of P50 suppression to complement the more typical ratio score analysis. Partial-eta2 (ηp2) is reported to reflect an ANOVA effect size. Zero-order correlations and hierarchical linear regression analyses were performed to examine P50 relationships with clinical ratings and cognitive performance. The Benjamini-Hochberg approach was used to control the false discovery rate (FDR) associated with multiple comparisons (24).
To examine potential confounds related to type and dosage of medication, all analyses were repeated with chlorpromazine (CPZ)-equivalent dosages included as a covariate for each schizophrenia patient and also in a sample restricted to the 45 patients receiving risperidone. To assess any confounding effects of nicotine, all analyses were repeated with the total number of cigarettes smoked in the past week as a covariate. To evaluate relationships with cognitive variables, all analyses were repeated to account statistically for SANS and SAPS symptom severity. Any deviations in results from the full sample are noted. Alpha level was set to 0.05, 2-tailed, for all statistical tests.
Results
Demographic and clinical characteristics are presented in Table 1. Because of group differences in age between schizophrenia patients and healthy comparison participants, relevant analyses were repeated using age as a covariate. The covariate did not alter any significant results, and group differences without age as a covariate are reported below. The two groups were matched for highest parental level of education. As might be expected, patients and healthy participants differed in years of education, given the likely influence of illness on the level achieved. Group comparisons were also repeated with years of education included as a covariate which again did not alter the results. Symptom levels were generally mild to moderate for patients.
Table 1.
Demographic, clinical, and performance characteristics of sample.
| Schizophrenia Patients (n = 52) |
Healthy Comparison Participants (n = 41) |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | % | n | % | Statistic | p | |
|
|
||||||
| Demographics | ||||||
| Gender (Female/Male) | 15/37 | 28.8/71.2 | 6/35 | 14.6/85.4 | χ2(1) = 2.65 | 0.136 |
| Cigarette Smoker (Yes/No) | 16/36 | 30.8/69.2 | 5/36 | 12.2/87.8 | χ2(1) = 4.52 | 0.033 |
| M | SD | M | SD | Statistic | p | |
|
|
||||||
| Age (years) | 26.10 | 8/17 | 30.22 | 7.07 | F(1,91) = 6.56 | 0.012 |
| Education (years) | 13.10 | 2.19 | 14.44 | 1.98 | F(1,91) = 9.40 | 0.003 |
| Parental Education (years) | 14.02 | 3.54 | 15.07 | 2.65 | F(1,91) = 2.52 | 0.116 |
| CPZ-Equiv. Dose(mg/day) | 204.17 | 116.26 | ||||
| Age of Psychosis Onset (years) | 22.59 | 3.89 | ||||
| Cigarettes in Past Week (no.) | 4.60 | 15.36 | 1.71 | 5.08 | F(1,91) = 1.33 | 0.251 |
| Symptoms (summary scores) | ||||||
| SAPS | 4.17 | 3.55 | ||||
| SANS | 8.98 | 4.34 | ||||
| Cognition (T-scores)a | ||||||
| Working Memory | 43.26 | 15.45 | ||||
| Speed of Processing | 32.26 | 12.00 | ||||
| Attention/Vigilanceb | 37.39 | 12.29 | ||||
| Verbal Learning | 42.41 | 10.16 | ||||
| Visual Learning | 38.59 | 11.27 | ||||
| Reasoning/Problem Solving | 38.67 | 9.03 | ||||
| Social Cognitionb | 41.34 | 12.49 | ||||
| MCCB Overall Compositec | 32.81 | 14.42 | ||||
| P50 Measures | ||||||
| S1 Amplitude (µV) | 2.88 | 1.65 | 3.50 | 1.84 | F(1,91) = 2.93 | 0.090 |
| S2 Amplitude (µV) | 1.56 | 1.11 | 1.44 | 1.21 | F(1,91) = 0.26 | 0.612 |
| S2/S1 Ratio Score | 0.60 | 0.41 | 0.39 | 0.28 | F(1,91) = 7.87 | 0.006 |
Note. Numbers and percentages of participants are reported for gender and cigarette smoking status, which was analyzed using a Pearson chi-square test. Group means (M) and standard deviations (SD) are reported for age, personal and parental years of education, and number of cigarettes consumed in the past week, which were analyzed using analysis of variance (ANOVA). Group mean (M) and standard deviation (SD) of the schizophrenia patient sample are reported for chlorpromazine (CPZ)-equivalent dosages, age of psychosis onset, total scores on the Scale for the Assessment of Positive Symptoms (SAPS) and on the Scale for the Assessment of Negative Symptoms (SANS), and norm-based T-scores on the MATRICS Consensus Cognitive Battery (MCCB). Mean P50 Stimulus 1 (S1) and Stimulus 2 (S2) amplitudes and S2/S1 Ratios are reported for both groups.
Data were collected from a subset of 39 schizophrenia participants who completed cognitive evaluation.
Data unavailable for 1 participant.
Data unavailable for 2 participants.
P50 Suppression
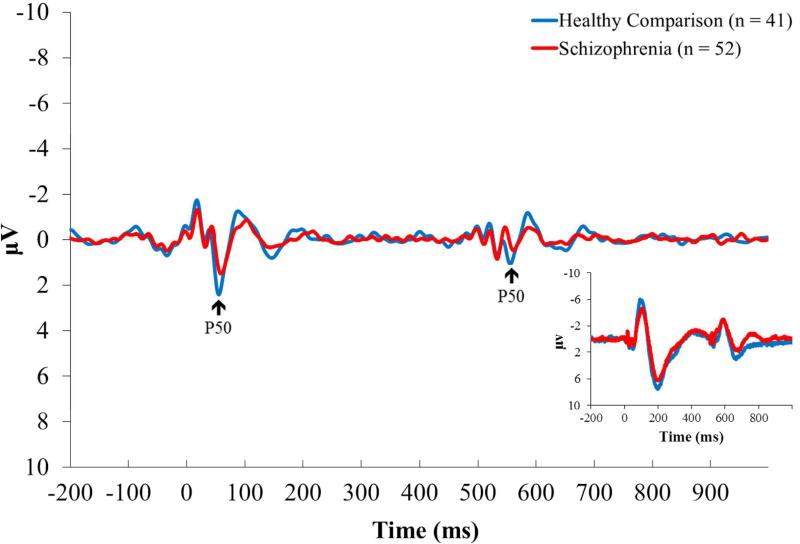
Grand-average ERP waveforms for each group are displayed in Figure 1. P50 mean amplitudes and suppression ratios are shown in Table 1. Consistent with prior reports, P50 ratio scores (Table 1) indicated poorer suppression in schizophrenia patients than in healthy comparison participants (p = 0.006). A main effect of stimulus, F(1,91) = 125.65, p < 0.001, ηp2 = 0.580, confirmed suppression and was qualified by a group by stimulus interaction, F(1,91) = 6.10, p = 0.015, ηp2 = 0.063. Post-hoc tests determined that P50 amplitude to S1 tended to be attenuated in patients relative to healthy participants, F(1,91) = 2.93, p = 0.090, ηp2 = 0.031. Although the magnitude of patients’ S2 amplitude response was larger than that of healthy individuals, the group difference was not statistically significant (p = 0.612).
Figure 1.
Grand average event-related potential waveforms at the Cz recording site, filtered 10–50Hz (inset: 0.5–200Hz). The P50 component is indicated with an arrow.
P50 and Clinical Symptoms
As shown in Table 2, a significant positive association was observed in patients between P50 ratio scores and the SANS summary score but not the SAPS summary score. Correlations with each SANS subscale measure indicated that the association with P50 suppression was only evident for the global inattention subscale. Consistent with an inhibitory gating deficit, this effect was restricted to P50 amplitude to S2, such that the significant association between poorer suppression and clinical ratings of greater attentional impairment (r = 0.338, p = 0.014) did not extend to P50 to S1 (r = 0.064, p = 0.654).
Table 2.
Correlations between P50 ratio scores and clinician-rated negative and positive symptoms in patients (N = 52).
| Symptom Scale Scores | r | p |
|---|---|---|
|
|
|
|
| SANS Summary | 0.315 | 0.023 |
| Affective flattening | 0.201 | 0.153 |
| Alogia | 0.112 | 0.428 |
| Avolition-apathy | 0.248 | 0.077 |
| Anhedonia-asociality | 0.143 | 0.312 |
| Inattentiona | 0.408 | 0.003 |
| SAPS Summary | 0.043 | 0.760 |
| Hallucinations | 0.079 | 0.577 |
| Delusions | 0.152 | 0.282 |
| Bizarre Behavior | −0.124 | 0.385 |
| Positive Formal Thought Disorder | −0.060 | 0.672 |
Note. SANS = Scale for the Assessment of Negative Symptoms, SAPS = Scale for the Assessment of Positive Symptoms.
Retained significance following False Discovery Rate (FDR) correction for multiple comparisons.
To address the specificity of the association between P50 suppression and clinical ratings of attentional difficulties in patients, a hierarchical linear regression assessed the contribution of the inattention score after accounting for all other SANS subscale scores. This analysis revealed that inattention (β = 0.394, p = 0.009) uniquely explained 12.8% of the variance in P50 ratio scores, above and beyond the 7.2% of the variation accounted for by the other SANS subscale scores.
P50 and Cognitive Performance
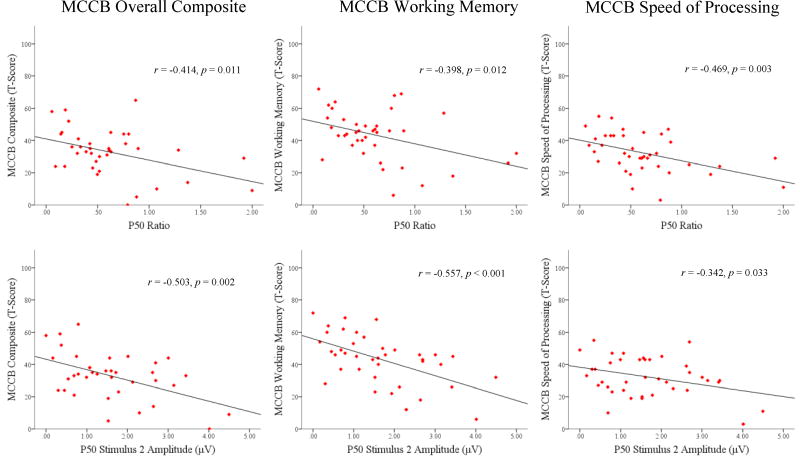
MCCB performance results are shown in Table 1. Zero-order correlations between P50 measures and MCCB cognitive variables are presented in Table 3 for the 39 schizophrenia patients who underwent cognitive testing. P50 ratios correlated with MCCB overall composite scores, indicating that impaired suppression was associated with poorer cognitive performance. As predicted, this effect was evident in significant associations with working memory and speed of processing performance (see Figure 2). Specifically, poorer P50 suppression was related to impaired performance during both verbal working memory (r = −0.330, p = 0.040) and visual working memory (r = −0.381, p = 0.017) tasks. There were no statistically significant associations between the P50 ratio score and the other MCCB domains (see Table 3).
Table 3.
Relationships between P50 ratio scores and MCCB composite score and domains in patients (N = 39).
| MCCB Measure | r | p |
|---|---|---|
|
|
|
|
| Working Memorya | −0.398 | 0.012 |
| Speed of Processinga | −0.469 | 0.003 |
| Attention/Vigilance | −0.104 | 0.533 |
| Verbal Learning | −0.262 | 0.107 |
| Visual Learning | −0.309 | 0.056 |
| Reasoning/Problem Solving | −0.270 | 0.096 |
| Social Cognition | −0.319 | 0.051 |
| Overall Composite Scorea | −0.414 | 0.011 |
Note: MCCB = MATRICS Consensus Cognitive Battery.
Retained significance following False Discovery Rate (FDR) correction for multiple comparisons involving the overall composite and all seven MCCB domains.
Figure 2.
Correlations between cognitive performance and P50 ratio scores and S2 amplitudes. The left column shows a significant association between the P50 ratio and MCCB composite score, which was largely explained by a significant relationship with S2 amplitude. The middle column shows a significant association between P50 ratios and MCCB working memory performance, accounted for by a relationship with S2 amplitude. The right column shows a significant association between P50 ratios and MCCB speed of processing performance, again attributable to a relationship with S2 amplitude.
An examination of the relative contributions of S1 and S2 amplitudes to the relationship of P50 ratios and cognitive performance revealed that a significant correlation with P50 to S2 drove this relationship for working memory (r = −0.557, p < 0.001) and speed of processing (r = −0.342, p = 0.033) performance, and for the MCCB overall composite (r = −0.503, p = 0.002). There were no significant associations involving P50 amplitude to S1 (working memory: r = −0.160 p = 0.331, speed of processing: r = 0.249, p = 0.126, overall composite: r = −0.056, p = 0.743).
Effects of Medication, Smoking, and Symptom Severity
When analyses were repeated and covaried for CPZ-equivalent dosages of antipsychotic medications and number of cigarettes smoked during the past week, all reported significant results remained significant. Similarly, all symptom relationships remained statistically significant when the sample was restricted to the 45 patients on risperidone. Furthermore, symptom severity did not account for any of the observed relationships between P50 and cognitive performance, as all associations with clinical and cognitive variables remained significant when adjusting statistically for SAPS and SANS global scores (all ps < 0.05).
Discussion
The present study investigated linkages between clinical symptoms, cognitive dysfunction, and pathophysiological mechanisms associated with auditory sensory processing deficits in schizophrenia. Current findings confirm the hypothesis that P50 suppression abnormalities are associated with such core clinical characteristics of schizophrenia as symptoms of inattentiveness, thereby clarifying the clinical significance of P50 suppression deficits. Although analogous results involving clinician-rated attentional impairment measures have been described previously (5, 8), present results are based on a larger patient sample and a more powerful statistical approach was achieved by retaining the continuous range of information offered by SANS and P50 ratio scores. Present results also underscore the specificity of the association between impaired P50 inhibitory processing and clinician-rated inattention in schizophrenia, as other negative symptom domains were not similarly implicated.
Beyond relating P50 suppression to a clinically-observed core feature of schizophrenia, results from the present study link auditory sensory dysfunction with impairments involving working memory and speed of information processing. These findings corroborate previous work (5, 13, 14, see 17) and also extend it by using standardized measures from the MCCB, a cognitive test battery on which schizophrenia patients reliably show compromised performance (18). These findings contrast with a recent report in which P50 ratio scores were unrelated to cognitive performance in similar domains in medicated, chronic schizophrenia patients (16). Illness duration and prolonged exposure to antipsychotic medications may account for the discrepancy.
The pattern of findings observed in the present study is substantiated by research into the neural mechanisms of P50 sensory impairments. Source localization studies implicate a neural network involving prefrontal cortex, particularly the dorsolateral prefrontal cortex (dlPFC), superior temporal gyrus (STG), hippocampus, and thalamus in the generation of P50 and its suppression (25, 26). Notably, dlPFC and hippocampus have been associated with working memory dysfunction in schizophrenia (27, 28), while prefrontal cortex, hippocampus, and STG structural and functional abnormalities have been implicated in efficient information processing (29, 30). Furthermore, present results parallel a recent report by Tregellas and colleagues (28) that hippocampal dysfunction is associated with poorer MCCB working memory performance, consistent with the suggestion that disrupted hippocampal activity contributes to impaired sensory information processing. Taken together, P50 suppression deficits appear to involve impaired interactions between frontal and temporal brain regions, and these early sensory processing difficulties may, in turn, contribute to cognitive symptoms in schizophrenia.
Another important consideration is the complex relationship between working memory and attention, which have been linked behaviorally and neurobiologically (31). Consistent with the present findings of an association between working memory and enhanced P50 suppression, individuals with high working memory capacity may be more successful in resisting attentional capture by salient but irrelevant stimuli than those with lower capacity (32). Optimal performance on working memory tasks utilized in the present investigation may also rely on attentional processes that influence maintenance of the material to be remembered (33). Likewise, tasks measuring attention often include working memory demands to varying degrees (34) and clinically rated inattention is likely to involve temporary disruptions in working memory. Furthermore, impairments on purported tests of processing speed (e.g., coding tasks) appear attributable in part to attention and working memory deficits, as efficient performance requires the ability to quickly bind representations in working memory to improve accurate speeded performance (35). Regardless, present results provide evidence for an important link between deficient P50 suppression and cognitive performance deficits in schizophrenia.
The absence of an association between P50 suppression and performance on the attention/vigilance task in the present study highlights the possibility that P50 is related more closely to efficient information processing and working memory than to short-term, focused sustained attention and vigilance as assessed, for example, by the CPT-IP. Specifically, P50 suppression may be more critical to tasks that involve rapid encoding, brief maintenance, and manipulation of stimuli, than to those that require ongoing monitoring and sustained attention. Similarly, the lack of an association between P50 and performance on the MCCB attention/vigilance task is not necessarily inconsistent with our finding of a relationship with SANS inattentiveness. Given that this global clinical rating is based on inattentiveness during social behavior and mental status testing, it may be that the construct better captures aspects of attention associated with speeded information processing and working memory rather than sustained attention and vigilance. This possibility is consistent with findings suggesting an absence of a relationship between P50 suppression and performance on the Degraded Stimulus CPT (16). In fact, the apparent divergence from studies reporting positive associations (5, 13) might be attributable to their reliance on tasks (i.e., Digit Vigilance and Gordon CPT) eliciting other dimensions of attention than in the present report (e.g., selective attention, vigilance, or orienting/shifting) or involving other task parameters (e.g., provision of feedback, time restrictions).
It is noteworthy that, in addition to the P50 ratio score, primary findings involved relationships between cognitive dysfunction and P50 S2 amplitude but not with the P50 S1 response. This pattern of findings is consistent with a dominant model of P50 sensory gating whereby S1 activates inhibitory neuronal mechanisms that suppress or inhibit the response to the identical S2, resulting in attenuation of P50 to S2 (3). Findings regarding the relative importance of a P50 S1 amplitude deficit have been debated in the literature. A meta-analysis demonstrated substantial heterogeneity in S1 amplitude group differences across studies and suggested that S1 amplitude alone does not differentiate patients and healthy subjects as reliably as the P50 ratio (2). Present results support the possibility that patients with schizophrenia, and particularly those with greater working memory deficits, have insufficient activation of P50 inhibitory mechanisms.
The present research did not include medication-free patients, as at the time of participation all patients were prescribed antipsychotic medications. Antipsychotic medications may affect some cognitive processes (36), although findings have been mixed (37). With respect to P50, neither first-generation nor second-generation antipsychotic medications, possibly excepting clozapine (38) and to a lesser extent risperidone (8), have been shown to improve sensory processing deficits in schizophrenia. Statistically accounting for CPZ-equivalent dosages and limiting the sample to recent-onset patients stabilized on risperidone did not modify the present results. Therefore, systematic impact of antipsychotic medications on study findings was likely minimal. In addition, although participants were assessed for substance abuse or dependence using the SCID, the present study is limited by the lack of urine toxicology screens to verify abstinence prior to EEG assessment.
Taken together, results of the present study implicate P50 inhibitory processing deficits with core features of schizophrenia. Furthermore, findings substantiate P50 as a promising indicator of early sensory processing abnormalities by confirming the presence of inhibitory P50 deficits in schizophrenia and by demonstrating their associations with clinically rated inattention and working memory and processing speed performance. These results are consistent with the RDoC initiative, and provide evidence that P50 suppression is a viable and promising manifestation of pathological mechanisms that can be used as a target for interventions that aim to improve cognitive impairments in schizophrenia (39). Indeed, there is encouraging evidence to suggest that inhibitory gating deficits in individuals with schizophrenia are modifiable by cognitive training (e.g., 40). Further research will help to clarify whether P50 suppression deficits are amenable to other forms of interventions while also determining whether such benefits extend to other aspects of cognition.
Acknowledgments
Dr. Nuechterlein reports he has received research grants from Janssen Research and Development, Genentech, and Posit Science, Inc, and has been a paid consultant to Astellas, Janssen Scientific Affairs, Takeda, and Teva. Dr. Ventura has received a research grant from Posit Science, Inc. and has been a paid consultant to Boehringer Ingelheim. Dr. Subotnik has been a paid consultant to Janssen and Teva. Dr. Williams is employed by and is a former shareholder with Allergan; he is a former employee of and past shareholder with Kythera Biopharmaceuticals; he is also a former employee of and past shareholder with Amgen.
This study was supported in part by Center grant P50 MH066286 and T32 MH014584 from the National Institute of Mental Health, Bethesda, MD, and the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. We are grateful for the many contributions provided by the patients and staff of the UCLA Aftercare Research Program.
References
- 1.Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria initiative: background, issues, and pragmatics. Psychophysiology. 2016;53:286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- 2.Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, Potkin S, Bunney WE., Jr P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry Res. 2008;158:226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- 4.Arnfred SM, Chen ACN. Exploration of somatosensory P50 gating in schizophrenia spectrum patients: reduced P50 amplitude correlates to social anhedonia. Psychiatry Res. 2004;125:147–160. doi: 10.1016/j.psychres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Erwin RJ, Turetsky BI, Moberg P, Gur RC, Gur RE. P50 abnormalities in schizophrenia: relationship to clinical and neuropsychological indices of attention. Schizophr Res. 1998;33:157–167. doi: 10.1016/s0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 6.Louchart-de la Chapelle S, Levillain D, Ménard J-F, Van der Elst A, Allio G, Haouzir S, Dollfus S, Campion D, Thibaut F. P50 inhibitory gating deficit is correlated with the negative symptomatology of schizophrenia. Psychiatry Res. 2005;136:27–34. doi: 10.1016/j.psychres.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Ringel TM, Heidrich A, Jacob CP, Pfuhlmann B, Stoeber G, Fallgatter AJ. Sensory gating deficit in a subtype of chronic schizophrenic patients. Psychiatry Res. 2004;125:237–245. doi: 10.1016/j.psychres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Yee CM, Nuechterlein KH, Morris SE, White PM. P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol. 1998;107:691–698. doi: 10.1037//0021-843x.107.4.691. [DOI] [PubMed] [Google Scholar]
- 9.Adler LE, Waldo MC, Tatcher A, Cawthra E, Baker N, Freedman R. Lack of relationship of auditory gating defects to negative symptoms in schizophrenia. Schizophr Res. 1990;3:131–138. doi: 10.1016/0920-9964(90)90046-a. [DOI] [PubMed] [Google Scholar]
- 10.Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- 11.Santos JL, Sánchez-Morla EM, Aparicio A, García-Jiménez MA, Villanueva C, Martínez-Vizcaíno V, Arango C. P50 gating in deficit and nondeficit schizophrenia. Schizophr Res. 2010;119:183–190. doi: 10.1016/j.schres.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Thoma RJ, Hanlon FM, Moses SN, Ricker D, Huang M, Edgar C, Irwin J, Torres F, Weisend MP, Adler LE, Miller GA, Canive JM. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr Res. 2005;73:311–318. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- 14.Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, McHaffie G, Jones AP, Paz RD, Miller GA, Cañive JM. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am J Psychiatry. 2010;167:1264–1275. doi: 10.1176/appi.ajp.2010.09071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoma RJ, Hanlon FM, Moses SN, Christopher Edgar J, Huang M, Weisend MP, Irwin J, Sherwood A, Paulson K, Bustillo J, Adler LE, Miller GA, Cañive JM. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Morla EM, Santos JL, Aparicio A, García-Jiménez MÁ, Soria C, Arango C. Neuropsychological correlates of P50 sensory gating in patients with schizophrenia. Schizophr Res. 2013;143:102–106. doi: 10.1016/j.schres.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 19.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen NC. Scale for the Assessment of Positive Symptoms. University of Iowa; 1984. [Google Scholar]
- 21.Andreasen NC. Scale for the Assessment of Negative Symptoms. University of Iowa; 1984. [Google Scholar]
- 22.Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R. Gating of auditory response in schizophrenics and normal controls: effects of recording site and stimulation interval on the P50 wave. Schizophr Res. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- 23.Subotnik KL, Casaus LR, Ventura J, Luo JS, Hellemann GS, Gretchen-Doorly D, Marder S, Nuechterlein KH. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72:822–829. doi: 10.1001/jamapsychiatry.2015.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 25.Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams TJ, Nuechterlein KH, Subotnik KL, Yee CM. Distinct neural generators of sensory gating in schizophrenia. Psychophysiology. 2011;48:470–478. doi: 10.1111/j.1469-8986.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2014;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 28.Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry. 2014;171:549–556. doi: 10.1176/appi.ajp.2013.13070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota Y, Toichi M, Shimizu M, Mason RA, Coconcea CM, Findling RL, Yamamoto K, Calabrese JR. Prefrontal activation during verbal fluency tests in schizophrenia—a near-infrared spectroscopy (NIRS) study. Schizophr Res. 2005;77:65–73. doi: 10.1016/j.schres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Rotrosen J, Wolkin A. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Res. 2002;116:1–23. doi: 10.1016/s0925-4927(02)00046-x. [DOI] [PubMed] [Google Scholar]
- 31.Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K, Vogel EK. Human variation in overriding attentional capture. J Neurosci. 2009;29:8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MM, Scholey KA. Interference in immediate spatial memory. Mem Cognit. 1994;22:1–13. doi: 10.3758/bf03202756. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- 35.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 38.Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, Hea R, Griffith J. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- 39.Yee CM, Javitt DC, Miller GA. Replacing DSM categorical analyses with dimensional analyses in psychiatry research: the Research Domain Criteria initiative. JAMA Psychiatry. 2015;72:1159–1160. doi: 10.1001/jamapsychiatry.2015.1900. [DOI] [PubMed] [Google Scholar]
- 40.Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol Psychiatry. 2011;69:465–471. doi: 10.1016/j.biopsych.2010.09.028. [DOI] [PubMed] [Google Scholar]