Abstract
Advances in technology have enabled the delivery of high doses of radiation therapy for pancreatic ductal adenocarcinoma (PDAC) with low rates of toxicity. Although the role of radiation for pancreatic cancer continues to evolve, encouraging results with newer techniques indicate that radiation may benefit selected patient populations. Imaging has been central to the modern successes of radiation therapy for PDAC. Here we review the role of diagnostic imaging, imaging-based planning, and image guidance in radiation oncology practice for PDAC.
I. Introduction
In contrast to the declines in cancer-related deaths from other malignancies (i.e., lung and bronchus, breast, colorectal and prostate cancer), progress in the management of pancreatic ductal adenocarcinoma (PDAC) has been slow, and the incidence of cancer-related deaths due to PDAC continues to rise[1]. Overall, PDAC is associated with a dire prognosis, and a 5-year survival rate of only 6%. As with many aggressive cancers, improved multi-modality treatment and management are needed for patients with PDAC, including radiotherapy, chemotherapy, and surgery. In each domain of therapeutic management of PDAC, diagnostic imaging plays an important role. This is especially true for radiation oncology. Although many patients die of distant metastasis, it is estimated that 30% of patients die due to local disease progression, emphasizing the importance of treatments that focus on the primary PDAC tumor like radiation and surgery [2].
While the role of radiotherapy for PDAC continues to evolve, the techniques of radiotherapy for this disease are improving. Indeed, diagnostic imaging and image guided radiotherapy have been important factors in modern successes with radiation for PDAC, and these successes are paving the way for new treatment approaches. Here we will review the different techniques of radiotherapy and describe the central role that imaging plays.
II. The role of radiation in different clinical situations
Changes to the staging for PDAC have recently been proposed [3]. Generally, clinical management depends upon the surgical resection status of the patient, grouping patients into those who are potentially resectable, borderline resectable, locally advanced, or metastatic. The role, approach, and timing of radiation in each of these clinical stage groups differs (Table).
Table.
Roles of radiation in the treatment of PDAC
| Clinical Stage | Possible role(s) of radiation, listed in order of approach with most supporting data |
|---|---|
| Resectable | 1. Chemoradiation after resection 2. Chemoradiation before resection 3. SBRT before resection 4. SBRT after resection |
| Borderline Resectable | 1. Chemoradiation before resection 2. Chemoradiation after resection 3. SBRT before resection 4. SBRT after resection |
| Locally advanced | 1. Chemoradiation after chemotherapy 2. SBRT after chemotherapy 3. Palliation |
| Metastatic | Palliation |
Potentially resectable disease
Patients with potentially resectable disease are candidates for upfront surgery followed by adjuvant therapy that begins with systemic chemotherapy. If patients have no evidence of recurrence after adjuvant chemotherapy, they may be candidates for adjuvant chemoradiation[4, 5]. Adjuvant stereotactic body radiotherapy (SBRT) has also been performed[6]. Patients with potentially resectable disease may also undergo neoadjuvant therapy [7] with chemoradiation, radiation alone, or SBRT, followed about 4 to 12 weeks by surgery [8]. Current National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant therapy on a clinical trial.
Borderline resectable disease
Borderline resectable PDAC is technically eligible for surgical removal of the primary tumor, but given the relationship of the tumor with adjacent vessels, there is a high propensity for R1 resections [9]. Therefore, patients with borderline resectable disease are now recommended to receive neoadjuvant therapy, according to the NCCN. The neoadjuvant regimens can be chemotherapy alone, chemoradiation, or sequential chemotherapy and radiotherapy (chemoradiation or SBRT). The use of chemoradiation and SBRT in this context continues to be investigated, including in an ongoing Alliance trial (A021501, clinicaltrials.gov NCT02839343). Radiotherapy may be used postoperatively if no radiation was given prior to surgery.
Locally advanced disease
Historically, patients would receive chemotherapy followed by chemoradiation or chemoradiation upfront followed by chemotherapy. The LAP07 trial evaluated chemotherapy with or without capecitabine-based chemoradiation. The study indicated no overall survival benefit with chemoradiation but there was a significant improvement in local control[10]. Prior studies indicated both local control and overall survival benefits of chemoradiation for locally advanced PDAC, but were limited by their retrospective nature and smaller numbers[11, 12]. Thus, in patients with locally advanced disease, most physicians use radiotherapy with a selective approach. For example, at MD Anderson Cancer Center, our medical oncologists try to maximize chemotherapy and use radiotherapy if the disease has been stable on chemotherapy after 4-6 months, chemo-limiting toxicity develops, or local disease problems emerge or are anticipated (e.g., obstruction, venous thrombosis). In general, the idea is to maximize chemotherapy and then incorporate radiation to prevent local progression. In select cases, patients may be considered for surgical resection [13].
Recent retrospective data suggest there may be a survival benefit to escalated radiation doses in selected patients with unresectable locally advanced disease [14]. These data show long-term survivors after definitive radiation. SBRT may also be an attractive option in this stage of disease, as it is well tolerated, safe and achieves median survival ranging from 10-20 months [13, 15].
Metastatic disease
Generally, radiotherapy does not play a role for stage IV disease. It is reserved for palliative purposes, including painful metastases in bone, liver, or other sites. Treatment of the primary tumor for a patient with metastatic disease is not typical, but may be considered if the primary tumor is causing local symptoms (bleeding from bowel invasion, obstruction, or venous thrombosis). Treatment of the primary tumor may also be considered in situations where the metastatic disease has significantly responded to chemotherapy [16].
III. Integration of imaging into Radiation Oncology clinical practice for pancreatic cancer
In this section, we will describe the use of imaging at different phases of the patient interaction for Radiation Oncology practice.
Consultation
Referral of a patient with PDAC to a radiation oncologist is generally tertiary. It is usually either a surgeon or medical oncologist who receives the initial consultation after a diagnosis or suspicion of PDAC. This referral will depend on whether the patient has localized or metastatic disease. When the patient comes to the radiation oncologist, the physician will review the baseline and follow up imaging to assess the local extent of disease. This will help determine whether radiotherapy is indicated, and if so, the dose and fractionation of radiotherapy. Different imaging modalities may aid in the radiation oncologist’s assessment.
CT is the recommended imaging modality for staging [17]. CT acquisition using a pancreatic protocol with thin sub-millimeter slices provides precise visualization of the pancreatic tumor in relation to the mesenteric vasculature, especially in conjunction with multi-planar reconstruction. This also allows detection of lung, liver and peritoneal deposits.
Recent advances in CT technology include dual energy CT, which improves conspicuity of PDAC in comparison to conventional CT images [18]. Dual energy has also been useful in evaluating bowel disease [19], potentially making the technology useful in determining if the tumor is invading the duodenum or stomach. This could influence the type of radiation that may be delivered.
Pancreatic protocol MRI may also be helpful in the delineation of disease due to the superior soft tissue contrast that MRI sequences may provide. This may be helpful for indeterminate liver lesions that are seen on CT and need further characterization [20]. MRI can also be helpful as a problem solver for those tumors which are difficult to visualize on CT [21, 22].
Positron Emission Tomography (PET) may be used as an adjunct imaging modality for PDAC, as it shows an increased sensitivity to detect metastatic disease compared to conventional imaging.[23, 24] Approximately 30% of primary pancreatic tumors are not avid on PET, however. This limits its usefulness in evaluation, but in patients for whom the lesion is avid on PET, prognostic information may be gleaned [25]. Specifically, PET aviditity was the most significant factor on multivariate analysis for survival following gemcitabine and radiation therapy [25, 26]. Also, on Positron Emission Tomography (PET) Response Criteria in Solid Tumors (PERCIST) analysis, metabolic tumor volume (MTV) and total lesion glycolysis were found to be predictive for outcome following chemotherapy and radiation.
On review of the diagnostic imaging, the radiation oncologist will review the extent of local, regional, and metastatic disease for a patient with PDAC. As described above, the radiation treatment options for each clinical stage of PDAC differ and multidisciplinary review is recommended.
How imaging helps in deciding between standard fractionation and SBRT
There is no randomized comparison for different radiation fractionation regimens for PDAC. The prospective data to date are difficult to compare due to changes in systemic therapy over time, differences in patient selection, and evolution of radiation techniques. In general, patients with adverse pathological features after surgery, including lymph node involvement or positive margins, would be eligible for standard fractionation radiotherapy to 50 Gy at 1.8 to 2.0 Gy per fraction. At MD Anderson Cancer Center, most patients with resectable or borderline resectable will receive preoperative radiotherapy at standard fractionation to 50 Gy, or to 30 Gy in 10 fractions, which was a regimen that was tested in two phase II clinical trials [27, 28]. SBRT or hypofractionated radiation therapy is also being tested as preoperative therapy in the ongoing Alliance A021501 trial [29].
A patient may be eligible for escalated-dose radiation (above 50.4 Gy in 28 fractions) if surgical resection is not an option due to the tumor being locally advanced or the patient being medically inoperable. The experience of dose escalation at MD Anderson Cancer Center showed a two year survival rate of 36% in patients who received radiation doses to a Biologically Effective Dose (BED) greater than 70 Gy, compared to 19% for those who received standard doses (BED 59.5 Gy) [14]. The selection of patients for escalated dose radiation was in cases where there was at least 1 cm of distance from the tumor to luminal bowel. However, recent advances have enabled the use of escalated radiation in almost any patient for whom resection is not an option. This method involves a simultaneous integrated boost of high dose radiation and simultaneous integrated protection of adjacent critical structures [30].
Considerations for patients who are eligible for SBRT include whether there is bowel invasion by the primary tumor and the size of the tumor, which may impact late gastrointestinal toxicity such as ulceration and bleeding. Diagnostic imaging often helps determine these factors, but direct tumor invasion is best documented using direct visualization by endoscopy. The concern with using high doses per fraction in patients with bowel invasion is that a gastrointestinal bleed may occur [31]. There is conflicting data on this, however. A single institution experience demonstrated no major bleeding events in patients who had duodenal invasion [32]. In situations of bowel invasion by the tumor, we advocate for using standard fractionation, rather than SBRT. SBRT could be delivered if subsequent surgery is planned.
The decision to use standard fractionation, dose escalation, or SBRT may also be based on physician preference, familiarity, and convenience. Many physicians are comfortable with standard fractionation since it has been done for decades. Due to the relatively new option of SBRT, some radiation oncologists do not yet have the expertise to perform this procedure. SBRT usually requires placement of radiopaque fiducials for target alignment using orthogonal plain films in the treatment position and/or a cone beam CT. An experienced endoscopist would be needed to place the fiducials (usually 3 are placed in a non-coplanar arrangement). Alignment to metal stents is possible, but it has been reported that these can move [33], making them less reliable for daily setup. We will discuss image-guided radiotherapy in a subsequent section.
Simulation to prepare for treatment planning
CT simulation
After deciding to treat a patient with PDAC, a CT simulation is performed. The objectives of the simulation are to (1) achieve a comfortable and reproducible position for the patient in the treatment position and (2) obtain CT images in this position so that targets can be defined and dose can be modeled. Prior to the simulation, the radiation oncologist must decide the dose, fractionation, target, and technique of radiotherapy.
Since the pancreas is adjacent to multiple luminal bowel structures (e.g., stomach, duodenum, jejunum and colon), patients are often instructed to be fasting for 2 to 4 hours prior to the CT simulation and each treatment, so that the internal anatomy is as consistent as possible throughout. If a patient is being simulated for SBRT and kV image guidance will be used, fiducials would have been placed prior to the CT simulation. CT on rails (and in some cases cone beam CT) image guidance may be used in cases where fiducials cannot be placed. The patient may drink 8 ounces of water just prior to the simulation and each treatment to better delineate the duodenum and stomach. If the patient is receiving postoperative treatment, scars or drains may be wired to help identify them on the scan.
During the simulation, the proper immobilization of the patient is critical (Fig. 1). Vacuum-locked cradles are usually used to conform to the patient’s anatomy and keep the upper extremities and torso in a consistent position. The arms are placed above the head so that they are not in the path of the radiation beams. The patient receives marks on the skin (either marker or tattoos) to triangulate their position using in-room laser guidance. The lasers are indexed to a point of reference on the CT simulation table, which is modified to be flat (as opposed to curved as for diagnostic CT tables). The skin marks are then placed in an anterior and two lateral positions to perform initial alignment to the linear accelerator for each treatment.
Figure 1.
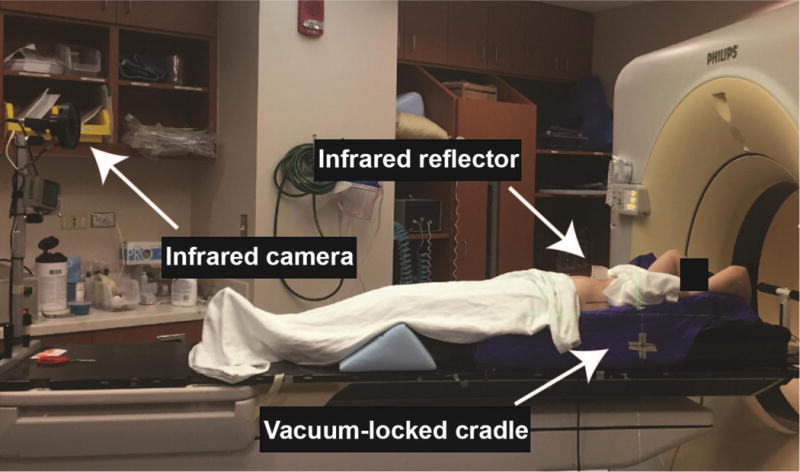
Immobilization and motion management during CT simulation
For standard fractionation, practice patterns vary regarding image acquisition. A 4D CT may be used to determine target motion and margins for treatment. An example of a 4D CT setup is shown in Figure 1, including the use of respiratory surrogates with an infrared reflector on the abdomen and infrared camera. Some radiation oncologists do not use the 4D CT and just use a free breathing scan for planning. Generally, it is not necessary to treat a patient with breath hold or gating techniques when standard fractionation is used. The coverage of regional lymph nodes is a topic of debate, where some will cover them but others just focus on the gross tumor volume (GTV) with a small margin.
For SBRT (5 fractions or less) or cases of dose escalation (using more than 5 fractions and to doses above 50 Gy in 1.8 to 2 Gy fractions), imaging acquisition typically involves respiratory management because the pancreas can move up to 2 cm during the breathing cycle. When high doses are used in this area, this movement can significantly impact target coverage and doses to critical structures like luminal bowel. Respiratory management can be achieved with a variety of approaches, including breath hold, gating, or abdominal compression. Each of these approaches has advantages and disadvantages. These approaches may require additional time for setup and/or treatment compared to treating with standard fractionation.
The use of contrast during a simulation is done in a compatible way with the technique of radiation. For example, if a patient is receiving dose escalation, the contrast images would be acquired in coordination with respiratory management such as breath hold. Contrast images can be acquired in free breathing, as is often done with diagnostic CT scans. Contrast images may also be acquired during a 4D CT procedure. Generally, the image acquisition would replicate a pancreatic protocol CT scan, helping to delineate the tumor and the local vascular anatomy around the pancreas [34].
MR Simulation
MR Simulation as compared with using a Diagnostic MRI
The objectives of an MR simulation are similar to CT, except the MRI is used for treatment planning [35]. The acquisition of an MRI in the treatment position enables normal organ and tumor delineation in the exact position in which a patient will be treated with radiation therapy. This is typically done in the same immobilization devices that are used during CT simulation. Similar to CT simulation, laser coordinate systems can be used to mark a patient on the MR simulator, or confirm existing alignment and positioning marks that were made during the process of CT simulation. These confirmatory marks can then be used on the linear accelerator to reproduce the patient’s exact position on a daily basis for radiation therapy delivery [35]. The three MR sequences that will typically be utilized for contouring the radiation targets are T2 (duodenal wall delineation), fat-suppressed T1 (normal gland delineation), and late arterial phase post-contrast, fat-suppressed T1 (tumor boundary and lymph node delineation; e.g. tumor appears dark, lymph nodes appear bright) because these sequences offer the best contrast resolution between tumor and normal pancreatic parenchyma.
Advantages of MR simulation over CT simulation
While CT simulation remains the standard for the process of external beam radiation therapy, MR simulation is an emerging area of interest. MR simulation has advantages over CT in the process of GTV delineation [36]. While CT is excellent in discriminating regions of the body with different electron densities, it is limited in its ability to identify and demarcate tissues in regions with similar electron densities. MR simulation offers particular advantages for pancreatic cancers as it is able to distinguish the pancreatic tumor (GTV) from the normal pancreas and the closely approximated pylorus and duodenum. Furthermore, MRI offers more options to help define the GTV, which can include both functional (diffusion weighted imaging [DWI], dynamic contrast enhanced [DCE] imaging) and morphologic (T1-weighted or T2-weighted imaging) information [35, 37].
Disadvantages and Precautions Associated With MR simulation
While MRI has advantages over CT simulation, there are also disadvantages and precautions that must be considered when implementing MR simulation. The use of MRI has several contraindications and can be considerably more dangerous when compared with CT simulation. This is particularly true if patients have implanted medical devices, metal fragments, or other contraindications to MRI [38]. The use of MRI requires careful review by MR technicians specially trained in assessing a patient’s candidacy for MRI. This can be logistically challenging for a Radiation Oncology department because radiation therapists and physicists are primarily trained in CT-based radiation delivery systems. Departments that adopt MR technology must hire or train the therapists and physicists, which can be a significant investment of time and resources. In addition, MR simulation is expensive and time consuming for the patient and health care system. The use of MRI requires a considerable amount of technical expertise from physics collaborators that must be familiar with optimal MR sequence development and acquisition. In addition, expensive equipment is required to be cross compatible between CT and MRI, and this equipment may require a substantial investment on the part of a radiation oncology department. This equipment can include MR compatible immobilization devices, flat tabletop modifications, along with additional software and licenses. Finally, the use of MR is subject to geometrical distortions that must be understood and accounted for when these images are acquired and used for radiation therapy planning and delivery [39–41]. In addition, motion management is extremely important with MRI as motion can significantly degrade the images. Accounting for these errors requires the use of a phantom and extensive quality assurance procedures to be done on the MR with an experienced physics group.
Defining tumor and normal structures on the planning scans
Once the CT or MR images are acquired, treatment planning can ensue. Often, contrast images from the simulation are all that is necessary to define the treatment volumes. However, in difficult cases, fusion with diagnostic scans, including CT, MRI, and PET, may be helpful. For standard fractionation, the radiation oncologist will define different treatment volumes during this process, including a gross tumor volume (GTV), clinical tumor volume (CTV), and planning tumor volume (PTV). The GTV is the pancreatic tumor and any associated nodes. The GTV includes tumor extension along blood vessels. The CTV may include the GTV, celiac artery, superior mesenteric artery and regional nodes with margin. Typical margins for CTV are about 1-2 cm around all of these structures. The PTV accounts for anticipated setup error. This is usually 3-5 mm (Fig. 2). The normal structures include all of the surrounding anatomy, such as kidneys, liver, stomach, duodenum, spinal cord, and bowels.
Figure 2.
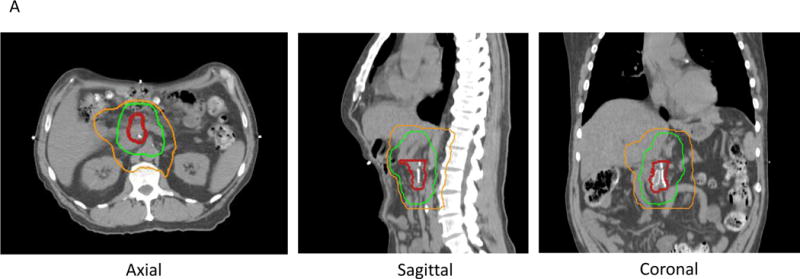
A. Postoperative radiation with IMRT treating tumor bed (red line) to 50 Gy (green line) and the regional lymphatics to 45 Gy (orange line) in 25 fractions.
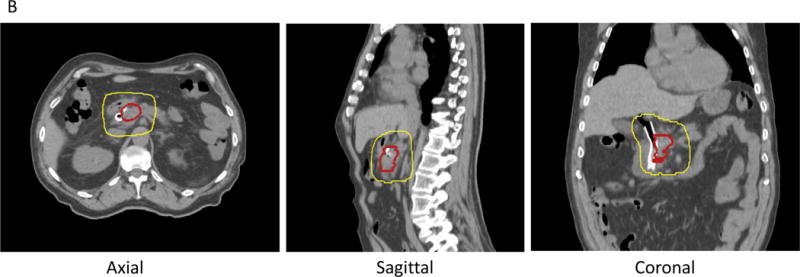
B. Preoperative radiation with 3D conformal technique (4 fields) treating tumor (red line) and regional lymphatics to 30 Gy (yellow line) in 10 fractions.
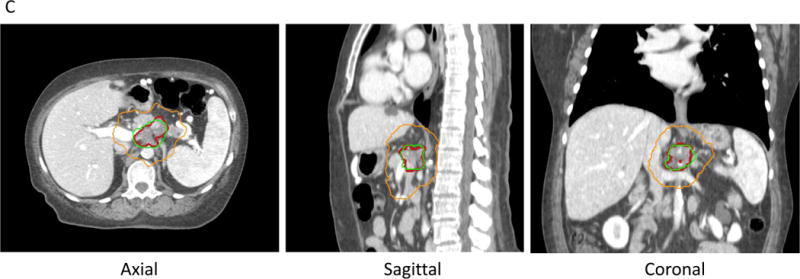
C. Escalated dose radiotherapy with IMRT, treating tumor (red line) to 67.5 Gy (green line) and regional lymphatics to 37.5 Gy (orange line) in 15 fractions.
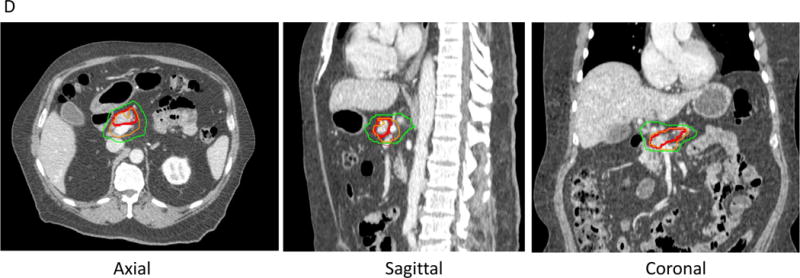
D. Stereotactic body radiation therapy (SBRT) for PDAC, treating tumor (red line) to 33 Gyand tumor-vessel interface to 36-40 Gy(orange line). With SBRT, there is a steep dose falloff. For example, a lower dose of 25 Gy is shown in the green line.
Target delineation for dose-escalated radiation is different than standard radiotherapy. While standard radiotherapy is usually prescribed to achieve fairly uniform doses throughout the target volume (+/− ~10%), dose-escalated radiation attempts to achieve much higher doses to the primary tumor and lower doses to regional areas at risk for nodal spread (Fig. 2). This is called “dose painting”. To achieve this effect, the radiation oncologist also will define avoidance structures so that there is a sharp dose falloff from the high dose region to organs at risk, such as bowel. This has been termed “simultaneous integrated boost with simultaneous integrated protection [30].”
Target delineation for SBRT is also different compared to standard and dose-escalated radiotherapy. For example, in the Alliance trial [29], in addition to the GTV, the tumor vessel interface (TVI) is defined. The luminal bowel is delineated carefully, just as with dose-escalated radiotherapy. Expansions of 3mm around the GTV and TVI are performed, but differential dosing may be performed to each of these structures, whereby higher doses are given in areas that are safely away from critical organs. Just as with dose-escalated radiotherapy, the radiation oncologist can “paint” the dose desired after defining targets and may prescribe a lower, safer dose in areas close to bowel (Fig. 2). Once the targets are delineated by a radiation oncologist, a dosimetrist designs the radiation treatment plan. The radiation oncologist will review the plan to make sure the modeled dose meets clinical standards, and a physicist will check that the plan is complete by performing multiple quality checks, including chart reviews and radiation quality assurance [42].
Image-guided radiotherapy
Once the radiation treatment plan is completed, the use of image-guidance is important to assure target alignment is consistent with the original simulation.
2D-2D matching
For standard fractionation, orthogonal kilovolt (kV) images are sufficient to align bony anatomy. For SBRT, orthogonal kV can be used to align to fiducials. In this case, on-board imaging is coordinated with gating or breath hold if those were used at simulation. Shifts are measured and recorded each day to make sure alignment and setup are consistent (Fig. 3).
Figure 3.
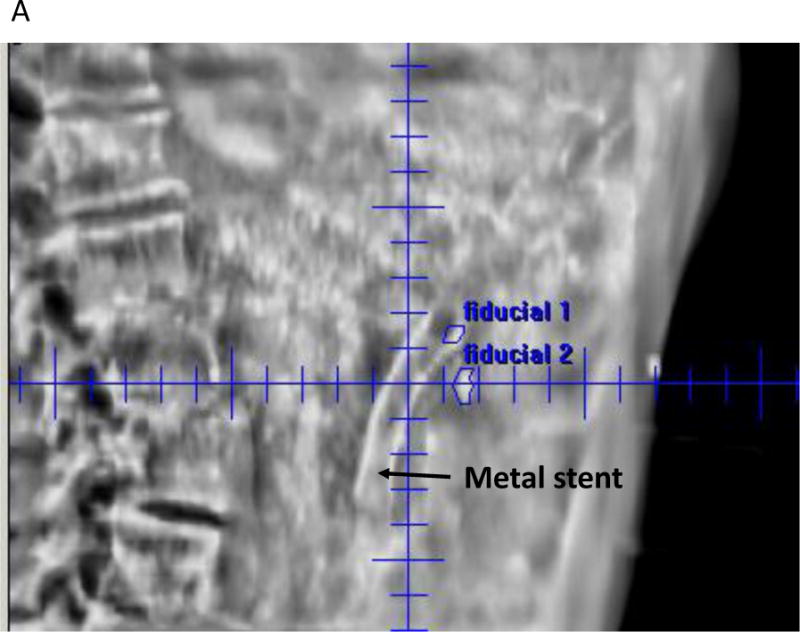
A. Fiducial alignment for SBRT using kV: Right lateral image shown for a patient with two fiducials in the pancreas. An orthogonal Anterior-Posterior (AP) image was also taken for alignment (not shown). A metal stent can also be seen.
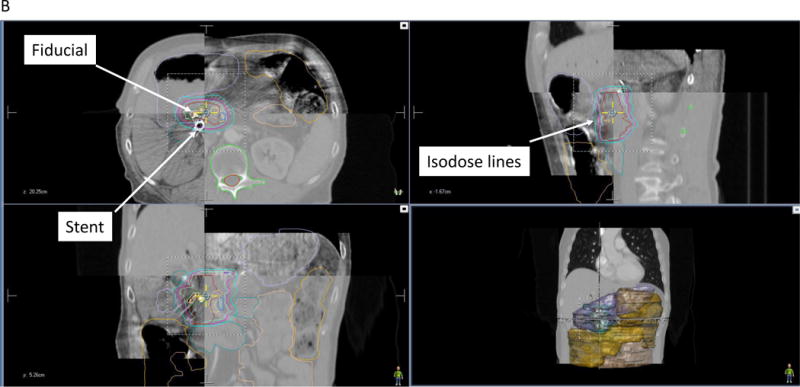
B. CBCT for 3D-3D matching during SBRT. The daily CBCT image (top left and bottom right quadrants of the axial, sagittal, and coronal images) are registered to the simulation CT scan (top right and bottom left quadrants of each view). Fiducials are identified for alignment and surrounding anatomy is identified in relation to radiation isodose lines.
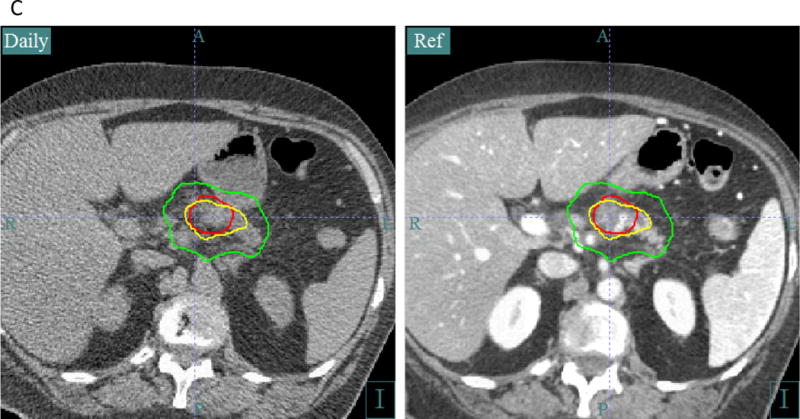
C. CT on rails image guidance for escalated dose radiotherapy. The axial image on the left is taken at the treatment machine using the in-room CT scan (labeled “Daily”). It is registered to the original CT simulation scan on the right (labeled “Ref”). The tumor contour (red line) and isodose lines are superimposed on the daily CT scan, allowing the radiation oncologist and therapists to verify proper alignment and assess changes in anatomy during treatment. Of particular importance is meeting dose constraints to the bowel and stomach. This patient received escalated dose radiotherapy to 67.5 Gy in 15 fractions. The bowel constraint is 45 Gy in 15 fractions. The yellow isodose line is 45 Gy, and the green isodose line is 37.5 Gy.
3D-3D matching
In cases of SBRT or dose escalation, 3D matching of the anatomy at the time of treatment with the anatomy at the time of simulation can be performed with more advanced image technologies. This includes cone-beam CT (CBCT) [43, 44], CT on rails [14, 45], or MRI (see next section) [46]. To achieve proper 3D-3D matching with CBCT and CT on rails, multiple quality checks must be done to ensure that the physical matching of the anatomy is accurate [47]. In complex cases, 3D-3D matching is necessary to avoid overdosing bowel [30] (Fig. 3). In some situations, adaptive planning can be done if the internal anatomy changes.
MR Guided Radiation Therapy
Radiation therapy is rapidly evolving, and has changed dramatically over the past decade. The ability to guide radiation therapy with the acquisition of daily pre- treatment CT images has considerably improved the accuracy by which radiation therapy can be delivered[48, 49]. In addition, frequent on board CT imaging enables an early understanding of changes in the size of the tumor or changes in a patient’s anatomy over the course of treatment. Such information can enable earlier adaption of treatment to account for changes in patient size or tumor. Until recently, this was taking place primarily with CT- based imaging that was acquired either with an onboard CT- based imaging device (cone beam), or a diagnostic CT on rails[48]. Though bone is readily visualized, one of the greatest limitations of cone beam CT imaging is the difficulty of seeing soft tissue while an actual radiation therapy treatment is being delivered. Over the past several years, additional imaging devices have emerged that have replaced CT on the treatment machine with an MRI. Moreover, these devices enable an MRI to be acquired while the radiation therapy is being delivered. This concept of “real time” tumor imaging enables visualization of both tumor and normal tissue motion while radiation is delivered. In addition, the change in tumor volume or MR signal can be monitored throughout the course of treatment and treatment adapted if necessary. The ability for MRI to offer superior soft tissue demarcation is an advantage of this technology [50]. To attempt to combine the benefits of these two technologies, there have been a total of four MR combined radiation therapy systems that have been developed.
The first of the hybrid MR guided radiation therapy systems to become commercially viable is known as the ViewRay system (ViewRay Inc, Oakwood Village, OH) [51, 52]. This system combines both MR imaging using a 0.35-T MRI and Co-60 sources that rotate around the patient and can deliver a conformal dose distribution through intensity modulated radiation therapy (IMRT)[53]. This system is currently commercially available and has been actively treating patients for several years at multiple institutions. Limitations to this system include the low MR field strength along with the Co-60 radiation therapy sources, which have inherent dosimetric limitations. The ViewRay company is also developing a linear accelerator equipped unit that will be combined with a 0.35 T MRI device[54].
There are several additional systems that combine MRI and radiation therapy delivery technology that are under development. The Linac-MR research group in Edmonton Canada, has published on the construction of a functional MR Linac system[55]. This system incorporates a Linac waveguide placed between open MR planes, or through the central opening of the planes. This consists of a 6 MV Linac that rotates concurrently with a 0.5-T MRI in the transverse plane[56]. This configuration has been proposed to reduce a potential for higher dose at the tissue-air interface and electron return effect[57]. While this system is interesting it has yet to be scaled to multiple institutions and made widely commercially available.
An Australian group has also developed an MR Linear accelerator which includes a 1.0 T open bore MRI system with a 6 MV Linac[58]. This is known as the Australian MR-Linac program and this system focuses on the development of a novel medical electron accelerator intended to be robustly operated within magnetic fields. The wide scale commercial viability of this implemental is yet to be seen[59].
Elekta, Philips, and University Medical Center Utrecht have collaborated to create a full field strength MR guided linear accelerator[46]. This device, known as the Unity, was announced at the 36th European Society for Radiotherapy and Oncology (ESTRO) meeting[60]. The Unity device combines a multi-leaf collimator (MLC) positioned on a ring gantry which rotates around an MR scanner. This unit has a full 1.5 T MRI manufactured by Phillips and has many of the same capabilities of diagnostic quality MR scanners. In addition, there are numerous sequences that can be acquired using this full field strength MR scanner. During the device rotation the treatment beam passes through the middle/inner ring of the MRI and is capable of IMRT [61]. It has also been shown that the images can be acquired during radiation therapy[62]. A research consortium has been formed by Elekta to design and execute the optimal use of the Elekta-Philips MR Linac and define the optimal use of this device.
IV. Summary
Imaging is central to the practice of radiation oncology. The radiation oncologist must understand diagnostic imaging to make treatment decisions at the time of consultation. During simulation, the acquisition of imaging is critical and is performed in conjunction with immobilization and consideration of the dose, fractionation, and technique of radiation. Image-guidance is also an important aspect as the role of radiation for PDAC evolves. This includes the use of 3D information using CT on rails, CBCT, and MRI units that are combined with the radiation machines. This ensures proper alignment to the target and adaptation of the radiation plan, if necessary. There is a trend toward using SBRT in multiple situations for PDAC, which heavily relies on image guidance. Dose escalation for PDAC is also a hot topic and may involve 5 fraction SBRT or more fractions to achieve higher doses. Proper image guidance and treatment planning in these more complex treatment procedures may enable higher efficacy and ensure safe radiation delivery.
Acknowledgments
Funding: This study was funded by the National Institutes of Health (NIH U54CA210181-01, U54CA143837, 1U01CA200468, and U01CA196403), the Pancreatic Cancer Action Network (14-20-25-KOAY and 16-65-SING), and the Radiological Society of North America (RSD1429). We gratefully acknowledge partial support from the Andrew Sabin Family Fellowship, Center for Radiation Oncology Research, the Sheikh Ahmed Center for Pancreatic Cancer Research, institutional funds from The University of Texas MD Anderson Cancer Center, GE Healthcare, Philips Healthcare, and Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute to MD Anderson.
Footnotes
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attiyeh MA, Fernandez-Del Castillo C, Al Efishat M, Eaton AA, Gonen M, Batts R, et al. Development and Validation of a Multi-Institutional Preoperative Nomogram for Predicting Grade of Dysplasia in Intraductal Papillary Mucinous Neoplasms (IPMNs) of the Pancreas: A Report from The Pancreatic Surgery Consortium. Ann Surg. 2016 doi: 10.1097/SLA.0000000000002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17(4):981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iott M, Neben-Wittich M, Quevedo JF, Miller RC. Adjuvant chemoradiotherapy for resected pancreas cancer. World J Gastrointest Surg. 2010;2(11):373–380. doi: 10.4240/wjgs.v2.i11.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rwigema JC, Heron DE, Parikh SD, Zeh HJ, 3rd, Moser JA, Bahary N, et al. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer. 2012;43(1):70–76. doi: 10.1007/s12029-010-9203-7. [DOI] [PubMed] [Google Scholar]
- 7.Mornex F, Girard N, Delpero JR, Partensky C. Radiochemotherapy in the management of pancreatic cancer–part I: neoadjuvant treatment. Semin Radiat Oncol. 2005;15(4):226–234. doi: 10.1016/j.semradonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Wei Q, Yu W, Rosati LM, Herman JM. Advances of stereotactic body radiotherapy in pancreatic cancer. Chin J Cancer Res. 2015;27(4):349–357. doi: 10.3978/j.issn.1000-9604.2015.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol. 2014;24(2):105–112. doi: 10.1016/j.semradonc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315(17):1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 11.Loehrer PJ, Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammel P, Huguet F, Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Comparison of chemoradiotherapy and chemotherapy in patients with a locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. J Clin Oncol suppl. 2013 abstr LBA4003. [Google Scholar]
- 13.Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol. 2015;22(7):2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan S, Chadha AS, Suh Y, Chen HC, Rao A, Das P, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94(4):755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman JM, Hoffman JP, Thayer SP, Wolff RA. Management of the Primary Tumor and Limited Metastases in Patients With Metastatic Pancreatic Cancer. J Natl Compr Canc Netw. 2015;13(5):e29–36. doi: 10.6004/jnccn.2015.0079. [DOI] [PubMed] [Google Scholar]
- 17.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Wagner-Bartak N, Jensen CT, Hui A, Wei W, Lertdilok P, et al. Dual-energy CT of pancreatic adenocarcinoma: reproducibility of primary tumor measurements and assessment of tumor conspicuity and margin sharpness. Abdom Radiol (NY) 2016;41(7):1317–1324. doi: 10.1007/s00261-016-0689-8. [DOI] [PubMed] [Google Scholar]
- 19.Fulwadhva UP, Wortman JR, Sodickson AD. Use of Dual-Energy CT and Iodine Maps in Evaluation of Bowel Disease. Radiographics. 2016;36(2):393–406. doi: 10.1148/rg.2016150151. [DOI] [PubMed] [Google Scholar]
- 20.Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009;20(1):3–9. doi: 10.1097/RMR.0b013e3181b48392. [DOI] [PubMed] [Google Scholar]
- 21.Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30(3):586–595. doi: 10.1002/jmri.21889. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Park SH, Yu ES, Kim MH, Kim J, Byun JH, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. 2010;257(1):87–96. doi: 10.1148/radiol.10100015. [DOI] [PubMed] [Google Scholar]
- 23.Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15(9):2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 24.Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CH. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol. 2014;40(7):794–804. doi: 10.1016/j.ejso.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Chirindel A, Alluri KC, Chaudhry MA, Wahl RL, Pawlik TM, Herman JM, et al. Prognostic Value of FDG PET/CT-Derived Parameters in Pancreatic Adenocarcinoma at Initial PET/CT Staging. AJR Am J Roentgenol. 2015;204(5):1093–1099. doi: 10.2214/AJR.14.13156. [DOI] [PubMed] [Google Scholar]
- 26.Dholakia AS, Chaudhry M, Leal JP, Chang DT, Raman SP, Hacker-Prietz A, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89(3):539–546. doi: 10.1016/j.ijrobp.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 28.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 29.Katz MH, Ou FS, Herman JM, Ahmad S, Wolpin B, Marsh R, et al. Alliance for Clinical Trials in Oncology (ALLIANCE) Trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer accepted. 2017 doi: 10.1186/s12885-017-3441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer. 2016;122(13):1974–1986. doi: 10.1002/cncr.29878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elhammali A, Patel M, Weinberg B, Verma V, Liu J, Olsen JR, et al. Late gastrointestinal tissue effects after hypofractionated radiation therapy of the pancreas. Radiat Oncol. 2015;10:186. doi: 10.1186/s13014-015-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murimwa G, Mellon EA, Frankes JM, Jin W, Hodul PJ, Pimiento JM, et al. Impact of duodenal invasion on outcomes in patients with pancreatic cancer treated with stereotactic body radiotherapy. J Clin Oncol. 2017;(4 suppl):408. [Google Scholar]
- 33.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey DJ, Patel BN, Adamson JD, Subashi E, Salama JK, Palta M. Triphasic contrast enhanced CT simulation with bolus tracking for pancreas SBRT target delineation. Pract Radiat Oncol. 2017 doi: 10.1016/j.prro.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Paulson ES, Erickson B, Schultz C, Allen Li X. Comprehensive MRI simulation methodology using a dedicated MRI scanner in radiation oncology for external beam radiation treatment planning. Med Phys. 2015;42(1):28–39. doi: 10.1118/1.4896096. [DOI] [PubMed] [Google Scholar]
- 36.Dirix P, Haustermans K, Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol. 2014;24(3):151–159. doi: 10.1016/j.semradonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Arivarasan I, Anuradha C, Subramanian S, Anantharaman A, Ramasubramanian V. Magnetic resonance image guidance in external beam radiation therapy planning and delivery. Jpn J Radiol. 2017 doi: 10.1007/s11604-017-0656-5. [DOI] [PubMed] [Google Scholar]
- 38.Panych LP, Madore B. The physics of MRI safety. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25761. [DOI] [PubMed] [Google Scholar]
- 39.Walker A, Liney G, Metcalfe P, Holloway L. MRI distortion: considerations for MRI based radiotherapy treatment planning. Australas Phys Eng Sci Med. 2014;37(1):103–113. doi: 10.1007/s13246-014-0252-2. [DOI] [PubMed] [Google Scholar]
- 40.Crijns SP, Bakker CJ, Seevinck PR, de Leeuw H, Lagendijk JJ, Raaymakers BW. Towards inherently distortion-free MR images for image-guided radiotherapy on an MRI accelerator. Phys Med Biol. 2012;57(5):1349–1358. doi: 10.1088/0031-9155/57/5/1349. [DOI] [PubMed] [Google Scholar]
- 41.Mizowaki T, Nagata Y, Okajima K, Kokubo M, Negoro Y, Araki N, et al. Reproducibility of geometric distortion in magnetic resonance imaging based on phantom studies. Radiother Oncol. 2000;57(2):237–242. doi: 10.1016/s0167-8140(00)00234-6. [DOI] [PubMed] [Google Scholar]
- 42.Basran PS, Woo MK. An analysis of tolerance levels in IMRT quality assurance procedures. Med Phys. 2008;35(6):2300–2307. doi: 10.1118/1.2919075. [DOI] [PubMed] [Google Scholar]
- 43.van der Horst A, Wognum S, Davila Fajardo R, de Jong R, van Hooft JE, Fockens P, et al. Interfractional position variation of pancreatic tumors quantified using intratumoral fiducial markers and daily cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2013;87(1):202–208. doi: 10.1016/j.ijrobp.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Lens E, van der Horst A, Kroon PS, van Hooft JE, Davila Fajardo R, Fockens P, et al. Differences in respiratory-induced pancreatic tumor motion between 4D treatment planning CT and daily cone beam CT, measured using intratumoral fiducials. Acta Oncol. 2014;53(9):1257–1264. doi: 10.3109/0284186X.2014.905699. [DOI] [PubMed] [Google Scholar]
- 45.Papalazarou C, Klop GJ, Milder MTW, Marijnissen JPA, Gupta V, Heijmen BJM, et al. CyberKnife with integrated CT-on-rails: system description and first clinical application for pancreas SBRT. Med Phys. 2017 doi: 10.1002/mp.12432. [DOI] [PubMed] [Google Scholar]
- 46.Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24(3):207–209. doi: 10.1016/j.semradonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Bissonnette JP, Balter PA, Dong L, Langen KM, Lovelock DM, Miften M, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys. 2012;39(4):1946–1963. doi: 10.1118/1.3690466. [DOI] [PubMed] [Google Scholar]
- 48.Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9(12):688–699. doi: 10.1038/nrclinonc.2012.194. [DOI] [PubMed] [Google Scholar]
- 49.Pereira GC, Traughber M, Muzic RF., Jr The role of imaging in radiation therapy planning: past, present, and future. Biomed Res Int. 2014;2014:231090. doi: 10.1155/2014/231090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kupelian P, Sonke JJ. Magnetic resonance-guided adaptive radiotherapy: a solution to the future. Semin Radiat Oncol. 2014;24(3):227–232. doi: 10.1016/j.semradonc.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Goddu S, Green OP, Mutic S. WE-G-BRB-08: TG-51 Calibration of First Commercial MRI-Guided IMRT System in the Presence of 0.35 Tesla Magnetic Field. Med Phys. 2012;39(6Part28):3968. doi: 10.1118/1.4736194. [DOI] [PubMed] [Google Scholar]
- 52.Jaffray D, Mutic S, Fallone B, Raaymakers B. MO-A-WAB-01: MRI-Guided Radiation Therapy. Med Phys. 2013;40(6Part23):390. [Google Scholar]
- 53.Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24(3):196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Tzeng CW, Fleming JB, Lee JE, Wang X, Pisters PW, Vauthey JN, et al. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB (Oxford) 2012;14(6):365–372. doi: 10.1111/j.1477-2574.2012.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St Aubin J, Steciw S, Fallone BG. The design of a simulated in-line side-coupled 6 MV linear accelerator waveguide. Med Phys. 2010;37(2):466–476. doi: 10.1118/1.3276778. [DOI] [PubMed] [Google Scholar]
- 56.Fallone BG. The rotating biplanar linac-magnetic resonance imaging system. Semin Radiat Oncol. 2014;24(3):200–202. doi: 10.1016/j.semradonc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Keyvanloo A, Burke B, St Aubin J, Baillie D, Wachowicz K, Warkentin B, et al. Minimal skin dose increase in longitudinal rotating biplanar linac-MR systems: examination of radiation energy and flattening filter design. Phys Med Biol. 2016;61(9):3527–3539. doi: 10.1088/0031-9155/61/9/3527. [DOI] [PubMed] [Google Scholar]
- 58.Keall PJ, Barton M, Crozier S, Australian Mri-Linac Program icfIIICCCLHSUUoNQSWS, and Wollongong The Australian magnetic resonance imaging-linac program. Semin Radiat Oncol. 2014;24(3):203–206. doi: 10.1016/j.semradonc.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Whelan B, Gierman S, Holloway L, Schmerge J, Keall P, Fahrig R. A novel electron accelerator for MRI-Linac radiotherapy. Med Phys. 2016;43(3):1285–1294. doi: 10.1118/1.4941309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aguilera KY, Rivera LB, Hur H, Carbon JG, Toombs JE, Goldstein CD, et al. Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(4):1032–1044. doi: 10.1158/0008-5472.CAN-13-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bol GH, Hissoiny S, Lagendijk JJ, Raaymakers BW. Fast online Monte Carlo-based IMRT planning for the MRI linear accelerator. Phys Med Biol. 2012;57(5):1375–1385. doi: 10.1088/0031-9155/57/5/1375. [DOI] [PubMed] [Google Scholar]
- 62.Raaymakers BW, Lagendijk JJ, Overweg J, Kok JG, Raaijmakers AJ, Kerkhof EM, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54(12):N229–237. doi: 10.1088/0031-9155/54/12/N01. [DOI] [PubMed] [Google Scholar]


