Abstract
OBJECTIVE
Pro-inflammatory cytokines have recently received considerable attention for their roles in suicidal behavior; however, how the expression of cytokine genes is regulated is not clearly known. Present study examined underlying mechanisms of critical cytokine gene TNF-α dysregulation in suicide brain.
METHODS
TNF-α expression was examined in dorsolateral prefrontal cortex (dlPFC) of suicide subjects with and without major depressive disorder (MDD) and non-suicide MDD subjects. Roles of putative miRNAs targeting TNF-α and RNA-binding protein HuR were tested using in-vitro and in-vivo approaches and by examining expression of TAR-RNA binding protein (TRBP). Genetic influence on TNF-α expression was determined by eQTL analysis and by genotyping three SNPs in promoter region of TNF-α gene. Promoter methylation of TNF-α was determined using MeDIP assay. Expression of miR-19a-3p and TNF-α was also determined in peripheral blood mono nuclear cells (PBMC) of 12 healthy controls and 12 MDD suicidal patients.
RESULTS
TNF-α expression was significantly higher in dlPFC of suicide subjects regardless of psychiatric diagnosis. Its expression level was also increased in MDD subjects who died by causes other than suicide. On the other hand, expression of miR-19a-3p was upregulated specifically in suicide subjects. In a preliminary observation, similar upregulation of TNF-α and miR-19a-3p was noted in PBMC of suicidal patients. Despite its ability to directly target TNF-α in-vitro, miR-19a-3p showed no interaction with TNF-α in dlPFC. HuR potentially stabilized TNF-α transcript presumably by sequestering its 3′UTR from miR-19a-3p-mediated inhibition. Furthermore, decreased TRBP expression supported abnormality in interaction between miR-19a-3p and TNF-α. Additionally, TNF-α transcriptional upregulation was associated with promoter hypomethylation, whereas no genetic influence was noted on altered TNF-α or miR-19a-3p expression in suicide subjects.
CONCLUSIONS
Our study provides mechanistic insights into the dysregulation of TNF-α gene in suicide brain, which could potentially be involved in suicidal behavior.
Keywords: TNF-α, miR-19a-3p, HuR, methylation, depression, suicide, human postmortem brain, SNP, eQTL, PBMC
INTRODUCTION
Approximately one million people die from suicide every year (1, 2). Thus, it is critical to explore the risk factors associated with suicidality. Several lines of evidence suggest that abnormalities in pro-inflammatory cytokines may be important contributory risk factor in suicidality (3, 4). Tumor necrosis factor (TNF)-α, a member of TNF superfamily, is one of the most important pro-inflammatory cytokines which plays a critical role in innate and adaptive immune responses (5). Studies have shown that not only TNF-α is increased in serum of depressed suicide attempters (6) but cerebrospinal fluid (CSF) TNF-α levels can serve as a predictor of suicidal ideation (7). This is supported by another study showing that a composite score of the inflammatory markers including TNF-α is associated with high suicidal ideation among patients with major depressive disorders (MDD) (8). Early-life adversity, a significant risk factor in suicidal behavior (9), is also associated with persistent high levels of TNF-α (10). We recently reported that expression of TNF-α is significantly increased in prefrontal cortex of adolescent suicide subjects (11).
Despite the evidence of increased TNF-α expression in suicide subjects, the underlying regulatory mechanisms are not well understood. Recent evidence suggests the involvement of several genetic and epigenetic mediators in the regulation of TNF-α expression (12, 13). For example, a genetic association study showed that frequency of G/G polymorphic locus at 308 position of TNF-α was increased in suicide completers (14) as well as suicide attempters in MDD patients (15). Besides, DNA methylation in promoter region of TNF-α appears to be crucial in neurodegenerative disorders (12). Additional layer of epigenetic regulation in TNF-α expression may occur at post-transcriptional level. This is evidenced by studies showing that miRNAs are involved in regulation of pro-inflammatory cytokines in cancer, age-related macular degeneration, and hemochromatosis (16–18). The repressive interaction mediated by miRNAs on its target genes is governed by several factors (19). One of them is derived from miRNA processing protein TRBP (20, 21). TRBP is an integral component of the RISC-loading complex. One of the functions of TRBP is to transfer the miRNA/miRNA* duplex from dicer to RISC, which eventually guides miRNA binding to 3′UTR of target genes (20, 22). An emerging concept of cis-acting structural RNA motif containing AU rich sequence element (ARE) on certain transcripts has also gained credence for their regulatory effects (23). ARE stabilizes transcripts by preventing the immature decay while interacting with specific set of RNA-binding proteins such as Hu antigen R (24, 25). In fact, enhanced expression of TNF-α has been shown to be associated with stabilization of its transcripts containing ARE in 3′UTR mediated by HuR (26). Endogenous depletion of HuR reverses this stabilization (27).
Given the multilayer regulation of TNF-α gene, the present study was undertaken not only to examine TNF-α gene expression but also to understand the precise molecular mechanisms associated with this dysregulation in suicide brain. For this, we determined TNF-α expression in dlPFC of suicide subjects with MDD, suicide subjects with other psychiatric disorders, and normal controls. The study was also performed in another cohort consisting of suicide subjects with MDD, MDD non-suicide, and normal controls. The underlying mechanisms of dysregulated TNF-α expression was investigated at genetic and epigenetic levels by examining eQTL, genetic polymorphisms, DNA methylation, miRNAs, TRBP, and HuR. Changes in expression of TNF-α and regulatory miRNA were also studied in a preliminary fashion in PBMCs of suicidal individuals.
METHODS
Methods are described briefly and are discussed in detail in the supplementary section. Also, the overall experimental design has been provided in Table S1. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham.
Human postmortem brain studies
Subjects
The study was performed in two cohorts: Quebec Suicide Brain Bank (referred as McGill cohort) and Maryland Brain Collection (referred as Maryland cohort). The Quebec cohort included 16 non-psychiatric controls (referred to as normal controls) and 43 suicide subjects (21 MDD-suicide and 22 suicides with other psychiatric disorders). Maryland cohort had 14 MDD-suicides, 12 MDD non-suicides, and 12 normal controls. The demographic and clinical characteristics of subjects from Quebec and Maryland cohorts are provided in Table S2 and Table S3, respectively. The psychiatric diagnoses were determined by the psychological autopsy method as detailed in the supplementary section. The study was performed in dlPFC since several studies have implicated this brain area in suicidal behavior (28–30) and we recently reported abnormalities in the expression of cytokine genes in this brain area of suicide subjects (31).
Relative transcript quantification of TNF-α, TRBP, HuR, miRNAs targeting TNF-α and members of miR-17-92 cluster
Total RNA was isolated following TRIzol® method and were screened based on their purity (260/280 nm) and RNA integrity number (>7). Since RNA integrity may affect the expression of genes and may pose a confounding factor, we measured pH and RNA Integrity Number (RIN) in all the samples included in this study. The mean pH and RIN are provided in Tables S2 AND 3. All the cases had RIN>7. Coding gene cDNA synthesis was performed with oligo (dT)18 method whereas miRNA specific cDNA was synthesized following poly A tailing method. Relative transcript expressions of all genes were determined using EvaGreen chemistry. miR-17-92 cluster contains 6 miRNAs (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92a-1). The coding units of these 6 miRNAs produce 12 mature miRNAs: miR-17-5p, miR-17-3p, miR-18a-5p, miR-18a-3p, miR-19a-5p, miR-19a-3p, miR-20a-5p, miR-20a-3p, miR-19b-1-5p, miR-19b-1-3p, miR-92-1-5p and miR-92-1-3p. According to miRBase 21, expression of miR-19a-5p variant is very low (number of reads 27 based on deep sequencing studies). Similar negligible expression of this variant has been reported in human brain as reported by miRmine, a human miRNA expression database (32) which appeared to be biologically irrelevant (33). Therefore, we studied expression of all the miRNAs from this cluster except miR-19a-5p. The primer sequences are listed in Table S4. The results were calculated using 2−ΔΔCt method and reported as fold change.
Genotyping
A case-control study including 35 controls and 60 cases was conducted to determine the genetic polymorphism in TNF-α gene in the genomic DNA isolated from dlPFC. The TNF-α gene is located on the short arm of chromosome 6 within the major histocompatibility complex, where genetic alterations in the TNF-α locus are involved directly in high TNF-α production (34). Several polymorphisms have been identified inside the TNF-α promoter [−1031 (T/C), −863 (C/A), −857 (C/A), −851 (C/T), −419 (G/C), −376 (G/A), −308 (G/A), −238 (G/A), −162 (G/A), and −49 (G/A)] (35). The allelic frequencies of TNF-α have been reported in various populations and are quite consistent (36), although the polymorphisms at −419, −163, −49 are rare in Caucasians (35). Three SNPs (−308G/A: rs1800629, −238G/A: rs361525, −1031T/C: rs1799964) were selected based on published studies in suicide or depression (15, 37, 38). PCR amplification products were purified and sequenced using BigDye terminator cycle sequencing kit v3.1. The primer sequences are listed in Table S4.
Mutation screening analysis
Since rare mutations within seed sequence of miR-19a-3p or TNF-α 3-UTR may directly affect their canonical bindings which may lead to altered transcript expression, presence of these mutation(s) were screened for both TNF-α 3′UTR and miR-19a-3p. DNA fragments of TNF-α 3′UTR and miR-19a-3p genes were amplified by PCR using specific primers (Table S4) and sequenced (described in supplementary method).
Methylated DNA immunoprecipitation assay (MeDIP) for TNF-α promoter element
A potential CpG island was identified within 1kb upstream region of TNF-α transcription start site and analyzed by MeDIP using monoclonal 5-mC antibody. To determine the methylation status, qPCR was used to amplify CpG island based on primer sequences listed in Table S4 and analyzed after normalization with input control.
RNP-IP assay for detecting HuR interaction with TNF-α AU rich element (ARE) in dlPFC
Relative enrichment of TNF-α 3′UTR harboring ARE was assessed following the RNP-IP method discussed in supplementary section. The primer sequences are mentioned in Table S4.
In vitro cell line based studies
Luciferase reporter assay for detecting miR-19a-3p interaction with TNF-α 3′UTR
Luciferase reporter assay was performed using the pMIR-REPORT™. pMIR-TNF-α plasmid engineered with a 192bp fragment from the 3′UTR of TNF-α containing miR-19a-3p binding sites was co-transfected in HEK-293 cells with miR-19a-3p mimics or negative controls, pMIR-TNF-α and pRL-TK. Forty eight hours later, Firefly/Renilla luciferase activities were measured.
Cell line-based TNF-α expression studies in response to miR-19a-3p manipulation
Transient transfections of miR-19a-3p mimic or scramble were performed in SH-SY5Y cells. Total RNAs were isolated and were reverse transcribed into 1st strand cDNA to determine the relative TNF-α expression after normalization with GAPDH.
Cell proliferation assay
Cell proliferation in transfected SH-SY5Y cells with miRNA oligonucleotide was measured by MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) assay. The Formazan crystals formed were dissolved in dimethyl sulfoxide and absorbance was measured at 570 nm.
Transient transfection of HEK-293 cells for HuR overexpression and knockdown
HuR siRNA, negative control siRNA, and HuR overexpression vector were transfected with lipofectamine 2000. After 48h of transfection, expression of TNF-α and HuR were analyzed at both mRNA and protein levels.
Luciferase reporter assay for HuR interaction with TNF-α AU-rich element
The pEZX-TNF-3′UTR containing 3′UTR full length fragments and HuR overexpression vector were co-transfected into HEK-293 cells. After 48h, Firefly/Renilla luciferase activity was measured.
RNP-IP assay detecting HuR interaction with TNF-α ARE in-vitro
HEK-293 cell-derived lysates were immunoprecipitated using HuR antibody. Immunocaptured RNA was used to prepare 1st strand cDNA following oligo (dT)18 method. HuR-mediated TNF-α 3′UTR enrichment was examined in cDNA following qPCR assay. The primer sequences are provided in Table S4.
Gene expression studies in PBMC of suicidal patients
In a pilot study, we examined expression of miR-19a-3p and TNF-α in 12 healthy controls and 12 MDD subjects with current serious suicidal ideation. The characteristics of patients are shown in Table S7. All participants were evaluated using the Mini International Neuropsychiatric Interview (39) or the Structured Clinical Interview for DSM-IV-TR (40). Depression severity was determined using the Montgomery-Åsberg Depression Rating Scale 4 (41). MDD suicidal patients had a MADRS item 10 score of >4. All MDD participants were psychotropic drug free prior to blood sampling. Healthy controls had no lifetime history of any mental disorder. Venous blood was collected (between 9 and 11 am) and processed immediately for PBMC isolation. PBMC pellets were processed for RNA isolation using TRIzol® method. mRNA- and miRNA-specific 1st strand cDNA synthesis and PCR amplifications were done using the primer sequences mentioned in Table S4.
Statistical Analysis
Data were analyzed using SPSS (v.23). The comparison between normal controls and suicide subjects were performed by independent-sample t-test. The correlations between miRNAs or mRNAs with covariates were determined by Pearson product-moment correlation analyses. Multiple group comparisons were performed using one-way ANOVA followed by Bonferroni corrections. We reported only those data that were normally distributed. For comparisons of genotypic and allelic frequencies and Hardy-Weinberg equilibrium within suicide and controls, χ2 tests was performed using SHEsis software (42). eQTL analysis accounted for sex and age as covariate was performed based on Matrix eQTL software on the R platform. To statistically measure the genotype effect on TNF-α expression, ANOVA was performed using function “aov” in R. The p-values ≤0.05 was considered statistical significant.
RESULTS
TNF-α expression studies
TNF-α expression in dlPFC: Effect of Suicide and MDD
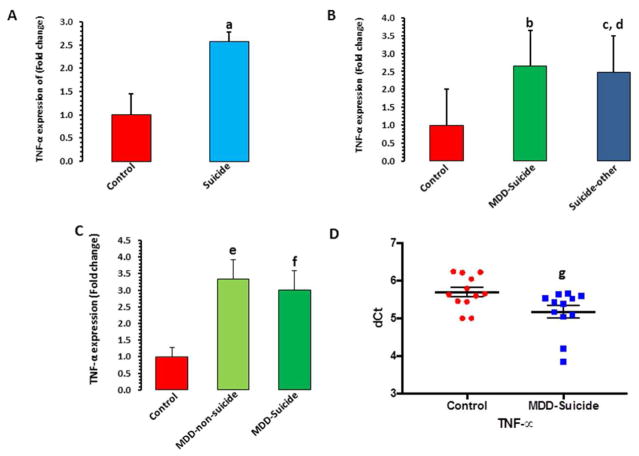
In McGill cohort, a statistically significant 2.5-fold greater expression (p=0.004) was found in dlPFC of suicide subjects compared with normal controls (Figure 1A). When suicide group was divided into MDD-suicide and suicide with other psychiatric illnesses, a significant overall difference (df=2,56, F=4.35, p=0.017) was noted. Individual comparisons showed that expression of TNF-α was significantly higher in both MDD-suicide (p=0.009) and suicide with other psychiatric illness (p=0.014) groups compared with normal controls without any significant difference between MDD-suicide and suicide with other psychiatric illness groups (p=0.83) (Figure 1B).
Figure 1. TNF-α expression profile in dlPFC and PBMC of suicide subjects and normal control.
A) TNF-α expression in PFC of normal controls (n = 16) and suicide subjects (n = 43). Data are represented as the mean ± SEM. The level of significance was determined with independent sample t-test. t = 2.97, df = 57, ap = 0.004 compared with normal controls.
B) Effect of psychiatric illnesses on the expression of TNF-α in dlPFC of suicide subjects. Data are represented as the mean ± SEM. Suicide subjects were divided into those who had MDD diagnosis (n = 21) and those with other psychiatric illnesses (n = 22) as described in Table S1. Overall one-way ANOVA between the 3 groups was: F = 4.35, df = 2,56, p = 0.017. bp = 0.009 compared with normal control; cp = 0.014 compared with normal control; dp = 0.83 compared with MDD-suicide.
C) Comparisons of TNF-α mRNA expression in dlPFC between MDD-non-suicide and MDD-suicide and normal controls. Data are represented as the mean ± SEM. Overall ANOVA analysis between the 3 groups was: F = 3.3, df = 2,35, p = 0.04. ep = 0.03 when MDD-suicide group was compared with normal controls; fp = 0.027 when MDD-non-suicide group was compared with normal controls and p = 0.83 when MDD-suicide group was compared with MDD-non-suicide group.
D) TNF-α mRNA expression in PBMC of normal controls (n = 12) and MDD-suicidal patients (n = 12). The level of significance was determined by independent sample t-test. t = 2.46, df = 22, gp = 0.02.
To further test if TNF-α increase was specifically associated with suicide, we examined its expression in MDD-suicide, MDD non-suicide, and normal control groups in Maryland cohort. Overall ANOVA showed a significant difference in the expression of TNF-α (df=2,35, F=3.3, p=0.04). When compared individually, MDD-suicide (p=0.03) and MDD non-suicide (p=0.027) groups were significantly different from the normal control group but these two groups were not significantly different among each other (p=0.83) (Figure 1C).
In both McGill and Maryland cohorts, the expression of TNF-α was not related to, PMI, brain pH, gender, antidepressant, alcohol, or substance abuse at the time of death, however, TNF-α showed significant correlation with age in the Maryland cohort but not in the McGill cohort (Figures S1, S2 and Table S5).
We also combined the data sets from both Maryland and McGill cohorts and analyzed the expression of TNF-α. A statistically significant (p=0.001; 4.6-fold) increase in TNF-α expression was noted in the suicide group (n=57) compared with normal controls (n=28) (Figure S7A).
TNF-α expression in PBMC of MDD suicidal patients
The expression levels of TNF-α was significantly higher (43%; p=0.02) in PBMCs of MDD patients with serious suicidal ideation compared with healthy controls (Figure 1D), which was not related to age, gender, or race (Figure S3).
MiRNA expression studies
Expression of miRNAs targeting TNF-α and miR-17-92 cluster miRNAs in dlPFC
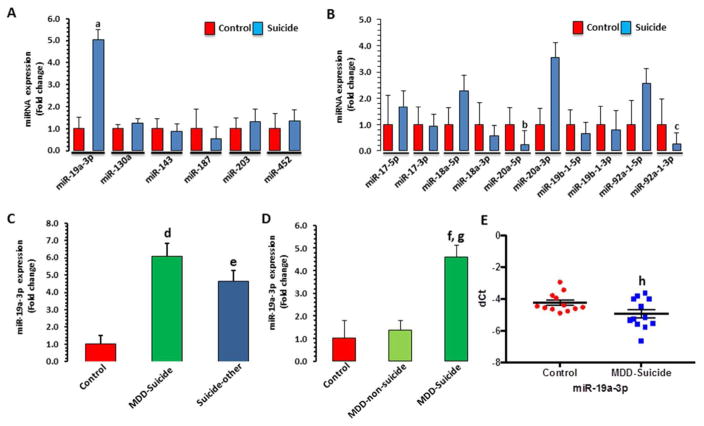
Based on the bioinformatics analysis and target prediction scores, 6 miRNAs (miR-130a-3p, miR-143-3p, miR-187-3p, miR-203a-3p, miR-452-5p and miR-19a-3p) were selected as potential repressors of TNF-α expression. qPCR analyses did not show any significant alteration in any miRNAs except miR-19a-3p, which was significantly higher (~5 fold) in suicide subjects (p=0.008) (Figure 2A).
Figure 2. miRNA expression profile in dlPFC and PBMC of suicide subjects and normal control.
A) Relative expression levels of 6 putative miRNAs, potentially targeting TNF-α mRNA expression in dlPFC of suicide subjects (n = 43) and normal controls (n = 16). Data are the mean ± SEM. The level of significance was determined by independent sample t-test. at = 2.74, df = 57, p = 0.008 compared with normal controls.
B) Expression of miRNA members (miR-17-5p, miR-17-3p, miR-18a-5p, miR-18a-3p, miR-20a-3p, miR-19b-1-5p, miR-19b-1-3p, miR-92-1-5p and miR-92-1-3p) of miR-17-92 cluster. Data are the mean ± SEM. bt = −2.1412.74, df = 57, p = 0.037008; ct = −2.089, df = 57, p = 0.041 when compared with normal controls.
C) Effect of psychiatric illnesses on the expression of miR-19a-3p in dlPFC. Suicide subjects were divided into those who had MDD diagnosis (n = 21) and those with other psychiatric illnesses (n = 22) as described in Table S1. Overall one-way ANOVA between the 3 groups was: F = 3.74, df = 2,56, p = 0.03. dp = 0.015 compared with normal controls; ep = 0.024 compared with normal controls; p = 0.80 compared with MDD-suicide.
D) Comparisons of miR-19a-3p expression levels in dlPFC between MDD-non-suicide (n = 12), MDD-suicide (n = 14) and normal controls (n = 12). Overall one-way ANOVA between the 3 groups was: F = 3.3, df = 2,35, p = 0.04. fp = 0.015 when MDD-suicide group was compared with normal controls; p = 0.61 when MDD-non-suicide group was compared with normal controls; gp = 0.04 when MDD-non-suicide and MDD-suicide groups were compared with each other.
E) miR-19a-3p expression in PBMC of MDD-suicidal patients (n = 12) compared with healthy controls (n = 12). The data were normalized using U6 gene expression values and represented as ± SEM. Level of significance was determined using independent-sample t-test (hdf = 28, f = 2.383, t = 4.349, p = 0.001).
Since miR-19a-3p is part of miR-17-92 cluster, expression levels of 10 other miRNAs (miR-17-5p, miR-17-3p, miR-18a-5p, miR-18a-3p, miR-20a-5p, miR-20a-3p, miR-19b-1-5p, miR-19b-1-3p, miR-92-1-5p and miR-92-1-3p) associated with this cluster (miR-17-92) were also examined. No significant changes in the expression of any of the cluster miRNAs were observed in dlPFC of suicide subjects except miR-20a-5p and miR-92a-1-3p whose expression levels were significantly downregulated (miR-20a-5p: p=0.037; miR-92a-1-3p; p = 0.041) by ~77% (0.23-fold) and 73% (0.27-fold), respectively (Figure 2B).
To examine whether observed change in miR-19a-3p was related to suicide, we divided suicide subjects into MDD-suicide and suicide with other psychiatric illnesses. Overall ANOVA showed significant group difference (df=2.56, F=3.74, p=0.03). When examined individually, the expression of miR-19a-3p was significantly higher in both MDD-suicide (p=0.015; ~6.2-fold) and suicide with other psychiatric illness (p=0.024; ~4.7-fold) groups compared with normal controls. There was no significant difference between MDD-suicide and suicide with other psychiatric illness groups (p=0.80) (Figure 2C).
Using dlPFC from Maryland cohort, we examined if the changes in miR-19a-3p expression were suicide specific. Overall ANOVA showed significant difference in the expression of miR-19a-3p when MDD-suicide, MDD non-suicide, and normal control groups were compared with each other (df=2,35, F=3.3, p=0.04). Individually, miR-19a-3p was significantly higher only in MDD-suicide group (p=0.015; ~4.6-fold) but not in the MDD-non-suicide group (p=0.61) compared with normal controls. On the other hand, MDD suicide group showed significant difference when compared with MDD-non-suicide group (p=0.04) (Figure 2D).
The changes in expression of miR-19a-3p in both the cohorts was not associated with, PMI, brain pH, and antidepressant toxicology except there was a significant correlation with age and miR-19a-3p (r=0.26, p=0.05) in McGill cohort (Figures S1, S2; Table S5).
Combing the data sets from Maryland and McGill cohorts showed similar significant upregulation (p = 0.0001; ~4.8-fold) of miR-19a-3p in dlPFC of suicide subjects compared with normal controls (Figure S7B).
Expression of miR-19a-3p in PBMC of MDD suicidal patients
When examined in PBMC, the expression of miR-19a-3p was significantly higher in MDD patients with serious suicidal ideation as compared to healthy controls (63%, p= 0.001; Figure 2E). The changes in miR-19a-3p expression were not correlated with gender, age or race (Figure S3).
In-vitro validation of TNF-α 3′UTR interaction with miR-19a-3p and its effects in dlPFC of suicide subjects
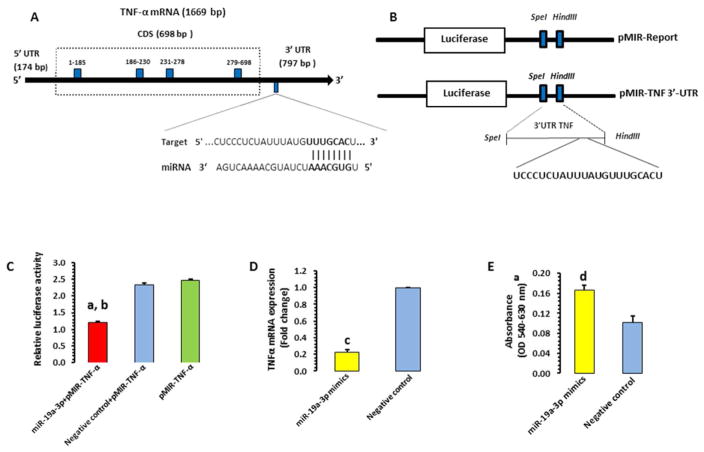
To examine whether miR-19a-3p directly interacts with TNF-α 3′UTR, HEK-293 cells were transfected with cloned TNF-α 3′UTR downstream of a luciferase reporter gene (Figure 3A, Figure 3B). Luciferase activity of HEK-293 cells co-transfected with miR-19a-3p mimic was significantly decreased compared with vector alone (~0.49-fold; p=0.0004) or negative control (0.5-fold; p=0.001) (Figure 3C). This suggests that TNF-α is a direct target of miR-19a-3p. Under in-vitro condition, functional regulation of miR-19a-3p on TNF-α gene was determined in SH-SY5Y cells by transfecting miR-19a-3p mimic or negative control and quantifying TNF-α expression. Transfection of miR-19a-3p significantly decreased the expression of TNF-α (p< 0.0001) whereas transfection with negative control had no effects (Figure 3D). We next examined whether overexpression of miR-19a-3p had proliferative potential for SH-SY5Y cells. MiR-19a-3p overexpression significantly stimulated cell growth compared with cells transfected with negative control (p=0.016; Figure 3E).
Figure 3. In-vitro validation of TNF-α 3′UTR interaction with miR-19a-3p.
A) Schematic diagram of human TNF-α coding gene with a focus on 797 bp of untranslated region at 3′ end. The representative sequence denotes the miR-19a-3p binding site on TNF-α 3′UTR.
B) A schematic representation of pMIR-report and pMIR-TNF-3′UTR vector constructs. A stretch of 192bp TNF-α 3′UTR was cloned into the multiple cloning sites downstream of the luciferase gene.
C) Bar diagram representing the ratio between firefly luciferase activity and Renilla luciferase activity. The overall group difference between miR-19a-3p mimics + pMIR-TNF-α, negative control, and pMIR-TNF-α is as follows: t = 23.64, df = 4, p <0.0001. ap < 0.00159 when miR-19a-3p mimics + pMIR-TNF-α co-transfection group was compared with negative control; bp = 0.0004 when miR-19a-3p mimics + pMIR-TNF-α co-transfection group was compared with vector alone; p = 0.16 when negative control was compared with vector alone.
D) Bar diagram representing the SH-SY5Y cell line based TNF-α expression status under miR-19a-3p oligo overexpression as compared to negative control. The expression level was normalized to GAPDH expression level. ct = 29.79, df = 4, p <0.0001 compared with negative control.
E) Effect of miR-19a-3p overexpression on the growth of SH-SY5Y cells as measured by MTT assay. miR-19a-3p mimics significantly enhanced cell proliferation. dt = 3.99, df = 4, p = 0.016 compared with negative control.
Regulation of TNF-α expression by TRBP and HuR in dlPFC
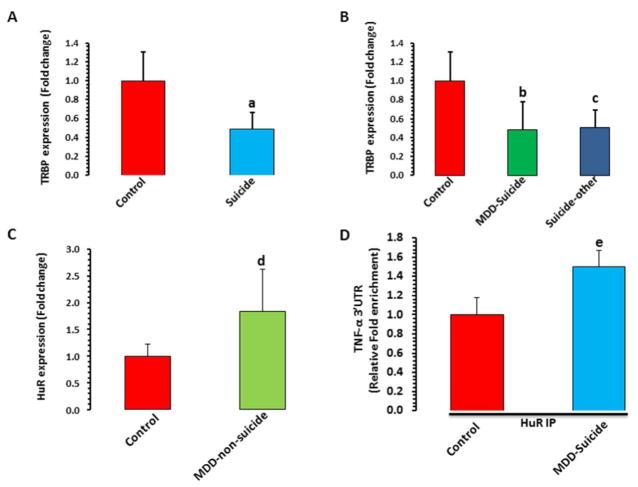
mRNA expression of TRBP was determined in the same RNA fraction in which miR-19a-3p and TNF-α were examined. It was observed that expression of TRBP was significantly lower in the suicide group compared with normal controls (p=0.007; ~0.45-fold; Figure 4A). This decrease was present in both MDD-suicide (p=0.021; ~0.44-fold) and suicide with other psychiatric illness (p=0.012; ~0.45-fold) groups compared with normal controls (Figure 4B).
Figure 4. Post-transcriptional regulation of TNF-α gene by TRBP and HuR.
A) Comparison of TRBP expression between normal controls (n = 16) and suicide subjects (n = 43). The two groups were compared by independent sample t test. Data are the mean ± SEM. The group differences are as follows: t = 2.8, df = 57, ap = 0.007.
B) Effect of psychiatric illnesses on the expression of TRBP in dlPFC. Suicide subjects were divided into those who had MDD diagnosis (n = 21) and those with other psychiatric illnesses (n = 22) as described in Table S1. Overall one-way ANOVA between the 3 groups was: F = 3.94, df = 2,56, p = 0.025. bp = 0.021 compared with normal controls; cp = 0.012 compared with normal controls; p = 0.84 compared with MDD-suicide.
C) Comparison of HuR mRNA expression in dlPFC between suicide (n=43) and normal controls (n=16). Data are the mean ± SEM. The group differences are as follows: dt = 2.16, df = 22, p = 0.04.
D) Interaction between HuR and TNF-α in dlPFC of normal controls (n= 8) and suicide subjects (n = 9) was examined by RNP-IP assay. Relative 3′ UTR enrichment was determined after normalizing with 10% input. Data are presented as ± SEM. Data was analyzed by independent sample t-test. ep = 0.028.
Next, mRNA expression of HuR was studied in dlPFC of suicide subjects. The expression of HuR was significantly higher (~1.8-fold; p=0.04) in the suicide group compared with normal controls (Figure 4C).
In order to examine the role of HuR protein in protecting the TNF-α transcript from repressive effect of miR-19a-3p, endogenous recruitment of HuR on putative AU-rich element of TNF-α 3′UTR was determined using RNP-IP assay. Differential binding analysis showed ~50% enrichment of HuR protein binding with 3′UTR of TNF-α mRNA in suicide subjects compared to normal controls (p=0.028; Figure 4D).
To further validate the protecting effect of HuR protein on TNF-α expression, HEK-293 cells were used. HuR mRNA was significantly up-regulated (p=0.0008; ~15-fold) in the HuR overexpression group compared to the negative control group, whereas HuR mRNA in the siRNA group was significantly decreased (p=0.001; ~0.3-fold) compared to negative control group (Figure S4A). Interestingly, TNF-α mRNA level in the overexpression group was increased corresponding to the up-regulated HuR expression (p=0.005; ~14-fold). On the contrary, the TNF-α mRNA level was decreased (p=0.004; ~0.6-fold) in the HuR knockdown group due to the down-regulation of HuR mRNA. This in-vitro assay reveals that TNF-α is positively regulated by HuR (Figure S4B).
To further confirm the transient effect of HuR overexpression and knockdown in HEK-293 cell line, the endogenous protein level of HuR was detected using immunoblotting. The overexpression group showed increased expression of endogenous HuR whereas the knockdown group showed depleted HuR compared to non-transfection control (Figure S4C).
Next, in-vitro luciferase reporter assay was used to determine the HuR-mediated direct regulation of TNF-α 3′UTR in HEK-293 cell line. As shown in Figure S4D, luciferase activity was significantly increased in TNF-α 3′UTR and GFP-HuR overexpression clone co-transfected group compared to GFP-HuR null transfected group (p=0.0002).
To assess HuR-mediated TNF-α transcript interaction, both ectopically expressed and endogenously depleted HEK-293 cells for HuR protein were used to measure HuR interaction with TNF-α ARE. Following the HuR-mediated RNP-IP approach, ~7 fold enrichment of TNF-α 3′UTR was observed in the overexpression group compared with no transfection control, whereas ~50% decrease was noticed in the knock-down group (Figure S4E).
TNF-α promoter methylation in dlPFC
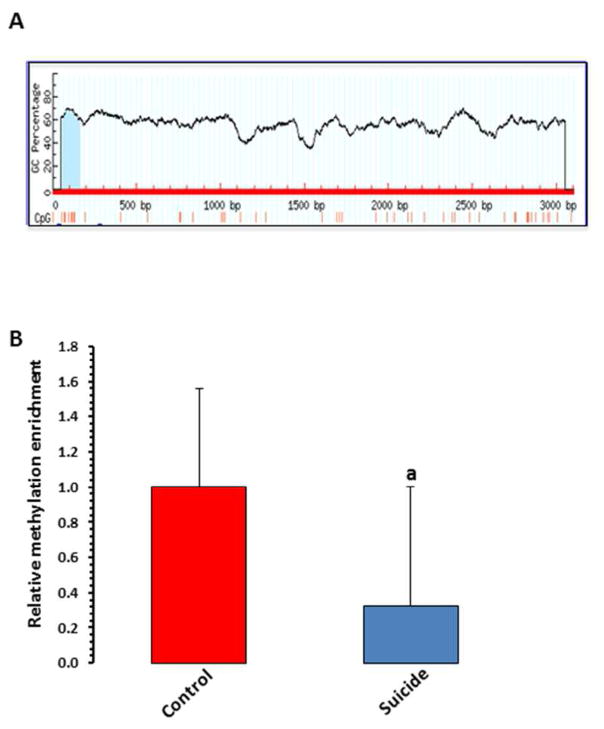
To examine the methylation associated epigenetic modification as putative cause of TNF-α upregulation, a CpG island comprised of 99 bases and ~50% GC contents was identified in TNF-α gene promoter (Figure 5A) and analyzed by MeDIP method. As shown in Figure 5B, the TNF-α promoter was significantly hypomethylated (~0.7-fold less enrichment) in the suicide group compared with the control group (p = 0.039).
Figure 5. In-vivo DNA methylation mediated epigenetic regulation of TNF-α transcription and putative model depicting the regulation of TNF-α gene in suicide brain.
A) Schematic diagram representing in-silico analysis for TNF-α proximal promoter (33kb upstream from transcription start site) on human chromosome 6. MethPrimer-based CpG site prediction algorithm identified a CpG island on TNF-α proximal promoter with more than 50% GC content.
B) Bar diagram showing relative methylation enrichment of TNF-α promoter in suicide subjects (n=16) compared with normal control (n=16). Represented data were normalized with 10% input and expressed as fold change over control group. Results are the mean ± SEM. ap = 0.039.
Genetic studies in dlPFC
To examine if genetic factors are involved in increased TNF-α expression in suicide subjects, 3 SNPs (rs361525, rs1800629, rs1799964) in TNF-α gene promoters were examined. Since there was no polymorphism for rs361525 in TNF-α gene, we excluded this SNP and focused only on rs1800629 and rs1799964. These two SNPs were in Hardy-Weinberg equilibrium. The allelic and genotypic frequencies are listed in the Table S6. There was no significant discrepancy in allelic or genotypic frequencies between suicide and normal control subjects (p >0.05, Table S6).
To detect the effect of each genotype on mRNA abundant, eQTL analysis was performed. The data demonstrated no significant difference between the three genotypes for both SNPs (rs1799964, p=0.19; rs18000629, p=0.54) (Figure S5).
To examine whether increased expression levels of miR-19a-3p and TNF-α were associated with duplication(s) or rare mutation(s), TNF-α 3UTR and miR-19a-3p seed DNA sequences were screened for structural variations. As can be seen in Figures S6A and 6B, no sequence differences for both miR-19-a-3p and TNF-α were observed between suicide subjects and normal controls.
DISCUSSION
To our knowledge, this is the first study of TNF-α expression regulation in brain of suicide subjects. We studied TNF-α expression in two different cohorts of brain samples and found that expression of TNF-α in dlPFC was consistently increased in all suicide subjects regardless of psychiatric diagnosis. A similar change was also noted in depressed individuals who died by causes other than suicide.
Interesting results emerged when we examined the molecular mechanisms associated with increased expression of TNF-α. We first studied the possible regulation of TNF-α by miRNAs, which belong to a novel class of small non-coding RNAs that regulate the gene expression at post-transcriptional level by binding to 3′UTR of the target genes guided by RNA-induced silencing complex (RISC). RISC is comprised of TAR-RNA binding protein (TRBP), Dicer, and Ago2 and is functionally responsible for miRNA processing and gene silencing (43). Based on sequence complementarity, prediction score, and experimentally validated target database, we selected 6 putative miRNAs (miR-130a-3p, miR-143-3p, miR-187-3p, miR-203a-3p, miR-452-5p, miR-19a-3p) potentially regulating TNF-α expression. When examined individually, miR-19a-3p was found to be the only miRNA that showed significant upregulation in dlPFC of suicide subjects. Interestingly, this upregulation was confined only to those individuals who died by suicide but not in those who died by causes other than suicide compared with normal controls. This suggests that change in miR-19a-3p expression in dlPFC is specific to suicide.
MiR-19a-3p is a member of miR-19a family, which is encoded by miR-17-92 cluster. Recently, it has been shown that miR-17-92 cluster is involved in inflammatory response in malignant human cancers which is primarily mediated by miR-19a-3p via TNF-α (17). Our in-vitro luciferase 3′UTR reporter assay in HEK293 cells and miR-19a-3p mimic transfection assay in neuroblastoma cells ascertain a direct binding of miR-19a-3p with TNF-α 3′UTR. However, contrary to our in-vitro study, we found that the conventional putative interaction was not observed under in-vivo conditions as expression levels of both TNF-α and miR-19a-3p were upregulated in dlPFC of suicide subjects. This indicates the possibility of sequestration of TNF-α 3′UTR from the inhibitory effect of miR-19a-3p under in vivo conditions.
MiR-19a-3p belongs to evolutionarily conserved miR-17-92 cluster, which is located within a locus of non-protein-coding gene MIR17HG (also known as also known as C13orf25) on chromosome 13. In order to test whether upregulation in miR-19a-3p was in consistent with other members of miR-17-92 cluster, we examined the expression of mature miRNAs belonging to this cluster. We found that none of the miRNAs were altered except miR-20a-5p and miR-92-1-3p, which showed significantly downregulation. This downregulation was in quite contrast to the observed change in miR-19a-3p, which was upregulated in suicide subjects. This suggests that miR-19a-3p expression is primarily governed by independent regulatory mechanism(s) despite being influenced by transcriptional mediators at miR-17-92 cluster. It is pertinent to mention that mapping of 3′UTR, even with less conservative target prediction scheme following TargetScan (v7.1) did not show any putative binding site for TNF-α gene for any of the miRNAs belonging to miR-17-92 cluster except miR-19a-3p (Figure S8).
To understand the underlying mechanism of no interaction of miR-19a-3p with TNF-α, we tested whether there are duplications or mutations in 3′UTR region of TNF-α or if there are mutations in the seed sequences of precursor-miR-19a. Sequencing of TNF-α 3′UTR showed no mutations. Also, no mutations or duplications were observed in the seed sequences of precursor-miR-19a. These results rule out the possibility that mutations or variations in TNF-α or precursor-miR-19a has any role in preventing the interaction between these two molecules.
Next, the role of TRBP was investigated. When examined, we found that expression of TRBP was significantly lower in dlPFC of suicide subjects. This raises an interesting possibility that less availability of TRBP may be critical in preventing miR-19a-3p binding to TNF-α. It is pertinent to note that recently we reported that pretreatment of rats with a flouoroquinolone compound, which stabilizes the complex between dicer and TRBP and enhances dicer-mediated precursor processing and/or loading onto RISC, can ameliorate depressive symptoms (44), suggesting that aberrant TRBP expression may have phenotypic consequences.
We further tested the possibility that miRNA response may be part of a homeostatic mechanism trying to pull down TNF-α expression but is being thwarted by changes in the accessibility of the TNF-α UTR through HUR binding. As mentioned in Introduction, HuR stabilizes specific transcript by interacting with AREs present in the 3′UTRs of protein coding genes (26). Interestingly, involvement of target mRNAs of HuR in inflammatory responses have been reported in various disorders (46). In the present study, HuR antibody-mediated RNP-IP assay was used to examine the interaction between HuR and 3′UTR of TNF-α transcript in in-vivo. We observed that this interaction was highly enriched in dlPFC of suicide subjects compared with normal controls, suggesting that increased interaction of HuR with TNF-α could probably be associated with its increased expression. This was further implied by our in-vitro HEK-293 cell studies where similar HuR:TNF-α transcript interaction was noted under ectopic overexpression of HuR protein, whereas a reduction in HuR-mediated TNF-α transcript interaction was identified in the HuR knockdown model. This mechanism could also be critical in preventing the binding of miRNAs to target genes. Thus, HuR can potentially hinder and competitively eliminate miR-19a-3p-mediated repressive effect on TNF-α transcript due to induction of a conformational change in RNA structure and subsequent obstruction of RISC-mediated interaction of miR-19a-3p with TNF-α.
Besides miRNA and HuR-mediated modifications, we also examined other epigenetic and genetic factors for their possible roles in TNF-α upregulation in suicide brain. For DNA methylation, we identified a potential CpG island within TNF-α promoter region and performed MeDIP analysis. We found that TNF-α gene was hypomethylated in dlPFC of suicide subjects compared with normal controls, suggesting that altered promoter methylation may additionally be involved in up-regulation of TNF-α in the suicide brain. For genetic analysis, a case-control study was designed. Based on the literature, three SNPs (−308G/A: rs1800629; −238G/A: rs361525; −1031T/C: rs1799964) in TNF-α promoter were selected for genotyping. No significant difference of allelic and genotypic frequency were found between suicide subjects and normal controls, suggesting that genetic polymorphisms may be not be critical in TNF-α expression changes in suicide brain.
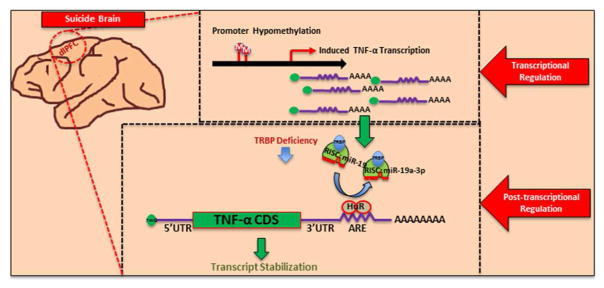
Altogether, our study shows that not only TNF-α is significantly upregulated in dlPFC of suicide subjects but there is a complex epigenetic regulatory mechanism that govern this increase. A summary of results and putative hypothesis of TNF-α upregulation in suicide brain is presented in Figure 6. It appears that several molecules may significantly contribute to this upregulation such as miR-19a-3p, TRBP, and HuR. HuR may affect TNF-α expression independently to miR-19a-3p. Epigenetic modification mediated by hypomethylation of the promoter region of TNF-α also appears to participate in this upregulation. Genetic factors presumably do not play a role regulation of TNF-α. Interestingly, in a pilot study, as with dlPFC, we observed that expression of both TNF-α and miR-19a-3p were significantly increased in the PBMCs of depressed individuals who had severe suicidal ideation. It indicates that both TNF-α and miR-19a-3p can be examined in blood tissues, which can possibly be associated with suicidality. However, this is a very preliminary observation which has limitations such that the power of the study is quite low and it lacks depressed patients without suicidal ideation. Overall, our study provides a novel concept in understanding the molecular basis of altered expression of TNF-α in suicidal behavior, which could potentially be involved in suicidal behavior.
Figure 6. Putative model depicting the regulation of TNF-α gene in suicide brain.
Schematic model showing epigenetic regulation of TNF-α expression at transcriptional and post-transcriptional levels in suicide brain. miR-19a-3p may be involved in this upregulation as evident by its no in-vivo interaction with TNF-α 3′UTR which may presumably be due to decreased TRBP expression and/or its possible elimination from binding to TNF-α by HuR. HuR may also affect TNF-α expression independently to miR-19a-3p. Additional transcriptional activation may possibly be attributed to DNA hypomethylation of the promoter region of TNF-α.
Supplementary Material
Acknowledgments
The research was supported by grants from National Institute of Mental Health (R01MH082802; 1R01MH101890; R01MH100616; 1R01MH107183-01), American Foundation for Suicide Prevention (SRG-1-042-14) to Dr. Dwivedi.
Footnotes
Authors report no conflict of interest financially or otherwise.
References
- 1.Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. Suicide and suicidal behavior. Epidemiologic reviews. 2008;30:133–154. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. Prevention of suicide: guidelines for the formulation and implementation of national strategies. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 3.Devorak J, Torres-Platas SG, Davoli MA, Prud’homme J, Turecki G, Mechawar N. Cellular and molecular inflammatory profile of the choroid plexus in depression and suicide. Frontiers in psychiatry. 2015:6. doi: 10.3389/fpsyt.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganança L, Oquendo MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology. 2016;63:296–310. doi: 10.1016/j.psyneuen.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. Journal of Allergy and Clinical immunology. 2010;125:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biological psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Monfrim X, Gazal M, De Leon PB, Quevedo L, Souza LD, Jansen K, Oses JP, Pinheiro RT, Silva RA, Lara DR. Immune dysfunction in bipolar disorder and suicide risk: is there an association between peripheral corticotropin-releasing hormone and interleukin-1 β? Bipolar disorders. 2014;16:741–747. doi: 10.1111/bdi.12214. [DOI] [PubMed] [Google Scholar]
- 8.O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, O’farrelly C, Malone KM. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depression and anxiety. 2013;30:307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- 9.Perez NM, Jennings WG, Piquero AR, Baglivio MT. Adverse Childhood Experiences and Suicide Attempts: The Mediating Influence of Personality Development and Problem Behaviors. Journal of youth and adolescence. 2016 doi: 10.1007/s10964-016-0519-x. [DOI] [PubMed] [Google Scholar]
- 10.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain, behavior, and immunity. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. Journal of psychiatric research. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pieper HC, Evert BO, Kaut O, Riederer PF, Waha A, Wullner U. Different methylation of the TNF-alpha promoter in cortex and substantia nigra: Implications for selective neuronal vulnerability. Neurobiology of disease. 2008;32:521–527. doi: 10.1016/j.nbd.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan KE, Reddy AB, Dietzmann K, Suriano AR, Kocieda VP, Stewart M, Bhatia M. Epigenetic regulation of tumor necrosis factor alpha. Molecular and cellular biology. 2007;27:5147–5160. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omrani MD, Bushehri B, Bagheri M, Salari-Lak S, Alipour A, Anoshae M-R, Massomi R. Role of IL-10– 1082, IFN-γ+ 874, and TNF-α– 308 Genes Polymorphisms in Suicidal Behavior. Archives of suicide research. 2009;13:330–339. doi: 10.1080/13811110903266418. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y-K, Hong J-P, Hwang J-A, Lee H-J, Yoon H-K, Lee B-H, Jung H-Y, Hahn S-W, Na KS. TNF-alpha– 308G> A polymorphism is associated with suicide attempts in major depressive disorder. Journal of affective disorders. 2013;150:668–672. doi: 10.1016/j.jad.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death & Differentiation. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, Wang Z, Yang S, Zhang W, He S, Hu C, Zhu H, Quan L, Bai J, Xu N. TNF-α is a novel target of miR-19a. International journal of oncology. 2011;38:1013–1022. doi: 10.3892/ijo.2011.924. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Z, Xiao-qin HH, Tian S-y, Lu Z-j, Zhang T-z, Bai Y-l. Down-regulation of microRNA-155 attenuates retinal neovascularization via the PI3K/Akt pathway. Molecular vision. 2015;21:1173. [PMC free article] [PubMed] [Google Scholar]
- 19.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature reviews Genetics. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Yeo J, Lee JH, Cho J, Seo D, Kim JS, Kim VN. Deletion of human tarbp2 reveals cellular microRNA targets and cell-cycle function of TRBP. Cell reports. 2014;9:1061–1074. doi: 10.1016/j.celrep.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RC, Tambe A, Kidwell MA, Noland CL, Schneider CP, Doudna JA. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Molecular cell. 2015;57:397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwerk J, Savan R. Translating the Untranslated Region. Journal of immunology (Baltimore, Md: 1950) 2015;195:2963–2971. doi: 10.4049/jimmunol.1500756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan CM, Steitz JA. HuR and mRNA stability. Cellular and molecular life sciences: CMLS. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng SS, Chen CY, Xu N, Shyu AB. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. The EMBO journal. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci U S A. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. The FASEB journal. 2006;20:2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 28.Dwivedi Y. The neurobiological basis of suicide. CRC press; 2012. [PubMed] [Google Scholar]
- 29.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre-and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain research. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 30.Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 31.Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y. Toll-like receptors in the depressed and suicide brain. Journal of psychiatric research. 2014;53:62–68. doi: 10.1016/j.jpsychires.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panwar B, Omenn GS, Guan Y. miRmine: a database of human miRNA expression profiles. Bioinformatics (Oxford, England) 2017;33:1554–1560. doi: 10.1093/bioinformatics/btx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eminaga S, Christodoulou DC, Vigneault F, Church GM, Seidman JG. Quantification of microRNA expression with next-generation sequencing. Current protocols in molecular biology. 2013;Chapter 4(Unit 4.17) doi: 10.1002/0471142727.mb0417s103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayley JP, Ottenhoff TH, Verweij CL. Is there a future for TNF promoter polymorphisms? Genes and immunity. 2004;5:315–329. doi: 10.1038/sj.gene.6364055. [DOI] [PubMed] [Google Scholar]
- 35.Elahi MM, Asotra K, Matata BM, Mastana SS. Tumor necrosis factor alpha -308 gene locus promoter polymorphism: an analysis of association with health and disease. Biochimica et biophysica acta. 2009;1792:163–172. doi: 10.1016/j.bbadis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Belfer I, Buzas B, Hipp H, Dean M, Evans C, Lorincz I, Max MB, Goldman D. Haplotype structure of inflammatory cytokines genes (IL1B, IL6 and TNF/LTA) in US Caucasians and African Americans. Genes and immunity. 2004;5:505–512. doi: 10.1038/sj.gene.6364118. [DOI] [PubMed] [Google Scholar]
- 37.Cerri A, Arosio B, Viazzoli C, Confalonieri R, Teruzzi F, Annoni G. −308 (G/A) TNF-α gene polymorphism and risk of depression late in the life. Archives of gerontology and geriatrics. 2009;49:29–34. doi: 10.1016/j.archger.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Misener V, Gomez L, Wigg K, Luca P, King N, Kiss E, Daroczi G, Kapornai K, Tamas Z, Mayer L. Cytokine Genes TNF, IL1A, IL1B, IL6, IL1RN and IL10, and childhood-onset mood disorders. Neuropsychobiology. 2008;58:71–80. doi: 10.1159/000159775. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 40.First MSRL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York, NY: Biometric Research, New York State Psychiatric Institute; 2002. Non-patient ed. [Google Scholar]
- 41.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry: the journal of mental science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Lin H. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell research. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 43.Smalheiser NR, Zhang H, Dwivedi Y. Enoxacin Elevates MicroRNA Levels in Rat Frontal Cortex and Prevents Learned Helplessness. Front Psychiatry. 2014;5:6. doi: 10.3389/fpsyt.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smalheiser NR, Zhang H, Dwivedi Y. Enoxacin elevates microRNA levels in rat frontal cortex and prevents learned helplessness. Frontiers in psychiatry. 2014;5:6. doi: 10.3389/fpsyt.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atasoy U, Curry SL, Lopez de Silanes I, Shyu AB, Casolaro V, Gorospe M, Stellato C. Regulation of eotaxin gene expression by TNF-alpha and IL-4 through mRNA stabilization: involvement of the RNA-binding protein HuR. Journal of immunology (Baltimore, Md: 1950) 2003;171:4369–4378. doi: 10.4049/jimmunol.171.8.4369. [DOI] [PubMed] [Google Scholar]
- 46.Srikantan S, Gorospe M. HuR function in disease. Frontiers in bioscience (Landmark edition) 2012;17:189–205. doi: 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.