Abstract
HIV-associated neurocognitive disorders (HAND) commonly feature verbal episodic memory impairment historically characterized by a retrieval deficit, consistent with a classic “subcortical” presentation; however, there are hints of a subtle shift toward a more “cortical” memory profile characterized by a primary encoding deficit. The current study evaluated this possibility by comparing the pattern of HAND-associated verbal episodic memory deficits to those of traditional “subcortical” (i.e., Huntington’s Disease; HD) versus “cortical” (i.e., left temporal lobe epilepsy with mesial temporal sclerosis; L-MTLE) profiles. Seventy-seven individuals with HAND, 47 individuals with HD, 21 individuals with L-MTLE, and 45 healthy participants were administered the California Verbal Learning Test – 2nd Edition (CVLT-II). CVLT-II profiles were classified as reflecting a primary encoding deficit, retrieval deficit, or a normal profile. Among participants with a deficit profile, the HAND group showed the highest rates of retrieval versus encoding profiles (71% vs. 29%), followed by HD (59% vs. 41%), L-MTLE (46% vs. 54%), and healthy (50% vs. 50%) groups. While significant profile heterogeneity was observed across clinical groups, findings suggest that HIV-associated verbal episodic memory impairments are most consistent with a traditional “subcortical,” retrieval deficit profile, consistent with the primary frontostriatal neuropathogenesis of HIV disease.
Introduction
While advances in antiretroviral therapy have led to remarkable improvements in immune health outcomes and increased lifespans for those living with HIV infection, HIV-associated neurocognitive disorders (HAND) remain highly prevalent, affecting approximately half of infected individuals (Heaton et al., 2010). While the incidence of frank HIV-associated dementia (HAD) has declined, the pattern of neurocognitive deficits observed in HAND is typically mild-to-moderate in nature (Reger et al., 2002). There is considerable evidence to suggest that the deficits observed in HAND are primarily a consequence of a preferential adverse structural and functional impact of HIV neuropathology on fronto-striato-thalamo-cortical (FSTC) loops (e.g., Ellis et al., 2009). However, unlike some other “hallmark” neurodegenerative disorders in which pathology is typically endogenous and has some affinity for specific brain regions (e.g., Huntington’s disease), HIV is not typically associated with such a pattern of regional specificity; rather, the pattern of neuropathology tends to show considerable heterogeneity (Everall et al., 2009), affecting widespread temporal (e.g., hippocampus) and parietal cortices (Thompson et al., 2005) in addition to FSTC loops.
These widespread effects of HIV neuropathology, particularly in frontostriatal and temporal regions, serve as the foundation for an ongoing controversy in the neuroAIDS literature; namely, to what extent the pattern of HIV-associated neurocognitive impairment maps on to more “subcortical” (e.g., retrieval deficits, executive dysfunction, bradyphrenia) versus “cortical” (e.g., rapid forgetting, visuoperceptual deficits, and a degradation of semantic memory) distinctions (e.g., Chan et al., 2014; Brew, 2016). While historically, the pattern of neurocognitive impairment in HIV has been broadly categorized as “subcortical” in nature (e.g., Ragin et al., 2005), akin to classic frontostriatal disorders such as Huntington’s disease (HD), some authors have argued that as the prevalence of older adults living with HIV infection is increasing in the era of combination antiretroviral therapy (cART; Scott et al., 2011), the pattern of impairment may be making subtle shifts toward a more “cortical” presentation, in line with prototypical disorders of the temporal cortex (e.g., Alzheimer’s disease; AD). This suggestion has been driven by pathological, neuroimaging, and neurobehavioral findings that are similar to what is observed in traditional “cortical” disorders. For example, neuropathological studies in HAND have reported decreased cerebrospinal fluid beta amyloid and increased tau concentrations (Brew, Pemberton, Blennow, Wallin, & Hagberg, 2005; cf. Ances et al., 2012), while neuroimaging studies have revealed smaller temporal lobe volumes in HIV-infected individuals (Jernigan et al., 2005). At the neurobehavioral level, support for this hypothesis has arisen from HIV-associated deficits on tests of visuoperception (e.g., Olesen, Schendan, Amick, & Cronin-Golomb, 2007) as well as verbal and visual episodic memory (e.g., Sacktor et al., 2007). However, those in favor of the HIV “subcortical” hypothesis argue that the pattern of HIV-associated deficits in these domains is more suggestive of frontostriatal pathology (e.g., impaired working memory and executive function; Scott et al., 2011; Iudicello, Woods, Deutsch, Grant, & The HNRP Group, 2012).
One neurocognitive domain that is particularly useful for examining “subcortical” versus “cortical” distinctions is episodic memory. Prototypical “subcortical” disorders are widely held to be most consistent with what is deemed a “retrieval” (sometimes called “mixed encoding/retrieval”) deficit profile of episodic memory. It is estimated that 30–40% of HIV-infected persons demonstrate this profile, which is characterized by poor immediate and delayed free recall in the setting of relatively intact recognition (Woods, Moore, Weber, & Grant, 2009). Individuals with this profile type occasionally also demonstrate mild encoding deficits, though such deficits are thought to be due to diminished use of higher level encoding strategies associated with executive dysfunction (e.g., semantic clustering; Gongvatana et al., 2007). By contrast, typically much smaller proportions of HIV-infected individuals demonstrate primary encoding deficit profiles, which are commonly associated with classic “cortical” disorders (e.g., AD, temporal lobe epilepsy; TLE). Encoding deficit profiles are characterized by a primary storage deficit resulting in poor performance on measures of recall and recognition, as well as elevated rates of intrusion errors. In line with the controversy noted above, some authors have suggested that HAND may be evolving in the cART era (e.g., Brew, 2004) such that individuals may be evidencing verbal memory deficits that are more consistent with cortical pathology (e.g., rapid forgetting).
As such, the present study was designed to examine the extent to which HAND-associated verbal episodic memory impairments map onto deficits associated with prototypical “subcortical” (i.e., primary retrieval deficit) versus “cortical” (i.e., primary encoding deficit) disorders. In doing so, we directly compared verbal episodic memory performance, as indexed by the California Verbal Learning Test – 2nd edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), across the following four groups: (1) HAND; (2) HD, a “subcortical” sample typically associated with a primary retrieval deficit; (3) left TLE with mesial temporal sclerosis (L-MTLE), a “cortical” sample typically associated with a primary encoding deficit (e.g., Messas, Mansur, & Castro, 2013; Jeyaraj et al., 2013); and (4) healthy comparison subjects. Using a previously established algorithm that has successfully discriminated between traditional “cortical,” “subcortical,” and “normal” groups (Massman et al., 1992; Filoteo, personal communication, 2016), individuals were classified as having an encoding deficit, retrieval deficit, or normal episodic memory profile. Given the literature reviewed above as well as the prominent frontostriatal neuropathology of HIV, we hypothesized that the HAND sample would show a similar pattern of deficits as the HD group, as evidenced by a larger proportion of retrieval deficit versus encoding deficit profiles. Alternately, the HAND group would evidence the opposite pattern as compared to the L-MTLE group, who we hypothesized would demonstrate a larger proportion of encoding deficit versus retrieval deficit profiles. Speaking to the magnitude of observed episodic memory impairments in HAND, we hypothesized that individuals with HAND would show mild-to-moderate deficits across primary CVLT-II indices, similar to the L-MTLE sample. The HD sample was hypothesized to demonstrate the most pronounced CVLT-II deficits, as prior research has shown that symptomatic HD patients demonstrate profound impairments in episodic memory as compared to asymptomatic HD patients and healthy controls (Montoya et al., 2006).
Method
Participants
A total of 190 participants were included in the current study: 77 individuals with HAND, 47 individuals with HD, 21 individuals with L-MTLE, and 45 healthy comparison subjects. All participants were drawn from larger parent studies conducted through the University of California, San Diego (UCSD), and are detailed below. The sample sizes differed across all 4 groups as a function of different funding-driven enrollment demands of the separate parent studies from which participants were drawn and our own selection procedures for this investigation, which prioritized sample comparability in key demographics (e.g., age, education, sex, and ethnicity).
Both the HAND and healthy comparison samples were drawn from an NIH-funded R01 studying the combined effects of age and HIV on neurocognition, which was conducted at the UCSD HIV Neurobehavioral Research Program (HNRP). These participants were recruited from local HIV clinics and the San Diego community, and HIV serostatus was determined via enzyme-linked immunosorbent assay and confirmatory Western blot or MedMira Multiplo rapid test (MedMira Inc., Nova Scotia, Canada). HIV+ and healthy comparison subjects who met any of the following screening criteria were not enrolled into the R01 parent study, due to the criterion’s high potential to affect neurocognitive and/or neuropsychiatric functioning: severe psychiatric (e.g., schizophrenia) or neurologic illness (e.g., seizure disorder, active CNS opportunistic infections); substance dependence within one month of evaluation (as determined by the Composite International Diagnostic Interview, CIDI version 2.1; World Health Organization, 1998); Breathalyzer test positive for alcohol (i.e., blood alcohol content >0.0 on two administrations) or urine toxicology screen positive for illicit drugs (excluding marijuana) on the day of evaluation.
This study focused on only those HIV+ who were diagnosed with HAND as we aimed to examine patterns of verbal episodic memory functioning solely in those who evidence HIV-associated neurocognitive impairment. HAND diagnoses were made in accordance with current Frascati criteria (Antinori et al., 2007), a method that is widely used and well-validated for diagnosing HAND (e.g., Heaton et al., 2010; cf. Gisslén, Price, & Nilsson, 2011). In brief, HAND diagnoses were based on the results of a comprehensive neuropsychological evaluation that assessed seven neurocognitive domains that are commonly affected in HAND (see Doyle et al., 2013 for details on specific assessments). For the purposes of this study, performance on the CVLT-II was excluded from the determination of HAND diagnoses. A global deficit score (GDS; see Carey et al., 2004) was calculated based on performance across the neurocognitive assessments. In this approach, individual raw scores from neurocognitive measures are converted to demographically-adjusted T scores using published normative data. Resulting T scores are then transformed into deficit scores, which range from 0 (T ≥ 40) to 5 (T < 20). GDS is calculated by averaging deficit scores across all measures, and a standard cutoff score of ≥0.5 (i.e., indicating that the participant demonstrated impaired performance on at least half of the neurocognitive assessments) indicated a diagnosis of HAND. Thirty-four of the HAND participants (77.3%) met criteria specifically for Asymptomatic Neurocognitive Impairment (ANI) and the remainder of participants (22.7%) met criteria for Minor Neurocognitive Disorder (MND). No participant was diagnosed with HIV-associated Dementia (HAD).
Individuals with HD were recruited from the Huntington’s Disease Clinical Research Program at UCSD. A diagnosis of HD was made by a senior staff neurologist. All HD patients were diagnosed with definite HD on the basis of unequivocal motor signs on the Unified Huntington’s Disease Rating Scale (UHDRS; Huntington Study Group, 1996) and a positive family history for HD. Based on the UHDRS motor exam, the neurologist assigned a diagnostic confidence rating representing the evaluator’s certainty that the presence of motor abnormalities were a manifestation of HD. Ratings are defined as: 0 = normal (no abnormalities), 1 = non-specific motor abnormalities (<50% confidence that the participant has sufficient motor abnormalities to warrant a diagnosis of HD), 2 = motor abnormalities that may be signs of HD (50–89% confidence), 3 = motor signs that are likely signs of HD (90–98% confidence), 4 = motor abnormalities that are unequivocal signs of HD (>99% confidence). All HD patients received a score of 4; therefore, they met the criteria for a diagnosis of manifest HD. In HD participants, the mean UHDRS Total Motor Score (TMS) was 33.8 (SD= 16.2) and the mean UHDRS Total Functional Capacity Score (TFC) was 7.4 (SD= 2.1). The UHDRS TMS ranges from 0–124, with higher scores suggestive of more severe motor impairment. The UHDRS TFC ranges from 0–13, with lower scores suggestive of increased disability. In addition, all HD participants had a CAG repeat length greater than 39, indicating that all carried the fully penetrant genetic mutation for HD. The mean number of CAG repeats in the HD group was 46.1 (SD= 3.6) Exclusion criteria included a history of neurological disorders (with the exception of HD), a formal diagnosis of a severe psychiatric disorder (e.g., psychosis prior to the onset of HD), or history of traumatic brain injury.
Within the MTLE sample, analysis was restricted to patients with medically refractory L-MTLE, as this subgroup, with visibly evident left hippocampal pathology, was expected to show the most classic verbal encoding deficit among patients with MTLE (e.g., Jeyaraj et al., 2013). Patients with L-MTLE were drawn from a NIH-funded R01 on the use of multimodal neuroimaging in preoperative planning for epilepsy surgery, conducted at the UCSD Multimodal Imaging Laboratory. At the time of enrollment, all patients were under evaluation for surgical treatment at the UCSD Epilepsy Center. They were diagnosed with medically refractory epilepsy by board-certified neurologists with expertise in epileptology, according to the criteria defined by the International League Against Epilepsy. Patients were classified as L-MTLE based on seizure onsets recorded by video-EEG monitoring and seizure semiology. Where clinically indicated, patients underwent Phase II video-EEG monitoring using 5-contact foramen ovale electrodes to exclude bilateral independent seizure onsets. MRIs were visually inspected by a board-certified neuroradiologist to confirm (1) the presence of unilateral left MTS in all patients and (2) the absence of dual pathology. No patients showed evidence of contralateral MTS or visible extra-hippocampal pathology on clinical MRI. L-MTLE patients with any comorbid neurological conditions, as well as those with a comorbid psychiatric disorder requiring inpatient hospitalization, were excluded from this study.
Basic demographic and disease-specific characteristics are outlined in Table 1. Study groups were comparable with respect to age and education, though consistent with the epidemiology of the clinical groups, significant group differences were observed for gender and race/ethnicity, such that the HAND group included a higher proportion of males as compared to the three other groups (ps<.05), and the HD group was comprised of a larger proportion of Caucasian participants as compared to the three other groups (ps<.05).
Table 1.
Demographic and disease-specific characteristics of the study samples.
| Healthy (n=45) |
HAND (n=77) |
TLE (n=21) |
HD (n=47) |
p | |
|---|---|---|---|---|---|
| Age | 41.7 (13.5) | 41.9 (11.8) | 39.0 (12.6) | 43.4 (7.1) | .704 |
| Education | 14.2 (2.0) | 13.4 (2.6) | 13.2 (1.7) | 13.9 (2.4) | .118 |
| Sex (% male)* | 57.8 | 77.9 | 38.1 | 59.6 | .003 |
| Ethnicity (% Caucasian) | 40.0 | 46.8 | 50.0 | 90.7 | <.001 |
| HAND Characteristics | |||||
| Estimated duration of infection (mos)a | – | 121 (38, 187) | – | – | – |
| Current CD4 Counta | – | 581 (372, 798) | – | – | – |
| Nadir CD4 Counta | – | 200 (42, 363) | – | – | – |
| Current cART (%) | – | 80.5 | – | – | – |
| AIDS (%) | – | 50.0 | – | – | – |
| Detectable plasma viral load (%) | – | 29.7 | – | – | – |
| TLE Characteristics | |||||
| Age Onseta | – | – | 9 (4, 30) | – | – |
| Seizures/Montha | – | – | 5 (3, 9) | – | – |
| No. Epilepsy Medications | – | – | 2.5 (0.7) | – | – |
| HD Characteristics | |||||
| CAG Repeats | – | – | – | 46.1 (3.6) | – |
| UHDRS – Total Motor Score | – | – | – | 33.8 (16.2) | – |
| UHDRS – Total Functional Capacity | – | – | – | 7.4 (2.1) | – |
Note.
Data represent median and interquartile range. HAND=HIV-associated neurocognitive disorders. TLE=Temporal lobe epilepsy. HD=Huntington’s disease. cART=Combination antiretroviral therapy. UHDRS = Unified Huntington’s Disease Rating Scale.
Materials and Procedure
These studies were approved by the human research protections program at UC San Diego, and all participants provided written, informed consent within their parent studies. The HAND, L-MTLE, and healthy comparison groups were paid a nominal fee for study participation. The HD group completed the neuropsychological evaluation as part of an annual exam, and were not paid for participation.
California Verbal Learning Test – 2nd Edition (CVLT-II)
As part of their respective parent studies, all participants completed the CVLT-II (Delis, Kramer, Kaplan, & Ober, 2000). The CVLT-II is a list-learning episodic verbal memory measure that consists of two 16-item word lists (lists A and B). The 16 words on each list belong to four different semantic categories; each category contains four words. List A is presented over five consecutive learning trials (i.e., verbal presentation and immediate recall), while List B is presented in a single learning trial after the final presentation of List A. Following the List B single learning trial, participants are asked to recall List A spontaneously (short-delay free recall) and then following semantic cues (short-delay cued recall). After a 20 minute delay, participants are again asked to recall List A spontaneously (long-delay free recall) and then with semantic cues (long-delay cued recall). Participants are then administered a recognition test in which they are presented with the 16 target words intermixed among 32 other non-target words, and are asked to respond “yes” if the word was from List A and “no” if it was not.
Here we leverage the most reliable and well-established aspects of the CVLT-II toward the aims of our study, which was focused on the classic cortical vs. subcortical profile of memory dysfunction. This approach used well-validated algorithms that take into account overall learning, intrusion errors, and recognition performance, which have shown to discriminate AD from HD. In considering whether to apply other CVLT-II indices in this regard, we were mindful of type I error risk, as was raised by prior reviewers. Particularly in the absence of any a priori hypotheses: That is, while other aspects of the CVLT-II provide useful process information, they do not necessarily help one to distinguish between traditional cortical vs. subcortical conditions any better than the primary well-validated metrics we used here. As such, we would argue that our study provides a more conservative, focused approach to assessing a complex question regarding HIV disease. For descriptive purposes, raw scores on primary CVLT-II variables across the four groups are displayed in Table 2. All other CVLT-II variables utilized in this study were either T or Z scores, for which the CVLT-II provides demographic adjustments for age and gender.
Table 2.
Raw scores on primary CVLT-II variables across the study samples.
| CVLT-II variable | Healthy (n=45) |
HAND (n=77) |
TLE (n=21) |
HD (n=47)d |
Pairwise Comparisons | Effect Size | Effect Size | Effect Size |
|---|---|---|---|---|---|---|---|---|
| a | b | c | d | a vs b | b vs c | b vs d | ||
| Total Trials 1–5 | 55.1 (9.8) | 43.4 (9.9) | 43.6 (11.5) | 33.9 (12.7) | a>b,c>d | −1.2 | −0.02 | 0.83 |
| Short-delay free recall | 11.8 (3.4) | 8.1 (3.2) | 8.1 (4.3) | 5.8 (3.7) | a>b,c.d; b>d | −1.1 | 0.00 | 0.66 |
| Short-delay cued recall | 12.5 (3.4) | 9.7 (3.0) | 8.9 (3.5) | 7.3 (3.9) | a>b,c,d; b>d | −0.87 | 0.25 | 0.69 |
| Long-delay free recall | 11.9 (3.4) | 8.5 (3.4) | 7.7 (4.3) | 5.9 (3.7) | a>b,c,d; b>d | −1.0 | 0.22 | 0.73 |
| Long-delay cued recall | 12.6 (3.4) | 9.7 (3.1) | 8.6 (4.2) | 6.9 (3.8) | a>b,c,d; b>d | −0.89 | 0.32 | 0.81 |
| Total recognition discrim. | 3.2 (0.8) | 2.7 (0.8) | 2.6 (1.1) | 1.8 (0.9) | a>b,c>d | −0.63 | 0.11 | 1.06 |
Note. All omnibus p-values < .001. Pairwise comparisons based off Tukey-Kramer HSD tests, p < .05. CVLT-II = California Verbal Learning Test – Second Edition. Discrim = discriminability. HAND = HIV-Associated Neurocognitive Disorder. TLE = temporal lobe epilepsy. HD = Huntington’s disease. Effect sizes represent unadjusted Hedges g values, comparing the HAND group to the other three study groups.
A discriminant function algorithm was used to classify participants into one of three memory profile groups (i.e., encoding deficit, retrieval deficit, or normal profile) according to a prior study from which it was derived (Massman et al., 1992). The algorithm, which was originally derived from the first version of the CVLT (Delis, Kramer, Kaplan, & Ober, 1987), was based on the performance of individuals with AD (i.e., “cortical” disorder group), HD (i.e., “subcortical” disorder group), and a healthy sample. The current study used an updated algorithm that was derived from performance on the CVLT-II (Filoteo, personal communication, 2016). The updated algorithm utilized the same following indices as the original Massman et al. (2012) algorithm, based on their ability to discriminate between the three patient groups: 1) Trials 1–5 Total T score; 2) Total Cued Recall Intrusions z-score; and 3) Total Recognition Discriminability z-score relative to the Trial 5 z-score. In the original Massman et al. (1992) study, the Trials 1–5 Total T score was found to discriminate between the healthy sample and the two clinical samples, mapping onto findings that have shown that learning tends to be deficient in both cortical and subcortical disorders relative to healthy adults (e.g., Delis et al., 1991). The Total Cued Recall Intrusions Z score discriminated performance of those with AD from those in the HD and healthy adult samples, in concordance with work that has shown that those with cortical disorders are more likely to demonstrate an encoding deficit by eliciting higher numbers of recall intrusions relative to controls and those with subcortical disorders (e.g., Delis et al., 1991). Finally, the Total Recognition Discriminability z-score was found to discriminate performance of those with HD from the AD and healthy adults, in concordance with studies that have shown that those with subcortical disorders are more likely to show a retrieval deficit by demonstrating greater improvement on recognition testing relative to free recall, as compared to healthy adults and those with cortical disorders (e.g., Delis et al., 1991). For each profile type, the three CVLT-II variables was multiplied by a coefficient that was established in the discriminant function analysis (Filoteo, personal communication, 2016). Resulting scores for each profile type were compared for each participant; their highest score determined what profile type they were designated. For example, a participant with a 50T on Trials 1–5, a z-score of 1 on Cued Recall Intrusions, and a z-score of 1.5 on Recognition Discriminability and a z-score of .5 Trial 5 would receive a Normal Profile score of 14.9 ([50 × 0.739] + [1.0 × 0.473] + [1.5−0.5−0 × 2.229] −24.75), an Encoding/Storage Deficit Profile of 11.7 ([50 × 0.375] + [1.0 × 1.819] + [1.5−0.5 × 0.484] − 9.343), and Retrieval Deficit Profile score of 13.2 ([50 × 0.361] + [1.0 × 0.421] + [1.5−0.5 × 1.7] − 6.951) and would thus be classified as having a “normal” profile. In the original Massman et al. (1992) study, the algorithm demonstrated a robust ability to differentiate between memory profiles associated with these neurological populations, as 90% of individuals across the study were correctly classified into their respective groups. The utility of this algorithm has been demonstrated in several other neurologic populations, including Parkinson’s disease (e.g., Filoteo et al., 1997; Weintraub et al., 2004), schizophrenia (Paulsen et al., 1995), and HIV disease (e.g., Obermeit et al., 2015).
In addition, we opted to examine performance on primary CVLT-II measures in an exploratory fashion.
Results
Table 2 details group outcomes and effect sizes on CVLT-II primary variables; Tukey HSD corrections were applied in an effort to limit Type I error due to multiple comparisons. Our total sample size of 190 afforded adequate power (1-B = .95) to detect medium omnibus effect sizes (f = .25) using a critical alpha of .05. A series of ANOVAs showed omnibus differences were observed across all primary CVLT-II indices (ps <.001). Post-hoc tests showed that the HAND group performed significantly worse as compared to the healthy comparison group (ps<.05), with large effect sizes across free recall measures and medium effects for recognition. The HAND group performed significantly better as compared to the HD group across all indices (ps<.05), with medium-to-large effect sizes on free recall and large effects for recognition.
The primary study hypothesis was tested via a multinomial regression analysis, with the three-level CVLT-II profile variable serving as the criterion and clinical group as the predictor. Demographic covariates in this model were selected for inclusion based on meeting the following criteria: (1) significant omnibus group differences on the variable as indicated in Table 1; and (2) the variable showed significant associations (via chi square or t-tests where appropriate) with the criterion (i.e., CVLT-II profile type) within the overall study sample. As such, ethnicity was included as a covariate in the regression model as it was significantly associated with CVLT-II profile type (χ2(2)=8.2, p=.02); however, gender was not included as the relationship between gender and CVLT-II profile type in the overall sample was not significant (χ2(2)=1.2, p=.56).
The overall multinomial regression model was significant (χ2(8)=44.9, p<.001), with clinical group emerging as a significant predictor of CVLT-II profile type (χ2(6)=36.7, p<.001). The effect of ethnicity was not significant (p=.47). Overall rates of CVLT-II deficit profiles (i.e., collapsed across deficit profile type) were such that the HD group evidenced the highest proportion of individuals with a CVLT-II deficit profile (87.3%), with the HAND (62.4%) and TLE (61.9%) groups falling intermediate to the healthy comparison group (22.2%). There were no significant differences in the CVLT-II deficit profiles of HAND participants with ANI versus those with MND (p = .815). Moreover, CVLT-II deficit profiles were not associated with any HIV disease or treatment variable listed in Table 1 (all ps > .10).
Restricting the sample to those who demonstrated a CVLT-II deficit profile (Healthy n = 10; HD n = 41; TLE n = 13, and HAND n = 48), Figure 1 displays the proportions of encoding and retrieval deficit profiles that were observed across the study groups. The HAND group evidenced higher rates of retrieval deficit (70.8%) versus encoding deficit (29.2%) profiles. This pattern was in the same direction as that which was observed in the HD group (58.5% retrieval deficit vs. 41.5% encoding deficit), and the HAND and HD groups did not significantly differ from one another (χ2(2)=1.5, p=.22, odds ratio = 1.7 [95% confidence interval 0.7, 4.1). Conversely, the L-MTLE group evidenced the opposite pattern of profile proportions (46.1% retrieval deficit vs. 53.9% encoding deficit), but did not differ significantly from the HAND group (χ2(2)=2.8, p=.10, odds ratio = 2.8 [95% confidence interval 0.8, 9.9). Finally, the healthy comparison group evidenced equal proportions of retrieval deficit (50%) and encoding deficit profiles (50%), which was not significantly different from the HAND group (χ2(2)=1.6, p=.20, odds ratio = 2.4 [95% confidence interval 0.6, 9.7). Figure 2 displays demographically-adjusted Z scores of primary CVLT-II indices across the study groups for descriptive purposes.
Figure 1.
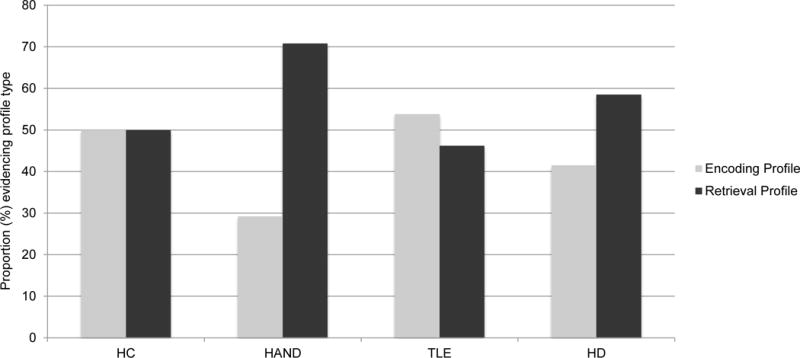
Distribution of CVLT-II deficit profile types across the study groups.
Note. HC=Healthy comparison (n=10). HAND=HIV-associated neurocognitive disorders (n=48). TLE=temporal lobe epilepsy (n=14). HD=Huntington’s disease (n=41). CVLT-II = California Verbal Learning Test – Second Edition.
Figure 2.
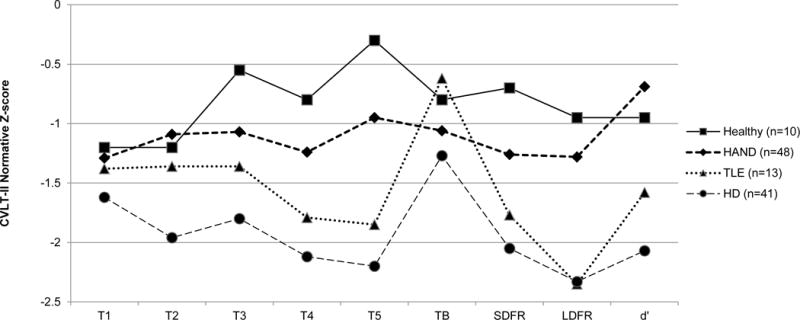
CVLT-II normative Z-scores by study group (only amongst those with CVLT-II deficit profiles).
Note. CVLT-II = California Verbal Learning Test-Second Edition; T=trial; SDFR = short-delay free recall; LDFR = long-delay free recall; d′ = recognition discriminability index. HC=Healthy comparison (n=10). HAND=HIV-associated neurocognitive disorders (n=48). TLE=temporal lobe epilepsy (n=14). HD=Huntington’s disease (n=41).
Discussion
One ongoing controversy in the neuroAIDS literature is the extent to which cART era neurocognitive deficits map onto a pattern of “subcortical” versus “cortical” distinctions. While historically, HAND has been broadly classified as most consistent with a “subcortical” pattern of deficits, more recent pathological and neurobehavioral studies have suggested increased “cortical” dysfunction in the era of cART, as HIV-infected individuals are now aging into later decades of life with what is now known as a chronic illness. The current study attempts to address this question by being the first study to our knowledge to directly compare verbal episodic memory performance in HAND to that of prototypical “subcortical” (i.e., HD) and “cortical” (i.e., MTLE) disorders. Consistent with previously reported prevalence rates, approximately 60% of individuals with HAND in the present study demonstrated impaired verbal episodic memory based on the results of a widely used algorithm that was developed to discriminate between “subcortical,” “cortical,” and “normal” memory profiles on the CVLT-II. In accordance with our primary hypothesis, among those with HAND who evidenced a CVLT-II deficit profile, a larger proportion of individuals demonstrated retrieval versus encoding deficit profiles, akin to the prototypical “subcortical” group. Specifically, this profile was characterized by better performance on the Recognition Discriminability index of the CVLT-II relative to performance on the Total Trials 1–5 and Cued Recall Intrusions. This distribution of profile types is consistent with a host of studies that have demonstrated that HIV neuropathology affects frontostriatal circuitry preferentially (e.g., Ellis et al., 2009), and is most consistent with a “subcortical” pattern of deficits. Also consistent with our hypotheses, the distribution of retrieval versus encoding deficit profiles in HAND was in opposition to that which was observed in the prototypical “cortical” (i.e., L-MTLE) group, who demonstrated higher proportions of encoding versus retrieval deficit profiles (however, the HAND and L-MTLE groups did not differ significantly from each other). This finding in the L-MTLE group is broadly consistent with the typical pattern of neuropathology seen in MTLE, which is characterized by prominent damage to the hippocampus, entorhinal, and parahippocampal cortices (Bonilha et al., 2007). However, the magnitude of HIV-associated deficits were similar to that which was observed in the L-MTLE group, with an overall pattern of mild-to-moderate deficits across CVLT-II primary variables. In turn, the HD group demonstrated large magnitude deficits across the majority of CVLT-II indices. Of note, these findings were not better explained by demographic characteristics.
While the observed patterns of CVLT-II deficit profile distributions occurred in the expected directions across the groups, there were no significant differences in deficit profile types between HAND and any of the comparison groups. In spite of this, there was significant profile type heterogeneity observed within all clinical groups, particularly within the classic “subcortical” (i.e., HD) and “cortical” (i.e., MTLE) groups, who evidenced roughly similar proportions of retrieval and encoding deficit profiles. Possible reasons for these observed patterns in the classic “subcortical” and “cortical” disorder groups include neuropathological variability (i.e., many MTLEs have associated subcortical pathology; Thom et al., 2009), measurement error, and the influence of secondary variables (e.g., cognitive reserve; medication effects) that were not obtained in the current study. While our findings largely converged with a host of studies providing evidence for a primary pattern of “subcortical” deficits in HAND continuing through the cART era (e.g., Iudicello et al., 2012; Scott et al., 2011), the HAND group also displayed a degree of profile heterogeneity, with nearly one third of individuals demonstrating a primary encoding deficit, characterized by elevated cued recall intrusion errors relative to poor performance on recognition discriminability and learning trials. A number of clinical researchers have postulated that older age in the context of HIV may confer an altered expression of HAND that is reflective of cortical pathology, or even an early AD process (Brew, Croww, Landay, Cysique, & Guillemin, 2009); however, a post hoc analysis revealed no association between age and verbal episodic memory profile type in the HAND sample (p=.95), consistent with prior studies that have examined the aging/HIV question and did not report evidence of an AD-like presentation in older HIV+ individuals (e.g., Scott et al., 2011). As with the other clinical groups in this study, it remains unclear what mechanisms may be driving the heterogeneous findings within the HAND group, and may be an appropriate question for future studies. However, it is of note that other studies have observed similar variability in verbal episodic memory profiles when using the same discriminant function algorithm, both within HIV (e.g., Obermeit et al., 2015) as well as other neurodegenerative populations (e.g., HD; Delis et al., 2005; Parkinson’s disease; Filoteo et al., 1997).
As displayed in Figure 2, the HAND group demonstrated a stable deficit pattern across Trials 1–5, Trial B, and both Short- and Long-Delay Free Recall trials. As expected, they showed an increase in performance on the Recognition Discriminability trial relative to the Free Recall trials, consistent with a primary retrieval deficit (i.e., poor recall in the setting of relatively better recognition). Both other clinical groups showed this pattern as well, which was an unexpected finding in the L-MTLE group; we had expected to observe similar performance on free recall and recognition discriminability trials in L-MTLE, consistent with a primary encoding deficit. However, this finding again speaks to the significant heterogeneity within patients with L-MTLE and new evidence indicating significant frontal-subcortical pathology in many patients with medically refractory L-MTLE, which may be reflect neurodevelopmental or neurodegenerative processes (McDonald et al., 2008; Kemmotsu et al., 2011). Another possible interpretation is that individuals with L-MTLE may be using compensatory mechanisms to boost memory performance, as prior work by Dupont and colleagues (2000) showed that L-MTLE patients demonstrated increased activation in various brain networks (particularly prefrontal regions) during episodic memory tasks.
Several limitations of the current study design are worth noting. First, this study focused exclusively on verbal episodic memory deficits, specifically via a list-learning task. Indeed, HIV-associated episodic memory deficits are readily observed on a variety of tasks, including both verbal (e.g., passages) and visual (simple and complex designs; see Woods et al., 2009 for a review) measures. Moreover, the current study utilized solely one measure of episodic verbal memory, the CVLT-II, as to our knowledge it is the only measure for which an established verbal episodic memory deficit profile algorithm has been established. Second, the drawing of samples from unrelated parent studies resulted in an inability to glean a host of sample characteristics on which we could compare groups, in particular, characteristics that theoretically could relate to the primary outcome variables in this study (e.g., lifetime psychiatric and substance use disorders, medical comorbidities, premorbid intellectual functioning, etc.). Moreover, global level of cognitive impairment could represent a confound to the interpretation of the present findings. Regretfully, because these study samples were drawn from different research protocols, we did not have a single screening measure that was common across the populations. Nevertheless, we took a conservative approach to this question by including Trial 1 of the CVLT-II in the primary multinomial regression model as a proxy for overall impairment. Despite the risk of criterion contamination in taking this approach, the model was still significant with independent contributions of group and CVLT-II Trial 1 (ps < .001). Of course, none of the participants met criteria for HAD, which might produce a different learning and memory profile, as previously shown by our group (e.g., Scott et al., 2006). Another limitation of these findings has to do with their generalizability to older HIV+ adults, who may arguably be at even greater risk of “cortical” deficits than their younger HIV+ counterparts. Nevertheless, there was no age effect on CVLT-II profiles in this relatively young HAND cohort (p = .94) and the rates of encoding vs. retrieval profiles among participants with CVLT-II deficits is comparable to those reported in much older HIV+ persons (e.g., Scott et al., 2010). Finally, because we limited our TLE cohort to well-characterized patients with medically-refractory L-MTLE (i.e., those with significant left hippocampal pathology), the sample size was small relative to the other groups. This may have limited our ability to detect pairwise differences for several CVLT-II variables outlined in Table 2.
In sum, the results reported herein suggest that HIV-associated verbal episodic memory impairment manifests in a manner similar to classic “subcortical” disorders, whereby there is a higher prevalence of retrieval as opposed to encoding deficits. Such findings are consistent with the prominent frontostriatal pathology of HIV infection. However, we observed significant heterogeneity (although group comparisons did not reach significance) in the distribution of profile types as well as unexpected performances on CVLT-II primary variables across all clinical groups. The source of such variability is unknown, and thus future investigations in this regard, in particular neuropathological and neuroimaging studies, are warranted. The clinical implications of these findings are two-fold. First, these data might help with differential diagnosis of HAND from other conditions, particularly as the HIV+ population ages and questions regarding possible Alzheimer’s disease and other common dementing disorders become more frequent. Second, these data may have ecological relevance; for example, recent work by Obermeit and colleagues (2015) found that retrieval deficits, but not encoding deficits, are associated with antiretroviral non-adherence. It is reasonable to posit that these learning and memory deficit types may map onto other distinct functional, health-related, and quality of life factors among persons with HAND.
References
- Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, Clifford DB. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Archives of Neuropsychology. 2012;69:72–77. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Alessio A, Rorden C, Baylis G, Damasceno BP, Min LL, Cendes F. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Human Brain Mapping. 2007;28:1376–1390. doi: 10.1002/hbm.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ. Has HIV-associated neurocognitive disorders now transformed into vascular cognitive impairment? AIDS. 2016;30:2379–2380. doi: 10.1097/QAD.0000000000001225. [DOI] [PubMed] [Google Scholar]
- Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18(Suppl 1):S75–S78. [PubMed] [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. Journal of Neuroimmune Pharmacology. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, The HNRC Group Predictive validity of global deficit scores for detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Current HIV/AIDS Rep. 2014;11:317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Adult Version Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – second edition Adult Version Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Wetter SR, Jacobson MW, Peavy G, Hamilton J, Gongvatana A, Salmon DP. Recall discriminability: Utility of a new CVLT-II measure in the differential diagnosis of dementia. Journal of the International Neuropsychological Society. 2005;11:708–715. doi: 10.1017/S1355617705050812. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Loft S, Morgan EE, Weber E, Cushman C, Johnston E, The HIV Neurobehavioral Research Program (HNRP) Group Prospective memory in HIV-associated neurocognitive disorders (HAND): The neuropsychological dynamics of time monitoring. Journal of Clinical and Experimental Neuropsychology. 2013;35:359–372. doi: 10.1080/13803395.2013.776010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Van de Moortele PF, Samson S, Hasboun D, Poline JB, Adam C, Baulac M. Episodic memory in left temporal lobe epilepsy: A functional MRI study. Brain. 2000;123:1722–1732. doi: 10.1093/brain/123.8.1722. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: A primer. Neuropsychology Review. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, National NeuroAIDS Tissue Consortium (NNTC) Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. Journal of NeuroVirology. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Rilling LM, Cole B, Williams BJ, Davis JD, Roberts JW. Variable memory profiles in Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology. 1997;19:878–888. doi: 10.1080/01688639708403768. [DOI] [PubMed] [Google Scholar]
- Gisslén M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infectious Diseases. 2011;11:356. doi: 10.1186/1471-2334-11-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Woods SP, Taylor MJ, Vigil O, Grant I, The HNRC Group Semantic clustering inefficiency in HIV-associated dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:36–42. doi: 10.1176/jnp.2007.19.1.36. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington Study Group. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Movement Disorders. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, Grant I, The HNRP Group Combined effects of aging and HIV infection on semantic verbal fluency: A view of the cortical hypothesis through the lens of clustering and switching. Journal of Clinical and Experimental Neuropsychology. 2012;34:476–488. doi: 10.1080/13803395.2011.651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Rivera-Mindt M, Marcotte T, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jeyaraj MK, Menon RN, Justus S, Alexander A, Sarma PS, Radhakrishnan K. A critical evaluation of the lateralizing significance of material-specific memory deficits in patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy and Behavior. 2013;28:460–466. doi: 10.1016/j.yebeh.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, McDonald CR. MRI analysis in temporal lobe epilepsy: Cortical thinning and white matter disruptions are related to side seizure onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massman PJ, Delis DC, Butters N, Renee M. The subcortical dysfunction hypothesis of memory deficits in depression: Neuropsychological validation in a subgroup of patients. Journal of Clinical and Experimental Neuropsychology. 1992;14:687–706. doi: 10.1080/01688639208402856. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A, Pelletier M, Menear M, Duplessis E, Richer F, Lepage M. Episodic memory impairment in Huntington’s disease: A meta-analysis. Neuropsychologia. 2006;44:1984–1994. doi: 10.1016/j.neuropsychologia.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Obermeit LC, Morgan EE, Casaletto KB, Grant I, Woods SP, The HNRP Group Antiretroviral nonadherence is associated with a retrieval profile of deficits in verbal episodic memory. The Clinical Neuropsychologist. 2015;29:197–213. doi: 10.1080/13854046.2015.1018950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Schendan HE, Amick MM, Cronin-Golomb A. HIV infection affects parietal-dependent spatial cognition: Evidence from mental rotation and hierarchical pattern perception. Behavioral Neuroscience. 2007;121:1163–1173. doi: 10.1037/0735-7044.121.6.1163. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, Jeste DV. The nature of learning and memory impairments in schizophrenia. Journal of the International Neuropsychological Society. 1995;1:88–99. doi: 10.1017/s135561770000014x. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. Journal of NeuroVirology. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani K, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of NeuroVirology. 2007;13:203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Carey CL, Weber E, Bondi MW, Grant I, The HNRC Group Neurocognitive consequences of HIV infection in older adults: An evaluation of the “cortical” hypothesis. AIDS and Behavior. 2011;15:1187–1196. doi: 10.1007/s10461-010-9815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Patterson K, Morgan EE, Heaton RK, Grant I, Marcotte TD, The HNRC Group Recency effects in HIV-associated dementia are characterized by deficient encoding. Neuropsychologia. 2006;44:1336–1343. doi: 10.1016/j.neuropsychologia.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Thom M, Eriksson S, Martinian L, Caboclo LO, McEvoy AW, Duncan JS, Sisodiya SM. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: Neuropathological features. Journal of Neuropathology and Experimental Neurology. 2009;68:928–938. doi: 10.1097/NEN.0b013e3181b05d67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15467–15562. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson’s disease. Cognitive and Behavioral Neurology. 2004;17:195–200. [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, version 2.1) Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]


