Abstract
Background
The effect of age on asthma severity is poorly understood.
Objectives
To compare the baseline features of severe and non-severe asthma in the SARP III cohort, and examine in cross section the effects of age on those features.
Methods
SARP III is an NIH/NHLBI multi-site 3 year cohort study conducted to investigate mechanisms of severe asthma. The sample included 188 children (111 severe, 77 non-severe) and 526 adults (313 severe, 213 non-severe) characterized for demographic features, symptoms, health care utilization, lung function, and inflammatory markers compared by age and severity.
Results
Compared to children with non-severe asthma, children with severe asthma had more symptoms and more historical exacerbations, but no difference in body weight, post-bronchodilator lung function, or inflammatory markers. After childhood, and increasing with age, the cohort had a higher proportion of women, less allergen sensitization, and overall fewer blood eosinophils. Enrollment of participants with severe asthma was highest in middle-age adults, who were older, more obese, with greater airflow limitation and higher blood eosinophils, but less allergen sensitization than adults with non-severe asthma.
Conclusions
The phenotypic features of asthma differ by severity and with advancing age. With advancing age, patients with severe asthma are more obese, have greater airflow limitation, less allergen sensitization, and variable type 2 inflammation. Novel mechanisms besides type 2 inflammatory pathways may inform the severe asthma phenotype with advancing age.
Keywords: Severe asthma, asthma phenotypes
Introduction
Patients with severe asthma require treatment with high-dose inhaled corticosteroids plus a second controller to maintain symptom control or have uncontrolled asthma despite therapy. 1 While these patients are estimated to represent 5–10% of the total asthma population in the U.S., they have frequent exacerbations, impaired quality of life, and account for more than half of the annual costs of asthma. 2 From 2001 to 2012 the NIH/NHLBI supported the Severe Asthma Research Program (SARP) I and II cohorts to study mechanisms differentiating severe from non-severe asthma. 3–7 SARP investigators characterized severe asthma as a heterogeneous syndrome with diverse molecular, biochemical, and cellular inflammatory features and structure-function abnormalities. 7 Adults and children with severe asthma were further categorized by un-biased statistical methods into clusters based on distinguishing clinical features. 5–6 Although older age was a determinant of treatment failure in asthma patients participating in the NIH Asthma Clinical Research Network therapeutic trials, these interventional studies did not target participants with severe asthma. 8 Furthermore age-related differences in the features of asthma were not studied in the original SARP I and II cohorts.
In November 2012 the NIH/NHLBI formed SARP III, a network of U.S. investigators and a data coordinating center (DCC) located at regional academic health centers involved in severe asthma research. The main objective of SARP III is to advance understanding of severe asthma through the integration of mechanistic studies with detailed phenotypic characterization. To accomplish this goal, a cohort of adults and children with severe and non-severe asthma was recruited for detailed characterization and enrollment into an observational study. This report highlights the baseline demographic, clinical, physiologic and inflammatory features of participants with severe compared to non-severe asthma and how these differences are influenced by age. The selection of specific features to examine with age was exploratory to generate hypotheses to be tested further in the longitudinal protocol.
Methods
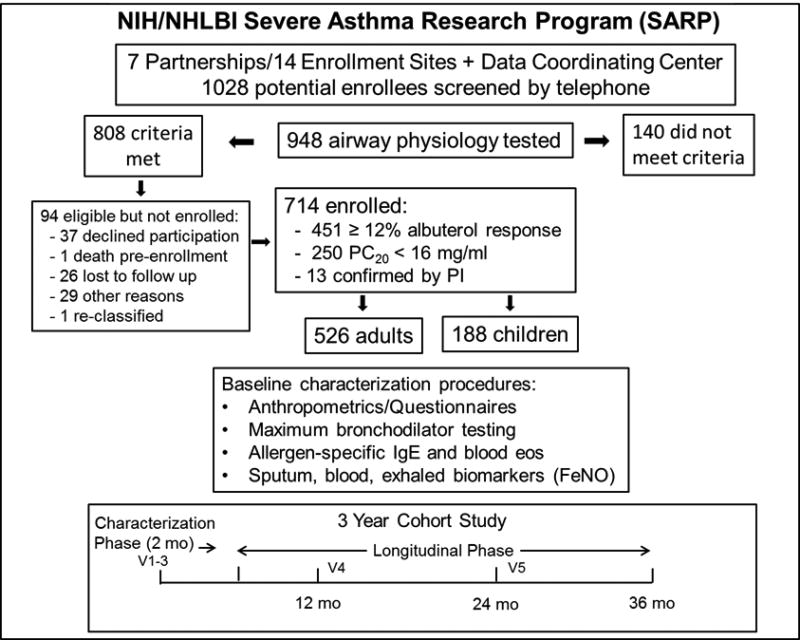
SARP clinical centers were tasked by NIH/NHLBI to recruit a sample that included 60% severe asthmatics, 50% women, 30% non-white minorities, and 25% children. Details of the screening and enrollment procedures are provided in a consort plot (Figure 1) and in the Online Repository (E-Methods and Tables EI and E2). Current tobacco smokers and those with significant past smoking history (> 5 pack years if they were < 30 years of age and > 10 pack years if they were > 30 years of age) were excluded. Participants maintained medications for asthma as prescribed by their care provider. Study procedures were approved by the IRB at each institution and an independent Data Safety Monitoring Board. All subjects provided informed consent and/or assent.
Figure 1.
SARP III screening, consort procedures, characterization procedures, and schematic of the longitudinal cohort study. Potential enrollees who did not complete the initial screening questionnaire are not counted among the 948 who underwent airway physiology screen testing. Potential enrollees with more than one screen failure are also not included.
Asthma was confirmed based on responsiveness to β agonist (≥ 12% increase in FEV1 from baseline post-albuterol, n = 451) or (if < 12%), a positive methacholine bronchoprovocation (n = 250). Thirteen enrollees with an FEV1 < 50% predicted and an albuterol response < 12% did not undergo methacholine bronchoprovocation. Severe asthma was defined according to a modification of the ERS/ATS consensus definition.1 Thresholds for high-dose inhaled corticosteroid therapy were ≥ 440 mcg of fluticasone equivalents per day for children 6–11 years of age and ≥ 880 mcg fluticasone equivalents per day for subjects 12 years of age and older. Enrollees treated with high-dose inhaled corticosteroids for at least 6 of the previous 12 months and the 3 months prior to enrollment were assigned to the severe sub-group and those who did not meet criteria for severe asthma were assigned to the non-severe sub-group. “Children” were defined as those participants < 18 years of age at enrollment.
Characterization procedures, adapted from SARP I and II, 3–6 included history and physical examination, vital signs, height, weight, and Tanner staging for children, characterization questionnaires, assessment of one week (ACQ6) and four week (ACT/cACT) symptom control, asthma-related quality of life scales (AQLQ(S) and PAQLQ(S)), spirometry with maximal β-agonist reversibility testing (dose escalation protocol at 180 mcg albuterol increments up to 720mcg), sputum induction (age 12 and older), exhaled nitric oxide (FeNO), and blood for CBC, total IgE, and allergen-specific IgE. Quality standards for spirometry were followed at each site, and results audited through the DCC. Medication adherence was measured by the Medication Adherence Report Scale (MARS-5). 9
Data Analysis
These included sex, body mass index (BMI), prevalence of obesity (defined as a BMI ≥ 30 kg/m2 for adults and > 95th percentile for children), pre- and maximum post-BD lung function, and prevalence of airflow limitation (FEV1/FVC ratio ≤ lower limit of normal (LLN) of the Global Lung Initiative (GLI) reference values, 10 maximum FEV1 BD response (both as absolute change and % change in FEV1 % predicted from baseline), prevalence of allergen sensitization, total blood eosinophil count, and FeNO. Allergen sensitization was defined as an elevated IgE (>0.35 IU/ml) to one of 15 allergen-specific IgE blood tests (Online Repository; E-Methods). Multiple sensitization was defined as ≥ 4 positive specific IgE tests, determined by the median of the number of positive blood allergen tests in the sample. Criterion for peripheral blood eosinophilia was ≥ 300 cells/µL, 11 and for increased FeNO ≥ 30 ppb. 12–13
The cohort features were stratified by asthma severity (severe and non-severe) and age group (children < 18 years and adults ≥ 18 years). Non-severe asthma was classified as mild intermittent, mild persistent, and moderate persistent (both treated and not treated with inhaled corticosteroids; On-line Repository, Table E3). The features of ineligible versus enrolled participants in the study sample are provided (Online Repository, Table E4).
Categorical data are presented as count and percentages grouped according to severe versus non-severe and tested with the Pearson chi-square test. Continuous data with approximately Gaussian distributions are presented as mean and standard deviation, and differences by severity tested with a t-test. Continuous data with skewed distributions are presented as median and quartiles and differences by severity tested with a Wilcoxon rank sum test. Supplemental analyses with and without 13 participants with a) FEV1 < 50% predicted, b) enrollees with a maximum bronchodilator response < 12.5% from baseline, and c) enrollees who did not undergo methacholine bronchoprovocation tests were compared. The analysis results did not differ when these sub-groups were excluded.
The relationship between age of enrollment and specific outcomes was modeled using cubic splines with appropriate distribution and link functions. In conducting the analysis, age at enrollment instead of duration of asthma was used as a dependent variable because duration of asthma does not correlate with risk factors for severity and is limited by recall bias. 14 Continuous outcomes with Gaussian distribution utilized the Normal distribution with identity link, binary outcomes utilized the Binomial distribution with logit link, and count outcomes utilized the Poisson distribution with log link. In addition to age, models also included severity and the interaction between age and severity to determine whether the shape of the age relationship differed by severity. P-values of < 0.05 indicated statistical significance. Whereas selection of the outcomes of interest was exploratory and not based on a priori hypotheses, no adjustment was made for multiple tests. All summary statistics, analyses, and graphs were performed via SAS/STAT ® and SAS/GRAPH ® software, Version 9.4 of the SAS System for Windows, Copyright 2012 SAS Institute, Cary, NC, USA.
Results
Differences between Severe and Non-severe Asthma
Children
General Features (Table I). 26.3% of the cohort were children < 18 years of age (n = 111 severe; n = 77 non-severe). The percentage of males exceeded females, and more African-American children (42%) were enrolled compared to adults (25%). Children with severe asthma had more symptoms and historical exacerbations, lower quality of life, and (by definition) took more controller medications and prednisone than children with non-severe asthma, but were no more obese. There was no significant difference in age at diagnosis of asthma or duration of asthma by severity in children. Mean medication adherence scores in children did not differ by asthma severity. Lung Function (Table II). Children with severe asthma had greater pre-BD airflow limitation than children with non-severe asthma, but post-BD airflow limitation was similar in severe and non-severe asthma. The mean post-BD absolute change in FEV1 % predicted was significantly greater in children with severe asthma. Inflammatory Markers and Allergen Sensitization (Table III). No inflammatory marker, neither blood or sputum eosinophils nor exhaled NO, was higher in children with severe asthma. Likewise, total serum IgE and the degree of allergen sensitization did not differ in children based on asthma severity.
Table I.
Features of the SARP III Cohort by Age and Asthma Severity: Demographics, Anthropometrics, Smoking History, Asthma Control, Quality of Life, Medication Adherence, and Treatment
| Children (< 18 yr.) | Adults | |||
|---|---|---|---|---|
|
| ||||
| Severe | Non- severe |
Severe | Non-severe | |
|
| ||||
| Sample n | 111 | 77 | 313 | 213 |
|
| ||||
| Age at Enrollment (years) | 11.5± 2.8 | 11.6±2.9 | 49.7± 12.8* | 44.5±14.6 |
| Mean ± SD** | ||||
|
| ||||
| Female, n (%)γ | 44 (39.6) | 27 (35.1) | 210 (67.1) | 142 (66.7) |
|
| ||||
| Race/Ethnicity, n (%) | ||||
| Caucasian | 39 (35.1) | 33 (42.9) | 194 (62.0) | 142 (66.7) |
| African-American | 49 (44.1) | 32 (41.6) | 85 (27.2) | 47 (22.1) |
| Other | 23 (20.7) | 12 (15.6) | 34 (10.9) | 24 (11.3) |
| Hispanic | 17 (15.3) | 11 (14.3) | 10 (3.2) | 9 (4.2) |
| Non-Hispanic | 94 (84.7) | 66 (85.7) | 303 (96.8) | 204 (95.8) |
|
| ||||
| BMI (kg/m2) Mean ± SD | 23.3±6.5 | 22.3±5.8 | 33.5± 8.5* | 31.0±7.9 |
|
| ||||
| Obese | ||||
| BMI ≥ 30 (18+ yrs) or BMI ≥ 95th percentile (<18 yrs), n (%) | 42 (37.8) | 23 (29.9) | 195* (62.1) | 96 (45.1) |
|
| ||||
| Smoking History in adults ≥ 30 years (pack years) Mean ± SD | n/a | n/a | 0.9±2.2 | 0.5±1.5 |
|
| ||||
| Asthma Symptom Control ACT ≤ 19 (≥ 12 yrs) n (%) | 29* (72.5) | 8 (26.7) | 240* (76.7) | 90 (42.3) |
| cACT ≤ 19 (age 6–11) n (%) | 51 (71.8)* | 18 (38.3) | n/a | n/a |
| ACQ(6) Mean ± SD | 1.3 ± 0.9* | 0.9 ± 0.8 | 1.9 ± 1.1* | 1.1 ± 0.9 |
| Age of Asthma Diagnosis (yrs) Mean ± SD | 3.0 ± 2.7 | 3.2 ± 2.8 | 20.1 ± 16.5 | 19.0 ± 15.3 |
| Years since onset of asthma symptoms Mean ± SD | 8.4 ± 3.4 | 8.4 ± 3.8 | 29.7 ±15.6* | 25.4 ± 15.6 |
| Years since asthma diagnosis Mean ± SD | 9.1 ± 3.2 | 9.3 ± 3.4 | 32.3 ±15.7* | 28.1 ± 15.9 |
| Number of Exacerbations (Mean ± SD, past 12 months) | 2.8 ±2.9* | 0.8 ±1.0 | 2.3 ± 2.8* | 0.6 ±1.2 |
|
| ||||
| Total AQLQ(S) Score (≥ 12 yrs) Mean ± SD | n/a | n/a | 4.6±1.3* | 5.5±1.1 |
|
| ||||
| Total PAQLQ(S) (age 6–11) Mean ± SD | 4.9 ±1.3 | 5.8±1.1 | n/a | n/a |
| Number Controller | 3.0 | 2.0 | 3.0 | 2.0 |
| Medications Median (IQR) | (2.0, 3.0)* | (1.0, 2.0) | (2.0, 4.0)* | (1.0, 2.0) |
|
| ||||
| Daily Oral Corticosteroids Current n (%) | 15 (13.5)* | 1 (1.3) | 70 (22.4)* | 1 (0.5) |
|
| ||||
| Medication Adherence Results Scale (MARS-5)γ Mean ± SD | 21.9 ± 2.9 | 21.4± 3.7 | 22.4 ± 2.6* | 21.4 ± 3.8 |
Table II.
Features of the SARP III Cohort by Age and Asthma Severity: Lung Function and Maximum Bronchodilator Response
| Children (< 18yr.) | Adults | |||
|---|---|---|---|---|
|
| ||||
| Severe | Non- severe |
Severe | Non-severe | |
|
| ||||
| Sample n | 111 | 77 | 313 | 213 |
|
| ||||
| Pre-BDπ FEV1 (% pred.) Mean ± SD** | 89.3±18.5* | 95.7±14.8 | 68.9±21.4* | 85.8±17.7 |
| Mean Z scoreα | −0.8±1.4* | −0.3±1.2 | −2.1±1.4* | −1.0±1.3 |
|
| ||||
| Pre-BD FVC (% pred.) Mean ± SD | 104.2±16.7 | 108.3±14.2 | 83.3±18.4* | 98.0±16.9 |
| Mean Z score | 0.4 ± 1.4 | 0.7 ± 1.2 | −1.2 ± 1.4* | −0.1 ± 1.3 |
|
| ||||
| Pre-BD FEV1/FVC (% pred.) Mean ± SD | 85.0 ± 11.0 | 88.1 ± 9.6 | 81.2 ± 13.8* | 87.3 ± 10.3 |
| Mean Z score | −1.8 ± 1.2 | −1.5 ± 1.1 | −2.1 ± 1.4* | −1.5 ± 1.2 |
|
| ||||
| Pre-BD FEV1/FVC < LLN µ n (%) | 66* (59.5) | 33 (42.9) | 194* (61.8) | 96 (45.1) |
|
| ||||
| Maximum Post- BD FEV1 (% pred.) Mean ± SD | 105.7 ± 18.0 | 108.4 ± 13.6 | 81.4 ± 20.8* | 96.8 ± 16.6 |
| Mean Z score (SD) | 0.5 ± 1.4 | 0.7 ± 1.1 | −1.2 ± 1.4* | −0.2 ± 1.2 |
|
| ||||
| Maximum Post- BD FEV1/FVC (% pred.) Mean ± SD | 94.8 ± 8.6 | 96.0 ± 7.5 | 86.5 ± 12.8* | 93.4 ± 9.4 |
|
| ||||
| Mean Z score | −0.6 ± 1.2 | −0.5 ± 1.0 | −1.5 ± 1.4* | −0.8 ± 1.1 |
|
| ||||
| Maximum Post- BD FEV−1/FVC < LLN n (%)γ | 20 (18.0) | 14 (18.2) | 141* (45.3) | 47 (22.1) |
|
| ||||
| FEV1 BD Response (absolute change) Mean ± SD | 16.4 ± 11.2* | 12.7 ± 7.9 | 12.5 ± 8.2* | 11.0 ± 8.1 |
BD, bronchodilator;
p < 0.05 severe versus non-severe;
SD, standard deviation;
Z scores from reference 10;
LLN, lower limits of normal defined by −1.6 Z scores;
% expressed per column.
Table III.
Features of the SARP III Cohort by Age and Asthma Severity: Markers of Inflammation
| Children (< 18 yrs) | Adults | |||
|---|---|---|---|---|
|
| ||||
| Severe | Non-severe | Severe | Non-severe | |
|
| ||||
| Sample n | 111 | 77 | 313 | 213 |
|
| ||||
| Sputum Differential n | 27 | 17 | 241 | 166 |
|
| ||||
| Sputum Cell Count (cells × 104/µl) Median (min, max) | 77.4 (23.7, 153.1) | 61.9 (9.5, 199.8) | 97.6 (0.0, 195.3) | 82.4 (34.9, 187.0) |
|
| ||||
| Sputum Eosinophil % Median (min, max) | 1.6 (0.0, 53.7) | 1.1 (0.0, 61.4) | 0.8 (0.0, 63.9) | 0.7 (0.0, 59.4) |
|
| ||||
| Sputum Neutrophil % Median (min, max) | 53.8 (9.4, 90.1) | 40.8 (8.3, 80.3) | 51.7 (1.5, 99.8) | 55.8 (0.5, 99.3) |
| FeNO (ppb) Median (quartiles) | 23.0 (12.0, 46.0) | 28.0 (12.0, 49.0) | 21.0 * (13.0, 37.0) | 24.0 (16.0, 43.0) |
|
| ||||
| Expired NO > 30 ppb n (%)γ | 40 (36.7) | 33 (44.0) | 96 (31.1)* | 87 (40.8) |
|
| ||||
| Serum IgE Median (quartiles) | 465 (164, 1207) | 490 (151, 834) | 163 (45, 384) | 141 (46, 374) |
|
| ||||
| At least 1/ of 15 positive blood IgE tests n (%) | 104 (94.5) | 67 (89.3) | 234 (75.2) | 173 (82.0) |
|
| ||||
| Number of positive (of 15) allergen-specific IgE tests Median (min, max) | 6.0 (3.0, 11.0) | 7.0 (3.0, 11.0) | 3.0* (0.5, 7.0) | 4.0 (2.0, 7.0) |
|
| ||||
| Highly sensitized ≥ 4/15 positive allergen tests n (%) | 74 (67.3) | 50 (66.7) | 115 (37.0)* | 101 (47.9) |
|
| ||||
| Blood eosinophils (%) Median (quartiles) | 5.4 (2.5, 9.0) | 5.6 (3.5, 8.3) | 3.0 (2.0, 5.7) | 3.0 (2.0, 5.0) |
|
| ||||
| Total blood eosinophils (cells/ul) Median (quartiles) | 324 (162,514) | 359 (208,575) | 228* (134,399) | 189 (111, 320) |
|
| ||||
| Blood eosinophilia ≥ 300 cells/µL n (%) | 60 (54.1) | 49 (63.6) | 120 (38.5)* | 60 (28.2) |
p < 0.05, severe versus non-severe;
% expressed per column.
Adults
General Features (Table I). Adults with severe (n=313) and non-severe (n=213) asthma had similar proportions of female and minority participants. Adults with severe asthma were significantly older, more likely to have a higher BMI, more symptoms, and had more historical exacerbations, longer duration of asthma, and lower quality of life despite treatment with higher doses of corticosteroids. Adherence to treatment and age at diagnosis did not differ by severity. Lung Function (Table II). Adults with severe asthma had greater pre- and post-BD airflow limitation than adults with non-severe asthma. The mean post-BD absolute change in FEV1 % predicted was significantly greater in adults with severe asthma. Inflammatory Markers and Allergen Sensitization (Table III). Adults with severe asthma had similar values for total and differential cell counts in induced sputum compared to adults with non-severe asthma, but adults with non-severe asthma had higher exhaled NO levels than severe asthma. Adults with severe asthma had higher total blood eosinophil counts and a higher prevalence of blood eosinophilia (> 300 cells/ul) than adults with non-severe asthma. Although total serum IgE levels did not differ significantly based on asthma severity, enrollees with severe asthma had a significantly lower number of positive allergen-specific IgE blood tests.
Effects of Age on the Features of Asthma
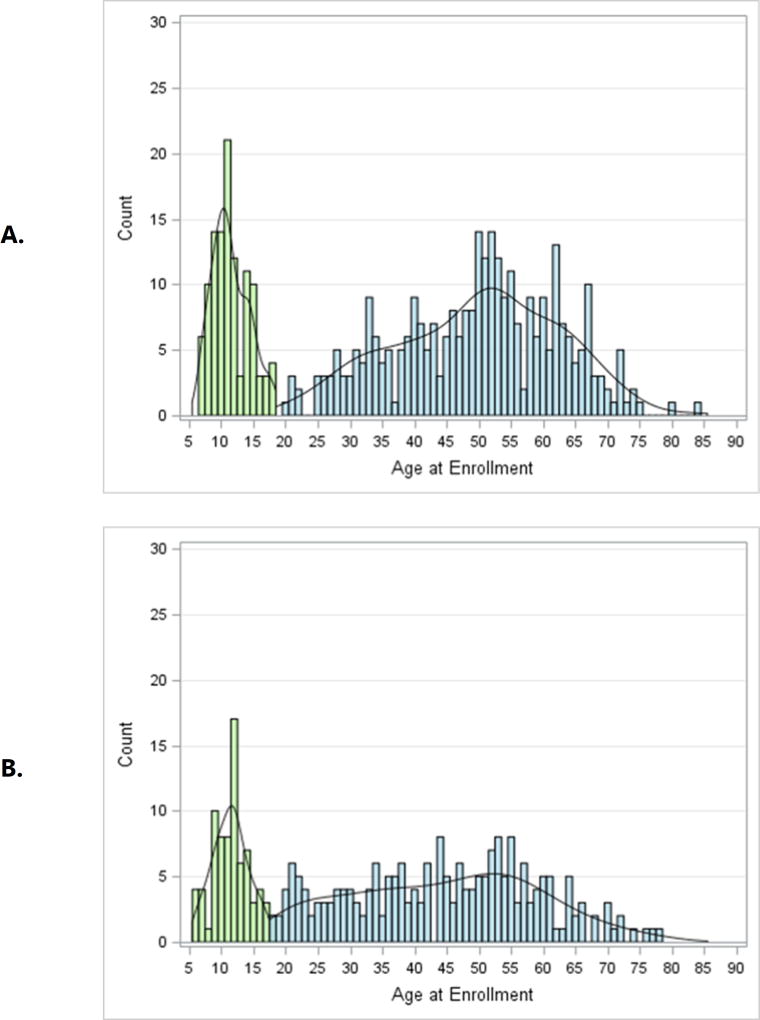
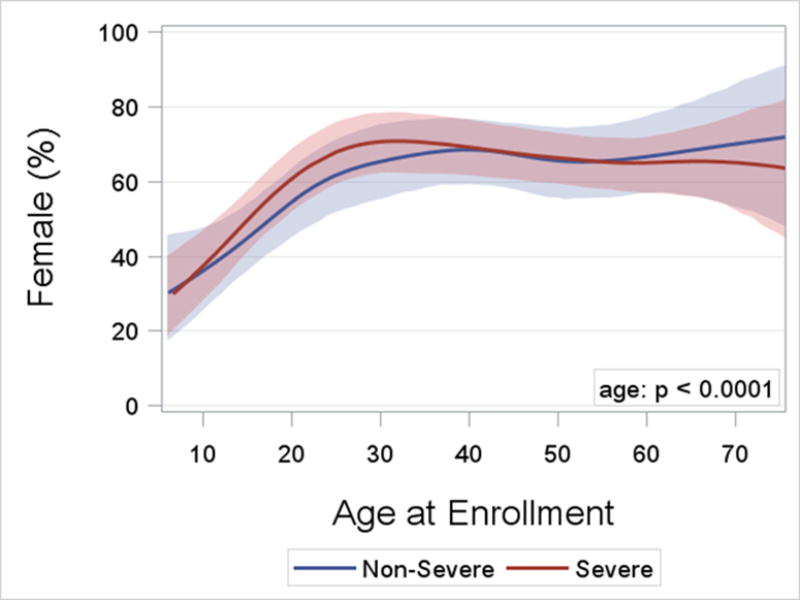
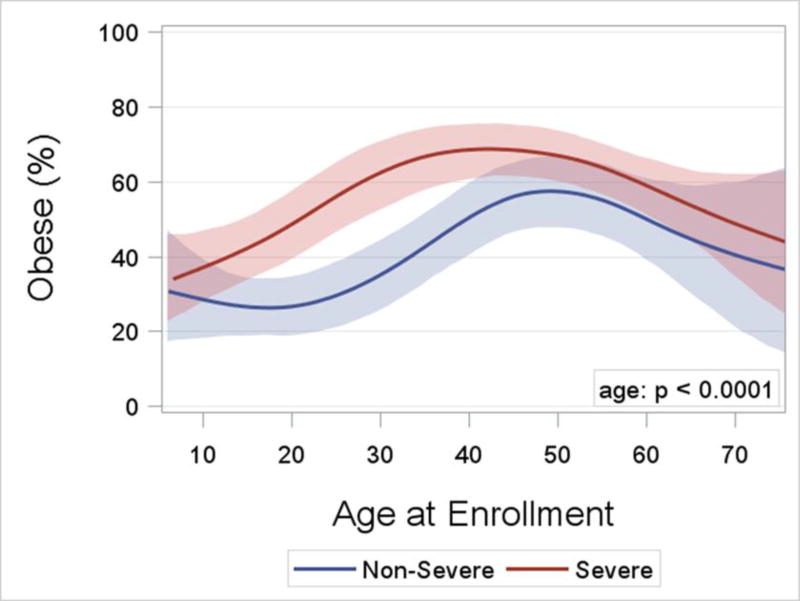
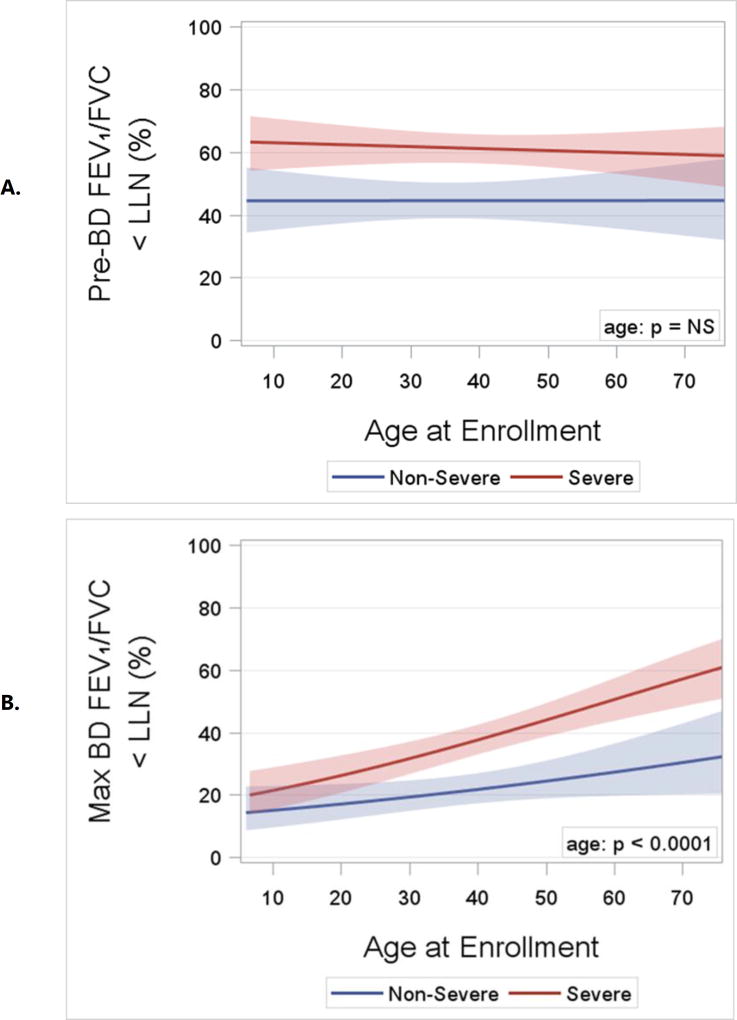
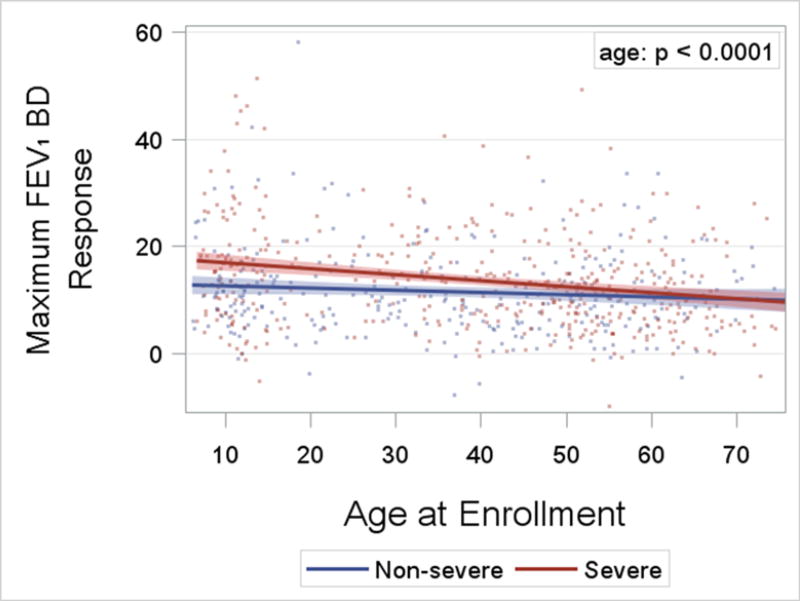
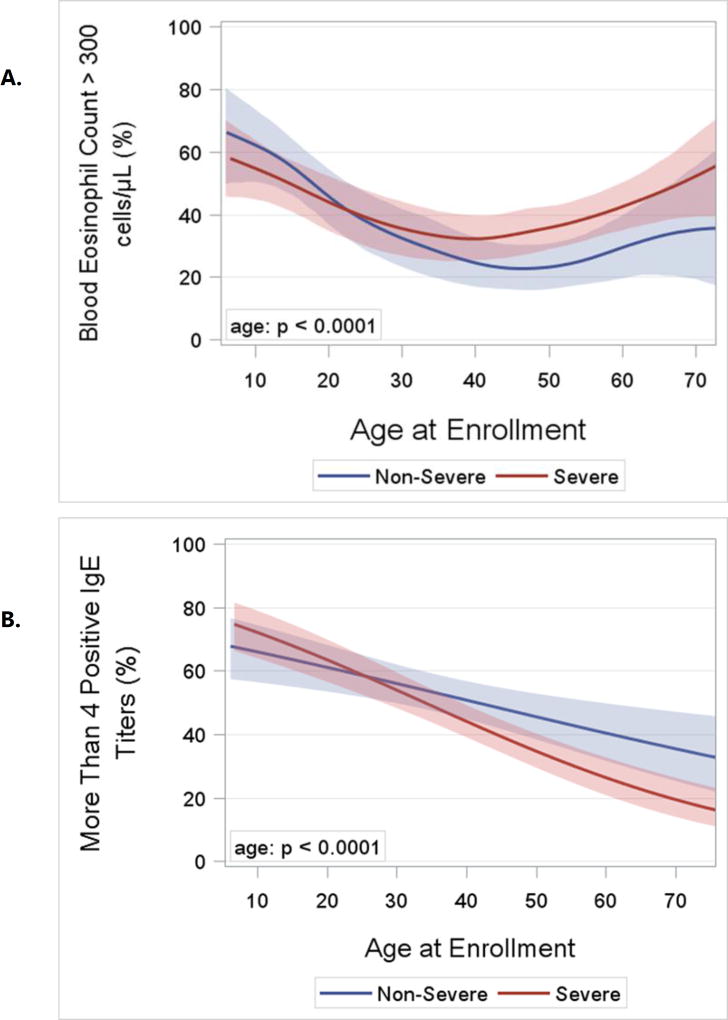
Asthma Severity. After 18 years of age the number of enrollees with severe asthma was higher and peaked in middle-age between 50 and 55 years (Figure 2). In contrast, the number of enrollees with non-severe asthma did not change appreciably after childhood. The distribution by age for adults with severe asthma differed from those with non-severe asthma (p = 0.002). Sex Ratio. Pre-pubescence the ratio of boys to girls favored boys, but from young adulthood onward the ratio of women was higher than men for both severe and non-severe asthma (p < 0.0001; Figure 3). BMI and Obesity. From childhood to middle age BMI was higher regardless of asthma severity, and was lower from 50 years of age onward (p < 0.0001; Online Repository, Figure E1). Accordingly the prevalence of obesity followed a similar pattern with advancing age (p < 0.0001; Figure 4) which likewise was no different based on severity. Lung Function. Both the pre- and post-BD FEV1/FVC % predicted were lower as age increased (p < 0.05 for both; Online Repository Figures E2A and E2B), but the percentage of enrollees who met the definition of pre-BD airflow limitation was not different with age (p = 0.60; Figure 5A). In contrast, the percentage of enrollees with post-BD airflow limitation was higher with age (p < 0.001; Figure 5B). The maximum absolute change in FEV1 % predicted post-BD was lower with age (p < 0.0001; Figure 6). Inflammatory Markers and Allergen Sensitization. Whereas peripheral blood eosinophil counts were approximately 50% higher in children than adults, blood eosinophilia was lower by middle age but then was higher in late middle-age enrollees (p < 0.0001; Figure 7A). Removing participants who took oral prednisone (n = 102; 96 severe, 6 non-severe) from the analysis had no effect on differences in blood eosinophilia with age. Exhaled NO values were significantly lower with age (p < 0.0001; Online Repository, Figure E3). Children had significantly greater number of positive allergen-specific IgE blood tests than adults (p < 0.0001, Figure 7B), and accordingly, serum IgE levels in children were 3–4-fold higher than adults (p < 00001; Online Repository Figure E4). Adherence. Adherence to treatment was not different with age.
Figure 2.
Histograms of enrollee counts and age for A) severe and B) non-severe asthma. Enrollees < 18 years of age are shaded in light green. Due to pre-specified recruiting goals of 25% children and equal numbers of children between 6 −11 years and 12–17 years, comparison of the empirical distributions for severe and non-severe asthma was limited to adult enrollees shaded in blue (p = 0.002) by the two-sample Kolmogorov-Smirnoff test.
Figure 3.
Logistic regression curves with cubic splines and bootstrap confidence limits for the percent of female participants by age and asthma severity. The age effect is significant (p < 0.0001; likelihood ratio test), but the shape of the curves with age do not differ significantly by severity (p= n.s. for age by severity interaction).
Figure 4.
Logistic regression curves with cubic splines and bootstrap confidence limits for the percentage of obesity by age at enrollment and asthma severity. The age effect is significant (p < 0.0001; likelihood ratio test), but the shapes of the curves do not differ by severity (p=ns for age by severity interaction).
Figure 5.
A. Logistic regression curves and confidence limits for the percentage of pre-BD airflow limitation by age and asthma severity. The age effect is not significant (n= ns; likelihood ratio test). B. Logistic regression curves and confidence limits for the percentage of post-BD airflow limitation by age and asthma severity. The age effect is significant (p < 0.0001; likelihood ratio test), but the slopes of the curves do not differ significantly by severity (p = ns for age by severity interaction).
Figure 6.
Linear regression lines and confidence limits for the absolute change in FEV1% post-BD by age and asthma severity. The age effect is significant (p< 0.0001; likelihood ratio test), and the slopes differ significantly by severity (p = 0.03; for age by severity interaction).
Figure 7.
A. Logistic regression curves with cubic splines and bootstrap confidence limits for the percentage of enrollees with total blood eosinophil count > 300 cells per ul of blood by age and asthma severity. The age effects is significant (p< 0.0001; likelihood ratio test), but the shapes of the curves do not differ significantly by severity (p = ns for age by severity interaction). B. Logistic regression curves and confidence limits for the percent of enrollees with four or more positive blood allergen-specific IgE tests by age and asthma severity. The age effect is significant (p < 0.0001; likelihood ratio test). Additionally the slopes differ by severity (p = 0.03 for age by severity interaction.
Interaction of Age and Asthma Severity on Specific Outcomes
The interaction of age and asthma severity was significant for three outcomes. The absolute BD response and prevalence of sensitization to four or more allergens were both significantly lower with age in severe than in non-severe asthma. Likewise, although exhaled FeNO levels were lower with age for the entire sample, exhaled NO values in severe asthma were relatively flat with age compared to the sharp decline from 20 to 50 years of age in FeNO in non-severe asthma (p < 0.0001).
Discussion
We report herein on the baseline characteristics of the SARP III cohort, a unique sample recruited according to pre-specified demographic features at academic health centers concentrated in urban settings. Thus the sample is enriched in patients with severe asthma, women, and non-white minorities. In this report we compare the baseline features of severe and non-severe asthma in cross-section and then study the effects of advancing age and severity on specific outcomes. We found that children with asthma, regardless of severity, were predominately male with normal body mass and normal lung function, but a relatively high level of blood eosinophilia and allergen sensitization. By contrast adults with asthma, also regardless of severity, were mostly female, with more obesity, and greater airflow limitation. The proportion of enrollees with allergen sensitization was high at all ages, although it was significantly lower in severe asthma by mid-adulthood. The prevalence of airflow limitation was significantly greater with age, and was accompanied by a lower maximum bronchodilator response. Standard markers of Th2 inflammation, both allergen sensitization and blood eosinophilia, were significantly lower after childhood.
These results are comparable with the features of severe asthma reported in previous characterization studies. 2–6, 15–16 Consistent among these reports, adults with severe asthma typically include a greater proportion of women, and have more symptoms, more exacerbations, and a greater degree of bronchodilator-resistant airflow limitation. Generally adults with severe asthma have relatively less allergen sensitization than their non-severe counterparts, whereas sputum and blood eosinophil counts vary significantly between cohorts. The SARP III cohort is most unique compared to other asthma cohorts in having a relatively greater prevalence of obesity and lower sputum eosinophils. 15–16 These findings may be attributable to intrinsic population differences, but also could be due to differences in recruiting practices by study investigators between Europe and the U.S. For example participants with severe asthma in U-BIOPRED, a large European characterization study, were required to have been under the care of a respiratory physician for at least 6 months preceding enrollment. 16 This was not an inclusion criterion for severe asthma in SARP III, and might account in part for the differences in the two cohorts.
A striking finding in the SARP III cohort was a complete reversal of the proportion of males to females after adolescence. This was the case for enrollees with severe and non-severe asthma. This result has been consistently found in other asthma cohort studies 2,15–17 and has been attributed to a greater degree of bronchial responsiveness in post-pubertal females 18 and potential deleterious effects of estrogen and progesterone on lung function 19 and beta adrenergic receptor function. 20 Alternatively, testosterone may confer protective effects on lung function. 21 Sex hormone and lung function changes with puberty will be studied in the longitudinal SARP III protocol so as to better understand the marked change in sex ratio from males to females that occurs post-adolescence in asthma.
The prevalence of obesity in the SARP III cohort was higher from childhood to middle age but then was lower after the fifth decade of life. Children had significant variability in body mass index but overall children were less obese than adults, wherein the prevalence of obesity in severe adults was nearly two-fold higher (62% adults versus 38% children). Factors which may explain the relatively high prevalence of obesity in the SARP III sample include the general increase in obesity in the U.S. population and enrichment of the cohort with urban African Americans, a particularly at risk minority group. 22–23 Obesity appears to predispose adult women more than it does men and appears to be at least as important as an asthma risk factor as the metabolic syndrome, if not more important. 24–25 Understanding of how obesity and metabolic syndrome affect asthma is broadened by the recent finding that metabolic syndrome is associated with more severe asthma but involves only a subset of obese patients. 26
The prevalence of post-bronchodilator airflow limitation in the cohort was significantly higher with advancing age. Whereas post-bronchodilator airflow limitation was no different by severity in children, airflow limitation associated with a lower maximum bronchodilator response was more common among adults with severe asthma. This was due in part to loss of the maximum bronchodilator response, which was significantly lower with advancing age, and the slope of this relationship was steeper in severe versus non-severe asthma. Differences in lung function with age were highly discordant with age-related differences in markers of Th2 inflammation, which improved as lung function deteriorated. This discordance is the basis for our speculation that alternate mechanisms, supplemental to Th2 inflammation, may favor the progression of airflow limitation with age.
Allergen sensitization, an important phenotypic feature of both severe and non-severe asthma, likewise was informed significantly by advancing age. The downward slope of the proportion of enrollees sensitized to four or more allergens with advancing age was particularly steep from childhood to middle age in adults with severe compared with non-severe asthma. Older age per se is associated with a reduction in the prevalence of allergen sensitization in the general population, both in healthy individuals and those with respiratory disease. 27 Thus a plausible reason for the lower proportions of adults with allergen sensitization in the SARP III cohort could be differential recruitment of more non-allergen sensitized adults compared to children. Likewise we would also consider heterogeneity of the severe asthma phenotype, particularly inclusion of a cluster of adult women identified in SARP I/II 5 and in Europe by Haldar and colleagues 28 with relatively low allergen sensitization but troublesome symptoms refractory to anti-inflammatory therapies. Even though allergen sensitization is lower with age in severe asthma, most adults with severe asthma and non-severe asthma are allergen-sensitized well into late middle-age.
The prevalence of peripheral blood eosinophilia was significantly higher in children than adults but was not differentiated by asthma severity. Although the slope of the prevalence of blood eosinophilia was sharply downward from childhood to middle age, it reached a plateau and then rose from late middle age onward. The relatively low number of enrollees in the sample older than 65 years of age might have skewed this result. Although the overall downward pattern in blood eosinophilia with age appeared to be attenuated in severe asthma, the interaction of age and severity on this outcome was not significant. Because of the relatively low number of acceptable sputum samples in children, we did not analyze the effects of advancing age on sputum eosinophilia. A striking feature of the SARP III cohort is the relatively low numbers of sputum eosinophils compared to those seen in severe asthmatics reported in European cohorts. 16, 29 We have no ready explanation for this except that it could be attributed to differences in recruiting practices, treatment, and sputum induction/quantification methods.
In the SARP III cohort FeNO was highest in young adults between 20 and 35 years of age, and was relatively higher in non-severe than it was in severe asthma. The effects of age on FeNO compared with peripheral blood eosinophilia were strikingly dissimilar, a result that suggests factors governing the level of FeNO in asthma are different from those governing blood eosinophilia. While the mechanisms for these differences are unclear, in trials of anti-IL-5, blood eosinophils were reduced without impacting FeNO, and in trials of anti-IL4rα antibodies, FeNO was reduced without decreasing blood eosinophils. 30–31 Mechanisms regulating FeNO include airway buffering capacity, 32 enhanced GSNOR activity, 33 and T-helper I inflammation. 34–35 Other factors that could have independently affected exhaled NO levels in the cohort include corticosteroid use and obesity. 36–38
In summary, we found significant differences in the baseline phenotypic features of severe and non-severe asthma in children and adults. Whereas children with asthma tend to be males with normal weight and lung function in the context of eosinophilia and frequent allergen sensitization, adults with asthma have more obesity and are more heterogeneous with regard to measures of lung function, eosinophilia, and allergen sensitization. Among adults with asthma, increasing age is significantly associated with greater airflow limitation, a decline and then greater eosinophilia by late middle age, and less allergen sensitization. Age-related differences in features affected by asthma severity were limited to the maximum BD response, allergen sensitization, and FeNO. The greatest number of enrollees in the SARP III cohort with severe asthma were in middle age, suggesting that gender effects, obesity, and novel immune perturbations that occur more commonly in middle age inform the severe asthma phenotype.
Supplementary Material
Clinical Implications/ Highlights Box.
-
What is already known about this topic?
Severe asthma has distinct phenotypic features in childhood, but whether the features of severe asthma are different with age has not been widely studied.
-
What does this article add to our knowledge?
With advancing age, asthma is more prevalent in women than men, and is associated with higher BMI and greater airflow limitation, but lower markers of Th2 inflammation.
-
How does this study impact current management guidelines?
Alternate therapies targeting non-Th2 mechanisms of inflammation need further study in the management of adult patients with severe asthma.
Acknowledgments
Principal/Co-Principal Investigators Funded by the NIH/NHLBI Severe Asthma Research Program:
Eugene R. Bleecker, PI, Wake Forest University, U10 HL109164
Mario Castro, PI, Washington University, U10 HL109257
John V. Fahy, PI, University of California San Francisco, U10 HL109146
Elliot Israel and Bruce Levy, Co-PI’s, Brigham and Women’s Hospital, U10 HL109172
Ben Gaston, PI, Case Western Univ. Virginia-Cleveland Consortium, U10 HL109250
Serpil Erzurum, Co-PI, Cleveland Clinic, Virginia-Cleveland Consortium, U10 HL109250
W. Gerald Teague, Co-PI, Univ. of Virginia, Virginia-Cleveland Consortium, U10 HL109250
Nizar N. Jarjour, PI, University of Wisconsin, U10 HL109168
Sally E. Wenzel, PI, University of Pittsburgh, U10 HL109152
David T. Mauger, PI, DCC, Penn State University, U10 HL109086-04
The authors acknowledge the contributions of the study coordinators and staff at each of the clinical centers and the Data Coordinating Center as well as all the study participants that have been integral to the success of the Severe Asthma Research Program. Spirometers used in SARP III were provided by nSpire Health (Longmont, CO). The authors appreciate the support of the Scientific Program Officers at the National Heart, Lung and Blood Institute (Dr. Patricia Noel, Dr. Tom Croxton, and Dr. Robert Smith) and input from the members of the Data Safety and Monitoring Board.
Abbreviations
- ACQ6
Asthma control quotient (6 item)
- ACT/cACT
Asthma Control Test, childhood Asthma Control Test
- ATS
American Thoracic Society
- BD
Bronchodilator
- BMI
body mass index
- CBC
complete blood count
- DCC
Data Coordinating Center
- DSMB
Data Safety and Monitoring Board
- EBC
exhaled breath condensate
- ENFUMOSA
European Network for Understanding Mechanisms of Severe Asthma
- ERS
European Respiratory Society
- FeNO
fraction expired nitric oxide
- FEV1
forced expired volume in 1 second
- FEV1/FVC
forced expired volume in 1 second/forced vital capacity ratio
- FVC
forced vital capacity
- IgE
immunoglobulin E
- IRB
Institution Review Board
- NIH
National Institutes of Health
- NHLBI
National Heart Lung Blood Institute
- SARP
Severe Asthma Research Program
- UBIOPRED
Unbiased BIOmarkers in PREDiction of respiratory disease outcomes
- µl
microliter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk P, Adcock IM, Bateman E, Bel E, Bleecker E, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jarjour N, Mauad T, Sorkness R, Teague WG. International ERS/ATS Consensus Definition, Mechanisms, Evaluation and Treatment of Severe Asthma. Eur Respir J. 2014;43(2):343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, Simons FE, Weiss ST, Wenzel SE, Borish L, Bleecker ER TENOR Study Group. Consistently very poorly controlled asthma as defined by impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2009;124:895–902. doi: 10.1016/j.jaci.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick AM, Gaston B, Erzurum S, Teague WG. Features of severe asthma in school age children: Atopy and increased exhaled NO. J Allergy Clin Immunol. 2006;118:1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore WC, Bleecker ER, Everett DC, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour N, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE for the National Heart Lung Blood Institute Severe Asthma Research Program (SARP). Characterization of the severe asthma phenotype by the National Heart Lung Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore WC, Meyers DA, Wenzel SE, Teague WG, Huashi L, Xingnan L, Castro M, Curran-Everett D, Fitzpatrick A, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SAA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum S, MD2, Bleecker ER for the National Heart Lung Blood Institute’s Severe Asthma Research Program: Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Castro M, Becharier L, Gaston BM, Bleecker ER, Moore WC. Heterogeneity of severe asthma in childhood: Confirmation by cluster analysis of children in the NIH/NHLBI Severe Asthma Research Network. J Allergy Clin Immunol. 2011;127:382–89. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SAA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, Fitzpatrick AM, Gaston BM, Israel E, Hastie A, Hoffman EA, Holguin F, Levy BD, Meyers DA, Moore WC, Peters SP, Sorkness RL, Teague WG, Wenzel SE, Busse WW. Severe Asthma: Lessons learned from the NHLBI Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–62. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn RM, Lehman E, Chinchilli VM, Martin RJ, Boushey HA, Israel E, Kraft M, Lazarus SC, Lemanske RF, Lugogo NL, Peters SP, Sorkness CA, Szefler S, Wechsler ME on behalf of the NHLBI Asthma Clinical Research Network. Impact of age and sex on response to asthma therapy. Am J Respir Crit Care Med. 2015;192:551–8. doi: 10.1164/rccm.201503-0426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tommelein E, Mehuys E, Van Tongelen I, Brusselle G, Bousserv K. Accuracy of the Medication Adherence Report Scale (MARS-5) as a quantitative measure of adherence to inhalation medications in patients with COPD. Ann Pharmacother. 2014;48:589–95. doi: 10.1177/1060028014522982. [DOI] [PubMed] [Google Scholar]
- 10.Quanjer PH, Sanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J. ERS Global Lung Function Initiative. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa K, Stoll SJ, Ahn J, Bittner JC, Camargo CA. Prevalence of eosinophilia in hospitalized patients with asthma exacerbation. Respir Med. 2015;109:130–2. doi: 10.1016/j.rmed.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, Taylor DR. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004;169:473–478. doi: 10.1164/rccm.200310-1376OC. [DOI] [PubMed] [Google Scholar]
- 13.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SAA, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Israel E, Jarjour N, Moore W, Peters S, Teague G, Gaston B, Erzurum SC for the National Heart Lung Blood Institute Severe Asthma Research Program (SARP) Use of exhaled nitric oxide measurement to identify a reactive, at- risk phenotype among patients with severe asthma. Am J Respir Crit Care Med. 2010;181(10):1033–41. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martyn M, Weaver AL, Jacobson RM, Juhn YJ. Characterization of the duration from onset of asthma symptoms to asthma disease. Ann Allergy Asthma Immunol. 2008;100:589–95. doi: 10.1016/S1081-1206(10)60059-2. [DOI] [PubMed] [Google Scholar]
- 15.The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicenter study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 16.Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, Pandis I, Bansal AT, Bel EH, Auffray C, Compton CH, Bisgaard H, Bucchioni E, Caruse M, Chanez P, Dahlen B, Dyson K, Frey U, Geiser T, Gerhardsson de Verdier M, Gibeon D, Guo Y, Hashimoto S, Hedlin G, Jeyasingham E, Hekking PW, Higenbottam T, Horvath I, Knox AJ, Krug N, Erpenbeck VJ, Larsson LX, Lazarinis N, Matthews JG, Middelveld R, Montuschi P, Musial J, Myles D, Pahus L, Sandstrom T, Seibold W, Singer F, Strandberg K, Vestbo J, Vissing N, von Garnier C, Adcock IM, Wagers S, Rowe A, Howarth P, Wagener AH, Djukanovic R, Sterk PJ, Chung KF on behalf of the U-BIOPRED Study Group. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 17.Zein JG, Dweik RA, Comhair SA, Bleecker ER, Moore WC, Peters SP, Busse WW, Jarjour NN, Calhoun WJ, Castro M, Chung KF, Fitzpatrick A, Israel E, Teague WG, Wenzel SE, Love TE, Gaston BM, Erzurum SC on behalf of the Severe Asthma Research Program. Asthma is more severe in older adults. PLoS ONE. 2015:e0133490. doi: 10.1371/journal.pone.0133490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research Group. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med. 2008;178(4):325–31. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YH, Lee E, Cho HJ, Yang SI, Jung YH, Kim HY, Seo JH, Kim HB, Lee SY, Song DJ, Kim WK, Jang GC, Shim JY, Kim EJ, Lee JS, Kwon JW, Hong SJ. Association between menarche and increased bronchial hyperresponsiveness during puberty in female children and adolescents. Pediatr Pulmonol. 2016;51:1040–47. doi: 10.1002/ppul.23433. [DOI] [PubMed] [Google Scholar]
- 20.Wheeldon NM, Newnham DM, Coutie WJ, Peters A, McDevitt DG, Lipworth BJ. Influence of sex-steroid hormones on the regulation of lymphocyte β2 adrenoceptors during the menstrual cycle. Br J Clin Pharmacol. 1994;37:583–88. doi: 10.1111/j.1365-2125.1994.tb04308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzel SE, Robinson CB, Leonard JM, Panettieri RA. Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010;31(6):461–471. doi: 10.2500/aap.2010.31.3384. [DOI] [PubMed] [Google Scholar]
- 22.Laurier D, Guiguet M, Chau NP, Wells JA, Valleron AJ. Prevalence of obesity: a comparative survey in France, the United Kingdom and the United States. Inter J Obesity and Related Metabolic Disorders. 1992;16:565–572. [PubMed] [Google Scholar]
- 23.Wang Y, Beydoun MA. The obesity epidemic in the United States - gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol Rev. 2007;29(1):6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 24.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–2050. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 25.Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M, Jacobs DR, Jr, Sood A. Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women: the longitudinal CARDIA study. Am J Respir Crit Care Med. 2013;188:319–326. doi: 10.1164/rccm.201303-0457OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. The Lancet Respir Med. 2016;4:574–84. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scichilone N, Callari A, Augugliaro G, Marchese M, Togias A, Bellia V. The impact of age on prevalence of positive skin prick tests and specific IgE tests. Respir Med. 2011;105:651–658. doi: 10.1016/j.rmed.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Haldar P, Pavord ID, Shaw DE, Berry MA, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Refractory eosinophilic airway inflammation in severe asthma: Effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004;170:601–605. doi: 10.1164/rccm.200404-440OC. [DOI] [PubMed] [Google Scholar]
- 30.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkessili S, Rocklin R, Bock B, Hamilton J, Ming JE, Radin A, Stahl N, Yancopoulos GD, Graham N, Pirozzi G. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 32.Gaston B, Kelly R, Urban P, Liu L, Henderson EM, Doctor A, Teague WG, Fitzpatrick A, Erzurum S, Hunt JF. Buffering airway acid decreases exhaled nitric oxide in asthma. J Allergy Clin Immunol. 2006;118:817–22. doi: 10.1016/j.jaci.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Marozkina NV, Wang XQ, Stsiapura V, Fitzpatrick A, Carraro S, Hawkins G, Bleecker E, Meyers D, Jarjour N, Fein S, Wenzel S, Busse WW, Castro M, Panettieri R, Moore W, McLaughlin D, Lewis SJ, Palmer LA, Altes T, de Lange E, Erzurum S, Teague WG, Gaston B. Phenotype of asthmatics with increased airway S-nitrosoglutathione reductase activity. Eur Resp J. 2015;45:87–97. doi: 10.1183/09031936.00042414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo FH, Uetani K, Haque SJ, Williams BR, Dweik RA, Thunnissen FB, Calhoun W, Erzurum SC. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100:829–38. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Ray A, Ray P, Erzurum SC, Busse WW, Zhao J, Trudeau JB, Wenzel SE. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. 2014;7:1175–85. doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 37.Uppalapati A, Gogineni S, Espiritu JR. Association between body mass index (BMI) and fraction of exhaled nitric oxide (FeNO) levels in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Obes Res Clin Prac. 2016 doi: 10.1016/j.orcp.2015.11.006. S1871-403X (15)00196-9 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Komakula S, Khatri S, Mermis J, Haque J, Savill S, Rojas M, Brown LA, Teague WG, Holguin F. In asthmatics body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes. Respir Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.