Abstract
Background
In a minority of patients with neuromyelitis optica spectrum disorder (NMOSD) and aqua-porin-4 antibodies (AQP4-IgG), the disease has a paraneoplastic origin. It is unknown whether these patients have distinctive clinical features.
Objective
To report the clinical features of a series of patients with paraneoplastic NMOSD and AQP4-IgG and to review previously reported cases.
Methods
Retrospective analysis of clinical records of 156 patients with NMOSD and AQP4-IgG and review of previously reported patients with paraneoplastic NMOSD and AQP4-IgG. Paraneoplastic patients were defined as those with cancer identified within 2 years of the diagnosis of NMOSD.
Results
Five (3.2%) of 156 patients had paraneoplastic NMOSD, and 12 previously reported patients were identified. The most common tumors were adenocarcinoma of the lung (five patients) and breast (five). Compared with the 151 non-paraneoplastic NMOSD patients, the 17 (5 current cases and 12 previously reported) were older at symptom onset (median age = 55 (range: 17–87) vs 40 (range: 10–77) years; p = 0.006), more frequently male (29.4% vs 6.6%; p = 0.009), and presented with severe nausea and vomiting (41.2% vs 6.6%; p < 0.001). The frequency of longitudinal extensive transverse myelitis (LETM) as heralding symptom was similar in both groups, but patients with paraneoplastic NMOSD were older than those with non-paraneoplastic NMOSD (median age: 63 (range: 48–73) vs 43 (range: 14–74) years; p = 0.001).
Conclusion
Patients, predominantly male, with NMOSD and AQP4-IgG should be investigated for an underlying cancer if they present with nausea and vomiting, or LETM after 45 years of age.
Keywords: Neuromyelitis optica spectrum disorders, paraneoplastic, AQP4 antibodies, cancer
Introduction
Antibodies against neural antigens are classified in two categories: those that almost always indicate the presence of an underlying cancer (onconeural antibodies) and therefore are used as biomarkers of paraneoplastic neurological syndromes (PNS), and those that associate with specific neurological syndromes regardless of the presence of cancer.1,2 Antibodies in this second category frequently target surface neural antigens and they are considered directly involved in the pathogenesis of the disease. The trigger of these antibodies is in many instances unknown, but in some patients (the frequency varies with the antibody type), the trigger is a tumor that expresses the neural antigen, and therefore, the neurological syndrome can be considered paraneoplastic.
Antibodies against aquaporin-4 (AQP4) are present in most patients with the neuromyelitis optica spectrum disorder (NMOSD), including neuromyelitis optica (NMO) and limited forms of single or relapsing optic neuritis or longitudinal extensive transverse myelitis (LETM).3 The presence of an underlying cancer has been reported only in a few patients with NMOSD andAQP4 antibodies,4 and therefore, it is yet unknown whether distinctive clinical features associate with a paraneoplastic origin. This information would allow selecting patients at risk for paraneoplastic NMOSD and include tumor screening as part of the initial work-up, which is currently not done in NMOSD patients.
To address this issue, we retrospectively examined a cohort of patients with NMOSD and AQP4 antibodies and compared the clinical features of those without cancer with those with cancer who fulfilled criteria of possible PNS according to published guidelines.1 In addition, we performed a systematic review of previously reported cases of AQP4 antibody–associated paraneoplastic NMOSD with the aim to provide relevant clinical characteristics of patients with NMOSD and AQP4 antibodies in which a tumor screening is warranted.
Methods
Patients
We retrospectively identified patients with NMOSD whose serum samples were sent to our laboratory and were found positive for AQP4 antibodies by routine immunohistochemistry on brain tissue and cell-based assay (CBA). Samples were obtained from three sources: (1) patients with NMOSD and positive AQP4 antibodies recruited from 59 centers through the multiple sclerosis (MS) study group of the Spanish Society of Neurology, from January 2013 to January 2015, with the initial aim to identify predictors of conversion to NMO,5 (2) patients whose serum was sent between 2005 and 2016 for the determination of either AQP4 antibodies or onconeural antibodies and routine immunohistochemistry on brain tissue revealed AQP4-like reactivity that was subsequently confirmed by CBA, and (3) patients with NMOSD and AQP4 antibodies who during the time period 2006–2016 appeared in a regional registry of epidemiological data (the Catalan Health Surveillance System) with a diagnosis of NMOSD.
The clinical features of paraneoplastic NMOSD patients with AQP4 antibodies were compared with those without cancer. Epidemiological data, including demographic, clinical, cerebrospinal fluid (CSF) (cell count, protein levels, and oligoclonal bands), magnetic resonance imaging (MRI) findings (number and extension of spinal cord lesions), treatment, and outcome, were obtained from medical records and information collected from referring physicians through a structured questionnaire designed for NMOSD. In 2017, referring physicians were contacted to confirm the oncological status of NMOSD patients with AQP4 antibodies. Median follow-up of patients finally defined as non-paraneoplastic was 94 months (range: 24–599 months). None of the patients underwent an extended diagnostic work-up to rule-out an occult tumor. The diagnosis of a possible paraneoplastic etiology was done according to the PNS Euronetwork criteria (detection of cancer within the first 2 years of diagnosis of NMOSD).1
In addition, we identified previously reported NMOSD patients with AQP4 antibodies who fulfilled the PNS Euronetwork criteria for a possible paraneoplastic etiology. Patients were identified through a comprehensive PubMed search (until 1 May 2017) using the terms “AQP4 antibodies, neuromyelitis optica, longitudinal extensive transverse myelitis, optic neuritis, AND cancer” or “paraneoplastic neuromyelitis optica.” Only cases published in English that included clinical information were selected.
Patients’ serum and CSF samples are archived in the collection of biological samples named “Neuroinmunología” registered in the Biobank of Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). Written informed consent for the storage and use of the samples for research purposes was obtained from all patients. The study was approved by the ethics committee of the Hospital Clinic of Barcelona, Spain.
Autoantibody assays
All serum samples were tested for AQP4 antibodies by immunohistochemistry of paraformaldehyde (PFA) fixed frozen rat brain sections and an in-house CBA with live human embryonic kidney 293 (HEK293) cells transfected with aquaporin-4-M23 isoform as previously reported.6 Briefly, 36 hours after transfecting HEK293 cells with AQP4-M23 (a gift of Dr Marignier), live cells were incubated at room temperature with serum (diluted 1:20) or CSF (1:2) for 30 minutes. Cells were fixed with 1% PFA for 15 minutes and permeabelized with 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 5 minutes. Cells were then immunolabeled with a rabbit polyclonal AQP4 antibody (1:500; Sigma-Aldrich) for 1 hour at room temperature, followed by the corresponding Alexa Fluor secondary antibodies against human and rabbit IgGs (1:1000; Molecular Probes, Invitrogen, Eugene, OR, USA).
To demonstrate the expression of AQP4 in the tumor, paraffin sections were deparaffinized and the antigen retrieved boiling the tissue sections in citrate buffer pH 6.0 for 20 minutes. After inhibition of endogenous peroxidase with 0.3% hydrogen peroxide in phosphate buffered saline (PBS) for 15 minutes, sections were incubated with AQP4 (Sigma-Aldrich) polyclonal antibody (diluted 1:400) overnight at 4°C, and developed with the avidin–biotin–peroxidase technique (Vector Labs, Burlingame, CA, USA).
Statistical analysis
We compared differences in categorical variables with Pearson’s chi-squared test or Fisher’s exact test when appropriated and continuous variables by Mann–Whitney U test. All p values were two-tailed and they were considered significant at p ≤ 0.050.
Results
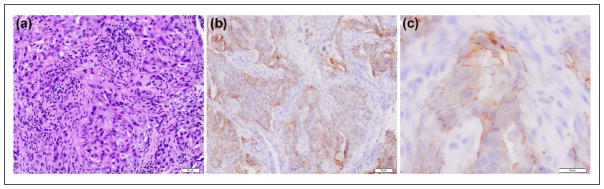
We identified 156 patients with NMOSD and AQP4 antibodies and only five (3.2%) of them fulfilled the criteria of possible PNS (Table 1). None of them had concurrent onconeural antibodies or other antibodies against neuronal surface antigens. Three paraneoplastic patients developed isolated LETM and two presented with a central nervous system (CNS) syndrome (severe nausea and vomiting, encephalopathy) followed in a few weeks by LETM. Three patients had adenocarcinoma of the lung, one breast cancer, and one squamous carcinoma of the oral cavity. Only one tumor (lung adenocarcinoma) could be tested for expression of AQP4 and it was found positive (Figure 1). Neurological symptoms developed after the diagnosis of cancer in two patients (median, 5 months; range: 1–10 months) and preceded the cancer diagnosis in the other two (median, 10 months; range: 1–19 months). In one patient, the diagnosis of cancer was made by the same time of neurological symptom onset. In the three patients without known cancer, the cause that led to the identification of the tumor was the presentation with encephalopathy in one, the study of severe vomiting in another, and the identification of an oral cavity lesion in the third.
Table 1.
Clinical findings of patients with paraneoplastic NMOSD and AQP4 antibodies.
| Patient | Age/sex | Tumor type (interval (months) NMOSD-tumor) | Presenting symptoms | Predominant neurologic syndrome | CSF | Spinal MRI: length of T2 lesion | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 49/M | NSCLC (+1) | Seizures rapidly followed by LETM | LETM | 157 WBC/μL, protein: 103 mg/dL OCB: negative |
medulla to L1 | Steroids, IvIG | Improved. Death due to tumor |
| 2 | 55/F | NSCLC (0) | Nausea and vomiting followed by LETM | LETM | 42 WBC/μL, protein: 127 mg/dL OCB: negative |
medulla to L1 | Steroids, chemotherapy | Worse, relapse at 3 months |
| 3 | 61/M | NSCLC (−1) | Myelopathy | LETM | 0 WBC/μL, protein: 140 mg/dL OCB: not done |
C3 to T8 | Steroids, IvIG, radiotherapy | Stable. Death due to tumor |
| 4 | 72/M | Squamous ca. oral cavity (+19) | Myelopathy | LETM | 9 WBC/μL, protein: 47 mg/dL OCB: negative |
C6 to T2 | Steroids, AZA, chemotherapy, radiotherapy | Worse, 3 relapses in 24 months. Death due to tumor |
| 5 | 73/F | Breast (−10) | Myelopathy | LETM | Unknown | C6 to T10 | Steroids, chemotherapy, radiotherapy | Worse, relapse at 8 months. Death due to tumor |
AQP4: aquaporin-4; AZA: azathioprine; ca: carcinoma; LETM: longitudinal extensive transverse myelitis; NMOSD: neuromyelitis optica spectrum disorder; NSCLC: non–small cell lung cancer; OCB: oligoclonal bands; WBC: white blood cells; IvIG: intravenous immunoglobulins; MRI: magnetic resonance imaging.
Figure 1.
(a) Paraffin section of a lung adenocarcinoma from a patient with LETM and AQP4 antibodies (hematoxylin eosin) and (b and c) AQP4 reactivity detected with a commercial polyclonal rabbit antibody against AQP4.
Our literature search identified 35 NMOSD patients with AQP4 antibodies and history of an underlying tumor.4,7–21 Twenty-three patients were excluded from analysis. In seven, the time period between the development of PNS and tumor diagnosis was unknown or longer than 2 years. Nine patients had minimal information available. Another five patients had no malignant neoplasms (e.g., monoclonal gammopathy, benign tumors), and in one, the diagnosis of lymphoma was unclear.4,7–9 Two additional cases were excluded: one because the patient had meningeal carcinomatosis and brain metastases, and the other because of the presence of concurrent onconeural antibodies.10,11
The clinical information of the remaining 12 patients with acceptable information and who fulfilled criteria of possible PNS is summarized in Supplementary Table S1.12–21 The most frequent presenting syndrome was severe nausea and vomiting in six patients, followed by LETM in four, and optic neuritis in two. The median age of the entire cohort was 46 years (range: 17–87 years), with female predominance (83%). Patients who presented with severe nausea and vomiting were younger than those presenting with optic neuritis or LETM (median age: 38.5 vs 60 years; p = 0.038). Four patients had breast cancer, two adenocarcinoma of the lung, two hematological neoplasms (acute myeloid leukemia and mature B-cell lymphoma), and one of each, papillary thyroid carcinoma, carcinoid of stomach, carcinoid of small-bowel, and prostate cancer. Five tumors were tested for APQ4 expression and four were found positive (two lung adenocarcinoma, one breast cancer, and one small-bowel carcinoid) (Supplementary Table S1).
Finally, we compared the clinical characteristics of the 151 patients with non-paraneoplastic NMOSD and AQP4 antibodies with those of the 17 paraneoplastic patients (5 from the current study and 12 previously reported). Results are summarized in Table 2. Paraneoplastic patients were older at onset of symptoms (median age: 55 (range:17–87) vs 40 (range: 10–77) years; p = 0.006), were more frequently male (29.4% vs 6.6%; p = 0.009), and 41.2% had initial onset of disease with brainstem symptoms, usually severe nausea and vomiting, a form of presentation that occurred only in 6.6% of non-paraneoplastic patients (p < 0.001). In contrast, optic neuritis or NMO was rarely the heralding syndrome in paraneoplastic patients (11.8% vs 42.4%; p = 0.017). The frequency of clinical presentation as LETM was similar in both groups. However, patients with paraneoplastic LETM were older (median age: 63 (range: 48–73) vs 43 (range: 14–74) years; p = 0.001). Response to immunotherapy was similar in both groups. Ten (59%) of the 17 paraneoplastic patients improved after treatment with immunotherapy, usually steroids (Table 1 and Supplementary Table S1). The number of deaths was higher in the paraneoplastic group due to cancer-related causes (Table 2).
Table 2.
Clinical and MRI features of patients with paraneoplastic and non-paraneoplastic NMOSD with AQP4 antibodies.
| Paraneoplastic (n = 17) | Non-paraneoplastic (n = 151) | p value | |
|---|---|---|---|
| Age in years, median (range) | 55 (17–87) | 40 (10–77) | 0.006 |
| Male/female | 5/12 | 10/141 | 0.009 |
| Autoimmune diseases | 1 | 39 | 0.172 |
| Presenting syndrome | |||
| Optic neuritis | 2 | 64 | |
| LETM | 7 | 60 | 0.001 |
| NMO | 0 | 16 | |
| Brainstem | 7a | 10 | |
| Other symptoms | 1b | 1c | |
| Spine MRI | |||
| No. of vertebral segments; median (range)d | 7 (3–20) | 5 (1–23) | 0.068 |
| CSF findings | |||
| WBC; abnormal (>10 cell/mm3) | 5/15 | 30/127 | 0.769 |
| Proteins; abnormal (>45 mg/dL) | 9/15 | 37/127 | 0.404 |
| Oligoclonal bands | 0/12 | 23/126 | 0.233 |
| Clinical outcome | |||
| Improved | 10 | 36 | 0.204 |
| Stable | 2 | 44 | |
| Worse | 5 | 71 | |
| Death | 5 | 8 | 0.005 |
LETM: longitudinal extensive transverse myelitis; NMO: neuromyelitis optica; WBC: white blood cell; MRI: magnetic resonance imaging; NMOSD: neuromyelitis optica spectrum disorder; AQP4: aquaporin-4.
Four patients rapidly presented an additional LETM.
Encephalitis rapidly followed by LETM.
Myelitis without LETM criteria.
Data collected from 12 paraneoplastic and 87 non-paraneoplastic NMOSD patients who developed myelitis.
Discussion
Our experience with paraneoplastic NMOSD and AQP4 antibodies along with that of previously reported patients provides several clinical clues that suggest when a patient with AQP4-antibody-positive NMOSD should be suspected to have an underlying cancer. The findings are clinically relevant because the frequency of paraneoplastic NMOSD is low, and therefore, the indiscriminate search for an underlying cancer is not indicated. We identified two clinical settings where the risk for cancer is higher: (1) patients who present with brainstem involvement, mainly nausea and vomiting, and (2) patients older than 45 years, usually male, presenting with LETM.
AQP4 is highly expressed in the area postrema, a brain region with a leaky blood-brain barrier, that may be a selective target of the autoimmune attack.22 In fact, severe vomiting and nausea as heralding symptoms of NMOSD was identified in 10%–12% of large series of patients with NMOSD and AQP4 antibodies, and none of the patients were reported to have cancer.23–25 In our series with a similar frequency (7%) of patients presenting with nausea and vomiting, only one of them (1/11; 9%) was paraneoplastic. However, our review of published case reports of paraneoplastic NMOSD shows that 6/12 (50%) patients (Supplementary Table S1) had this type of disease onset indicating that among this relatively small subgroup of patients, nausea and vomiting are unexpectedly high, and their presentation at disease onset suggest that cancer search is warranted.
LETM is the presenting clinical syndrome in up to 47% of the patients with NMOSD and AQP4 antibodies,26 and it occurs mostly in female (83%–89%) patients with a median age below 50 years.27 In contrast, paraneoplastic patients with LETM are more frequently older males, suggesting that an underlying cancer should be considered in this clinical setting. This is important because in patients who present with LETM, the detection of AQP4 antibodies may lead to believe that the cause is idiopathic and the search for an underlying tumor is not even considered.
Other forms of paraneoplastic-isolated myelopathies are rare, can associate with onconeural antibodies, or be seronegative, and the clinical or MRI features may overlap with those of AQP4-antibody-positive LETM.28 In the largest series described of 31 patients with AQP4-antibody-negative paraneoplastic myelopathies, the median age was 62 years (range: 37–79 years) and 65% were female. Clinical onset was subacute in 52% of the patients, spinal cord MRI revealed extensive involvement (>3 vertebral segments) in 70%, and onconeural antibodies, mainly CRMP5 and amphiphysin, were detected in 81% of the patients.29 Given the important overlap of clinical and MRI features between patients with paraneoplastic LETM, with and without AQP4 antibodies, screening for onconeural antibodies is indicated in elderly patients with LETM and negative AQP4 antibodies because of the higher risk for cancer in this age group. Response to immunotherapy was poor; only 31% of patients improved and 52% were wheelchair-bound at the last visit. In contrast, 59% of patients with paraneoplastic AQP4-antibody-positive NMOSD improved, which is in line with the favorable response to immunotherapy in neurological diseases associated with antibodies against neural surface antigens. However, we have to take these results with caution because in many of the reported paraneoplastic NMOSD patients, the follow-up was short and it is unclear whether the initially observed response to immunotherapy persisted over time. In any case, this observation emphasizes the importance of looking for AQP4 antibodies to correctly classify the paraneoplastic myelopathies according to the associated immune response.
Unlike some PNS that preferentially associate with a specific cancer type, paraneoplastic AQP4-antibody-positive NMOSD occurs with a wider variety of cancers, the most common being lung and breast adenocarcinomas.10–21 Lung and breast cancers are also the most common tumors found in paraneoplastic myelopathies not associated with AQP4 antibodies with the difference that in this setting, the most common lung cancer is the small-cell type.29 If this difference is related to the variable expression of AQP4 among tumor types is presently unclear. AQP4 is highly expressed in lung adenocarcinomas but it is low in breast cancer and there are no studies on small cell lung cancer.30 As in other PNS, antigen expression by the underlying tumor is expected to contribute in triggering the immune response but the genetic background of the patient may play also a crucial role. For example, antibodies against AQP4 were not detected in serum of patients without neurological symptoms but with lung adenocarcinomas, known to express AQP4.31
Overall, the current study on paraneoplastic NMOSD with AQP4 antibodies suggests that this association is rare but there are specific clinical settings, including presentation with nausea and vomiting or older, predominantly male, patients with LETM, where search for cancer is warranted. Moreover, in NMOSD patients with known cancer, the detection of AQP4 antibodies may indicate an initial better response to treatment.
Supplementary Material
Acknowledgments
The authors thank all physicians who have contributed by providing clinical information of their patients, and all patients for their generous contribution to research. They also thank Dr Ellen Gelpi for her advice in the evaluation in the AQP4 expression of tumors. M.S. and N.S.-V have contributed equally for this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant RO1NS077851, RO1MH094741 (J.D.), grants 11/01780, (J.D.) from the Fondo Investigaciones Sanitarias, grant SLT002/16/00354, Generalitat de Catalunya (M.S.), and Fundació La Marató TV3 (2014183 F.G., A.S.) Spain.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Sepulveda received speaker honoraria from Genzyme and Novartis. Dr Dalmau receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and from Euroimmun for the use of NMDA, GABAB receptor, GABAA receptor, DPPX, and IgLON5 as autoantibody tests; he has received an unrestricted research grant from Euroimmun. Dr Graus received a licensing fee from Euroimmun for the use of IgLON5 as an autoantibody test. Dr Sola-Valls, Dr Escudero, Dr Rojc, Dr Barón, Dr Hernández-Echebarría, Dr Gómez, and Dr Saiz report no disclosures.
Contributor Information
Maria Sepúlveda, Service of Neurology, Hospital Clinic, University of Barcelona, Barcelona, Spain Neuroimmunology Program, Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
Nuria Sola-Valls, Service of Neurology, Hospital Clinic, University of Barcelona, Barcelona, Spain Neuroimmunology Program, Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
Domingo Escudero, Service of Neurology, Hospital Clinic, University of Barcelona, Barcelona, Spain Neuroimmunology Program, Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
Bojan Rojc, Service of Neurology, General Hospital Izola, Izola, Slovenia.
Manuel Barón, Service of Neurology, Hospital Universitario Fundación Alcorcón, Madrid, Spain.
Luis Hernández-Echebarría, Service of Neurology, Hospital de León, León, Spain.
Begoña Gómez, Service of Neurology, Hospital Universitario Puerto Real, Cádiz, Spain.
Josep Dalmau, Neuroimmunology Program, Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain/Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain/Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA.
Albert Saiz, Service of Neurology, Hospital Clinic, University of Barcelona, Barcelona, Spain Neuroimmunology Program, Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
Francesc Graus, Service of Neurology, Hospital Clinic, University of Barcelona, Barcelona, Spain Neuroimmunology Program, Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
References
- 1.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97:839–887. doi: 10.1152/physrev.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittock SJ, Lennon VA. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch Neurol. 2008;65:629–632. doi: 10.1001/archneur.65.5.629. [DOI] [PubMed] [Google Scholar]
- 5.Sepúlveda M, Armangué T, Sola-Valls N, et al. Neuromyelitis optica spectrum disorders: Comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm. 2016;3:e225. doi: 10.1212/NXI.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höftberger R, Sabater L, Marignier R, et al. An optimized immunohistochemistry technique improves NMO-IgG detection: study comparison with cell-based assays. PLoS ONE. 2013;8:e79083. doi: 10.1371/journal.pone.0079083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ontaneda D, Fox RJ. Is neuromyelitis optica with advanced age of onset a paraneoplastic disorder? Int J Neurosci. 2014;124:509–511. doi: 10.3109/00207454.2013.854208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasquet M, Bataller L, Torres-Vega E, et al. Longitudinally extensive transverse myelitis with AQP4 antibodies revealing ovarian teratoma. J Neuroimmunol. 2013;263:145–147. doi: 10.1016/j.jneuroim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Miocinovic S, Greenberg BM. Neuromyelitis optica spectrum disorder associated with autoimmune hemolytic anemia and lymphoma. Neurologist. 2015;20:33–34. doi: 10.1097/NRL.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 10.De Santis G, Caniatti L, De Vito A, et al. A possible paraneoplastic neuromyelitis optica associated with lung cancer. Neurol Sci. 2009;30:397–400. doi: 10.1007/s10072-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 11.Soelberg K, Larsen SR, Moerch MT, et al. Aquaporin-4 IgG autoimmune syndrome and immunoreactivity associated with thyroid cancer. Neurol Neuroimmunol Neuroinflamm. 2016;3:e25. doi: 10.1212/NXI.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai G, He D, Chu L, et al. Paraneoplastic neuromyelitis optica spectrum disorders: three new cases and a review of the literature. Int J Neurosci. 2016;126:660–668. doi: 10.3109/00207454.2015.1054481. [DOI] [PubMed] [Google Scholar]
- 13.Moussawi K, Lin DJ, Matiello M, et al. Brainstem and limbic encephalitis with paraneoplastic neuromyelitis optica. J Clin Neurosci. 2016;23:159–161. doi: 10.1016/j.jocn.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Al-Harbi T, Al-Sarawi A, Binfalah M, et al. Paraneoplastic neuromyelitis optica spectrum disorder associated with stomach carcinoid tumor. Hematol Oncol Stem Cell Ther. 2014;7:116–119. doi: 10.1016/j.hemonc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Verschuur CV, Kooi AJ, Troost D. Anti-aquaporin 4 related paraneoplastic neuromyelitis optica in the presence of adenocarcinoma of the lung. Clin Neuropathol. 2015;34:232–236. doi: 10.5414/np300855. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa M, Guo Y, Tselis A, et al. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastatic carcinoid expressing aquaporin-4. JAMA Neurol. 2014;71:495–498. doi: 10.1001/jamaneurol.2013.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama-Ichiyama S, Yokote T, Hiraoka N, et al. A paraneoplastic neuromyelitis optica spectrum disorder associated with a mature B-cell neoplasm. Leuk Res. 2011;35:e111–113. doi: 10.1016/j.leukres.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Armağan H, Tüzün E, Içöz S, et al. Long extensive transverse myelitis associated with aquaporin-4 antibody and breast cancer: favorable response to cancer treatment. J Spinal Cord Med. 2012;35:267–269. doi: 10.1179/2045772312Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller S, Dubal DB, Josephson SA. A case of paraneoplastic myelopathy associated with the neuromyelitis optica antibody. Nat Clin Pract Neurol. 2008;4:284–288. doi: 10.1038/ncpneuro0765. [DOI] [PubMed] [Google Scholar]
- 20.Iorio R, Damato V, Mirabella M, et al. Distinctive clinical and neuroimaging characteristics of longitudinally extensive transverse myelitis associated with aquaporin-4 autoantibodies. J Neurol. 2013;260:2396–2402. doi: 10.1007/s00415-013-6997-9. [DOI] [PubMed] [Google Scholar]
- 21.Kitazawa Y, Warabi Y, Bandoh M, et al. Elderly-onset neuromyelitis optica which developed after the diagnosis of prostate adenocarcinoma and relapsed after a 23-valent pneumococcal polysaccharide vaccination. Intern Med. 2012;51:103–107. doi: 10.2169/internalmedicine.51.5636. [DOI] [PubMed] [Google Scholar]
- 22.Popescu BF, Lennon VA, Parisi JE, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–1237. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apiwattanakul M, Popescu BF, Matiello M, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol. 2010;68:757–761. doi: 10.1002/ana.22121. [DOI] [PubMed] [Google Scholar]
- 24.Long Y, Liang J, Wu L, et al. Different phenotypes at onset in neuromyelitis optica spectrum disorder patients with aquaporin-4 autoimmunity. Front Neurol. 2017;8:62. doi: 10.3389/fneur.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin X, Pei S, Liu Y, et al. Clinical analysis of neuromyelitis optica presenting as intractable nausea, vomiting and hiccups. Int J Neurosci. 2016;16:1–5. doi: 10.1080/00207454.2016.1269090. [DOI] [PubMed] [Google Scholar]
- 26.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao Y, Fryer JP, Lennon VA, et al. Aquaporin-4 IgG serostatus and outcome in recurrent longitudinally extensive transverse myelitis. JAMA Neurol. 2014;71:48–54. doi: 10.1001/jamaneurol.2013.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urai Y, Matsumoto K, Shimamura M, et al. Paraneoplastic necrotizing myelopathy in a patient with advanced esophageal cancer: an autopsied case report. J Neurol Sci. 2009;280:113–117. doi: 10.1016/j.jns.2009.02.324. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan EP, McKeon A, Lennon VA, et al. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology. 2011;76:2089–2095. doi: 10.1212/WNL.0b013e31821f468f. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos MC, Saadoun S. Key roles of aquaporins in tumor biology. Biochim Biophys Acta. 2015;1848:2576–2583. doi: 10.1016/j.bbamem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Jarius S, Warth A, Wandinger KP, et al. Antibodies to aquaporin-4 in non-small cell lung cancer: a study on 50 patients. Neurol Sci. 2010;31:871–872. doi: 10.1007/s10072-010-0290-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.