Abstract
Background
Liver dysfunction is common in pregnancy but its association with adverse pregnancy outcomes such as preterm birth (PTB) remains unclear.
Methods
A prospective cohort of HBV-infected or uninfected pregnant women attending antenatal care was recruited at Nantong Maternal and Child Health Hospital between January 1, 2012, and June 30, 2016. Liver function tests (LFTs) were monitored through pregnancy. The primary outcomes were PTB and very PTB (delivery prior 37 and 32 weeks' gestation respectively). Poisson regression was used to estimate adjusted risk ratios (RR) for women with HBV infection and LFT abnormalities.
Results
Among 36,755 pregnant women (1,113 HBV carriers and 35,642 non-HBV subjects), 3,519 (9.57%) had abnormal LFTs. The commonest cause for liver dysfunction during pregnancy was non-alcoholic fatty liver diseases (NAFLD, 51.3%). Abnormal aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT) and two folds upper limit of normal total bilirubin (RR and 95%CI: 2.73, 1.30–5.76; 2.24, 1.35–3.31; 2.01, 1.22–3.31 respectively), rather than HBsAg positivity, were identified as independent risk factors for preterm birth. Besides, GGT abnormality was associated with increased risk of very PTB.
Conclusions
We suggest that surveillance of LFTs among pregnant women should be warranted, given the increased risk of PTB.
Keywords: Preterm birth, Liver function tests, Hepatitis B virus, Cohort study
Highlights
-
•
Among a cohort of 36,755 pregnant women (1,113 HBV carriers, 35,642 non-HBV controls), 3,519 (9.57%) had abnormal LFT results.
-
•
Abnormal AST, GGT and total bilirubin, rather than HBsAg positivity, were independent risk factors for preterm birth.
-
•
The commonest cause for liver dysfunction during pregnancy was non-alcoholic fatty liver diseases (NAFLD).
Most previous studies showed that HBV carrier status may be an independent, but only weak risk factor for preterm birth. In this prospective cohort study, we suggest that liver dysfunction, rather than HBsAg positivity, is associated with risk for preterm birth. The commonest cause for liver dysfunction during pregnancy was non-alcoholic fatty liver diseases (NAFLD). Our findings are not contradictory to previous findings, as HBV infection status may have a synergistic effect in case of premature birth. This study improves our understanding on the importance of surveillance for liver function tests among pregnant women.
1. Introduction
Preterm birth (PTB, delivery prior 37 weeks' gestation) or premature birth remains a leading cause for neonatal morbidity and mortality, as well as a wide array of long-term sequelae (Saigal and Doyle, 2008). Worldwide, an estimated 15 million babies are born premature annually and the number is increasing, with rates varying from 5% to 18% across countries (Goldenberg et al., 2008).
Chronic hepatitis B virus (HBV) infection is highly endemic in China. The seroprevalence rate of hepatitis B surface antigen (HBsAg) was reported 6.71% among pregnant women in our province (Zhang et al., 2010). Several studies, including our previous studies, explored the incidence of PTB in women with HBV infection, but the results are inconsistent (Chen et al., 2015, Connell et al., 2011, Cui et al., 2016a, Cui et al., 2016b, Salemi et al., 2014, Sirilert et al., 2014). Recently, a large-scale population-based cohort study suggested that pre-pregnancy HBV infection may be associated with increased risk for preterm delivery (Liu et al., 2017). Moreover, three reviews suggested minimally but significantly increased risk of preterm birth among HBV carriers (Cui et al., 2016b, Huang et al., 2014, Ma et al., 2017). However, It should be stressed that chronic HBV infection is also a major cause of liver dysfunction (e.g. rise in serum aminotransferase and bilirubin), which may have serious consequences by itself (Than and Neuberger, 2013). The impact of liver dysfunction on preterm birth has not been systematically documented and remains unclear.
In the current study, we conducted a hospital-based cohort study to determine whether liver function test (LFT) abnormalities, as well as chronic HBV infection, were associated with increased risk of PTB.
2. Methods
2.1. Study Cohort
A prospective cohort was recruited at the Nantong Maternal and Child Health Hospital affiliated to Nantong University, China between January 1, 2012 and June 30, 2016. The medical history as well as supporting clinical and laboratory information were collected at baseline. All pregnant women aged 18 to 50 years were screened. Exclusion criteria were (1) multiple pregnancy; (2) positivity for antibodies against hepatitis C virus (HCV), human immunodeficiency virus (HIV), toxoplasma (TOX), rubella virus (RV), cytomegalovirus (CMV) or herpes simplex virus (HSV-1/2), positivity for rapid plasma reagin test/RPR (indicating active syphilis infection); (3) pre-existing chronic diseases such as diabetes mellitus (DM), hypertension or heart diseases; (4) spontaneous or induced abortion; (5) lost to follow-up or incomplete data. HBV carriers were defined as those who had positive HBsAg for at least 6 month.
This study was performed according to the Declaration of Helsinki and approved by the Institutional Review Board of the Nantong Maternal and Child Health Hospital affiliated to Nantong University, China. Written informed consents were obtained from all participants.
2.2. Procedures
This study was conducted during January 2012 to April 2017. Data on maternal demographic characteristics were collected from questionnaires completed by women at the first antenatal visit. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT) and total bilirubin (TBiL) were measured with an automatic biochemical analyzer (AU2700, Olympus, Japan). Serological tests for HBsAg and hepatitis B e antigen (HBeAg) were done by enzyme linked immunoassays on a random-access analyzer (Architect i2000; Abbott Diagnostics, USA). Liver function tests were assessed as routine in all pregnancies. All recruited women had baseline LFT performed and at least one subsequent test. Additional LFTs were requested by the obstetric staff or general clinical practitioners. The medical aspects of the pregnancy were followed-up as clinically appropriate. Other investigations included hepatobiliary ultrasonography, serum bile acids, hepatitis A, C, E serology, autoantibodies, etc.
Abnormal LFT was defined as at least one result of the four markers (ALT, AST, GGT, total bilirubin) greater than its upper limit of normal (ULN), i.e. ALT > 40 U/L or AST > 40 U/L or GGT > 50 U/L or total bilirubin > 17.1 μmol/L. Diagnoses of non-alcoholic fatty liver diseases (NAFLD), intrahepatic cholestasis of pregnancy (ICP), pre-eclampsia/eclampsia, haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, and acute fatty liver of pregnancy (AFLP) were made according to relevant guidelines (Chalasani et al., 2012, Tran et al., 2016).
2.3. Outcomes
The primary outcome was preterm birth. The definitions of PTB, very PTB and extremely PTB were spontaneous or medically indicated labor occurring at < 37, 32, and 28 weeks of gestation respectively, according to the World Health Organization (WHO) guidelines (WHO, 2015). The participants were followed-up until the end of pregnancy.
2.4. Statistical Analysis
Statistical analyses were conducted using Stata 14.0 (Stata Corp., TX, USA). Continuous or categorical data were compared using Student's t-test or Chi-square test respectively. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated using the ‘cs’ command in Stata to describe the association between each outcome category and potential risk factors. All tests were two-sided, and the significance level was set at 0.05.
According to hepatitis B virus infection status, participants were divided into two or three groups. HBsAg negative indicated no infection with HBV (control group); HBsAg positive women (exposure group) were further divided into HBeAg negative (exposure group 1) and HBeAg positive (exposure group 2) subgroups. Poisson regression models, as we described elsewhere (Qin et al., 2016), were used to estimate RRs of PTB for women with HBV infection or LFT abnormality. In model 1, we adjusted for age categories (18–24 years, 25–29 years, 30–34 years, 35–39 years, or 40–50 years). In model 2, we additionally adjusted for pre-pregnancy BMI categories (< 18.5 kg/m2, 18.5–23.9 kg/m2, 24.0–27.9 kg/m2, or ≥ 28.0 kg/m2), levels of education (high school or under, college or above), history of pregnancy (first gestation) and history of adverse pregnancy outcomes (spontaneous or induced abortion, preterm birth or stillbirth). In model 3, in addition to those factors included in model 2, we also adjusted for HBV infection status (HBsAg negative, HBsAg positive and HBeAg negative, HBsAg and HBeAg double positive), LFT abnormality categories.
2.5. Role of the Funding Source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Participant Characteristics and Pregnancy Outcomes
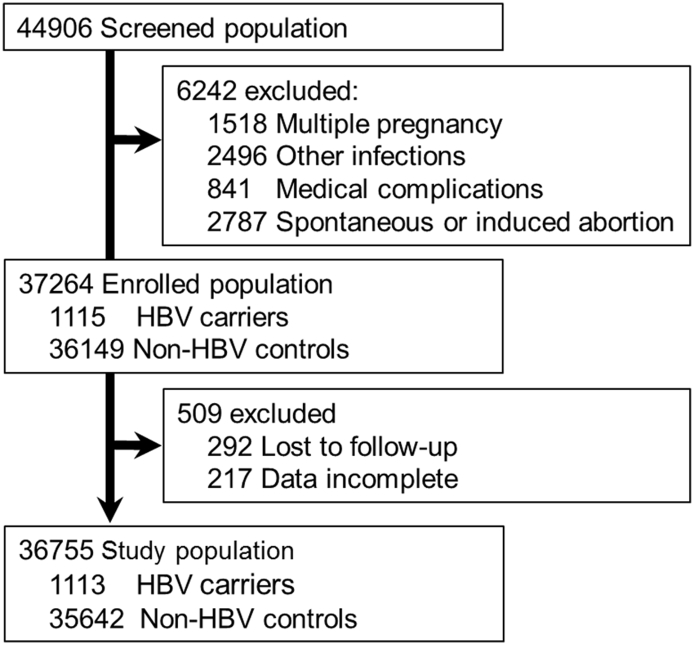
A total of 44,906 pregnant women were screened during their first trimester of pregnancy at Nantong Maternal and Child Health Hospital affiliated to Nantong University, China. Of these, 6,242 were excluded: due to multiple pregnancy in 1,518 subjects; due to other infection in 2,496 subjects; due to concurrent medical complications (DM, hypertension or heart diseases) in 841 subjects, due to spontaneous or induced abortion in 2,787 subjects. Thereafter, 37,264 pregnant women were recruited in this study, including 1,115 HBV carriers and 36,149 non-HBV subjects. Most of the enrolled subjects received at least three health examinations and were followed-up until delivery. Five hundred and nine subjects were excluded because of lost to follow-up (292 subjects) or incomplete data (217 subjects). Finally, 36,755 subjects, with 1,113 HBV carriers and 35,642 non-HBV subjects, were included (Fig. 1).
Fig. 1.
Flowchart of participant selection for the study cohort.
The demographic and clinical characteristics were listed in Table 1. The median age of all participants was 26 years (IQR 25–29). 6.38% of the subjects were aged 35 years or more, 62.04% had an education level of college or above, and 61.41% were in their first pregnancy. However, baseline characteristics for the HBV carrier and non-HBV groups were not comparable in terms of history of gestation, abortion.
Table 1.
Characteristics, pregnancy outcomes and complications of the study population.
| Characteristic | Non-HBV | HBV carrier | Pa | Total | PTB | Very PTB | Extremly PTB |
|---|---|---|---|---|---|---|---|
| n | 35,642 | 1,113 | 36,755 | 2,875 (7.82%) | 379 (1.03%) | 55 (0.15%) | |
| Ethnic origin | 0.872 | ||||||
| Han | 35,503 | 1,109 | 36,612 | 2,863 (7.82%) | 746 (2.04%) | 55 (0.15%) | |
| Others | 139 | 4 | 143 | 12 (8.39%) | 4 (2.80%) | 0 | |
| Maternal age (y) | 27.18 ± 4.06 | 28.28 ± 4.28 | < 0.001 | 27.22 ± 4.07 | |||
| 18–24 | 8,818 | 182 | < 0.001 | 9,000 | 730 (8.11%) | 78 (0.87%) | 6 (0.07%) |
| 25–29 | 18,710 | 579 | 19,289 | 1,320 (6.84%) | 181 (0.94%) | 35 (0.18%) | |
| 30–34 | 5,876 | 246 | 6,122 | 529 (8.64%) | 74 (1.21%) | 9 (0.15%) | |
| 35–39 | 1,931 | 94 | 2,025 | 232 (11.46%) | 32 (1.58%) | 3 (0.15%) | |
| 40–50 | 307 | 12 | 319 | 64 (20.06%) | 14 (4.39%) | 2 (0.63%) | |
| Pre-pregnancy BMI | 22.41 ± 5.74 | 22.15 ± 5.66 | 0.137 | 22.40 ± 5.74 | |||
| < 18.5 | 2,889 | 84 | 0.098 | 2,973 | 243 (8.17%) | 33 (1.11%) | 6 (0.20%) |
| 18.5–24.9 | 28,133 | 871 | 29,004 | 2,058 (7.10%) | 272 (0.94%) | 34 (0.12%) | |
| 25–29.9 | 4,446 | 147 | 4,593 | 547 (11.91%) | 69 (1.50%) | 14 (0.30%) | |
| ≥ 30 | 174 | 11 | 185 | 27 (14.59%) | 5 (2.70%) | 1 (0.54%) | |
| Education | |||||||
| High school/under | 13,532 | 420 | 0.876 | 13,952 | 1,122 (8.04%) | 143 (1.02%) | 22 (0.16%) |
| College/above | 22,110 | 693 | 22,803 | 1,753 (7.69%) | 236 (1.03%) | 33 (0.14%) | |
| History of pregnancy | |||||||
| First gestation | 21,975 | 596 | < 0.001 | 22,571 | 1,560 (6.91%) | 201 (0.89%) | 34 (0.15%) |
| Prior abortion | 2,710 | 114 | 0.001 | 2,824 | 345 (12.22%) | 53 (1.88%) | 10 (0.35%) |
| Prior preterm birth | 629 | 23 | 0.135 | 652 | 71 (10.89%) | 11 (1.69%) | 2 (0.31%) |
| Prior stillbirth | 22 | 1 | 0.712 | 23 | 3 (13.04%) | 0 | |
| Pregnancy outcomes | |||||||
| Preterm birth | 2,767 | 108 | 0.018 | 2875 | |||
| Very PTB | 362 | 17 | 0.096 | 379 | |||
| Extremely PTB | 53 | 2 | 0.792 | 55 | |||
| Full-term birth | 32,305 | 988 | 0.275 | 33,293 | |||
| Postterm birth | 537 | 15 | 0.668 | 552 | |||
| Stillbirth | 33 | 2 | 0.354 | 35 | |||
| GDM | 2,605 | 98 | 0.060 | 2703 | 225 (8.32%) | 28 (1.04%) | 2 (0.07%) |
Abbreviations: PTB, preterm birth; BMI, body mass index; LFT, liver function test; HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; TBiL, total bilirubin; GDM, gestational diabetes.
Compared with t-test, or χ2 test or Fish's test.
The main pregnancy outcomes were also listed in Table 1. The incidence of preterm birth was significantly higher in HBV carrier group than that in the non-HBV group (9.70% vs 7.16%, P = 0.018). Meanwhile, for incidence of very PTB and extremely PTB, there was no significant difference between the two groups.
3.2. Distribution of Preterm Birth Cases in Women With LFT Abnormality
Of these 36,755 women, 3,519 (9.57%) had at least one abnormal liver function test result. The commonest liver disease observed in pregnancy here was NAFLD (1,805/36,755, 4.91%). The onset of liver dysfunction occurred mostly during the first and third trimesters. The incidence of ICP, preeclampsia/eclampsia and HELLP syndrome were 1.37%, 3.14% and 0.02% respectively. Only one case of AFLP was diagnosed.
HBV carriers were more likely than non-HBV subjects to have abnormal LFT results (15.81% vs. 9.38%, P < 0.001). Women with normal and abnormal test results were grouped according to individual LFT for analysis. Preterm birth was more common in women with abnormal ALT, AST, GGT or total bilirubin (P < 0.05). For subgroups of women with normal, > 1 × ULN and ≤ 2 × ULN, > 2 × ULN and ≤ 5 × ULN, > 5 × ULN of ALT, the incidence of PTB were 7.66%, 8.15%, 12.07% and 26.92% [P (trend) < 0.05]. Women with > 2 × ULN of total bilirubin had higher incidence of PTB than those with lower levels. Similar trends were observed for the incidence of very PTB (Table 2).
Table 2.
Association of LFT abnormality with preterm birth in the study population.
| Characteristic | Non-HBV | HBV carrier | Pa | Total | PTB | Very PTB | Extremely PTB |
|---|---|---|---|---|---|---|---|
| n | 35,642 | 1113 | 36,755 | 2,875 (7.82%) | 379 (1.03%) | 55 (0.15%) | |
| LFT | |||||||
| Abnormalb | 3,343 | 176 | < 0.001 | 3,519 | 358 (10.17%) | 57 (1.62%) | 5 (0.14%) |
| 1st trimester | 1,225 | 56 | 1,281 | ||||
| 2nd trimester | 519 | 28 | 547 | ||||
| 3rd trimester | 1,589 | 92 | 0.412 | 1,691 | |||
| Etiology | |||||||
| NAFLD | 1,493 | 61 | 1,805 | 217 (12.02) | 22 (1.22%) | 3 (0.17%) | |
| Pre-eclampsia/eclampsia | 1,114 | 40 | 1,154 | 313 (27.12%) | 32 (2.77%) | 1 (0.09%) | |
| HELLP | 7 | 0 | 7 | 5 (71.43%) | 4 (57.14%) | 0 | |
| ICP | 466 | 39 | 505 | 117 (23.17%) | 11 (2.18%) | 1 (0.20%) | |
| AFLP | 1 | 0 | 1 | 0 | 0 | 0 | |
| Others | 262 | 36 | < 0.001 | 298 | |||
| ALT (U/L) | |||||||
| 0–40 | 33,406 | 987 | 34,393 | 2,634 (7.66%) | 345 (1.00%) | 52 (0.15%) | |
| 41–80 | 1,536 | 83 | 1,619 | 132 (8.15%) | 22 (1.36%) | 2 (0.12%) | |
| 81–200 | 581 | 32 | 613 | 74 (12.07%) | 11 (1.79%) | 1 (0.16%) | |
| 201– | 119 | 11 | < 0.001 | 130 | 35 (26.92%) | 1 (0.77%) | 0 |
| AST (U/L) | |||||||
| 0–40 | 34,335 | 1025 | 35,360 | 2677 (7.57%) | 353 (1.00%) | 53 (0.15%) | |
| 41–80 | 989 | 57 | 1,046 | 123 (11.76%) | 19 (1.82%) | 1 (0.10%) | |
| 81–200 | 260 | 24 | 284 | 55 (19.37%) | 6 (2.11%) | 1 (0.35%) | |
| 201– | 58 | 7 | < 0.001 | 65 | 20 (30.77%) | 1 (1.54%) | 0 |
| GGT (U/L) | |||||||
| 0–50 | 35,063 | 1,085 | 36,148 | 2,759 (7.63%) | 356 (0.98%) | 52 (0.14%) | |
| 51–100 | 466 | 23 | 489 | 91 (18.61%) | 20 (4.09%) | 3 (0.61%) | |
| 101– | 113 | 5 | 0.069 | 118 | 25 (21.19%) | 3 (2.54%) | 0 |
| TBiL (μmol/L) | |||||||
| 0–17.1 | 34,859 | 1,070 | 35,929 | 2,804 (7.80%) | 364 (1.01%) | 53 (0.15%) | |
| 17.2–34.2 | 730 | 40 | 770 | 53 (6.88%) | 12 (1.56%) | 2 (0.26%) | |
| 34.3– | 53 | 3 | 0.001 | 56 | 18 (32.14%) | 3 (5.36%) | 0 |
Abbreviations: PTB, preterm birth; BMI, body mass index; LFT, liver function test; HBV, hepatitis B virus; NAFLD, nonalcoholic fatty liver diseases; HELLP syndrome, hemolysis, elevated liver enzymes, and low platelets with or without preeclampsia; ICP, intrahepatic cholestasis of pregnancy; AFLP, acute fatty liver of pregnancy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; TBiL, total bilirubin.
Compared with t-test, or χ2 test or Fish's test.
At least one result of the four markers (ALT, AST, GGT, TBiL) was greater than its upper limit of normal.
3.3. Association of LFT Abnormality With HBV Infection and Preterm Birth
Table 3 shows unadjusted and multivariate-adjusted RRs and 95% CIs of PTB associated with HBV infection and LFT abnormality. Unadjusted Poisson analysis suggested that the HBV infection status (HBsAg and HBeAg double positive) and liver function test abnormalities (ALT > 80 U/L, AST > 40 U/L, GGT > 50 U/L and total bilirubin > 34.2 μmol/L) were significantly associated with risk of preterm birth. Abnormal AST and GGT results showed stable association with risk of preterm birth in all three models (model 1, model 2 and model 3). Besides, the trends were significant (P < 0.001). Two folds ULN of total bilirubin levels were also an independent risk factor for PTB. In contrast, adjusting for different covariates substantially influenced the estimates for HBV status and ALT abnormality. Women with HBV infection, abnormal ALT or total bilirubin results seemed to be at higher risk for preterm birth, according to age- and multivariate-adjusted analyses (model 1 and model 2). The fully adjusted model (model 3) showed that they were not independent risk factors for preterm birth.
Table 3.
Relative risks of preterm birth in subjects with HBV infection or LFT abnormality.
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| RR (95%CI) | P for trend | RR (95%CI) | P for trend | RR (95%CI) | P for trend | RR (95%CI) | P for trend | |
| HBV status | ||||||||
| HBsAg − | 1 | 1 | 1 | 1 | ||||
| HBsAg + HBeAg − | 1.20 (0.96–1.50) | 1.16 (0.93–1.45) | 1.13 (0.91–1.42) | 1.10 (0.88–1.38) | ||||
| HBsAg + HBeAg + | 1.43 (1.01–2.06) | 0.015 | 1.44 (1.00–2.08) | 0.021 | 1.44 (1.00–2.07) | 0.030 | 1.34 (0.93–1.94) | 0.091 |
| ALT (U/L) | ||||||||
| 0–40 | 1 | 1 | 1 | 1 | ||||
| 41–80 | 1.06 (0.89–1.27) | 1.06 (0.89–1.26) | 1.07 (0.90–1.28) | 0.76 (0.62–1.05) | ||||
| 81–200 | 1.58 (1.25–1.99) | 1.57 (1.24–1.57) | 1.59 (1.26–2.00) | 0.71 (0.50–1.01) | ||||
| 201– | 3.52 (2.52–4.91) | < 0.001 | 3.59 (2.57–5.02) | < 0.001 | 3.63 (2.60–5.06) | < 0.001 | 1.02 (0.55–1.88) | 0.054 |
| AST (U/L) | ||||||||
| 0–40 | 1 | 1 | 1 | 1 | ||||
| 41–80 | 1.55 (1.30–1.86) | 1.55 (1.29–1.86) | 1.56 (1.31–1.87) | 1.82 (1.41–2.35) | ||||
| 81–200 | 2.56 (1.96–3.34) | 2.54 (1.94–3.31) | 2.52 (1.93–3.29) | 2.42 (1.59–3.67) | ||||
| 201– | 4.06 (2.62–6.31) | < 0.001 | 4.07 (2.62–6.32) | < 0.001 | 4.04 (2.60–6.27) | < 0.001 | 2.73 (1.30–5.76) | < 0.001 |
| GGT (U/L) | ||||||||
| 0–50 | 1 | 1 | 1 | 1 | ||||
| 51–100 | 2.44 (1.98–3.00) | 2.46 (2.00–3.03) | 2.44 (1.98–3.01) | 2.00 (1.60–2.49) | ||||
| 101– | 2.78 (1.87–4.12) | < 0.001 | 2.71 (1.83–4.02) | < 0.001 | 2.69 (1.81–3.99) | < 0.001 | 2.24 (1.35–3.31) | < 0.001 |
| TBiL (μmol/L) | ||||||||
| 0–17.1 | 1 | 1 | 1 | 1 | ||||
| 17.2–34.2 | 0.88 (0.67–1.16) | 0.86 (0.65–1.13) | 0.85 (0.65–1.11) | 0.74 (0.56–1.02) | ||||
| 34.3– | 4.12 (2.59–6.55) | 0.022 | 3.91 (2.46–6.22) | 0.043 | 3.91 (2.46–6.21) | 0.055 | 2.01 (1.22–3.31) | 0.874 |
RR, risk ratio; CI, confidence interval; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; TBiL, total bilirubin.
Model 1: adjustment was made for age.
Model 2: adjustment was made for age, BMI, education, history of pregnancy (first gestation, spontaneous or induced abortion, preterm birth and stillbirth).
Model 3: adjustment was made for the variables used in Model 2 and for HBV status and the other liver function tests.
Unadjusted, model 1 and model 2 analyses suggested that ALT, AST, GGT and TBiL abnormalities were associated with very PTB. However, the fully adjusted model (model 3) only identified GGT abnormality as an independent risk factor for very PTB (Table S1).
4. Discussion
Pregnancy is sometimes complicated by specific or non-specific liver diseases (Joshi et al., 2010). It has been estimated that liver function test abnormalities occur in around 3%–5% of pregnancies (Pan and Perumalswami, 2011). Its incidence in China has received little attention. Our prospective study has shown that liver dysfunction in pregnancy is more common (9.57%). The commonest cause for abnormal liver function tests during pregnancy here is NAFLD (1,805/3,519, 51.3%). Actually, the number of patients with NAFLD is rising at an alarming rate in China and worldwide (Wang et al., 2014). It is biologically plausible that pregnancy may reveal previously subclinical diseases such as NAFLD, given the insulin resistance in pregnancy (Page and Girling, 2011). In terms of the incidence of the four pregnancy-related liver diseases (ICP, preeclampsia/eclampsia, HELLP syndrome and AFLP), our results were similar to study performed in UK (Ch'ng et al., 2002).
The liver plays critical roles in regulating inflammation by controlling local as well as systemic inflammatory responses via different molecular mechanisms (Marra and Tacke, 2014). Meanwhile, the pathophysiology of PTB remains unclear. It has been implicated that local and systemic inflammation may be independent etiological factors of PTB. According to previous studies, a significant proportion of preterm births were associated with overproduction of pro-inflammatory cytokines (Wei et al., 2010).
It is not always easy to distinguish the cause of abnormal LFT results, due to chronic HBV infection or other causes, because they share similar biochemical (ALT, AST, GGT, total bilirubin levels) characteristics. Although most studies show that HBV carrier status is an independent, but only weak risk factor for preterm birth, our study demonstrates that HBV carriers who tend to have more preterm birth are also more likely to have abnormal LFT. Our findings are not contradictory to previous findings (Liu et al., 2017), as HBV carrier status might have a synergistic effect in case of premature birth. To the best of our knowledge, this study for the first time identified the association of maternal LFT abnormality with preterm birth.
Admittedly, there are several limitations in the study. First, the study was undertaken solely in a tertiary maternal and child health hospital. The hospital serves nearly half of the city's population and guarantees good patient representative. Secondly, we have not examined hepatitis B viral activity (e.g. HBV DNA) which might have certain effects on pregnancy outcomes. Meanwhile, HBeAg in serum indicates active viral replication in hepatocytes and thus represents a surrogate marker for HBV DNA (Yang et al., 2002). Thirdly, LFTs are only one set of variables that may be associated with adverse pregnancy outcomes and their results may change over time. Future research that examines the association of changes in LFT results more meticulously will be necessary. Last, some diseases such as gall bladder disease, drug-induced liver injury (e.g., estrogen or progesterone) have not listed as separate causes of abnormal LFTs.
In conclusion, our cohort study indicates that pregnant women with LFT abnormalities may be a clinically important group given the increased risk of preterm birth. Surveillance of liver function tests among pregnant women, especially HBV carriers, is warranted. In the future, pathophysiological studies are warranted to clarify the causality and the possible mechanisms involved.
The following is the supplementary data related to this article.
Relative risks of very PTB in subjects with HBV infection or LFT abnormality.
Declaration of Interests
All authors declare no competing interests.
Funding
This work was partly supported by grants from Jiangsu provincial Department of Science and Technology, China (no. BE2015655), from Jiangsu Provincial Department of Health, China (no. LGY2017039), from National Natural Science Foundation of China (NSFC, no. 81370520, 81373060), and from Nantong Municipal Bureau of Science and Technology, Jiangsu Province, China (no. HS2016002).
Acknowledgments
Acknowledgments
We thank all participants in this study and the staff from the Department of Obstetrics, Nantong Maternal and Child Health Hospital affiliated to Nantong University for their work on patients enrollment and follow-up.
Author Contribution
G.Q. and X.Z. designed the study. A.-M.C., X.-Y.C. and G.Q. counseled the women, recruited the participants or did the follow-up. H.-B.L did the laboratory work. Q.W., Y.S. and S.Z. did the biostatistics analysis and modeling. G.Q. and W.-H.C. wrote the manuscript. All authors read and approved the final version of the manuscript.
References
- Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang S., Zhou Y.H., Xu B., Hu Y. Minimal adverse influence of maternal hepatitis B carrier status on perinatal outcomes and child's growth. J. Matern. Fetal Neonatal Med. 2015;28(18):2192–2196. doi: 10.3109/14767058.2014.981805. [DOI] [PubMed] [Google Scholar]
- Ch'ng C.L., Morgan M., Hainsworth I., Kingham J.G. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. 2002;51(6):876–880. doi: 10.1136/gut.51.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell L.E., Salihu H.M., Salemi J.L., August E.M., Weldeselasse H., Mbah A.K. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31(8):1163–1170. doi: 10.1111/j.1478-3231.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- Cui A.M., Cheng X.Y., Shao J.G. Maternal hepatitis B virus carrier status and pregnancy outcomes: a prospective cohort study. BMC Pregnancy Childbirth. 2016;16(1):87. doi: 10.1186/s12884-016-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui A.M., Shao J.G., Li H.B. Association of chronic hepatitis B virus infection with preterm birth: our experience and meta-analysis. J. Perinat. Med. 2016 doi: 10.1515/jpm-2016-0201. [DOI] [PubMed] [Google Scholar]
- Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.T., Wei S.S., Zhong M. Chronic hepatitis B infection and risk of preterm labor: a meta-analysis of observational studies. J. Clin. Virol. 2014;61(1):3–8. doi: 10.1016/j.jcv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Joshi D., James A., Quaglia A., Westbrook R.H., Heneghan M.A. Liver disease in pregnancy. Lancet. 2010;375(9714):594–605. doi: 10.1016/S0140-6736(09)61495-1. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang S., Liu M., Wang Q., Shen H., Zhang Y. Maternal pre-pregnancy infection with hepatitis B virus and the risk of preterm birth: a population-based cohort study. Lancet Glob. Health. 2017;5(6):e624–e632. doi: 10.1016/S2214-109X(17)30142-0. [DOI] [PubMed] [Google Scholar]
- Ma X., Sun D., Li C., Ying J., Yan Y. Chronic hepatitis B virus infection and preterm labor(birth) in pregnant women-an updated systematic review and meta-analysis. J. Med. Virol. 2017 doi: 10.1002/jmv.24927. [DOI] [PubMed] [Google Scholar]
- Marra F., Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147(3):577–594. doi: 10.1053/j.gastro.2014.06.043. (e571) [DOI] [PubMed] [Google Scholar]
- Page L.M., Girling J.C. A novel cause for abnormal liver function tests in pregnancy and the puerperium: non-alcoholic fatty liver disease. BJOG. 2011;118(12):1532–1535. doi: 10.1111/j.1471-0528.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- Pan C., Perumalswami P.V. Pregnancy-related liver diseases. Clin. Liver Dis. 2011;15(1):199–208. doi: 10.1016/j.cld.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Qin G., Shao J.G., Zhu Y.C. Population-representative incidence of acute-on-chronic liver failure: a prospective cross-sectional study. J. Clin. Gastroenterol. 2016;50(8):670–675. doi: 10.1097/MCG.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Salemi J.L., Whiteman V.E., August E.M., Chandler K., Mbah A.K., Salihu H.M. Maternal hepatitis B and hepatitis C infection and neonatal neurological outcomes. J. Viral Hepat. 2014;21(11):e144–153. doi: 10.1111/jvh.12250. [DOI] [PubMed] [Google Scholar]
- Sirilert S., Traisrisilp K., Sirivatanapa P., Tongsong T. Pregnancy outcomes among chronic carriers of hepatitis B virus. Int. J. Gynaecol. Obstet. 2014;126(2):106–110. doi: 10.1016/j.ijgo.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Than N.N., Neuberger J. Liver abnormalities in pregnancy. Best Pract. Res. Clin. Gastroenterol. 2013;27(4):565–575. doi: 10.1016/j.bpg.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Tran T.T., Ahn J., Reau N.S. ACG clinical guideline: liver disease and pregnancy. Am. J. Gastroenterol. 2016;111(2):176–194. doi: 10.1038/ajg.2015.430. (quiz 196) [DOI] [PubMed] [Google Scholar]
- Wang F.S., Fan J.G., Zhang Z., Gao B., Wang H.Y. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S.Q., Fraser W., Luo Z.C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 2010;116(2 Pt 1):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- WHO . 2015. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. (Geneva) [PubMed] [Google Scholar]
- Yang H.I., Lu S.N., Liaw Y.F. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N. Engl. J. Med. 2002;347(3):168–174. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li R.T., Wang Y., Liu Q., Zhou Y.H., Hu Y. Seroprevalence of hepatitis B surface antigen among pregnant women in Jiangsu, China, 17 years after introduction of hepatitis B vaccine. Int. J. Gynaecol. Obstet. 2010;109(3):194–197. doi: 10.1016/j.ijgo.2010.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative risks of very PTB in subjects with HBV infection or LFT abnormality.