Abstract
Ischemic stroke remains one of most common causes of death and disability worldwide. Stroke triggers a cascade of events leading to rapid neuronal damage and death. Neuroprotective agents that showed promise in preclinical experiments have failed to translate to the clinic. Even after decades of research, tPA remains the only FDA approved drug for stroke treatment. However, tPA is effective when administered 3–4.5 hours after stroke onset and the vast majority of stroke patients do not receive tPA therapy. Therefore, there is a pressing need for novel therapies for ischemic stroke. Since stroke induces rapid cell damage and death, neuroprotective strategies that aim to salvage or replace injured brain tissue are challenged by treatment time frames. To overcome the barriers of neuroprotective therapies, there is an increasing focus on neurorestorative therapies for stroke. In this review article, we provide an update on neurorestorative treatments for stroke using cell therapy such as bone marrow derived mesenchymal stromal cells (BMSCs), human umbilical cord blood cells (HUCBCs) and select pharmacological approaches including Minocycline and Candesartan that have been employed in clinical trials. This review article discusses the present understanding of mechanisms of neurorestorative therapies and summarizes ongoing clinical trials.
Keywords: Stroke, Neurorestoration, Neurovascular remodeling, White matter remodeling, Synaptogenesis, Stem Cell therapy, Mesenchymal stromal cells, Umbilical cord blood cells, MicroRNA, Minocycline, Candesartan
1. Introduction
Ischemic stroke is a global health concern that often leads to lifelong disability or death of patients (Lackland et al., 2014). Stroke patients and their families incur steep social and medical burdens. To date, tissue plasminogen activator (tPA) is the only pharmacological agent approved by the US Food and Drug Administration to treat ischemic stroke. However, the use of tPA is limited by its narrow therapeutic time window of 3 to 4.5 hours from ischemic stroke onset, and a large majority of patients do not receive timely medical attention needed for tPA administration. Recent advances in mechanical clot retrieval strategies such as mechanical thrombectomy for treatment of large artery stroke have enabled rapid and effective recanalization using endovascular approaches, and patients with salvageable tissues benefit from improved outcomes (Linfante and Cipolla, 2016). However, only a small population of stroke patients are eligible for acute endovascular intervention (Linfante and Cipolla, 2016). There has been a research focus on developing novel and effective treatments for stroke in the last few decades. While there has been significant improvement in stroke awareness, care and rehabilitation, effective treatments for the management of ischemic stroke remain constrained. The rapid damage and death of brain cells after ischemic stroke onset has limited the time window for initiation of neuroprotective treatments. Several factors have hindered clinical translation of experimental therapeutics including a narrow time window for treatment, widespread use of healthy young male animals in preclinical research, and lack of including co-morbidities such as diabetes and hypertension (Jolkkonen and Kwakkel, 2016; Pennypacker et al., 2017). In the wake of understanding the limitations of neuroprotective strategies, there has been a paradigm shift in research strategies towards neurorestorative therapies (Cramer and Chopp, 2000) either as a stand-alone treatment or as an adjunct therapy to improve stroke outcome. Neurorestorative therapies for stroke typically have wide therapeutic window of days to weeks after stroke onset and aim to amplify endogenous brain repair mechanisms and improve neurological functional outcome after stroke by promoting restorative mechanisms such as neurovascular remodeling, white matter remodeling and attenuating local and systemic inflammatory and immune responses. Neurorestorative agents target intact parenchymal cells such as neurons, glial cells and endothelial cells to promote brain remodeling or repair of damaged tissue. Neurorestorative therapies for stroke aim to improve neurovascular remodeling, white matter remodeling and synaptogenesis, and decrease inflammatory and immune responses, which are discussed below and summarized in Figure 1.
Figure 1.
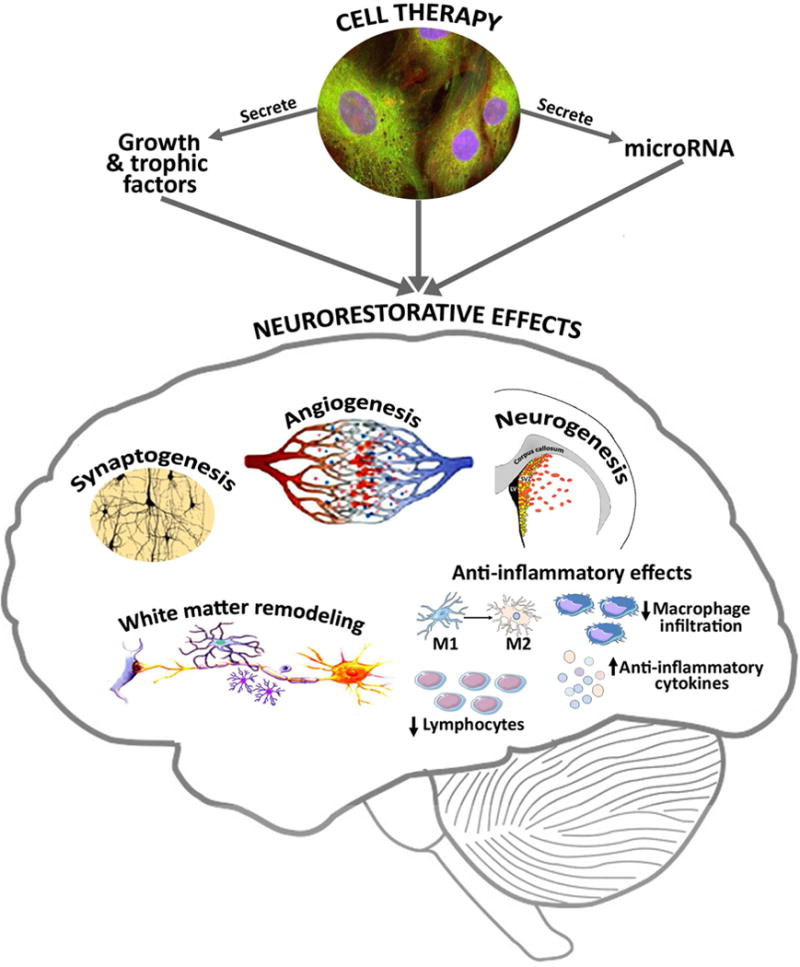
Neurorestorative mechanisms underlying cell therapy after stroke.
1.1 Neurovascular remodeling
The neurovascular unit includes the anatomical and functional interactions between neurons, glial cells and vascular cells (endothelial cells, pericytes) in the brain and controls brain homeostasis (Venkat et al., 2016). The cross-talk among these cells regulates complex brain functions under normal and pathological conditions. Interactions between the various components of the neurovascular unit support blood brain barrier (BBB) integrity and function. Neurovascular uncoupling and disruption of the BBB during neurological diseases such as stroke, aggravate inflammatory responses and exacerbate brain damage (Abbruscato and Davis, 1999). Neurorestorative therapies promote neuronal plasticity, glial cell proliferation, neovascularization, angiogenesis and arteriogenesis to initiate repair of damaged tissue and importantly, facilitate structural and functional reorganization of the neurovascular unit, which is critical to improve neurological functional outcome after stroke (Chen et al., 2003b; Liu et al., 2007).
Angiogenesis is the process of sprouting new blood vessels from pre-existing blood vessels. Angiogenesis includes a series of events such as endothelial cells proliferation, migration, sprouting, branching and recruitment of the pericytes and smooth muscle. Angiogenesis is regulated by a variety of growth factors and angiogenic genes and proteins are upregulated as early as one hour after initial ischemic injury and remain elevated for subsequent days to weeks after stroke (Hayashi et al., 2003; Liu et al., 2007). The ischemic penumbra is the partially damaged tissue adjacent to the ischemic core and is a region where recovery mechanisms are actively occurring after ischemic stroke. Death of the penumbra due to ischemic reperfusion injury or poor recovery mechanisms contributes to infarct expansion and secondary injury after stroke. The hypoxic conditions of ischemic stroke stimulate angiogenesis within hours in the ischemic penumbra (Krupinski et al., 2003). Angiogenesis in the ischemic brain has been associated with improvement of neurological function in experimental stroke as well as in patients (Chen et al., 2003b; Krupinski et al., 1994; Liu et al., 2007). In patients with thrombo-embolic stroke, post mortem studies revealed higher microvessel density in the ischemic penumbra compared to contra lateral (normal) tissue and was correlated with longer survival time and functional improvement (Krupinski et al., 1994). In addition to increasing blood supply to ischemic brain tissue, the newly formed blood vessels following angiogenesis may also facilitate removal of necrotic tissue (Manoonkitiwongsa et al., 2001). In rats subject to ischemic stroke, increased microvessel density in the ischemic penumbra was associated with increased macrophage infiltration which could then facilitate removal of necrotic debris from the infarct region (Manoonkitiwongsa et al., 2001).
The vascular and neuronal components of the neurovascular units are tightly coupled during the process of angiogenesis and neurogenesis (Chopp et al., 2007; Teng et al., 2008). Neurogenesis is the proliferation and differentiation of neural stem cells (NSCs) and neural progenitor cells (NPCs) into mature parenchymal cells. In the adult brain, neurogenesis occurs in mainly in the subgranular zone of dentate gyrus, the subventricular zone and olfactory bulb (Kaplan and Hinds, 1977; Lois et al., 1996). Under normal physiological conditions, the progenitor cell population maintains balance between cell apoptosis and proliferation (Favaro et al., 2009). In response to hypoxic conditions of stroke, there is an increased recruitment of NSCs in the subventricular zone and rapid repopulation of depleted neuroblasts (Zhang et al., 2004). The newly generated neuroblasts form tight chains along blood vessels that may serve as scaffolds, and migrate from the subventricular zone to peri-infarct regions (Kojima et al., 2010; Zhang et al., 2004). Angiogenesis stimulates endothelial cells to secrete a number of growth factors that can regulate the biological activity of NPCs and influence their proliferation, migration and differentiation (Shen et al., 2004). In turn, NPCs also promote angiogenesis and regulate capillary blood flow (Lacar et al., 2012).
In the brain, NPCs can differentiate into glial cells including oligodendrocytes, astrocytes and ependymal cells as well as modulate microglial activation, proliferation and phagocytosis (Egawa et al., 2016; Mosher et al., 2012). Astrocytes closely interact with neurons and endothelial cells, and play key roles in pathological angiogenesis and neurogenesis by releasing neurotrophins, cytotrophic factors and vascular growth factors (Goss et al., 1998; Weidemann et al., 2010). The strategic anatomical positioning of astrocytes can mediate neurotransmission and translate neuronal activity to cerebral vessels, thereby maintaining the coupling of cerebral blood flow and metabolism (Venkat et al., 2016). Astrocytes have high plasticity and can alter their morphological (astrogliosis) and biochemical states in association with increased glial fibrillary acidic protein (GFAP) expression in response to neurological injury (Choudhury and Ding, 2016). During the early phase of stroke, formation of a glial scar in the ischemic penumbra demarcates the infarct core from intact normal tissue and protects the penumbra from the local inflammatory responses and free radical scavenging that is initiated in the core (Huang et al., 2014). Therefore, reactive astrocytes in the initial stages of ischemic injury exert neuroprotective effects such as taking up excess glutamate and acting as a diffusion barrier to limit the spread of neurotoxic molecules (Roitbak and Sykova, 1999). Reactive astrocytes also increase endothelial progenitor cell adherence and migration to promote re-vascularization and stabilization of vessels as well as direct NPC migration towards ischemic tissue (Becerra-Calixto and Cardona-Gómez, 2017). After reactive astrocytes, reactive microglia/macrophages are the second most predominant cell type in glial scar (Huang et al., 2014). Under normal physiological conditions, microglia are in a resting phase and constantly surveying their microenvironment, however, under ischemic stress they become activated and have altered migration patterns and cell surface protein expression (Nimmerjahn et al., 2005). In the chronic phase of stroke, reactive astrocytes are known to have deleterious effects and hinder neurological recovery by secreting growth-inhibitory molecules that chemically prevent axonal and white matter remodeling (Abeysinghe et al., 2016). The glial scar may also hinder neurological recovery due to poor connectivity of surviving neural pathways and neurovascular uncoupling (Abeysinghe et al., 2016). Therefore, in the chronic phase of stroke it is beneficial to decrease reactive astrocytes and promote polarization of macrophages and microglia to their anti-inflammatory M2 phenotype.
1.2 White matter remodeling and synaptogenesis
An important aspect of neurorestorative mechanisms after stroke is white matter remodeling and synaptogenesis which are required to restore and enhance neuronal connectivity and communication, which are vital for improvement of neurological function (Jiang et al., 2006). White matter remodeling includes axonal remodeling, oligodendrogenesis and remyelination. White matter remodeling after ischemic stroke in rodents begins early after stroke and has been observed at 28 days later (Liu et al., 2010). The corticospinal tract (CST) neuronal pathway mediates voluntary movements and is required for motor functions. The severity of motor impairment sustained by ischemic stroke patients are highly correlated with the extent of ischemic injury to the CST in all phases of stroke, with stronger correlation in outcome phases (Maraka et al., 2014; Zhu et al., 2010). In rodents subject to stroke, CST axonal remodeling in the spinal cord and pyramidal neuronal reorganization in the bilateral cortices promote neuronal communication and contribute at least in part to spontaneous functional recovery observed in chronic phase of stroke (Liu et al., 2009). Focal cortical stroke induces structural alterations in the peri-infarct cortex and loss of thalamic connections, following which surviving cortical neurons develop new horizontal cortical connections via axonal sprouting (Carmichael et al., 2001).
In the adult brain, oligodendrocyte progenitor cells (OPCs) are present in the corpus callosum, striatum, and cortex and they proliferate and differentiate into mature oligodendrocytes (OLs) which form myelin sheath around axons. Owing to limited blood supply in the white matter, OLs are highly vulnerable to ischemic injury (Zhang et al., 2010). Mature OLs do not proliferate and injured OLs do not form new myelin; therefore, oligodendrogenesis is essential to form myelin sheaths around the new sprouting axons after stroke. An increase in OPCs number has been reported in the peri-infarct area after stroke in rodents (Tanaka et al., 2003). In addition to the resident OPCs in the gray and white matter of brain, some NPCs in the subventricular zone also generate OPCs, which then migrate to the ischemic brain and differentiate into mature OLs (Zhang et al., 2012). Following white matter damage, astrocytes may also promote oligodendrogenesis by secreting brain derived neurotrophic factor (BDNF) (Miyamoto et al., 2015), and oligodendrogenesis associated neurorestorative effects have been demonstrated (Zhang et al., 2010).
Synaptogenesis is the formation of synapses between neurons. Synaptophysin is a presynaptic vesicle protein present in all nerve terminals and is frequently used as a marker for synaptogenesis (Stroemer et al., 1995). Hypoxia stimulates undamaged neurons in the peri-infarct area to sprout and establish new synaptic connections (Stroemer et al., 1995). Dendritic spines are actin rich protrusions from dendrites that contribute to synaptic plasticity by facilitating synaptic transmission and improve inter-neuronal communication by increasing axodendritic contact points (Stroemer et al., 1995; Yang et al., 2009). Stroke decreases the number of pre-synaptic vesicles and post-synaptic dendritic spines in the ischemic rat brain (Sun et al., 2008). Therapies that increase post stroke synaptogenesis have been associated with improved neurological recovery in rodents (Chen et al., 2003c; Stroemer et al., 1995).
1.3 Immune and inflammatory responses
Immune response and inflammation are important factors that have a profound impact on stroke progression and outcome (Gendron et al., 2002; Offner et al., 2006). In the early stages of stroke, local inflammatory responses initiated in the brain parenchyma include activation of endothelial and glial cells (microgliosis and astrogliosis), and subsequent release of pro-inflammatory mediators, cytokines and chemokines that induce secondary cell damage and create an inhospitable environment for neurovascular plasticity. Ischemic injury to the brain leads to an increased availability of free radicals, reactive oxygen species, oxidative stress and mitochondrial dysfunction which disrupts the BBB and leads to secondary brain damage (Flamm et al., 1978). Activated microglia, resident and infiltrating macrophages can assume either a pro-inflammatory M1 phenotype and secrete a variety of pro-inflammatory factors, or an anti-inflammatory M2 phenotype. Soon after ischemia, the local and infiltrating macrophages assume M2 phenotype to protect neurons from further damage and to aid in removal of cellular debris (Hu et al., 2012). Injured brain cells then release factors that favor M1 polarization, and microglia/macrophage persist in the M1 state and release pro-inflammatory factors for subsequent days to weeks (Zhao et al., 2015).
Inflammatory responses at the systemic level involve many immune cell types, circulating signals, systemic activation of T and B lymphocytes, all of which have important implications for post stroke infection and secondary complications (Gendron et al., 2002). Peripheral immune response to ischemic stroke is largely regulated by the spleen, which releases lymphocytes and proinflammatory cytokines and chemokines such as tumor necrosis factor (TNF-α), monocyte chemoattractant protein-1 (MCP-1), Interferon gamma (IFN-γ) and interleukins (IL)-2, 6, in the systemic circulation, which can aggravate overall inflammation in the acute phase after stroke (Offner et al., 2006). Delayed ischemic brain damage has been attributed to the influx of T lymphocytes. Influx begins about 48 hours after initial injury and promotes detrimental inflammatory cascades in the brain (Liesz et al., 2011). Excessive leukocyte infiltration to the ischemic brain tissue can increase intracranial pressure leading to increased mortality or poor outcome of stroke (Heuschmann et al., 2004).
Extending the initially acquired M2 phenotype of macrophages and microglia and delaying their transit into M1 phenotype may promote neurorestorative effects in the late stages of stroke (Liu et al., 2016; Zhao et al., 2015). T helper cells 1 and 2 (Th1 and Th2) cytokines control macrophage polarization and mediate antigen specific autoimmune response (Becker et al., 2011). Increase in anti-inflammatory cytokines such as IL-4 induces M2 polarization in microglia/macrophages which in turn can secrete additional anti-inflammatory mediators and promote effective cleanup of ischemic debris and improve long-term neurological outcomes after stroke (Liu et al., 2016; Zhao et al., 2015). M2 macrophage polarization has been associated with increased axonal growth in injured mouse spinal cord (Kigerl et al., 2009). Interventions that can regulate immune responses after stroke have been reported to limit uncontrolled inflammatory responses in the brain, decrease edema and BBB permeability, as well as improve neurological functional outcome after stroke (Zhu et al., 2015). Therefore, proper inflammatory milieu provides a suitable microenvironment for neurovascular and white matter remodeling (Xiong et al., 2016). Cell therapy for stroke has been reported to regulate neuroinflammation and mediate functional recovery after stroke which is discussed in the subsequent sections of this review.
2. Neurorestorative treatment of stroke with cell-based therapy
Cell based therapy for ischemic stroke includes administration of stem cells derived from various sources, including adipose, human fetal/embryonic tissue, bone marrow, peripheral, and umbilical cord blood. Human umbilical cord blood cells (HUCBCs) and mesenchymal stromal cells (MSCs) have emerged as promising treatment options for patients with ischemic stroke. We summarize the present knowledge of advantages and disadvantages of cell therapy from different sources for ischemic stroke treatment in table 1. In a recent meta-analysis of stem cell therapies for patients with brain ischemia, Chen et al concluded that stem cell therapy significantly enhanced neurological functions and quality of life (Chen et al., 2016b). However, additional investigation is required to provide further support for the safety and efficacy of stem cell transplantation in stroke patients. A number of clinical trials investigating safety, efficacy, optimal timing for treatment initiation, dose of cells, etc. have already begun worldwide, and are summarized in table 2. In this section, we will discuss the potential mechanisms underlying cell based therapy with HUCBC and MSCs induced neurorestorative effects after stroke.
Table 1.
Advantages and disadvantages of cell therapy from different sources
| Cell Sources And Types | Advantages | Disadvantages |
|---|---|---|
| Bone marrow, autologous |
|
|
| Bone marrow, allogeneic |
|
|
| Umbilical cord, mononuclear cells |
|
|
| Human umbilical cord, stem cell |
|
|
| Embryonic stem cell |
|
|
| Induced-pluripotent stem, cell autologous |
|
|
| Induced pluripotent stem cell, allogeneic |
|
|
Table 2.
Select ongoing clinical trials
| Study phase | Mode of delivery | Cell type | Time from onset | Primary Outcome measure | Secondary Outcome measure | Clinical identifier |
|---|---|---|---|---|---|---|
| I | IC | CTX0E03 neural stem | 6 mo–5 year | safety | Function outcome | NCT01151124 |
| I/II | IC | Modified stem, SB623 | 6–12 mo | safety | Function outcome | NCT01287936 |
| II | IC | Autologous peripheral CD34+ stem | >6 mo, <60 mo | Function outcome | Function outcome; Imaging | NCT00950521 |
| I | IV; IT | Umbilical cord, mesenchymal | IV, 7–14 d; IT, | Function outcome | Function outcome; MRI changes | NCT01389453 |
| I/IIa | IV | Autologous bone marrow mononuclear | 24–72 h | Safety; Feasibility | Function outcome | NCT00859014 |
| I/IIa | IV | Autologous mesenchymal stem | Within 6 wk | Safety; Feasibility | Function outcome | NCT00875654 |
| I/II | IA | Autologous bone marrow CD 34 + stem | 7 d | safety | Function outcome | NCT00535197 |
Abbreviations: IA=intra-arterial; IC=intracerebral; IT=intrathecal.
2.1 Human umbilical cord blood cells
There are several advantages in using HUCBCs as a neurorestorative agent for stroke, such as easy isolation without technical and ethical challenges and their suitability for autologous transplantation as well as allogeneic transplantation without requiring full human leukocyte antigen (HLA) matching (Chen et al., 2001b; Newcomb et al., 2006). In preclinical studies, it has been demonstrated that treatment of ischemic stroke using HUCBCs promotes neurorestorative mechanisms in the ischemic brain and dose-dependently improves neurological function when administered intravenously at 1, 2 or 7 days after stroke onset (Chen et al., 2001b; Newcomb et al., 2006; Vendrame et al., 2004; Willing et al., 2003). HUCBCs delivered intravenously have been reported to be more effective compared to striatal delivery with respect to producing long-term neurological functional recovery after stroke in rodents (Willing et al., 2003).
Several mechanisms underlying the neurorestorative effects and improvement of functional outcome after post stroke HUCBC transplantation have been put forward based on pre-clinical experiments. Intravenously administered HUCBCs were found to pass the BBB, enter and survive in the ischemic brain as well as differentiate into neurons and astrocytes (Chen et al., 2001b; Vendrame et al., 2004). However, the fraction of HUCBCs that follow this cell replacement pathway was very low, and it has become evident in recent years that these HUCBCs act as “factories” producing growth factors, trophic factors and anti-inflammatory factors which then promote neurorestorative effects and improve stroke outcome (Chen et al., 2001b; Vendrame et al., 2004; Yasuhara et al., 2010).
In addition, some studies report that the efficacy of HUCBCs can be augmented using combination therapy with Simvastatin or Mannitol (Cui et al., 2012; Yasuhara et al., 2010). Combination therapy of stroke with Simvastatin significantly increased the number of engrafted HUCBCs in the ischemic brain and improved NPCs proliferation and migration from the subventricular zone to ischemic brain regions (Cui et al., 2012). Simvastatin and HUCBC combination therapy also enhanced HUCBC treatment induced synaptic plasticity, axonal and neurite outgrowth, as well as BDNF expression in the ischemic brain (Cui et al., 2012). In a neonatal hypoxic ischemic injury rat model, growth and trophic factors such as glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF) and BDNF were increased after intravenous administration of mononuclear fraction of HUCBC 7 days after ischemia and amplified when administered together with Mannitol (Yasuhara et al., 2010). Therefore, pre-clinical studies suggest that cell therapy with HUCBCs alone or in combination with other pharmacological treatments can be employed to improve stroke outcome.
HUCBCs trigger strong immunomodulatory and anti-inflammatory responses in the host after administration (Nikolic et al., 2008; Vendrame et al., 2006). In rats subject to ischemic stroke, HUCBC treatment administered 48 hours after stroke onset, significantly decreased infiltration of granulocytes and monocytes, and reduced astrocytic and microglial activation in the ischemic brain parenchyma (Newcomb et al., 2006). In rodents subject to stroke, administration of HUCBCs attenuated pro-inflammatory Th1 response and increased anti-inflammatory Th2 responses (Nikolic et al., 2008; Vendrame et al., 2004). After ischemic stroke in rats, a gross reduction of spleen size and number of spleen leucocytes by day 7, and a return to normal count by days 14–28 after stroke have been reported (Gendron et al., 2002). HUCBC treatment administered 1 day after stroke in rats, rescued stroke induced reduction of spleen size 48 hours after stroke (Vendrame et al., 2006). HUCBC administered intravenously were found to home to spleen parenchyma (in addition to the ischemic brain) where they may modulate immune response (Vendrame et al., 2004).
In addition to improving stroke outcome in healthy, wild type rodents, HUCBCs also improve neurological functional outcome in diabetic or hypertensive animals subject to stroke (Chen et al., 2016a; Riegelsberger et al., 2011; Yan et al., 2015; Yan et al., 2014). In type 1 diabetic (T1DM) or type 2 diabetic (T2DM) rodents subject to stroke, HUCBCs administered intravenously at 1 or 3 days after stroke significantly increased growth and trophic factor production, enhanced neurovascular and white matter remodeling, decreased BBB leakage, attenuated neuroinflammation and induced M2 macrophage polarization compared to control diabetic rats subject to stroke (Yan et al., 2015; Yan et al., 2014). HUCBC treatment increases Angiopoietin-1 (Ang-1), an important protein in the regulation of angiogenesis, vascular maturity and stabilization and concomitantly decreases inflammatory factor receptor for advanced glycation end-products (RAGE) expression in the ischemic brain of type one diabetic stroke rats (Yan et al., 2014). In type 2 diabetic mice subject to stroke and treated with HUCBCs 3 days later, improvement in stroke outcome and neurorestorative effects such as vascular and axonal/white matter remodeling were found to be mediated at least in part by microRNA-126 (Chen et al., 2016a). MicroRNAs are small non-coding RNA sequences that can regulate several genes, pathways, and complicated cellular networks by acting either alone or in concert with other microRNAs (Chen et al., 2014). MicroRNA-126 is a key regulator in angiogenesis and maintenance of endothelial cell function, and has been reported to be altered in diabetes and stroke (Chen et al., 2016a; Wang et al., 2008; Zampetaki et al., 2010). Inhibition of microRNA-126 in HUCBCs significantly attenuated HUCBC induced neurorestorative effects, suggesting that increasing microRNA-126 may partially contribute to HUCBC treatment induced neurorestorative effects in type two diabetic stroke mice (Chen et al., 2016a). Further studies are required to optimize HUCBC treatment for stroke and for a clear understanding of the multifaceted mechanisms of action.
2.2 Mesenchymal stromal cells
MSCs are a heterogeneous subset of multipotent precursors that reside in the stromal fraction of many adult tissues. MSCs can differentiate into osteoblasts, adipocytes, chondroblasts, myocytes and neurons (Jiang et al., 2002). MSCs can be found and isolated from a variety of tissue types (such as bone marrow, adipose tissue, cord blood, brain, kidney, lung, muscle) of adult rodents suggesting that MSCs are present in all postnatal organs. The bone marrow is a major source of MSCs and has been widely studied as a treatment option for stroke. The International Society for Cell Therapy proposed a criteria to define and differentiate MSCs from other stem cells (Dominici et al., 2006). MSCs can be distinguished from other hematopoietic stem cells based on the presence of surface molecules such as CD73, CD90, and CD105 and the absence of CD34, CD45, HLA-antigen D Related, CD14 or CD11b, CD79a, or CD19 (Dominici et al., 2006).
Since a majority of studies using MSC therapy for stroke repair have used bone marrow derived MSCs, we will focus the present review on BMSCs. BMSCs are harvested from bone marrow, and employing BMSCs derived from HLA matched donor, full or partial HLA matched patient’s relative, or employing the patient’s own BMSCs may decrease the risk of immune rejection (Anasetti et al., 2012; Hamidieh et al., 2015). Intravenous, intra-arterial, intra-carotid or intra-striatal administration of BMSCs initiated at 1 day after stroke, dose dependently improved functional outcome after stroke in rodents (Chen et al., 2001a; Shen et al., 2006). The various routes of transplantation have their own advantages and disadvantages. For instance, intravascular delivery of BMSCs is non invasive and can yield a wide distribution of BMSCs in the brain; but it requires early transplantation since inflammation drives homing of cells to ischemic brain tissue, this route carries a risk of thrombus formation, and it is likely that the majority of transplanted cells may home to peripheral organs such as the lungs (Fischer et al., 2009; Guzman et al., 2008). While intracranial/intracerebral delivery of BMSCs leads to a greater fraction of cells homing to ischemic brain tissue, it is invasive, may not be clinically feasible and carries a risk of damaging healthy tissue (Jin et al., 2002). A delayed BMSC treatment initiation time point of 7 days post stroke was also found to significantly improve long term functional outcome in middle aged rats (Li et al., 2005). However, BMSC treatment initiated 1 day after experimental stroke was found to be more beneficial in improving functional outcome compared to 7 day treatment initiation time point (Yang et al., 2010). In fact, BMSC transplantation at 12 hours after stroke induced optimal neurological functional recovery, least ischemic damage, maximum trafficking of transplanted BMSCs to ischemic region, and lowest caspase-3 activity in the ischemic brain (Hosseini et al., 2015).
BMSC treatment initiated at 1 day after stroke decreased apoptosis, improved BBB integrity, increased axonal growth and sprouting, remyelination in the cortical penumbra and corpus callosum, and improved neurological function in experimental stroke rats (Chen et al., 2003a; Chen et al., 2001a; Shen et al., 2006; Zacharek et al., 2007). BMSC treatment initiated one day after stroke in rats significantly promoted angiogenesis in the ischemic boundary zone, indicated by increased number of vascular endothelial growth factor (VEGF) and Notch-1 positive microvessels (Dao et al., 2013; Yang et al., 2010). In vitro studies indicate that BMSCs co-cultured with astrocytes significantly increases VEGF and Ang1/Tie2 expression in astrocytes, and BMSCs conditioned medium significantly improves capillary tube formation demonstrating the potential of BMSCs to induce angiogenesis (Zacharek et al., 2007). Compared to vehicle treated rats that only exhibit unilateral activation of sensorimotor cortex, rats treated with BMSCs at 6 hours after stroke exhibit activation of the ischemic as well as contralateral hemispheres upon electrical stimulation of the left forepaw during functional MRI which was associated with greater motor function recovery (Suzuki et al., 2013). Increased activation of microglia, and persisting reactive astrogliosis in response to BMSC treatment after stroke may contribute to white matter remodeling effects such as enhanced axonal regeneration aiding long term functional recovery (Li et al., 2005; Yang et al., 2010). BMSC treatment for stroke significantly increased synaptophysin, synapse density, number of myelinated axons and neurite outgrowth in the ischemic border zone (Ding et al., 2013). In the adult brain, Nogo-A is primarily expressed by OLs on the axonal and outer myelin membrane and inhibits neurite growth (Mahmood et al., 2014). BMSCs administered after traumatic brain injury or stroke in rats inhibits Nogo-A expression in the ischemic regions and induces axonal regeneration (Mahmood et al., 2014).
Several mechanisms of action for BMSC induced neuroprotection and neurorestoration have been put forth including release of growth and trophic factors, mediating anti-inflammatory responses, decreasing glial scar formation and mediating restorative mechanisms via release of microRNA (Chen et al., 2001a; Chen et al., 2003b; Yang et al., 2010). Although transplanted cells are known to traffic to the injured parenchyma in response to ischemic and chemotactic stimuli, a number of studies have found that after systemic administration, a majority of the transplanted BMSCs home to peripheral organs such as the spleen, lung, kidney and intestine, and the fraction of cells that home to the ischemic brain and differentiate into neuronal cells to replace injured/lost cells is very small (Chen et al., 2001a; Chen et al., 2003b; Yang et al., 2010).
The most widely accepted mechanism of BMSC induced neurorestorative effects after stroke is via the release of trophic and growth factors such as VEGF, BDNF and bFGF (basic fibroblast growth factor) (Chen et al., 2001a; Yang et al., 2010). Post stroke BMSC treatment increased the expression of many growth and trophic factors and their receptors in the circulation (Yang et al., 2010). BMSC induced increase of cytokines and growth factors exert both autocrine and paracrine activities which mediate differentiation, proliferation, and cell survival (Matsuda-Hashii et al., 2004). These growth factors play a crucial role in maintaining and augmenting neurorestorative process such as neurogenesis, angiogenesis, white matter remodeling and synaptic plasticity. Therefore, BMSCs behave as small biochemical and molecular “factories” and catalysts, producing a wide array of trophic and growth factors that induce angiogenesis and vascular stabilization in the ischemic boundary, where the majority of BMSCs that survive in the brain are located.
BMSC treated stroke rats exhibit accelerated glial scar formation, decreased inflammatory responses in the ischemic penumbra and reduction of the ischemic lesion volume (Pavlichenko et al., 2008). BMSCs also exert immunosuppressive and immunomodulatory effects in stroke rats without altering stroke induced immune cell dysfunction or systemic inflammatory cytokine expression levels (Scheibe et al., 2012). Transplantation of human BMSCs into the hippocampus of adult mice at 1 day after transient global ischemic stroke, significantly decreased cell death of hippocampal neurons, promoted M2 macrophage/microglial polarization, induced Th2 immune bias with increased ratio of IL-4/IFNγ or IL-4/TNFα and down regulated pro-inflammatory genes (Ohtaki et al., 2008).
Several studies have manipulated BMSCs and investigated their effectiveness in stroke treatment. BMSC conditioned medium derived from normal or ischemic rats administered immediately after stroke was found to improve functional outcome, enhance neurogenesis and decrease microglia/ macrophage infiltration into the ischemic penumbra (Tsai et al., 2014). Combination therapy of BMSCs with pharmacological agents, growth factors, or gene modified BMSCs such as FGF-2 or BDNF gene modified BMSCs, have been used to extend therapeutic time window and amplify neurorestorative effects after stroke (Chen et al., 2011a; Ikeda et al., 2005; Kurozumi et al., 2004).
MicroRNAs can interact with BMSCs to impact neurorestorative effects after stroke. For example, the microRNA-145 and its target ATP-binding cassette transporter (ABCA1)/ Insulin-like growth factor 1 (IGFR1) pathway may contribute to diabetic-BMSCs induced functional outcome improvement and neurorestorative effects in T1DM stroke rats (Cui et al., 2016). MicroRNA-34a mediates neuronal precursor motility that is crucial for homing of stem cells to target tissue (Chang et al., 2011). In addition, microRNA-96, microRNA-124 and microRNA-199a regulate gene expression important for BMSC differentiation and were differentially expressed during osteogenic, adipogenic, and chondrogenic induction of human BMSCs (Laine et al., 2012). In addition, it has been demonstrated that transfer of microRNA-133b from BMSCs to astrocytes and neurons regulates gene expression and promotes neurite remodeling and neurological recovery after stroke (Xin et al., 2012; Xin et al., 2013). Knockdown of microRNA-133b in BMSCs attenuated BMSC induced neurorestorative effects after stroke (Xin et al., 2013). MicroRNAs regulate neurogenesis, mediate trans-differentiation of MSCs into functional neurons and are also useful in restoration of lost or damaged neurons in neurological disorders (Shen and Temple, 2009). MicroRNAs, with their varied biological functions and regulatory capabilities, open new avenues and strategies for BMSC therapy and manipulation with potential therapeutic benefits.
Currently clinical trials to evaluate the safety, feasibility, and efficacy of BMSCs treatment for stroke patients are underway and have been summarized later in this review. In addition, the optimal cell type, time window of administration, delivery route and dose are yet to be determined.
3. Pharmacological agent treatment of stroke
There are several pharmacological treatments under investigation both in preclinical experiments and clinical trials to promote neurorestorative effects after ischemic stroke such as, 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors (statins) (Chen et al., 2003c), erythropoietin (EPO) (Wang et al., 2004), phosphodiesterase type 5 (PDE-5) inhibitors (Zhang et al., 2002), Niaspan (Shehadah et al., 2010; Yan et al., 2012; Yan et al., 2013), Minocycline, and Candesartan. Statins, EPO and PDE-5 inhibitors and Niaspan have been discussed in our previous review (Chen and Chopp, 2006; Chen et al., 2014). In this review, we focus on Minocycline and Candesartan which are currently under clinical trials. The proposed basic mechanisms underlying Minocycline and Candesartan derived neuroprotective and neurorestorative effects are discussed in the following sections and summarized in figure 2.
Figure 2.
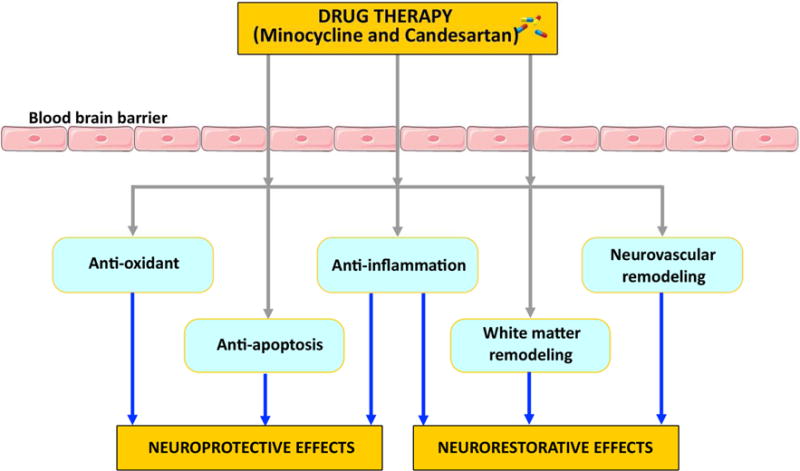
Summary of basic mechanisms underlying Minocycline and Candesartan derived neuroprotective and neurorestorative effects after stroke.
3.1 Neuroprotective and neurorestorative effects of Minocycline for stroke treatment
Minocycline is a second-generation, semi-synthetic tetracycline which has been applied in the treatment of acne vulgaris and some sexually transmitted diseases (Ma et al., 2015). Drug resistance and the emergence of mounting novel agents have led to the decline of clinical applications of Minocycline as an antibiotic. In addition to its antibiotic effects, Minocycline has anti-inflammatory and anti-apoptotic properties. These effects have been confirmed in various diseases including atherosclerosis, multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease (Garrido-Mesa et al., 2011; Metz et al., 2017; Ohshima et al., 2010; Tilley et al., 1995).
Minocycline is a potentially novel pharmacologic therapy for stroke as it is highly lipophilic and can easily pass the BBB (Ma et al., 2015; Naderi et al., 2017; Soliman et al., 2015; Zou et al., 2016). Increasing studies indicate that Minocycline may induce neuroprotective effects in experimental stroke and clinical stroke patients (Lampl et al., 2007; Yang et al., 2015b). The results of an open-labeled evaluator-blinded clinical trial that included 152 patients indicated that oral Minocycline administrated within 6 to 24 hours after acute stroke at a dosage of 200 mg/day for 5 days significantly improved stroke outcome, as evaluated by National Institutes of Health Stroke Scale and modified Rankin Score compared with placebo group (Lampl et al., 2007). A similar study confirmed the safety, efficacy, dose range and feasibility of Minocycline for stroke treatment (Padma Srivastava et al., 2012). An open labeled, dose finding study with 60 patients employing intravenous administration of Minocycline within 6 hours of stroke found that Minocycline treatment was well tolerated up to doses of 10 mg/kg/day alone or in combination with tPA without severe hemorrhagic complications (Fagan et al., 2010). Combination of Minocycline with tPA not only extended the time window for tPA treatment for ischemic stroke, but also decreased the incidence of hemorrhage, improved neurologic outcome and lowered mortality (Machado et al., 2009; Murata et al., 2008). The combination treatment induced benefit effects may due to BBB protection rather than compromising clot lysis (Machado et al., 2009). A delayed combinatorial treatment of Flavopiridol with Minocycline provides synergistic brain plasticity after ischemic stroke for 10 weeks (Iyirhiaro et al., 2008). Sequential therapy of Minocycline and Candesartan in rats subjected to stroke, significantly improved neurobehavioral outcome, and reduced infarct size (Soliman et al., 2015). About 90% of meningococcal meningitis patients who were treated with Minocycline exhibited side effects such as nausea, vertigo and mild dizziness, within the first 72 hours after Minocycline administration and quickly (48 hours) disappeared after stopping Minocycline therapy (Fanning and Gump, 1976).
The mechanisms of Minocycline induced neuroprotective effects are not clear. Minocycline induced neuroprotective effects after stroke are most likely due to its anti-inflammatory, antioxidant, anti-autophagy, and anti-apoptotic effects (Naderi et al., 2017; Wu et al., 2016). Minocycline treatment at 3 hours after stroke reduced brain tissue loss and improved functional outcome by promoting neurovascular remodeling, enhancing tight junction protein expression and alternative activation of microglia/macrophages in the ischemic brain (Yang et al., 2015b). Minocycline inhibits hypoxia-inducible factor (HIF-1α) mediated cellular responses and protects BBB integrity (Yang et al., 2015a), reduces microglial activation and matrix metalloproteinase (MMP) expression (Machado et al., 2006), and decreases neuronal apoptosis (Matsukawa et al., 2009). Minocycline treatment (50 mg/kg, i.p., twice a day for 2 days starting at 2 hours after stroke) in adult rats subject to experimental striatal stroke inhibited microglial activation, decreased apoptotic cell death, induced significant neuroprotection and improved functional outcome compared to saline treated stroke rats (Souza et al., 2017). Minocycline derived neuroprotection may be derived from attenuation of neuroinflammation and inhibition of inflammatory processes in microglia and brain endothelial cells after stroke (Souza et al., 2017; Yu et al., 2017).
Minocycline not only regulates neuroprotective effects, but also promotes neurorestorative effects including white matter remodeling, remyelination and neurogenesis after ischemic injury to the brain (Jalal et al., 2015). Minocycline administered intraperitoneally one day after stroke for 14 days, dose dependently improved neurologic score, motor coordination and survival (Hayakawa et al., 2008). Spontaneously hypertensive/stroke prone rats (SHR/SP) subject to unilateral common carotid artery occlusion and treated with Minocycline initiated one day after stroke every other day for 9 weeks significantly reduced lesion size, improved cerebral blood flow and white matter remodeling, and extended survival (Jalal et al., 2015). In a randomized single-blinded open-label clinical trial, patients who received oral Minocycline (200 mg/day for 5 days) showed significant improvement in functional outcome without increasing mortality rate compared to placebo group (Padma Srivastava et al., 2012). These data suggest that Minocycline has long term restorative effects in the chronic phase post stroke.
The mechanisms of Minocycline induced neurorestorative effects may be related with the inhibition of activated microglia expressing high-mobility group box1 (HMGB1) (Hayakawa et al., 2008), Minocycline also decreases MMP-9-mediated infiltration of leukocytes, and reduced levels of TNF-α and IL-1β, and increased levels of anti-inflammatory factors transforming growth factor-β (TGF-β) and IL-10 and YM1 (M2 macrophage/microglia) in the damaged brain (Jalal et al., 2015; Xing et al., 2012; Yang et al., 2015b). Minocycline can induce the proliferation of OPCs in the subventricular zone, which promotes remyelination and improves functional outcome (Ma et al., 2015). Collectively, the above findings indicate that Minocycline shows neuroprotective and neurorestorative effects and can improve neurological outcome after ischemic stroke, although there are still many problems to resolve (Padma Srivastava et al., 2012).
3.2 Neuroprotective and neurorestorative effects of Angiotensin Receptor Blockers (ARBs)
Candesartan is an Angiotensin II receptor agonist that is widely used in cardiovascular disorders, renal disease, metabolic syndrome, diabetes, as well as brain injury. When administered systemically, Candesartan can enter the brain, protect cerebral blood flow, decrease BBB leakage, decrease cerebral hemorrhage, reduce neuro-inflammation and decrease neuronal injury in animal models of stroke (Ishrat et al., 2015; Soliman et al., 2015), traumatic brain injury (Villapol et al., 2015; Villapol et al., 2012), Alzheimer’s disease (Elkahloun et al., 2016) and other brain conditions. In this review, we will focus discuss Candesartan therapeutic effects in stroke.
Treatment with low dose of Candesartan either pre-stroke (Barakat et al., 2014) or in the acute phase after stroke (Ishrat et al., 2015) significantly reduced infarct volume and edema (Groth et al., 2003), protected BBB integrity (Pelisch et al., 2011), and improved neurological outcome (Brdon et al., 2007; Soliman et al., 2015). Low dose Candesartan treatment (0.1–0.3mg/kg) increases the expression of active MMP-3, laminin, angiopoietin-1, VEGF, BDNF, synaptophysin, and postsynaptic density protein 95 (PSD-95) expression in the ischemic brain (Ishrat et al., 2015). Candesartan also decreases leukocyte infiltration and inflammatory factor expression such as toll like receptor-2 (TLR-2) and TLR-4, Myd88, TIR-domain-containing adapter-inducing interferon-β (TRIF) and interferon regulatory transcription factor 3 (IRF-3) and TNF-α, IL-1β, IL-6 and NF-kB (Barakat et al., 2014; Benicky et al., 2011). Low dose oral administration of Candesartan after transient focal ischemia reduces infarct volume without decreasing blood pressure (Omura-Matsuoka et al., 2009). Neurovascular protection induced by Candesartan may be derived from the inhibition of Rho-kinase in brain microvessels (Omura-Matsuoka et al., 2009). A single high dose of 1 mg/kg Candesartan administered intravenously after ischemia-reperfusion injury improved vascular remodeling and provided long-term benefits (Kozak et al., 2009). In combination with tPA, high dose Candesartan (1mg/kg) ameliorated brain hemorrhage, and improved neurological outcome in embolic stroke rats when compared to rats treated with tPA alone (Ishrat et al., 2013).
The ACCESS (Acute Candesartan Cilexetil Therapy in Stroke Survivors) clinical trial demonstrated the safety of Candesartan and its benefits in stroke treatment even though it did not lower blood pressure (Doggrell et al., 2004; Schrader et al., 2003). However, in a randomized double-blind Scandinavian Candesartan Acute Stoke Trial (SCAST) with 2029 patients recruited within 30 hours of acute ischemic or hemorrhagic stroke, Candesartan treatment showed no significant benefit on daily activities at 6 months compared to control group (Hornslien et al., 2015; Sandset et al., 2011). Candesartan therapeutic effects may differ according to different types of acute ischemic stroke (Sandset et al., 2015). These studies indicated a significant trend toward a better effect of Candesartan in patients with larger infarcts than in patients with smaller lacunar infarcts (Sandset et al., 2015). Therefore, several studies suggest neuroprotective effects of Candesartan treatment for ischemic stroke.
Candesartan is also tightly associated with neurorestorative processes, especially angiogenesis which can promote other events such as neuroplasticity and synaptogenesis (Soliman et al., 2014; Villapol et al., 2012). A low, sub-hypotensive dose of Candesartan promotes neuroplasticity and functional recovery via the over-expression of VEGF, BDNF and its receptors (Ishrat et al., 2015). In vivo and in vitro experiments have demonstrated that Candesartan provides long-term benefits including vascular protection and angiogenic remodeling post stroke (Kozak et al., 2009; Soliman et al., 2014). Candesartan treatment decreases microglial activation, astrogliosis and pro-inflammatory responses, while protecting cerebral blood flow, at 1 to 3 days post brain injury (Villapol et al., 2015; Villapol et al., 2012). In addition, Candesartan also promotes peroxisome proliferator-activated receptor gamma (PPARγ) signaling activity (Villapol et al., 2015; Villapol et al., 2012). The neurorestorative effects of Candesartan were reduced by concomitant administration of the PPARγ antagonist which indicates that Candesartan induced neurorestorative effects may be associated with the PPARγ pathway (Villapol et al., 2015; Villapol et al., 2012). Collectively, these results indicate that Candesartan, which may have both neuroprotective and neurorestorative effects, and is a potential neuroprotective and neurorestorative therapy for the treatment of acute ischemic stroke (Villapol et al., 2015). A recent study reported that in rats subjected to experimental stroke, sequential combination therapy rather than simultaneous treatment with Minocycline and Candesartan improved long term functional recovery and preserved Candesartan’s proangiogenic activity (Soliman et al., 2015). Future studies are warranted to investigate the effectiveness and optimization of combination therapies for stroke.
4. Conclusions and future directions
In this review, we provided an update on the role of HUCBC, BMSCs and select pharmacological agents such, Minocycline and Candesartan in neurorestorative therapy for stroke. Each of these therapies has their own advantages, and emerging evidence indicates their potential to serve as novel treatment strategies for stroke. The mechanisms underlying these beneficial therapies are being elucidated. We have briefly discussed some of the mechanisms which mediate neurorestorative effects after stroke including neurovascular remodeling and white matter remodeling, synaptogenesis and anti-inflammatory effects, which contribute to the amplification of the brain plasticity and subsequent improvement of the neurological outcome. It is extremely likely that these neurorestorative events are coupled, and future studies are required to investigate mechanisms that temporally and spatially coordinate these neurorestorative events. Future studies are required to identify the key therapeutic roles played by microRNAs and to optimize their manipulation to enhance therapeutic efficacy of neurorestorative agents.
Additional experimental considerations and preclinical studies are required to properly and effectively translate treatments for stroke from the bench to the bedside. Among issues that should be addressed in order to facilitate clinical application is the widespread use of only healthy young male animals in preclinical research, and lack of attention to co-morbidities such as diabetes and hypertension which alter and aggravate stroke pathology, and impact response to therapeutic interventions. Additionally, sex differences in stroke pathology and therapeutic response are understudied. Over the past few decades, while there have been several novel neuroprotective agents to treat stroke that showed promise in pre-clinical evaluation, very few progressed towards clinical testing and tPA still remains the only FDA approved drug for stroke treatment. However, tPA has disadvantages including narrow treatment time window and contra-indications for stroke patients with diabetes (Demchuk et al., 1999). Adverse effects such as poor functional outcome, haemorrhage and arteriosclerosis were reported when BMSC treatment was initiated 24 hours after stroke in type one diabetic rats (Chen et al., 2011b). Recent studies have evaluated delayed stroke treatment (1–3 days after stroke) using cell therapy with HUCBC or MSC in rodents with or without co-morbidity with type 2 diabetes and hypertension, and reported short and long term beneficial effects (Calio et al., 2014; Yan et al., 2016). Neurorestorative treatments with wide treatment time windows are critical for successful clinical translation. Neurorestorative agents and re-purposing of FDA approved drugs for stroke treatment hold translational potential and promise as therapeutic agents for stroke. In addition, the use of cell therapy alone or in combination with pharmacological treatments may promote synergistic and additive neurorestorative effects which hold promise to improve stroke outcome. The safety and efficacy of these treatments in stroke patients need to be demonstrated by clinical trials.
Highlights.
Cell therapy for stroke has a wide treatment window of days to weeks after stroke
Cell therapy for stroke improves stroke outcome and neurological function
Neurorestorative agents promote neurovascular and white matter remodeling
Minocycline and Candesartan promote neuroprotection and neurorestoration after stroke
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (R01NS083078, R01NS099030-01, R01NS097747, R01NS088656) and American Heart Association (17POST33410580).
Abbreviations
- ABCA1
ATP-binding cassette transporter
- Ang-1
Angiopoietin-1
- ARBs
Angiotensin Receptor Blockers
- BBB
Blood brain barrier
- BDNF
Brain derived neurotrophic factor
- bFGF
Basic fibroblast growth factor
- CST
Corticospinal tract
- EPO
Erythropoietin
- GDNF
Glial cell-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- HIF-1α
Hypoxia-inducible factor
- HLA
Human leukocyte antigen
- HMGB1
High-mobility group box1
- HUCBC
Human umbilical cord blood cells
- IFN-γ
Interferon gamma
- IGFR1
Insulin-like growth factor 1
- IL
Interleukin
- MCP-1
Monocyte chemoattractant protein-1
- MMP
Matrix metalloproteinase
- MSC
Mesenchymal stromal cell
- NGF
Nerve growth factor
- NPCs
Neural progenitor cells
- NSCs
Neural stem cells
- OL
Oligodendrocyte
- OPC
Oligodendrocyte progenitor cell
- PDE-5
Phosphodiesterase type 5
- PPARγ
Peroxisome proliferator-activated receptor gamma
- RAGE
Receptor for advanced glycation end-products
- SHR/SP
Spontaneously hypertensive/stroke prone
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TGF-β
Transforming growth factor-β
- Th
T helper cell
- TLR
Toll like receptor
- TNF-α
Tumor necrosis factor
- tPA
Tissue plasminogen activator
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruscato TJ, Davis TP. Combination of Hypoxia/Aglycemia Compromises In Vitro Blood-Brain Barrier Integrity. Journal of Pharmacology and Experimental Therapeutics. 1999;289:668–675. [PubMed] [Google Scholar]
- Abeysinghe HCS, Phillips EL, Chin-Cheng H, Beart PM, Roulston CL. Modulating Astrocyte Transition after Stroke to Promote Brain Rescue and Functional Recovery: Emerging Targets Include Rho Kinase. Int J Mol Sci. 2016;17:288. doi: 10.3390/ijms17030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, Ehninger G, Johnston L, Maziarz RT, Pulsipher MA, Porter DL, Mineishi S, McCarty JM, Khan SP, Anderlini P, Bensinger WI, Leitman SF, Rowley SD, Bredeson C, Carter SL, Horowitz MM, Confer DL. Peripheral-Blood Stem Cells versus Bone Marrow from Unrelated Donors. New England Journal of Medicine. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat W, Safwet N, El-Maraghy NN, Zakaria MN. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur J Pharmacol. 2014;724:43–50. doi: 10.1016/j.ejphar.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Becerra-Calixto A, Cardona-Gómez GP. The Role of Astrocytes in Neuroprotection after Brain Stroke: Potential in Cell Therapy. Frontiers in Molecular Neuroscience. 2017;10 doi: 10.3389/fnmol.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, Hadwin J, Carter KT, Shibata D, Cain KC. Autoimmune Responses to Brain Following Stroke are Associated with Worse Outcome. Stroke; a Journal of Cerebral Circulation. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdon J, Kaiser S, Hagemann F, Zhao Y, Culman J, Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J Hypertens. 2007;25:187–196. doi: 10.1097/01.hjh.0000254376.80864.d3. [DOI] [PubMed] [Google Scholar]
- Calio ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calio PL, Reis LA, Simoes Mde J, Lisboa-Nascimento T, Ferreira AT, Bertoncini CR. Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med. 2014;70:141–154. doi: 10.1016/j.freeradbiomed.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Weng SL, Hsieh JY, Wang TY, Chang MDT, Wang HW. MicroRNA-34a modulates genes involved in cellular motility and oxidative phosphorylation in neural precursors derived from human umbilical cord mesenchymal stem cells. BMC Medical Genomics. 2011;4:65. doi: 10.1186/1755-8794-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cheng Y, Chen J. Transfection of Noggin in bone marrow stromal cells (BMSCs) enhances BMSC-induced functional outcome after stroke in rats. Journal of Neuroscience Research. 2011a;89:1194–1202. doi: 10.1002/jnr.22662. [DOI] [PubMed] [Google Scholar]
- Chen J, Chopp M. Neurorestorative treatment of stroke: Cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, Venkat P, Zhang Y, Chopp M. MiR-126 Contributes to Human Umbilical Cord Blood Cell-Induced Neurorestorative Effects After Stroke in Type-2 Diabetic Mice. Stem Cells. 2016a;34:102–113. doi: 10.1002/stem.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011b;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003c;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang G, Khan AA, Guo X, Gu Y. Clinical Efficacy and Meta-Analysis of Stem Cell Therapies for Patients with Brain Ischemia. Stem Cells International. 2016b;2016:6129579. doi: 10.1155/2016/6129579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- Choudhury GR, Ding S. Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiology of disease. 2016;85:234–244. doi: 10.1016/j.nbd.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- Cui C, Ye X, Chopp M, Venkat P, Zacharek A, Yan T, Ning R, Yu P, Cui G, Chen J. miR-145 Regulates Diabetes-Bone Marrow Stromal Cell-Induced Neurorestorative Effects in Diabetes Stroke Rats. Stem Cells Transl Med. 2016;5:1656–1667. doi: 10.5966/sctm.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Shehadah A, Zacharek A, Kuzmin-Nichols N, Sanberg CD, Dai J, Zhang C, Ueno Y, Roberts C, Chen J. Therapeutic Benefit of Treatment of Stroke With Simvastatin and Human Umbilical Cord Blood Cells: Neurogenesis, Synaptic Plasticity, and Axon Growth. Cell transplantation. 2012;21:845–856. doi: 10.3727/096368911X627417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M, Tate CC, McGrogan M, Case CC. Comparing the angiogenic potency of naive marrow stromal cells and Notch-transfected marrow stromal cells. J Transl Med. 2013;11:81. doi: 10.1186/1479-5876-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan AM. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 2013;33:1015–1024. doi: 10.1038/jcbfm.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggrell SA, Scope, Trials, A. Candesartan for the prevention and treatment of stroke - results of the SCOPE and ACCESS trials. Expert Opin Pharmacother. 2004;5:687–690. doi: 10.1517/14656566.5.3.687. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Egawa N, Lok J, Arai K. Mechanisms of cellular plasticity in cerebral perivascular region. Prog Brain Res. 2016;225:183–200. doi: 10.1016/bs.pbr.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahloun AG, Hafko R, Saavedra JM. An integrative genome-wide transcriptome reveals that candesartan is neuroprotective and a candidate therapeutic for Alzheimer’s disease. Alzheimers Res Ther. 2016;8:5. doi: 10.1186/s13195-015-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning WL, Gump DW. Distressing side-effects of minocycline hydrochloride. Arch Intern Med. 1976;136:761–762. [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978;9:445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N, Camuesco D, Arribas B, Comalada M, Bailon E, Cueto-Sola M, Utrilla P, Nieto A, Zarzuelo A, Rodriguez-Cabezas ME, Galvez J. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res. 2011;63:308–319. doi: 10.1016/j.phrs.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Gendron A, Teitelbaum J, Cossette C, Nuara S, Dumont M, Geadah D, du Souich P, Kouassi E. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Research. 2002;955:85–97. doi: 10.1016/s0006-8993(02)03368-1. [DOI] [PubMed] [Google Scholar]
- Goss JR, O’Malley ME, Zou L, Styren SD, Kochanek PM, DeKosky ST. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998;149:301–309. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- Groth W, Blume A, Gohlke P, Unger T, Culman J. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J Hypertens. 2003;21:2175–2182. doi: 10.1097/00004872-200311000-00028. [DOI] [PubMed] [Google Scholar]
- Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, Schaar B, Steinberg G. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- Hamidieh AA, Dehaghi MO, Paragomi P, Navaei S, Jalali A, Eslami GG, Behfar M, Ghavamzadeh A. Efficiency of allogeneic hematopoietic SCT from HLA fully-matched non-sibling relatives: A new prospect of exploiting extended family search. Bone Marrow Transplant. 2015;50:545–552. doi: 10.1038/bmt.2014.307. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Iwasaki K, Fujiwara M. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke. 2008;39:951–958. doi: 10.1161/STROKEAHA.107.495820. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Heuschmann PU, Kolominsky-Rabas PL, Misselwitz B, Hermanek P, Leffmann C, Janzen RW, Rother J, Buecker-Nott HJ, Berger K. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German Stroke Registers Study Group. Arch Intern Med. 2004;164:1761–1768. doi: 10.1001/archinte.164.16.1761. [DOI] [PubMed] [Google Scholar]
- Hornslien AG, Sandset EC, Igland J, Terent A, Boysen G, Bath PM, Murray GD, Berge E. Effects of candesartan in acute stroke on vascular events during long-term follow-up: results from the Scandinavian Candesartan Acute Stroke Trial (SCAST) Int J Stroke. 2015;10:830–835. doi: 10.1111/ijs.12477. [DOI] [PubMed] [Google Scholar]
- Hosseini SM, Farahmandnia M, Razi Z, Delavarifar S, Shakibajahromi B. 12 hours after cerebral ischemia is the optimal time for bone marrow mesenchymal stem cell transplantation. Neural Regeneration Research. 2015;10:904–908. doi: 10.4103/1673-5374.158354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huang L, Wu ZB, ZhuGe Q, Zheng W, Shao B, Wang B, Sun F, Jin K. Glial Scar Formation Occurs in the Human Brain after Ischemic Stroke. International Journal of Medical Sciences. 2014;11:344–348. doi: 10.7150/ijms.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, Kimura F, Dezawa M, Coffin RS, Otsuki Y, Kuroiwa T, Miyatake S. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36:2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Pillai B, Ergul A, Hafez S, Fagan SC. Candesartan reduces the hemorrhage associated with delayed tissue plasminogen activator treatment in rat embolic stroke. Neurochem Res. 2013;38:2668–2677. doi: 10.1007/s11064-013-1185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Pillai B, Soliman S, Fouda AY, Kozak A, Johnson MH, Ergul A, Fagan SC. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol Neurobiol. 2015;51:1542–1553. doi: 10.1007/s12035-014-8830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyirhiaro GO, Brust TB, Rashidian J, Galehdar Z, Osman A, Phillips M, Slack RS, Macvicar BA, Park DS. Delayed combinatorial treatment with flavopiridol and minocycline provides longer term protection for neuronal soma but not dendrites following global ischemia. J Neurochem. 2008;105:703–713. doi: 10.1111/j.1471-4159.2007.05166.x. [DOI] [PubMed] [Google Scholar]
- Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab. 2015;35:1145–1153. doi: 10.1038/jcbfm.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, Silver B, Zhang L, Meng H, Lu M, Pourabdillah-Nejed DS, Wang L, Savant-Bhonsale S, Li L, Bagher-Ebadian H, Hu J, Arbab AS, Vanguri P, Ewing JR, Ledbetter KA, Chopp M. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–1089. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jin HK, Carter JE, Huntley GW, Schuchman EH. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase–deficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest. 2002;109:1183–1191. doi: 10.1172/JCI14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen J, Kwakkel G. Translational Hurdles in Stroke Recovery Studies. Translational Stroke Research. 2016;7:331–342. doi: 10.1007/s12975-016-0461-y. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. The Journal of Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, Abdelsaid M, Wiley DC, Fagan SC. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke. 2009;40:1870–1876. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Stroemer P, Slevin M, Marti E, Kumar P, Rubio F. Three-dimensional structure and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport. 2003;14:1171–1176. doi: 10.1097/01.wnr.0000075304.76650.29. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Lacar B, Herman P, Platel JC, Kubera C, Hyder F, Bordey A. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. J Neurosci. 2012;32:16435–16448. doi: 10.1523/JNEUROSCI.1457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A, American Heart Association Stroke, C., Council on, C., Stroke, N., Council on Quality of, C., Outcomes, R., Council on Functional, G., Translational, B. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine SK, Alm JJ, Virtanen SP, Aro HT, Laitala-Leinonen TK. MicroRNAs miR-96, miR-124, and miR-199a regulate gene expression in human bone marrow-derived mesenchymal stem cells. Journal of Cellular Biochemistry. 2012;113:2687–2695. doi: 10.1002/jcb.24144. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- Linfante I, Cipolla MJ. Improving Reperfusion Therapies in the Era of Mechanical Thrombectomy. Translational Stroke Research. 2016;7:294–302. doi: 10.1007/s12975-016-0469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, Ji X, Leak RK, Gao Y, Chen J, Hu X. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the Corticospinal Innervation and Spontaneous Behavioral Recovery After Ischemic Stroke in Adult Mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang J, Hou WW, Wu XH, Liao RJ, Chen Y, Wang Z, Zhang XN, Zhang LS, Zhou YD, Chen Z, Hu WW. Early treatment of minocycline alleviates white matter and cognitive impairments after chronic cerebral hypoperfusion. Sci Rep. 2015;5:12079. doi: 10.1038/srep12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado LS, Sazonova IY, Kozak A, Wiley DC, El-Remessy AB, Ergul A, Hess DC, Waller JL, Fagan SC. Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke. 2009;40:3028–3033. doi: 10.1161/STROKEAHA.109.556852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Wu H, Qu C, Mahmood S, Xiong Y, Kaplan D, Chopp M. Down-regulation of Nogo-A by collagen scaffolds impregnated with bone marrow stromal cell treatment after traumatic brain injury promotes axonal regeneration in rats. Brain Research. 2014;1542:41–48. doi: 10.1016/j.brainres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21:1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- Maraka S, Jiang Q, Jafari-Khouzani K, Li L, Malik S, Hamidian H, Zhang T, Lu M, Soltanian-Zadeh H, Chopp M, Mitsias PD. Degree of corticospinal tract damage correlates with motor function after stroke. Annals of Clinical and Translational Neurology. 2014;1:891–899. doi: 10.1002/acn3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda-Hashii Y, Takai K, Ohta H, Fujisaki H, Tokimasa S, Osugi Y, Ozono K, Matsumoto K, Nakamura T, Hara J. Hepatocyte growth factor plays roles in the induction and autocrine maintenance of bone marrow stromal cell IL-11, SDF-1 alpha, and stem cell factor. Exp Hematol. 2004;32:955–961. doi: 10.1016/j.exphem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, Kaneko Y, Ojika K, Hess DC, Borlongan CV. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. Bmc Neuroscience. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz LM, Li DKB, Traboulsee AL, Duquette P, Eliasziw M, Cerchiaro G, Greenfield J, Riddehough A, Yeung M, Kremenchutzky M, Vorobeychik G, Freedman MS, Bhan V, Blevins G, Marriott JJ, Grand’Maison F, Lee L, Thibault M, Hill MD, Yong VW. Trial of Minocycline in a Clinically Isolated Syndrome of Multiple Sclerosis. New England Journal of Medicine. 2017;376:2122–2133. doi: 10.1056/NEJMoa1608889. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. The Journal of Neuroscience. 2015;35:14002–14008. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi Y, Sabetkasaei M, Parvardeh S, Moini Zanjani T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol. 2017;28:214–222. doi: 10.1097/FBP.0000000000000297. [DOI] [PubMed] [Google Scholar]
- Newcomb JD, Ajmo CT, Jr, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006;15:213–223. doi: 10.3727/000000006783982043. [DOI] [PubMed] [Google Scholar]
- Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Fujimoto S, Petrov A, Nakagami H, Haider N, Zhou J, Tahara N, Osako MK, Fujimoto A, Zhu J, Murohara T, Edwards DS, Narula N, Wong ND, Chandrashekhar Y, Morishita R, Narula J. Effect of an Antimicrobial Agent on Atherosclerotic Plaques. Journal of the American College of Cardiology. 2010;55:1240–1249. doi: 10.1016/j.jacc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura-Matsuoka E, Yagita Y, Sasaki T, Terasaki Y, Oyama N, Sugiyama Y, Okazaki S, Sakoda S, Kitagawa K. Postischemic administration of angiotensin II type 1 receptor blocker reduces cerebral infarction size in hypertensive rats. Hypertens Res. 2009;32:548–553. doi: 10.1038/hr.2009.69. [DOI] [PubMed] [Google Scholar]
- Padma Srivastava MV, Bhasin A, Bhatia R, Garg A, Gaikwad S, Prasad K, Singh MB, Tripathi M. Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India. 2012;60:23–28. doi: 10.4103/0028-3886.93584. [DOI] [PubMed] [Google Scholar]
- Pavlichenko N, Sokolova I, Vijde S, Shvedova E, Alexandrov G, Krouglyakov P, Fedotova O, Gilerovich EG, Polyntsev DG, Otellin VA. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;3:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, Matsumoto M, Kohno M, Nishiyama A. Blockade of AT1 receptors protects the blood-brain barrier and improves cognition in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2011;24:362–368. doi: 10.1038/ajh.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker KR, Bix G, Fraser JF. Correcting the Trajectory of Stroke Therapeutic Research. Translational Stroke Research. 2017;8:65–66. doi: 10.1007/s12975-016-0517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegelsberger UM, Deten A, Pösel C, Zille M, Kranz A, Boltze J, Wagner DC. Intravenous human umbilical cord blood transplantation for stroke: Impact on infarct volume and caspase-3-dependent cell death in spontaneously hypertensive rats. Experimental neurology. 2011;227:218–223. doi: 10.1016/j.expneurol.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Roitbak T, Sykova E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28:40–48. doi: 10.1002/(sici)1098-1136(199910)28:1<40::aid-glia5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, Murray GD, Richter PS, Roine RO, Terent A, Thijs V, Berge E, Group, S.S. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- Sandset EC, Jusufovic M, Sandset PM, Bath PM, Berge E. Effects of blood pressure-lowering treatment in different subtypes of acute ischemic stroke. Stroke. 2015;46:877–879. doi: 10.1161/STROKEAHA.114.008512. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Ladhoff J, Huck J, Grohmann M, Blazej K, Oersal A, Baeva N, Seifert M, Priller J. Immune effects of mesenchymal stromal cells in experimental stroke. J Cereb Blood Flow Metab. 2012;32:1578–1588. doi: 10.1038/jcbfm.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Luders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhaupl K, Diener HC, Dominiak P, Acute Candesartan Cilexetil Therapy in Stroke Survivors Study, G. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Zacharek A, Cui Y, Ion M, Roberts C, Kapke A, Chopp M. Niaspan treatment induces neuroprotection after stroke. Neurobiol Dis. 2010;40:277–283. doi: 10.1016/j.nbd.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial Cells Stimulate Self-Renewal and Expand Neurogenesis of Neural Stem Cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Temple S. Fine control: microRNA regulation of adult neurogenesis. Nat Neurosci. 2009;12:369–370. doi: 10.1038/nn0409-369. [DOI] [PubMed] [Google Scholar]
- Soliman S, Ishrat T, Fouda AY, Patel A, Pillai B, Fagan SC. Sequential Therapy with Minocycline and Candesartan Improves Long-Term Recovery After Experimental Stroke. Transl Stroke Res. 2015;6:309–322. doi: 10.1007/s12975-015-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman S, Ishrat T, Pillai A, Somanath PR, Ergul A, El-Remessy AB, Fagan SC. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: role of vascular endothelial growth factors A and B. J Pharmacol Exp Ther. 2014;349:444–457. doi: 10.1124/jpet.113.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza CC, da Silva MC, Lopes RT, Cardoso MM, de Souza LL, Santos AG, dos Santos IR, Franco ECS, Gomes-Leal W. Comparative Therapeutic Effects of Minocycline Treatment and Bone Marrow Mononuclear Cell Transplantation following Striatal Stroke. Oxidative Medicine and Cellular Longevity. 2017;2017:1976191. doi: 10.1155/2017/1976191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Neocortical Neural Sprouting, Synaptogenesis, and Behavioral Recovery After Neocortical Infarction in Rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- Sun MK, Hongpaisan J, Nelson TJ, Alkon DL. Poststroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc Natl Acad Sci U S A. 2008;105:13620–13625. doi: 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Sasaki M, Harada K, Bando M, Kataoka Y, Onodera R, Mikami T, Wanibuchi M, Mikuni N, Kocsis JD, Honmou O. Bilateral cortical hyperactivity detected by fMRI associates with improved motor function following intravenous infusion of mesenchymal stem cells in a rat stroke model. Brain Res. 2013;25:15–22. doi: 10.1016/j.brainres.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley BC, Alarcon GS, Heyse SP, Trentham DE, Neuner R, Kaplan DA, Clegg DO, Leisen JC, Buckley L, Cooper SM, Duncan H, Pillemer SR, Tuttleman M, Fowler SE. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann Intern Med. 1995;122:81–89. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, Tsai SK, Hu BR, Liou DY, Huang SL, Huang MC, Huang WC, Cheng H, Huang SS. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. Journal of Biomedical Science. 2014;21:5–5. doi: 10.1186/1423-0127-21-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, Willing AE. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Chen J. New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J. 2016;57:223–228. doi: 10.3325/cmj.2016.57.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Balarezo MG, Affram K, Saavedra JM, Symes AJ. Neurorestoration after traumatic brain injury through angiotensin II receptor blockage. Brain. 2015;138:3299–3315. doi: 10.1093/brain/awv172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an angiotensin II AT(1)-receptor blocker and PPAR-gamma agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology. 2012;37:2817–2829. doi: 10.1038/npp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. Journal of Neuroscience Research. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- Wu Z, Zou X, Zhu W, Mao Y, Chen L, Zhao F. Minocycline is effective in intracerebral hemorrhage by inhibition of apoptosis and autophagy. J Neurol Sci. 2016;371:88–95. doi: 10.1016/j.jns.2016.10.025. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Levchenko T, Guo S, Stins M, Torchilin VP, Lo EH. Delivering minocycline into brain endothelial cells with liposome-based technology. J Cereb Blood Flow Metab. 2012;32:983–988. doi: 10.1038/jcbfm.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Yan T, Chopp M, Ye X, Liu Z, Zacharek A, Cui Y, Roberts C, Buller B, Chen J. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol Dis. 2012;46:157–164. doi: 10.1016/j.nbd.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. Neurorestorative Therapy of Stroke in Type 2 Diabetes Mellitus Rats Treated With Human Umbilical Cord Blood Cells. Stroke. 2015;46:2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Roberts C, Zhang Y, Lu M, Chen J. Neurorestorative Responses to Delayed Human Mesenchymal Stromal Cells Treatment of Stroke in Type 2 Diabetic Rats. Stroke. 2016;47:2850–2858. doi: 10.1161/STROKEAHA.116.014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther. 2014;20:935–944. doi: 10.1111/cns.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Ye X, Chopp M, Zacharek A, Ning R, Venkat P, Roberts C, Lu M, Chen J. Niaspan attenuates the adverse effects of bone marrow stromal cell treatment of stroke in type one diabetic rats. PLoS One. 2013;8:e81199. doi: 10.1371/journal.pone.0081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Zhou L, Wang D, Wang Z, Huang QY. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1alpha through SIRT-3/PHD-2 degradation pathway. Neuroscience. 2015a;304:250–259. doi: 10.1016/j.neuroscience.2015.07.051. [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Iacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010;19:1073–1084. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015b;12:015–0245. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali MM, Masuda T, Yu SJ, Bae EK, Hayashi T, Matsukawa N, Kaneko Y, Kuzmin-Nichols N, Ellovitch S, Cruz EL, Klasko SK, Sanberg CD, Sanberg PR, Borlongan CV. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14:914–921. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Kuo PC, Yen JH, Paraiso HC, Curfman ET, Hong-Goka BC, Sweazey RD, Chang FL. A Combination of Three Repurposed Drugs Administered at Reperfusion as a Promising Therapy for Postischemic Brain Injury. Transl Stroke Res. 2017 doi: 10.1007/s12975-017-0543-5. [DOI] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Zhang RL, Wang L, Zhang J, Wang Y, Toh Y, Santra M, Lu M, Zhang ZG. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, Lu M, Zhang ZG. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One. 2012;7:e48141. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion Load of the Corticospinal Tract Predicts Motor Impairment in Chronic Stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Wu Z, Zhu W, Chen L, Mao Y, Zhao F. Effectiveness of minocycline in acute white matter injury after intracerebral hemorrhage. J Neurosurg. 2016:1–8. doi: 10.3171/2016.5.JNS152670. [DOI] [PubMed] [Google Scholar]


