Abstract
Background
Nexvax2® is a novel, peptide-based, epitope-specific immunotherapy intended to be administered by regular injections at dose levels that increase the threshold for clinical reactivity to natural exposure to gluten and ultimately restore tolerance to gluten in patients with celiac disease. Celiac disease patients administered fixed intradermal doses of Nexvax2 become unresponsive to the HLA-DQ2·5-restricted gluten epitopes in Nexvax2, but gastrointestinal symptoms and cytokine release mimicking gluten exposure, that accompany the first dose, limit the maximum tolerated dose to 150 μg. Our aim was to test whether stepwise dose escalation attenuated the first dose effect of Nexvax2 in celiac disease patients.
Methods
We conducted a randomized, double-blind, placebo-controlled trial at four community sites in Australia (3) and New Zealand (1) in HLA-DQ2·5 genotype positive adults with celiac disease who were on a gluten-free diet. Participants were assigned to cohort 1 if they were HLA-DQ2·5 homozygotes; other participants were assigned to cohort 2, or to cohort 3 subsequent to completion of cohort 2. Manual central randomization without blocking was used to assign treatment for each cohort. Initially, Nexvax2-treated participants in cohorts 1 and 2 received an intradermal dose of 30 μg (consisting of 10 μg of each constituent peptide), followed by 60 μg, 90 μg, 150 μg, and then eight doses of 300 μg over six weeks, but this was amended to include doses of 3 μg and 9 μg and extended over a total of seven weeks. Nexvax2-treated participants in cohort 3 received doses of 3 μg, 9 μg, 30 μg, 60 μg, 90 μg, 150 μg, 300 μg, 450 μg, 600 μg, 750 μg, and then eight of 900 μg over nine weeks. The dose interval was 3 or 4 days. Participants, care providers, data managers, sponsor personnel, and study site personnel were blinded to treatment assignment. The primary outcome was the number of adverse events and percentage of participants with adverse events during the treatment period. This completed trial is registered with ClinicalTrials.gov, number NCT02528799.
Findings
From the 73 participants who we screened from 19 August 2015 to 31 October 2016, 24 did not meet eligibility criteria, and 36 were ultimately randomized and received study drug. For cohort 1, seven participants received Nexvax2 (two with the starting dose of 30 μg and then five at 3 μg) and three received placebo. For cohort 2, 10 participants received Nexvax2 (four with starting dose of 30 μg and then six at 3 μg) and four received placebo. For cohort 3, 10 participants received Nexvax2 and two received placebo. All 36 participants were included in safety and immune analyses, and 33 participants completed treatment and follow-up; in cohort 3, 11 participants were assessed and included in pharmacokinetics and duodenal histology analyses. Whereas the maximum dose of Nexvax2 had previously been limited by adverse events and cytokine release, no such effect was observed when dosing escalated from 3 μg up to 300 μg in HLA-DQ2·5 homozygotes or to 900 μg in HLA-DQ2.5 non-homozygotes. Adverse events with Nexvax2 treatment were less common in cohorts 1 and 2 with the starting dose of 3 μg (72 for 11 participants) than with the starting dose of 30 μg (91 for six participants). Adverse events during the treatment period in placebo-treated participants (46 for nine participants) were similar to those in Nexvax2-treated participants when the starting dose was 3 μg in cohort 1 (16 for five participants), cohort 2 (56 for six participants), and cohort 3 (44 for 10 participants). Two participants in cohort 2 and one in cohort 3 who received Nexvax2 starting at 3 μg did not report any adverse event, while the other 33 participants experienced at least one adverse event. One participant, who was in cohort 1, withdrew from the study due to adverse events, which included abdominal pain graded moderate or severe and associated with nausea after receiving the starting dose of 30 μg and one 60 μg dose. The most common treatment-emergent adverse events in the Nexvax2 participants were headache (52%), diarrhoea (48%), nausea (37%), abdominal pain (26%), and abdominal discomfort (19%). Administration of Nexvax2 at dose levels from 150 μg to 900 μg preceded by dose escalation was not associated with elevations in plasma cytokines at 4 h. Nexvax2 treatment was associated with trends towards improved duodenal histology. Plasma concentrations of Nexvax2 peptides were dose-dependent.
Interpretation
We show that antigenic peptides recognized by CD4-positive T cells in an autoimmune disease can be safely administered to patients at high maintenance dose levels without immune activation if preceded by gradual dose escalation. These findings facilitate efficacy studies that test high-dose epitope-specific immunotherapy in celiac disease.
Keywords: Celiac disease, Nexvax2, Immunotherapy, T cell, Peptide, Randomized controlled trial
Highlights
-
•
Maximum tolerated doses of peptide-based epitope-specific immunotherapy for celiac disease are increased by dose escalation.
-
•
Starting dose rather than maximum dose during up-dosing determine tolerability of Nexvax2 in celiac disease patients.
-
•
After up-dosing, systemic exposure to antigenic peptides in Nexvax2 is not associated with cytokine release or symptoms.
Many autoimmune diseases including celiac disease are linked to activation of CD4 + T cells by peptides from disease-specific antigens. The antigenic peptides in celiac disease are well defined, which has enabled the design of a peptide-based therapeutic vaccine intended to induce immune unresponsiveness to dietary gluten. For this targeted immunotherapy, high doses are expected to be more effective, but phase 1 studies revealed the first dose of Nexvax2 caused immune activation. We found escalating from low doses prevents symptoms and cytokine release with later “high” doses even at levels that result in measurable plasma peptide concentrations.
1. Introduction
“Immune tolerance” has been defined as “a state of indifference or non-reactivity towards a substance that would normally be expected to excite an immunological response” (Medawar, 1961). In patients with celiac disease, immunological tolerance to dietary gluten is replaced by a T cell-mediated hypersensitivity reaction that results in small intestinal injury and digestive symptoms (Sollid and Jabri, 2013). Quarantining the immune system with a life-long, strict, gluten-free diet is currently the mainstay of management for celiac disease (Ludvigsson et al., 2014). Gluten-free diet for six months or more usually results in normalisation of serum antibodies specific for gluten-derived peptides and autoantibodies specific for transglutaminase, but signs of ongoing intestinal injury persist in many patients (Ludvigsson et al., 2014). Recurrent digestive symptoms on gluten-free diet are common, and the risk of acute symptoms that can follow within hours of accidental gluten exposure is ever present (See et al., 2015). The shortcomings of a gluten-free diet highlight a substantial unmet need that is being addressed by clinical development of agents that may enhance the effectiveness of dietary therapy (Kurada et al., 2016). However, overcoming the gluten-specific adaptive immune response and ultimately restoring immune tolerance without global immunosuppression is the long-term goal of pharmacotherapy for autoimmune diseases, including celiac disease (Sabatos-Peyton et al., 2010).
Antigen-specific CD4-positive T cells are implicated in many human autoimmune diseases that have strong associations with genes in the class II region of the major histocompatibility complex. Celiac disease stands out as a candidate for development of peptide-based immunotherapy because 90% of patients carry genes encoding human leukocyte antigen (HLA)-DQ2·5 (HLA-DQA1*05 and HLA-DQB1*02) and there is clear understanding of the antigenic peptides (epitopes) recognized by disease-associated (gluten-specific) CD4-positive T cells (Karell et al., 2003, Anderson and Jabri, 2013, Tye-Din et al., 2010). Following systemic peptide administrations, CD4-positive T cells repeatedly exposed to the epitope recognized by their T cell antigen receptor become unresponsive to further antigenic stimulation and may be deleted, rendered anergic or adopt regulatory properties (Pape et al., 1998, McPherson et al., 2014). Therefore, short, soluble peptides containing epitopes for CD4-positive T cells that cause autoimmune disease could have the potential to control or abort destructive autoreactivity (Larche and Wraith, 2005).
Early-stage clinical trials of adjuvant-free peptide immunotherapies for multiple sclerosis and type-1 diabetes have shown promising clinical results, but dose selection in clinical trials has been empirical (Streeter et al., 2015, Alhadj et al., 2017). Nexvax2® is a peptide-based, epitope-specific immunotherapy under development for celiac disease (Goel et al., 2017). Nexvax2 is customized for HLA-DQ2·5-positive patients and is a simple mixture of three synthetic peptides dissolved in normal saline that is administered by intradermal injection. The peptides in Nexvax2 (NPL001, NPL002, and NPL003) each contains 15 or 16 amino acids and encompass at least five HLA-DQ2.5-restricted epitopes frequently recognized by gluten-reactive CD4 positive T cells (Goel et al., 2017, Tye-Din et al., 2010). Phase 1 clinical trials of Nexvax2 in celiac disease have provided insight into the safety and therapeutic potential of peptide immunotherapy as well as the immunological effects of small antigenic peptides that are recognized by a discrete population of gut-homing or gut-located, memory CD4 positive T cells (Ráki et al., 2007). As well as having the potential to modify gluten-specific immune responses, injections of Nexvax2 are effectively systemic “gluten epitope challenges” that test the responsiveness of gluten-reactive CD4 positive T cells specific for epitopes in Nexvax2.
Previous phase 1b studies have assessed the safety, tolerability, and bioactivity of Nexvax2 in HLA-DQ2·5 positive participants with celiac disease following a gluten-free diet (Brown et al., 2011, Goel et al., 2017); Nexvax2 has been assessed in fixed, repeat doses from 9 μg to 300 μg for up to eight weeks with dose intervals as short as 3 to 4 days. A course of intradermal injections with Nexvax2, particularly at the highest tolerated dose of 150 μg, results in unresponsiveness to the gluten epitopes in Nexvax2, but the first administration at dose levels above 30 μg has sometimes been associated with clinical symptoms similar to those experienced by patients with celiac disease on a gluten-free diet when they consume gluten (Brown et al., 2011, Goel et al., 2017). Participants in these phase 1 studies who were HLA-DQ2·5 homozygotes and had completed a 3-day gluten challenge a month before dosing were particularly susceptible to acute gastrointestinal symptoms, which occurred 2 to 5 h after the first administration of Nexvax2. The first dose of Nexvax2 also caused immune activation as early as 2 h, which was demonstrated by elevations in plasma interleukin (IL)-2, IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1 or CCL2), interferon gamma-induced protein 10 (IP-10 or CXCL10), and IL-8 that peaked at 4 to 6 h (Goel et al., 2016). These features have not been observed with the first dose of antigenic peptides in multiple sclerosis or type-1 diabetes (Streeter et al., 2015, Alhadj et al., 2017), but bare some similarities to the first dose effects of immunosuppressive biologics that initially activate T cells (Chatenoud et al., 1990), and also to the timing of isolated late asthmatic reaction elicited by T-cell stimulatory allergen-derived peptide (Haselden et al., 1999).
Although higher maintenance dose levels of peptide immunotherapy are hypothesized to be more efficacious (Sabatos-Peyton et al., 2010), adverse events associated with the first dose of Nexvax2 prevented further evaluation of Nexvax2 at levels of 300 μg or higher in fixed dose regimens (Goel et al., 2017). Studies in genetically modified mice with clonal T cell populations indicate that systemic cytokine release caused by CD4-positive T cell activation after subcutaneous administration of antigenic peptide is attenuated by gradual escalation in dose (or stepwise “up-dosing”) from a low starting dose similar to that employed in specific immunotherapy for allergy (Burton et al., 2014, Burks et al., 2013). However, the relevance of findings in young mice with over 90% of their CD4 + T cells specific for a single peptide to patients with autoimmune disease established for many years and polyclonal memory CD4 + T cells is uncertain (Burton et al., 2014). Having characterized the effects of fixed dose regimens in phase 1 studies, we wanted to know if up-dosing could allow us to test higher maintenance dose levels of Nexvax2 in future efficacy studies. In the present study, our objective was to determine the safety and tolerability of Nexvax2 administered at maintenance dose levels of 300 μg or 900 μg when preceded by dose titrations from a low, well tolerated starting dose in patients with celiac disease on a gluten-free diet.
2. Materials and Methods
2.1. Investigational Drug Product
CS Bio (Menlo Park, California, USA) manufactured the peptides NPL001, NPL002, and NPL003 according to current Good Manufacturing Practice (cGMP). Grand River Aseptic Manufacturing (Grand Rapids, Michigan, USA) formulated and filled vials and syringes with Nexvax2 or sterile USP 0.9% sodium chloride according to cGMP. Sterile equimolar solutions of NPL001, NPL002, and NPL003 were prepared with sterile USP 0.9% sodium chloride as excipient. Total peptide concentration was 1.5 mg/mL in Nexvax2 vials, and 3.0 mg/mL in Soluvia™ syringes (Becton-Dickinson, Franklin Lakes, New Jersey, USA). Pre-filled syringes were designed for intradermal injection of the total injection volume of 0.1 mL.
2.2. Study Design
We evaluated the safety and tolerability of Nexvax2 in participants with celiac disease on a gluten-free diet. Nexvax2 was administered by stepwise dose escalation followed by a high maintenance dose in this randomized, double-blind, placebo-controlled phase 1 study (Fig. 1). This study was conducted at four community sites in Australia (3) and New Zealand (1) listed in the appendix. CPR Pharma Services (Thebarton, SA, Australia) managed the study. All participants provided written informed consent before enrolment. Approval was granted by local ethics committees listed in the appendix. This study was conducted in accordance with the International Conference on Harmonisation's Good Clinical Practice.
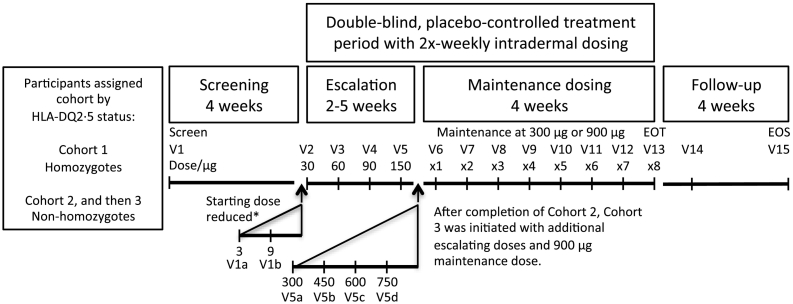
Fig. 1.
Study schematic. *Escalation was amended for all cohorts by including 3 μg and 9 μg doses when one participant in Cohort 1 withdrew with gastrointestinal adverse events graded moderate or severe after 30 μg and 60 μg doses. V14 was 1 week after V12. EOS, end of study; EOT, end of treatment; V, visit.
2.3. Participants
Participants were required to be between 18 and 70 years old, have a celiac disease diagnosis on the basis of intestinal histology demonstrating villous atrophy, and possess each allele encoding HLA-DQ2·5. At the screening visit, participants were excluded if they had not maintained a gluten-free diet for at least one year, had elevated serology for both transglutaminase 2 IgA and deamidated gliadin peptide IgG, or had a score of more than 12 on the Celiac Dietary Adherence Test (CDAT) consistent with reduced adherence to gluten-free diet (Leffler et al., 2009). Full eligibility criteria are provided in the appendix. Because of the potential for gene dose influencing susceptibility to adverse events caused by Nexvax2, eligible participants were allocated to cohort 1 if they had HLA-DQA1*05 and HLA-DQB1*02 alleles and no other HLA-DQA or HLA-DQB alleles (HLA-DQ2·5 “homozygotes”), whereas other eligible participants (HLA-DQ2·5 “non-homozygotes”) were enrolled in cohort 2 or, subsequently, in cohort 3.
2.4. Randomization and Masking
Study site personnel provided a summary of documentation supporting celiac disease diagnosis to the sponsor for review and approval for screening. Following completion of participant eligibility, study site personnel faxed a Randomization Request Form to CPR Pharma Services to obtain the participant's unique randomization number and study drug assignment. An unblinded statistician assigned randomization and kit numbers, which were forwarded to Catalent Pharma Solutions (Tullamarine, VIC, Australia) who were responsible for shipment of study drug to the study site. Manual central randomization without blocking was used for each cohort. The randomization schedule was generated with SAS v9·3 (SAS Institute Inc., Cary, NC, USA) and remained sequestered until database lock. Participants were randomized to receive Nexvax2 or placebo 8:3 in cohorts 1 and 2, and 10:2 in cohort 3. Replacements were allowed, and they received identical treatment as the participant being replaced. Study drug supplies were shipped to the study site in double-blind treatment kits according to the randomization assignment. Study site personnel and sponsor received only the unique randomization number, the date of randomization, and the treatment kit assignment. The appearance of vials, the drug product, the volume injected, and the number of injections for Nexvax2 and placebo treatments were identical within each cohort. Study participants, care providers, data managers, sponsor personnel, and study site personnel remained blinded to study treatment assignment until database lock for each cohort.
2.5. Clinical Procedures
At the screening visit, participant eligibility was determined by assessing the level of compliance to a gluten-free diet (CDAT) and according to the results of a physical examination, electrocardiogram, and blood tests, including celiac disease serology and HLA-DQA and HLA-DQB genotype. Digestive symptoms over the previous week were assessed at the screening visit and weekly until after the treatment period using the Gastrointestinal Symptom Rating Scale (GSRS) (Svedlund et al., 1988). Participants in cohort 3 also had an upper gastrointestinal endoscopy to assess second part duodenal histology. The full set of assessments in the screening period is shown in the appendix (Fig. S1 in the Supplementary Appendix). Within four weeks of the screening visit, eligible participants were randomized and began the treatment period.
The masked site pharmacist prepared the desired dose using Soluvia syringes prefilled with Nexvax2 300 μg or placebo in 0.1 mL, and/or by loading 0.1 mL of study drug suitably diluted with sterile USP 0.9% sodium chloride into fixed needle 1-mL allergy syringes (#30550; Becton-Dickinson). A single injection was used for each of the escalation doses in cohorts 1 and 2, and for the first six escalation doses in cohort 3. Subsequent escalation doses in cohort 3 were administered using the Soluvia syringe prefilled with Nexvax2 300 μg or placebo alone or in combination with the allergy syringe prefilled with Nexvax2 150 μg or placebo. Maintenance doses were administered in cohorts 1 and 2 as two injections of 150 μg or placebo in pre-filled allergy syringes, and in cohort 3 as three injections of 300 μg or placebo in pre-filled Soluvia syringes. Intradermal injections were administered at the study site staff using Mantoux injection technique for allergy syringes fitted with a West Intradermal Adapter (#5070206; West Pharmaceutical Services Inc., Exton, Pennsylvania, USA), or by injection perpendicular to the skin using Soluvia syringes according to the manufacturer's instructions. Injections were administered to the abdomen at the level of the waist alternating between the right and left of the body twice per week (3- or 4-day intervals) for up to nine weeks.
The treatment period was divided between an up-dosing phase and a four-week maintenance phase when eight doses of Nexvax2 were administered at 300 μg in cohorts 1 and 2, or at 900 μg in cohort 3 (Fig. 1). The up-dosing regimen for cohorts 1 and 2 was initially 30, 60, 90, and 150 μg, but was subsequently amended to 3 μg, 9 μg, 30 μg, 60 μg, 90 μg, and 150 μg. The up-dosing regimen for cohort 3 was 3 μg, 9 μg, 30 μg, 60 μg, 90 μg, 150 μg, 300 μg, 450 μg, 600 μg, and 750 μg. Dose levels below 300 μg could be administered only once, whereas dose levels from 450 μg to 750 μg could be administered up to a total of three times. Down-dosing to the next lowest dose was allowed if dose levels from 450 μg to 900 μg were poorly tolerated after three administrations. Criteria for down-dosing and study halting rules are listed in the appendix.
Safety assessments during the treatment period included vital signs, clinical pathology, and adverse event monitoring (Fig. S1 in the Supplementary Appendix). Adverse events were recorded at each visit, which were graded by site staff according to Common Terminology Criteria for Adverse Events (CTCAE) v4·03. Pharmacodynamics assessments included a 38plex assay to profile cytokine and chemokine concentrations in plasma before and up to 10 h post-treatment at visits corresponding to administration of Nexvax2 at the previously determined maximum tolerated dose (150 μg) and at each of the higher dose levels. The percentage of leukocytes in whole blood that corresponded to T cells or helper, cytotoxic, regulatory, or activated (CCR6-positive) T cell subsets was estimated using epigenetic cell counting before and after dosing during the treatment period at indicated times (Fig. S1 in the Supplementary Appendix). Pharmacokinetics of the three constituent peptides in Nexvax2 were assessed pre-treatment and 45 min post-treatment in cohort 3 at visits corresponding to dose levels 300 μg and above. Serum levels of anti-Nexvax2 antibodies were also assessed in cohort 3 (Fig. S1 in the Supplementary Appendix). In the four-week observational post-treatment period, participants in cohort 3 had an upper gastrointestinal endoscopy to assess second part duodenal histology within one week of completing the treatment period.
2.6. Outcomes
All outcomes were centrally assessed. The pre-specified primary outcome was the number and percentage of adverse events during the treatment period. The following pre-specified secondary outcomes were also assessed: 1) weekly GSRS scores during the treatment period; 2) in cohort 3, pharmacokinetics of Nexvax2 at the first administration of 300, 450, 600, 750, and 900 μg doses and at the end of treatment; 3) in cohort 3, the effect of Nexvax2 at 900 μg on duodenal histology, as determined by the change in villous height to crypt depth ratio from baseline screening to end of treatment; and 4) relative change in the concentration of plasma cytokines and chemokines after sequential doses of Nexvax2.
2.7. Statistical Analysis
A sample size of 34 participants was planned for this study, including randomization of approximately 22 participants for cohorts 1 and 2 and randomization of approximately 12 participants for cohort 3. The sample size was chosen pragmatically to permit assessment of safety and tolerability of Nexvax2 while limiting unnecessary exposure. The following study populations were used in the statistical analyses: the safety population included all participants who received a dose of Nexvax2 or placebo (analysed according to treatment actually received); the gastrointestinal symptom score population included all participants who received a dose of Nexvax2 or placebo and had at least one assessment of the GSRS after dosing (analysed according to treatment actually received); the pharmacokinetics population included all participants in cohort 3 who received a dose of at least 300 μg of Nexvax2.
Descriptive statistics was used to summarise demographic data and baseline participant characteristics. Adverse events were presented as numbers and percentage of participants. Pharmacokinetics of Nexvax2 peptides was summarised by dose level and presented as mean (95% CI) plasma concentrations; Pearson correlation coefficients were used to compare the plasma concentrations of the Nexvax2 peptides. The paired, non-parametric Wilcoxon's signed-rank test was used to compare GSRS scores over time and between treatment groups and to compare the change in villous height to crypt depth ratio between treatment groups. Cytokine data were presented as median fold change from pre-treatment levels. Data from cohorts 1 and 2 were analysed separately according to the Nexvax2 starting dose levels of 3 μg or 30 μg. Data were collected by investigators and managed by CPR Pharma Services, and statistical analyses were performed by PROMETRIKA, LLC (Cambridge, MA, USA). SAS v9·4 and Prism v6 (GraphPad Software, Inc., La Jolla, CA, USA) were used for statistical analyses. An independent data safety monitoring board oversaw the study. This trial was registered with ClinicalTrials.gov (NCT02528799).
2.8. Laboratory Procedures
Clinical pathology assessments, including routine hematology, blood chemistry, coagulation, urinalysis, and urinary pregnancy test (β-hCG) for all female participants were performed by Dorevitch Pathology (Heidelberg, Victoria, Australia). IgA specific for recombinant human transglutaminase 2 and IgG specific for deamidated gliadin peptide were measured in serum by Dorevitch Pathology using commercial kits manufactured by INOVA Diagnostics (San Diego, California, USA). HLA-DQA and HLA-DQB alleles were assessed in blood collected into a K2 EDTA tube by Sonic Genetics (Sonic Healthcare Ltd., Macquarie Park, New South Wales, Australia) using polymerase chain reaction with sequence-specific oligonucleotides (Gen-Probe, Hologic Inc., Bedford, Massachusetts, USA). Participants with HLA-DQA1*05 (including all alleles whose numerical code commences with 05 such as HLA-DQA1*0501 or HLA-DQA1*0505) and HLA-DQB1*02 (including all alleles whose numerical code commences with 02 such as HLA-DQB1*0201 or HLA-DQB1*0202) were considered positive for HLA-DQ2·5, and non-homozygotes if they possessed additional HLA-DQA and HLA-DQB alleles. Participants who were HLA-DQ2·5 + and had no other HLA-DQA or HLA-DQB alleles were defined as HLA-DQ2·5 homozygotes.
Anti-Nexvax2 serology was analysed by Blue Stream Laboratories, Inc., a Charles River Company (Woburn, Massachusetts, USA) according to methods previously described (Goel et al., 2017). The upper cutoff level of 1194 for IgG and 5754 for IgA specific for Nexvax2 peptides were defined by the 95th percentile for assessments made on sera from 50 individuals provided by HemaCare Corporation (Van Nuys, California, USA; BioreclamationIVT, Hicksville, New York, USA). These “healthy-donor” sera were confirmed seronegative for recombinant human tTG-specific IgA and deamidated gliadin peptide-specific IgG and IgA.
Pharmacokinetics of NPL001, NPL002 and NPL003 were assessed using blood collected 30 min before and 45 min after study drug administration. Blood was collected into K2 EDTA tubes and within 10 min was centrifuged at 1100–1300 g for 10 min. Pharmacokinetics assays were performed by Charles River Laboratories Ashland, LLC (Ashland, Ohio, USA). Plasma was thawed and spiked with a known amount of a mixture of isotopically labelled Nexvax2 peptides (Pepscan, Lelystad, The Netherlands). A solid phase extraction procedure was performed with samples volumes of 0.3 mL. Nexvax2 peptide concentrations were measured in reconstituted sample extracts analysed with an ultra-high performance liquid chromatography-mass spectrometry/mass spectrometry (UHPLC-MS/MS) method in the positive electron ionization mode using a Waters Acquity® UPLC Peptide BEH C18 Column, 300 Å, 1.7-μm particle-size, 2.1 × 50 mm column (Waters Corporation, Milford, Massachusetts, USA). The peak area ratios of NPL001, NPL002, and NPL003, and internal standards and the theoretical concentrations of the calibration samples were fit to a linear regression function with 1/x weighting, excluding the origin. The method was validated over the concentration range of 2.00 to 100 ng/mL of human plasma using a 0.3 mL sample.
Plasma cytokines and chemokines were assessed in blood collected into K2 EDTA tubes and immediately placed on wet ice. Within 30 min of collection, blood was centrifuged at 1100–1300 RCF for 10 min, and plasma was aliquotted and frozen. Concentrations of 38 cytokines and chemokines were assessed by ImmusanT, Inc. (Cambridge, MA, USA) using a multiplex magnetic bead assay according to the manufacturer's instructions (Milliplex® MAP Human Cytokine/Chemokine Magnetic Bead Panel; EMD Millipore Corp., Billerica, MA, USA and Luminex® MAGPIX® System xPONENT®, Luminex Corporation, Austin, TX, USA). Final concentrations were the average of triplicate measurements. Pre-treatment cytokine and chemokine concentrations in plasma were compared with post-treatment levels on the same day.
Lymphocyte subsets in blood were assessed using epigenetic immune cell counting by Epiontis GmbH (Berlin, Germany). Blood was collected into K2 EDTA tubes and frozen at − 20 °C within 60 min. Percentage of leukocytes that were T cells (CD3-positive lymphocytes), helper T cells (CD4-positive), cytotoxic T cells (CD8-positive), CCR6-positive T cells, or regulatory T cells (CD3-positive, CD4-positive, CD25-positive, FOXP3-positive) were determined using epigenetic real time PCR based analyses that were unique and highly specific for the cell type of interest.
Duodenal histology was assessed by digital histomorphometry. Four biopsies were collected from the 2nd part of the duodenum using a single pass of the biopsy forceps for each tissue sample. Biopsy samples taken from the distal duodenum were immersed in PAXgene fixative for 1–4 h and transferred to the proprietary storage solution in PAXgene dual chamber containers (QIAGEN, Hilden, Germany). The central pathologist (JiLab Inc., Tampere, Finland) processed and evaluated biopsies. Samples were processed as paraffin blocks using a standard formalin-free protocol. Tissue sections (3–4 μm) were cut on SuperFrost Plus slides for hematoxylin and eosin staining. Biopsies were embedded and sections were cut orthogonally to the luminal surface. Immunohistochemistry was performed using a standard protocol consisting of antigen retrieval (incubation at 98 °C for 15 min in 0.01 Tris-EDTA buffer, pH 9.0), blocking of endogenous peroxidase (3% H2O2 for 5 min at RT), primary antibody incubation (60 min at RT), anti-mouse or anti-rabbit peroxidase polymer (RTU, 30 min at RT, Nichirei Biosciences, Tokyo, Japan), and diamino benzidine chromogen (Nichirei). Slides were counterstained with hematoxylin. Mouse anti-CD3 (clone SP7; Thermo Fisher Scientific, Waltham, Massachusetts, USA) diluted 1:100 was used to evaluate intra-epithelial lymphocyte frequencies. Stained slides were scanned as whole slide images using SlideStrider digital slide scanner at resolution 0.28 μm per pixel (Jilab Inc.). Images were stored as JPEG2000 files and viewed with a dedicated web-based Coeliac Slide Viewer (Jilab Inc.). Two independent readers made at least three replicate measurements of villus height and adjacent crypt depth, and the average of the two readers' assessments was used as the final result for villous height to crypt depth ratio. CD3 positive intraepithelial lymphocytes (IELs) in at least 300 enterocytes were enumerated to obtain the IEL count per 100 enterocytes.
2.9. Role of the Funding Source
The funder of the study was involved in the study design, data collection, data analysis, data interpretation, and the writing of this report. AT, JM, LJW, and RPA had full access to all the data in the study. RPA had final responsibility for the decision to submit for publication.
3. Results
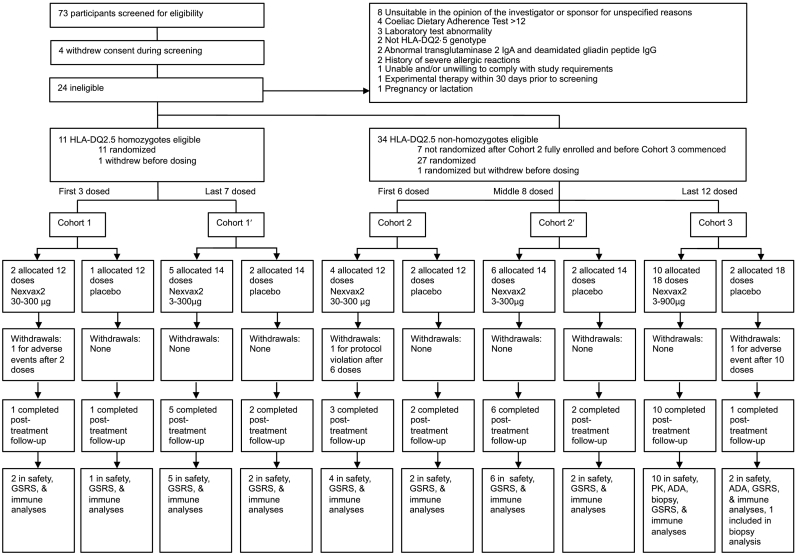
Between August 19, 2015 and October 31, 2016, 73 volunteers were screened for eligibility (Fig. 2). Four (5%) volunteers withdrew before completing screening, 24 (33%) were ineligible, and 45 (62%) were eligible. The commonest reasons for exclusion were being unsuitable in the opinion of the investigator or sponsor for unspecified reasons (8, 11%), Coeliac Dietary Adherence Test score over 12 (4, 5%), and having a laboratory test abnormality (3, 4%). Two (3%) participants were eligible but withdrew after randomization and were replaced before dosing commenced, and 7 participants who had been screened after completion of cohort 2 and met all eligibility criteria were found to be non-homozygotes and not randomized because cohort 3 was not yet open for enrolment. Recruitment was slower for cohort 1 because HLA-DQ2·5 homozygotes constitute only about 20% of patients diagnosed with celiac disease (Murray et al., 2007). Altogether, 36 ultimately received investigational product. By November 16, 2015, three participants had been recruited into cohort 1 (2 randomized to Nexvax2 and 1 randomized to placebo), while 6 had been recruited to cohort 2 (4 randomized to Nexvax2 and 2 randomized to placebo). For these participants, the Nexvax2 starting dose was 30 μg and their assigned treatment included a total of 12 doses with four in the up-dosing phase. For participants enrolled after November 16, 2015, the dosing regimen was amended with the aim of improving tolerability of the starting dose. For the seven subsequent participants in cohort 1 (5 randomized to Nexvax2 and 2 randomized to placebo) and 8 participants in cohort 2 (6 randomized to Nexvax2 and 2 randomized to placebo), the Nexvax2 starting dose was 3 μg and their assigned treatment included a total of 14 doses with six in the up-dosing phase. By December 3, 2015, the last of 15 participants was enrolled into cohort 2 (10 receiving Nexvax2 and 5 placebo), and ten months later, October 31, 2016, the last of 11 participants were entered into cohort 1 (8 receiving Nexvax2 and 3 placebo). After interim analysis of findings from cohort 2, screening for cohort 3 began June 21, 2016 and the last of 12 participants was enrolled in this cohort on October 10, 2016 (10 receiving Nexvax2 and two placebo).
Fig. 2.
Trial profile. For cohort 1 and cohort 2, the Nexvax2 starting dose was 30 μg; for cohort 1′ and cohort 2′, the Nexvax2 starting dose was 3 μg.
The mode for total Nexvax2 exposure was 2742 μg over 14 doses in cohort 1 (n = 4), 2742 μg over 14 doses in cohort 2 (n = 6), and 9642 μg over 18 doses in cohort 3 (n = 8) (Table S1 in the Supplementary Appendix). Six participants who commenced treatment did not complete the assigned number of doses, for 2 participants (1 receiving Nexvax2 and 1 placebo) this was due to early withdrawal with adverse events, and for 1 participant receiving Nexvax2 discontinuation was due to a protocol violation (gluten exposure). In addition, 2 participants missed one or two consecutive maintenance doses of 300 μg or 900 μg, respectively, and 1 participant repeated the 600 μg dose during escalation.
One of two participants enrolled in the initial group in cohort 1 who received Nexvax2 starting at 30 μg withdrew consent after the second dose in the up-dosing phase following adverse events considered to be study drug related. At 4·25 h after the initial 30 μg Nexvax2 starting dose, this participant had onset of upper abdominal pain graded severe, which lasted for 1 h and was associated with mild nausea. Three days later, at 3·75 h after the second dose of Nexvax2 (60 μg), there was onset of abdominal pain and nausea both graded moderate, which were accompanied by arthralgia, mental `fogginess`, and perspiring, each graded mild. The protocol was revised following this participant's withdrawal so that the up-dosing phase began with Nexvax2 doses of 3 μg and 9 μg. One participant in cohort 2 received six doses of Nexvax2 including two doses at 300 μg before being discontinued from the study because of a protocol violation of unintended non-adherence to gluten-free diet. Approximately 7 h after the fifth dose, food containing gluten was consumed inadvertently, which was followed between 2 and 3 h later by abdominal pain graded moderate and fatigue, nausea, vomiting, and diarrhoea, each graded mild. One participant in cohort 3 who received 10 doses of placebo withdrew from the study due to an intervertebral disc protrusion graded severe and unrelated to study drug. One replacement participant was enrolled in cohort 1 and randomized to Nexvax2. Two replacement participants were enrolled in cohort 2 (1 randomized to placebo and 1 randomized to Nexvax2). Altogether, 33 participants completed treatment out of 36 participants who received at least one dose of Nexvax2 or placebo; all 36 participants were included in the primary outcome safety population analyses.
Median age of the 36 participants who received at least one dose of Nexvax2 or placebo was 41·0 years (25th–75th percentiles: 32·0–52·8), and 25 (69%) were women (Table 1). Median age at celiac disease diagnosis was 33·5 years (25th–75th percentiles: 27·5–41·0); median time since diagnosis was 6·5 years (25th–75th percentiles: 3·8–12·3); and median time on a gluten-free diet was 5·5 years (25th–75th percentiles: 3·0–11·5). Participants in each cohort of the Nexvax2 (n = 27) and placebo (n = 9) groups displayed similar demographics, baseline celiac disease-specific serology, and gene dose for the alleles that code HLA-DQ2·5 (Table 1).
Table 1.
Demographics and baseline characteristics.
| Treatment |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Placebo |
Any |
|---|---|---|---|---|---|---|---|---|
| Starting dose, μg |
30 |
30 |
3 |
3 |
3 |
All |
All |
|
| Maintenance dose, μg |
300 |
300 |
300 |
300 |
900 |
All |
All |
|
| Cohort | 1 | 2 | 1 | 2 | 3 | All | All | All |
| n | 2 | 4 | 5 | 6 | 10 | 27 | 9 | 36 |
| Age (years) | 28 (27–29) |
42 (36–43) |
32 (24–45) |
35 (32–40) |
53 (43–60) |
41 (32–49) |
43 (32–57) |
41 (32–53) |
| Sex | ||||||||
| Male | 0 (0%) | 0 (0%) | 1 (20%) | 2 (33%) | 6 (60%) | 9 (33%) | 2 (22%) | 11 (31%) |
| Female | 2 (100%) | 4 (100%) | 4 (80%) | 4 (67%) | 4 (40%) | 18 (67%) | 7 (78%) | 25 (69%) |
| Race | ||||||||
| White | 2 (100%) | 4 (100%) | 5 (100%) | 6 (100%) | 10 (100%) | 27 (100%) | 9 (100%) | 36 (100%) |
| Age at diagnosis (years) | 23 (21–24) |
35 (28–39) |
20 (18–36) |
30 (28–31) |
39 (35–46) |
33 (27–40) |
37 (30–42) |
34 (28–41) |
| Time since diagnosis (years) | 6 (6–7) |
4 (3–6) |
9 (4–14) |
8 (3 − 11) |
7 (5–12) |
7 (4–13) |
6 (2 − 11) |
7 (4–12) |
| Time on gluten-free diet (years) | 6 (6–7) |
4 (3–6) |
9 (4–14) |
6 (3 − 10) |
7 (5–12) |
6 (4–12) |
5 (2–11) |
6 (3 − 12) |
| Body mass (kg) | 78 (71–85) |
61 (56–66) |
84 (78–89) |
74 (60–85) |
79 (69–108) |
73 (64–90) |
66 (60–77) |
71 (62–87) |
| Height (cm) | 169 (167–170) |
163 (160–164) |
169 (168–175) |
168 (162–177) |
175 (169–181) |
169 (163–178) |
169 (165–171) |
169 (163–175) |
| Body-mass index (kg/m2) | 27 (25–29) |
24 (22–25) |
29 (29–30) |
25 (21 − 30) |
27 (26–30) |
26 (23 − 30) |
22 (22–26) |
26 (22 − 30) |
| Abnormal serologya | 0 (0%) | 1 (25%) | 2 (40%) | 1 (17%) | 1 (10%) | 5 (19%) | 2 (22%) | 7 (19%) |
| Homozygote for HLA-DQ2·5 alleles | ||||||||
| Both | 2 (100%) | 0 (0%) | 5 (100%) | 0 (0%) | 0 (0%) | 7 (26%) | 3 (33%) | 10 (28%) |
| HLA-DQB1*02 only | 0 (0%) | 3 (75%) | 0 (0%) | 1 (17%) | 4 (40%) | 8 (30%) | 1 (11%) | 9 (25%) |
| HLA-DQA1*05 only | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 1 (4%) | 0 (0%) | 1 (3%) |
| Neither | 0 (0%) | 1 (25%) | 0 (0%) | 5 (83%) | 5 (50%) | 11 (41%) | 5 (56%) | 16 (44%) |
Data are median (25th–75th percentiles) or n (%).
Deamidated gliadin peptide IgG or transglutaminase 2 IgA.
The total number of treatment-emergent adverse events in the 27 participants who received Nexvax2 was 207 compared with 46 in 9 participants who received placebo (Table 2). Overall, 24 (89%) of the 27 participants receiving Nexvax2 experienced at least one treatment-emergent adverse event compared with 9 (100%) of 9 participants who received placebo (Table 3). There was no particular dose level consistently associated with increased frequency of adverse events (Fig. 3). In the Nexvax2-treated participants, 136 (66%) of the 207 treatment-emergent adverse events were considered related to the study drug compared with 25 (54%) of the 46 treatment-emergent adverse events in placebo-treated participants. There were two serious adverse events (somnolence and intervertebral disc protrusion), both of which affected placebo-treated participants. Participant vital signs were measured before and after dosing; there were no remarkable findings in the vital signs of participants in the Nexvax2 or placebo groups, and treatment with Nexvax2 did not result in any treatment-related changes in ECG readings or physical examination (data not shown).
Table 2.
Overall adverse events summary for participants starting at 3 μg or 30 μg of Nexvax2.
| Treatment |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Placebo |
|---|---|---|---|---|---|---|
| Starting dose, μg |
30 |
30 |
3 |
3 |
3 |
|
| Maintenance dose, μg |
300 |
300 |
300 |
300 |
900 |
|
| Cohort | 1 | 2 | 1 | 2 | 3 | All |
| Participants, n | 2 | 4 | 5 | 6 | 10 | 9 |
| Participants with any adverse events | 2 (100%) | 4 (100%) | 3 (60%) | 6 (100%) | 9 (90%) | 9 (100%) |
| Participants with any drug-related adverse events | 2 (100%) | 4 (100%) | 3 (60%) | 6 (100%) | 7 (70%) | 8 (89%) |
| Participants with any adverse events graded at least moderate in severity | 2 (100%) | 3 (75%) | 2 (40%) | 5 (83%) | 6 (60%) | 4 (44%) |
| Participants with any adverse events graded at least moderate in severity and drug-related | 1 (50%) | 2 (50%) | 1 (20%) | 4 (67%) | 2 (20%) | 2 (22%) |
| Participants who withdrew due to adverse events | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) |
| Participants with any serious adverse events | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (22%) |
| Adverse events | 34 | 57 | 16 | 56 | 44 | 46 |
| Adverse events drug-related | 21 | 45 | 9 | 41 | 20 | 25 |
| Adverse events graded at least moderate in severity | 7 | 5 | 3 | 17 | 12 | 7 |
| Adverse events graded at least moderate in severity and drug-related | 5 | 2 | 1 | 13 | 2 | 4 |
| Adverse events leading to withdrawal | 1 | 0 | 0 | 0 | 0 | 1 |
| Serious adverse events | 0 | 0 | 0 | 0 | 0 | 2 |
Data are n (%).
Table 3.
Adverse events by system organ class for participants starting at 3 μg or 30 μg of Nexvax2.
| Treatment |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Nexvax2 |
Placebo |
|---|---|---|---|---|---|---|
| Starting dose, μg |
30 |
30 |
3 |
3 |
3 |
|
| Maintenance dose, μg |
300 |
300 |
300 |
300 |
900 |
|
| Cohort | 1 | 2 | 1 | 2 | 3 | All |
| Participants, n | 2 | 4 | 5 | 6 | 10 | 9 |
| Any adverse events | 2 (100%) 34 | 4 (100%) 57 | 3 (60%) 16 | 6 (100%) 56 | 9 (90%) 44 | 9 (100%) 46 |
| Gastrointestinal disorders | 2 (100%) 11 | 4 (100%) 28 | 3 (60%) 5 | 6 (100%) 26 | 7 (70%) 13 | 6 (67%) 14 |
| Diarrhoea | 1 (50%) 1 | 2 (50%) 2 | 1 (20%) 1 | 5 (83%) 9 | 4 (40%) 5 | 1 (11%) 1 |
| Nausea | 2 (100%) 4 | 3 (75%) 10 | 1 (20%) 1 | 2 (33%) 3 | 2 (20%) 2 | 3 (33%) 4 |
| Abdominal pain | 1 (50%) 2 | 1 (25%) 1 | 0 (0%) 0 | 3 (50%) 6 | 2 (20%) 3 | 0 (0%) 0 |
| Abdominal pain upper | 1 (50%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Abdominal pain lower | 0 (0%) 0 | 1 (25%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Abdominal discomfort | 0 (0%) 0 | 2 (50%) 3 | 1 (20%) 1 | 2 (33%) 3 | 0 (0%) 0 | 2 (22%) 4 |
| Gastroesophageal reflux | 1 (50%) 2 | 1 (25%) 1 | 1 (20%) 1 | 0 (0%) 0 | 1 (10%) 1 | 0 (0%) 0 |
| Flatulence | 0 (0%) 0 | 1 (25%) 1 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 1 (11%) 1 |
| Abdominal distension | 1 (50%) 1 | 1 (25%) 3 | 1 (20%) 1 | 1 (17%) 1 | 0 (0%) 0 | 2 (22%) 2 |
| Eructation | 0 (0%) 0 | 2 (50%) 5 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Vomiting | 0 (0%) 0 | 1 (25%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (11%) 1 |
| Constipation | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (11%) 1 |
| Nervous system disorders | 1 (50%) 3 | 4 (100%) 8 | 2 (40%) 3 | 4 (67%) 11 | 6 (60%) 9 | 3 (33%) 6 |
| Headache | 0 (0%) 0 | 2 (50%) 3 | 2 (40%) 2 | 4 (67%) 9 | 6 (60%) 8 | 1 (11%) 1 |
| Migraine | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (17%) 1 | 0 (0%) 0 | 0 (0%) 0 |
| Tension headache | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 0 (0%) 0 |
| Dizziness | 1 (50%) 2 | 1 (25%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (11%) 1 |
| Dysgeusia | 0 (0%) 0 | 2 (50%) 2 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Lethargy | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (17%) 1 | 0 (0%) 0 | 2 (22%) 2 |
| Syncope | 1 (50%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| General disorders & administration site conditions | 2 (100%) 13 | 3 (75%) 15 | 1 (20%) 4 | 3 (50%) 4 | 2 (20%) 4 | 3 (33%) 11 |
| Fatigue | 1 (50%) 2 | 2 (50%) 6 | 1 (20%) 1 | 1 (17%) 2 | 0 (0%) 0 | 2 (22%) 4 |
| Injection site reactions | 2 (100%) 6 | 2 (50%) 6 | 1 (20%) 1 | 2 (33%) 2 | 2 (20%) 2 | 2 (22%) 3 |
| Injection site erythema | 1 (50%) 4 | 2 (50%) 5 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 2 (22%) 2 |
| Injection site pruritus | 1 (50%) 1 | 1 (25%) 1 | 0 (0%) 0 | 1 (17%) 1 | 0 (0%) 0 | 0 (0%) 0 |
| Injection site pain | 1 (50%) 1 | 0 (0%) 0 | 1 (20%) 1 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 |
| Injection site reaction | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (17%) 1 | 1 (10%) 1 | 0 (0%) 0 |
| Injection site bruise | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (11%) 1 |
| Skin & subcutaneous tissue disorders | 2 (100%) 4 | 1 (25%) 1 | 0 (0%) 0 | 2 (33%) 3 | 4 (40%) 4 | 0 (0%) 0 |
| Ecchymosis | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (17%) 2 | 1 (10%) 1 | 0 (0%) 0 |
| Infections and infestations | 1 (50%) 1 | 1 (25%) 1 | 1 (20%) 1 | 1 (17%) 1 | 4 (40%) 8 | 1 (11%) 1 |
| URTI | 0 (0%) 0 | 1 (25%) 1 | 0 (0%) 0 | 0 (0%) 0 | 2 (20%) 5 | 1 (11%) 1 |
| Musculoskeletal & connective tissue disorders | 1 (50%) 1 | 1 (25%) 2 | 0 (0%) 0 | 1 (17%) 1 | 3 (30%) 3 | 5 (56%) 8 |
| Arthralgia | 1 (50%) 1 | 1 (25%) 1 | 0 (0%) 0 | 1 (17%) 1 | 1 (10%) 1 | 1 (11%) 1 |
| Back pain | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (10%) 1 | 2 (22%) 2 |
| Musculoskeletal pain | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 2 (22%) 3 |
| Injury, poisoning, & procedural complications | 0 (0%) 0 | 0 (0%) 0 | 1 (20%) 2 | 3 (50%) 3 | 0 (0%) 0 | 3 (33%) 4 |
| Contusion | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 1 (17%) 1 | 0 (0%) 0 | 2 (22%) 2 |
| Vascular disorders | 0 (0%) 0 | 1 (25%) 1 | 0 (0%) 0 | 2 (33%) 2 | 1 (10%) 1 | 0 (0%) 0 |
| Phlebitis | 0 (0%) 0 | 0 (0%) 0 | 0 (0%) 0 | 2 (33%) 2 | 0 (0%) 0 | 0 (0%) 0 |
Data are n (%) and total number adverse events. Treatment-emergent adverse events are shown only if reported by more than one participant.
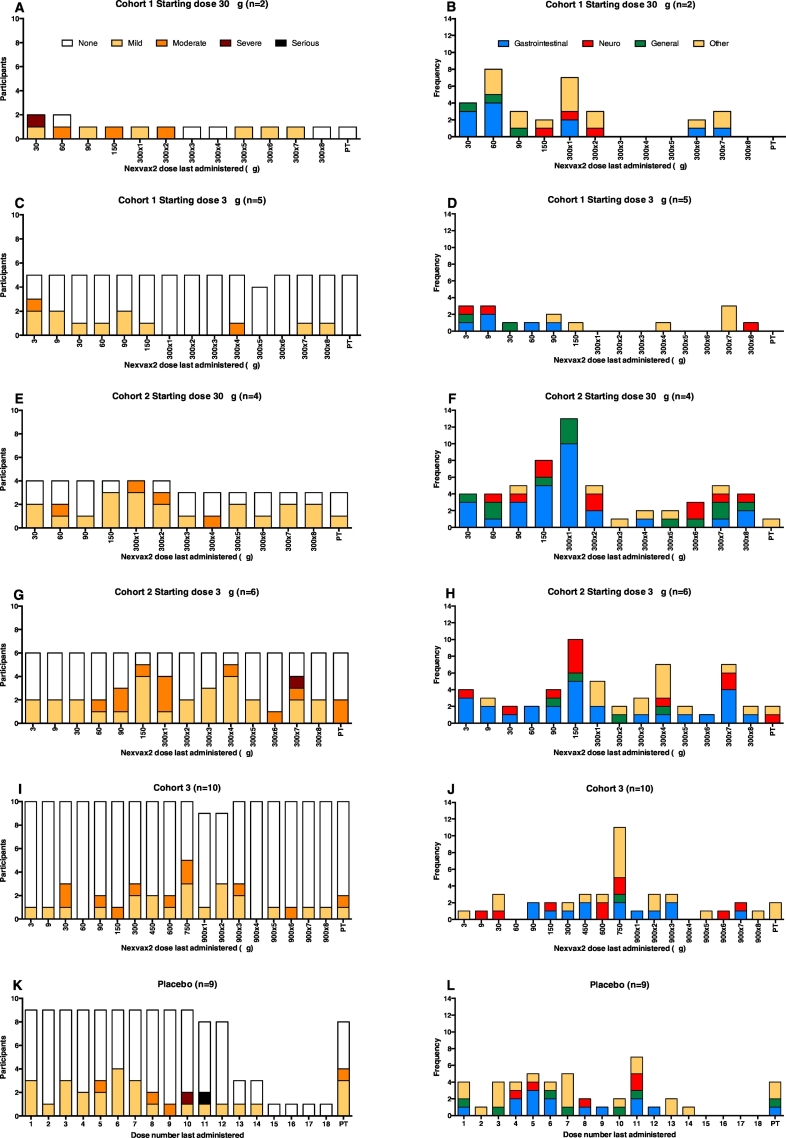
Fig. 3.
Incidence, severity, and organ class of treatment-emergent adverse events after each dose. Treatment-emergent adverse events after each dose of Nexvax2 or placebo are shown as the number of participants who experienced no, mild, moderate, severe, or serious treatment-emergent adverse events in (A), (C), (E), (G), (I), and (K) and as the total number of treatment-emergent adverse events classified by organ system in (B), (D), (F), (H), (J), and (L). PT, post-treatment.
In cohort 1, the two participants who had shorter duration up-dosing, and the higher Nexvax2 starting dose of 30 μg, accounted for 34 (68%) of all adverse events reported for Nexvax2-treated participants in this cohort (Fig. 3 and Table 2), even though one of these two participants discontinued after only 2 doses. The four (40%) participants in cohort 2 who had shorter duration up-dosing and the higher Nexvax2 starting dose of 30 μg, including one participant who had an inadvertent gluten exposure, contributed 57 (50%) of the treatment-emergent adverse events in cohort 2 (Table 2).
Altogether there were 50 treatment-emergent adverse events in the 7 participants who received Nexvax2 in cohort 1, 113 in the 10 participants who received Nexvax2 in cohort 2, 44 in the 10 participants who received Nexvax2 in cohort 3, and 46 in the 9 participants who received placebo (Table 3). Treatment-emergent adverse events affecting the gastrointestinal system accounted for 83 (40%) of the 207 treatment-emergent adverse events in the 27 participants who received Nexvax2 compared with 14 (30%) of 46 treatment-emergent adverse events in the 9 participants who received placebo (Table 3). Altogether there were 16 treatment-emergent gastrointestinal adverse events in the 7 participants who received Nexvax2 in cohort 1, 54 in the 10 participants who received Nexvax2 in cohort 2, and 13 in the 10 participants who received Nexvax2 in cohort 3. Five (71%) of seven participants who received Nexvax2 in cohort 1 reported at least one episode of a treatment-emergent gastrointestinal adverse event, as did 10 (100%) of 10 who received Nexvax2 in cohort 2, 7 (70%) of 10 who received Nexvax2 in cohort 3, and 6 (67%) of 9 who received placebo. Treatment-emergent adverse events affecting the nervous system were second most common overall and accounted for 34 (16%) of the 207 treatment-emergent adverse events in the 27 participants who received Nexvax2 compared with 6 (13%) of 46 treatment-emergent adverse events in the 9 participants who received placebo.
The most common individual treatment-emergent adverse events reported for Nexvax2-treated participants were headache in 14 (52%), diarrhoea in 13 (48%), nausea in 10 (37%), abdominal pain in 7 (26%), abdominal discomfort in 5 (19%), and fatigue in 5 (19%) (Table 3). In the Nexvax2 group, the only instance of treatment-emergent vomiting was in one participant in cohort 2 who inadvertently consumed gluten after the first maintenance dose. Adverse events classified as injection site reactions were all graded mild and affected 2 (22%) of 9 participants who received placebo and 9 (33%) of 27 participants who received Nexvax2 (Table S2 in the Supplementary Appendix). Among those participants who experienced injection site reactions, there were 5 (24%) of 21 Nexvax2-treated participants who had a starting dose of 3 μg (each experienced one injection site reaction) and 4 (67%) of 6 who had a starting dose of 30 μg, who accounted for 12 (71%) of the 17 injection site reaction adverse events in Nexvax2-treated participants.
For the six participants in cohorts 1 and 2 whose Nexvax2 starting dose was 30 μg, on average, 4 (67%) experienced adverse events after each of the first 5 Nexvax2 administrations concluding with the first 300 μg maintenance dose, with 31 (48%) out the total of 65 adverse events during this phase affecting the gastrointestinal system (Fig. 3). For the four Nexvax2-treated participants in cohorts 1 and 2 who received more than two 300 μg maintenance doses and whose starting dose was 30 μg, on average, 2 (50%) experienced adverse events after each of the last seven 300 μg maintenance doses.
Overall, in Nexvax2-treated participants whose starting dose was 3 μg, there was no specific dose level or dose number that was poorly tolerated (Fig. 3) or caused discontinuation; thus, no maximum tolerated dose was determined. There was one instance during the up-dosing phase when the same dose was repeated because of an adverse event; 1 participant in cohort 3 experienced arthralgia graded mild after receiving 600 μg of Nexvax2, which did not recur with repeat or higher doses. For the 21 participants in cohorts 1, 2, and 3 whose Nexvax2 starting dose was 3 μg, six (29%) experienced adverse events after each of the first seven Nexvax2 administrations up to 300 μg, with 17 (43%) out the total of 40 adverse events during this phase affecting the gastrointestinal system (Fig. 3). Adverse events following subsequent doses of Nexvax2 were similar to those observed in the placebo group. For the 9 participants in cohorts 1, 2, and 3 who received placebo, on average, 3 (33%) experienced adverse events after each of the first 7 placebo administrations with 8 (28%) out the total of 29 adverse events during this phase affecting the gastrointestinal system (Fig. 3). For the 11 participants in cohorts 1 and 2 whose starting dose was 3 μg, on average, 3 (27%) experienced adverse events after each of the last seven 300 μg doses. For the 10 participants in cohort 3, on average, 3 (30%) experienced adverse events after each of the 4 Nexvax2 doses from 450 μg up to 900 μg; on average, 1 (10%) experienced adverse events after each of the subsequent seven 900 μg maintenance doses.
The average GSRS score was used to measure participant's digestive symptoms over the previous week (Fig. S2 in the Supplementary Appendix). For the 9 participants who received placebo, three had lower average GSRS scores after six weeks of treatment than at baseline; of the remaining participants, 3 had the same scores and 3 had higher scores, resulting in a median difference between average GSRS scores between baseline and six weeks of zero (25th–75th percentiles: − 0.27-0.05). For the 21 participants who had a Nexvax2 starting dose of 3 μg and completed seven weeks of treatment in cohorts 1 and 2 or nine weeks of treatment in cohort 3, the average GSRS scores were lower at the end of treatment than at baseline in 13, the same in 3, and higher in 5 participants. In cohort 3, participants who received Nexvax2 showed the highest median change in GSRS scores between baseline and end of treatment (− 0·13, 25th–75th percentiles:-0·18–0·02), compared with cohort 1 (− 0·07, 25th–75th percentiles:-0·13–0·06) and cohort 2 (− 0·04, 25th–75th percentiles:−0·12-0).
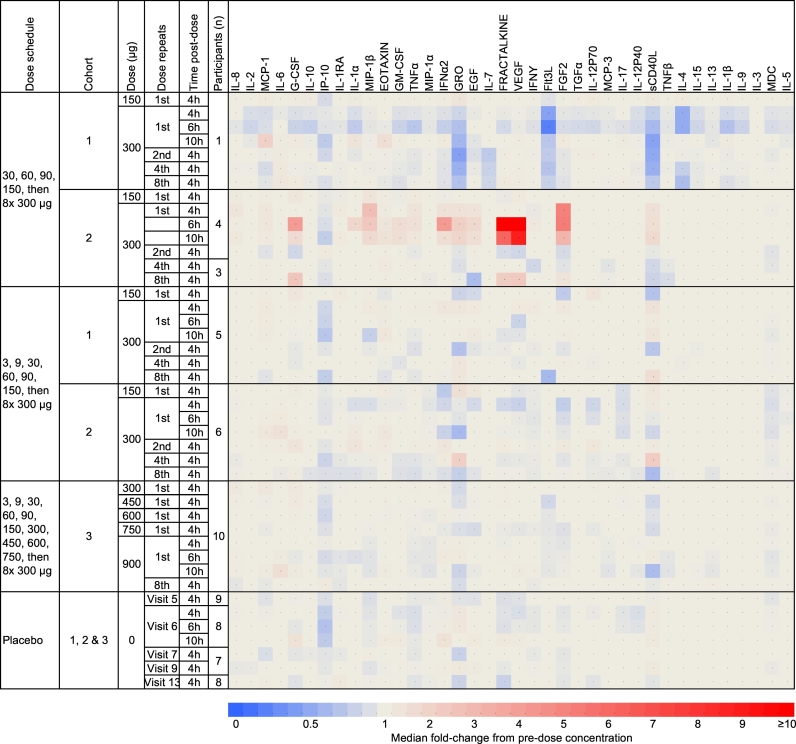
Relative change in the concentration of plasma cytokines and chemokines after sequential doses of Nexvax2 was a secondary endpoint. We have previously observed acute elevations in plasma IL-8, IL-2, MCP-1, IL-6, IL-10, and IP-10 after the first 150 μg dose of Nexvax2 in fixed dose regimen studies (Goel et al., 2016). In participants who had a Nexvax2 starting dose of 3 μg, the first administrations of Nexvax2 at 150 μg, 300 μg, or 900 μg were not associated with acute elevations in plasma cytokines or chemokines (Fig. 4 and Fig. S3 in the Supplementary Appendix).
Fig. 4.
Median fold change in plasma cytokines and chemokines following administration of Nexvax2. Assessments were made during the escalation phase, at 150 μg of Nexvax2 (previously defined maximum tolerated dose), and after the first, second, forth, and eighth administrations at the 300 μg and 900 μg maintenance doses. Plasma cytokines and chemokines were measured pre-treatment, and at 4, 6, and 10 h post-treatment.
We also assessed changes in duodenal histology in 10 participants following up-dosing and maintenance of Nexvax2 at 900 μg, and in one placebo-treated participant over the nine-week treatment period. The number of participants was insufficient to infer any beneficial effect of Nexvax2, but overall, for Nexvax2-treated participants, duodenal morphology assessments were stable or showed trends towards improvement (Table S3 in the Supplementary Appendix). Median villous height to crypt depth ratio before treatment with Nexvax2 was 1·62 (25th–75th percentiles: 1·33–1·98) and post-treatment 1·78 (25th–75th percentiles: 1·55–1·88; p = 0·9688, Wilcoxon's signed-rank test); median villus height before Nexvax2 treatment was 300·0 μm (25th–75th percentiles: 275·4–338·4) compared with post-treatment 343·7 μm (25th–75th percentiles: 302·3–357·3; p = 0·156), and the median value for the sum of paired villus height and crypt depth measurements before treatment was 484·3 μm (25th–75th percentiles: 473·8–528·2) compared with post-treatment 540·3 μm (25th–75th percentiles: 528·4–569·9; p = 0·065). Crypt depth, and the frequency of intraepithelial lymphocytes were stable in Nexvax2-treated participants.
For participants in cohort 3, serum assessments of transglutaminase 2-specific IgA and deamidated gliadin peptide-specific IgG were repeated at the end of the treatment period (Table S3). These assessments were in the normal range except in 2 participants who had elevated deamidated gliadin peptide-specific IgG, which in one case was not elevated before treatment but was not accompanied by change in quantitative histology (1·8 before and 1·8 after treatment). In addition, for participants in cohort 3, serum levels of IgG and IgA specific for Nexvax2 were assessed. Participants in cohort 3 who received Nexvax2 had serum levels of IgG and IgA specific for Nexvax2 before and after the treatment period that were below the 95% cut off levels established with sera from unaffected donors (Fig. S4 in the Supplementary Appendix). Median levels of IgG and IgA specific for Nexvax2 were stable in cohort 3 over the 60-day treatment period.
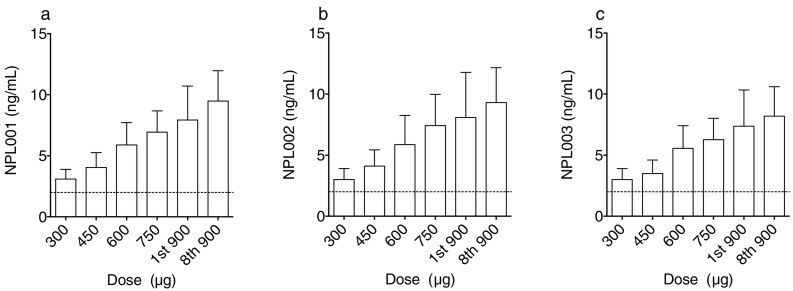
Previous phase 1 studies of Nexvax2 peptides demonstrated detectable plasma levels from 10 min to 2 h after administration of 300 μg of Nexvax2, albeit at concentrations below levels of quantitation (5 ng/mL) (Goel et al., 2017). An improved pharmacokinetics assay was developed with lower levels of quantitation of 2 ng/mL for each peptide. In almost all participants, plasma concentrations of NPL001, NPL002, and NPL003 were above the limits of quantification in plasma at 45 min after treatment at levels above 300 μg (Fig. 5). The three Nexvax2 peptides were not detected pre-treatment, and at 45 min post-treatment, displayed similar plasma concentrations that were consistent with dose-proportional kinetics. In addition, the 45-min post-treatment concentrations of each Nexvax2 peptide correlated significantly with one another (Fig. S5 a–c in the Supplementary Appendix), were stable, and correlated significantly between the first and last 900 μg doses (Fig. S5 d–f). No significant correlations were found between serum Nexvax2-specific IgG and IgA concentrations and the concentrations of the three Nexvax2 peptides (Fig. S6 in the Supplementary Appendix).
Fig. 5.
Plasma concentrations of Nexvax2 peptides. Plasma concentrations of NPL001, NPL002, and NPL003 peptides at 45 min after intradermal administration of Nexvax2 in cohort 3 (n = 10). Mean (95% CI) concentrations are shown for NPL001 (A), NPL002 (B), and NPL003 (C) after escalating doses of Nexvax2, and at the maintenance dose of 900 μg. The lower level of quantitation (LLOQ) for each peptide was 2 ng/mL indicated by the dashed line; readings below the LLOQ were assigned 2 ng/mL. Pre-treatment plasma concentrations of Nexvax2 peptides were below the LLOQ for each of the indicated doses in all participants.
The relative change in T cell frequencies in whole blood during the treatment period was an exploratory endpoint. Epigenetic cell counting demonstrated that the percentages of leukocytes defined as T cells, and the subsets of T cells that were defined as regulatory, helper, CCR6-positive, and cytotoxic were stable from the first to last day of the treatment period in participants treated with Nexvax2 or placebo (Table S4 in the Supplementary Appendix). T cell subset frequencies were also stable from pre-treatment to 4 h or 10 h after the first maintenance dose and from pre-treatment to 4 h after the last maintenance dose.
4. Discussion
This study provides the clinical evidence supporting the effectiveness of up-dosing in reducing adverse effects and in enabling higher maintenance dose levels for epitope-specific immunotherapy in a T-cell mediated autoimmune disease. We found that stepwise, intradermal up-dosing from a low, well tolerated starting dose allowed Nexvax2 to be administered without any increase in adverse effects at a maintenance dose 300 × higher than the starting dose that was also 6 × higher than the previously determined maximum tolerated dose. The frequency and severity of adverse events appeared to be more strongly influenced by the starting dose of Nexvax2 (3 μg or 30 μg) than by the maximum dose administered (300 μg or 900 μg). Dose inflexions during up-dosing were tolerated without any particular dose level being associated with an excess of adverse events. We found that the adverse event profile during up-dosing from 3 μg to 300 μg was similar in HLA-DQ2·5 homozygotes (cohort 1) and non-homozygotes (cohorts 2 and 3). HLA-DQ2·5 non-homozygotes in cohort 3 also tolerated further up-dosing from 300 μg to the maintenance dose of 900 μg, although this was not tested in HLA-DQ2·5 homozygotes due to their slower rate of recruitment. Self-reported gastrointestinal symptom scores were similar for treatment with Nexvax2 and placebo.
HLA-DQ2·5 positive volunteers with celiac disease participating in previous studies frequently experienced acute gastrointestinal symptoms after the first administration of Nexvax2 in regimens with fixed doses ranging from 60 μg to 300 μg (Goel et al., 2017). In these studies, elevated plasma levels of IL-2 (a cytokine released by activated T cells), IL-6, IL-10, and the chemokines IL-8, MCP-1, and IP-10 were observed between two and 6 h after the first dose (Goel et al., 2016). In keeping with the milder adverse event profile in the present study, no cytokine signature was observed up to 10 h post-treatment with Nexvax2 from 150 μg to 900 μg. Occasional, but inconsistent, alterations in plasma chemokines were observed in some Nexvax2-treated participants who commenced up-dosing at 30 μg, which included one participant who inadvertently consumed gluten after receiving the first 300 μg dose.
Although we have previously detected the constituent Nexvax2 peptides in plasma after intradermal administration of Nexvax2, their levels were below limits of quantitation (Goel et al., 2017). In the present study, we show that plasma concentrations of Nexvax2 peptides 45 min after intradermal injection are dose-dependent, which confirms systemic bioavailability that would facilitate engagement of cognate T cells at distant sites, including the gut, within 45 min of administration. Thus, the pharmacokinetics of Nexvax2 is consistent with other intradermally administered peptides that show dose-dependent pharmacokinetics and achieve maximal concentrations within 1 h, and dose exposure similar to subcutaneous administration (Milewski et al., 2015). Plasma concentrations of each of the three Nexvax2 peptides were similar at 45 min post-treatment. No difference was found in Nexvax2 pharmacokinetics after the first and eighth maintenance dose at 900 μg, which was associated with no change in serum Nexvax2-specific IgG and IgA levels.
Duodenal morphology was a safety measure to assess whether repeated administrations of “high” doses of Nexvax2 could mimic the deleterious effects of gluten exposure. We found that two-times weekly up-dosing over five weeks and maintenance for four weeks with Nexvax2 at the highest dose of 900 μg was associated with duodenal histology showing no overall deterioration, and with trends for villus and villus-crypt axis lengthening. However, only one placebo-treated participant was available for comparison, precluding further interpretation of changes in duodenal histology.
Although one limitation of this study was the small cohort sizes with fewer participants receiving placebo than Nexvax2, they were typical of phase 1 clinical studies, and participant demographics in these cohorts was consistent with the general population that suffers from celiac disease, which is primarily white, non-Hispanic women (Kim et al., 2016). In addition, although we have drawn comparisons between Nexvax2 fixed dosing and up-dosing regimens, we did not examine fixed dosing regimens in this study. However, because a maximum tolerated Nexvax2 dose of 150 μg had been previously established in participants similar to those in the present study, we could not justify arms in this study receiving fixed doses of 300 μg or 900 μg to establish their inferior safety profiles compared to up-dosing. Previous clinical trials of peptide-based immunotherapy have not stratified recruitment according to gene dose for the restriction element of the epitopes being administered. Because recruitment of volunteers homozygous for HLA-DQ2.5 was considerably slower than non-homozygotes, we elected to test the effects of up-dosing to the high maintenance dose of 900 μg only in HLA-DQ2.5 non-homozygotes. Notably, vomiting was the commonest adverse event after the first dose of Nexvax2 and affected 9 of 26 participants in these previous studies when they received 150 μg in a fixed dose regimen (Goel et al., 2017). In the present study, none of 26 participants experienced vomiting when up-dosing preceded their first dose at 150 μg and at 300 μg, as well as none of 10 who received doses of 450 μg, 600 μg, 750 μg, and 900 μg preceded by up-dosing. Later maintenance doses of Nexvax2 at 300 μg and 900 μg were also tolerated without excess adverse events, similar to placebo in the present study. In parallel with improved tolerability, Nexvax2 first administered at doses of 150 μg to 900 μg preceded by up-dosing was not associated with elevations in plasma IL-8, IL-2, MCP-1, IL-6, IL-10, and IP-10, which contrasted with the increases in these cytokines after the first dose at 60 μg to 300 μg in fixed dose regimens (Goel et al., 2016).
Patients with coeliac disease having no excess of adverse events and no increasing plasma cytokine levels after dosing with Nexvax2 at dose levels as high as 900 μg supports the potential use of Nexvax2 maintenance treatment to protect against the effects of dietary gluten exposure. Our recent findings in patients with celiac disease on a gluten-free diet indicate that the plasma cytokine signature associated with bolus administration of Nexvax2 is qualitatively and temporally indistinguishable from that following ingestion of gluten (Tye-Din et al., 2017). Consuming a 3 g bolus of gluten elicits about 1/8th the elevation in plasma IL-2 as intradermal administration of a single dose of Nexvax2 150 μg in HLA-DQ2.5 positive celiac disease volunteers adhering to a gluten-free diet (Tye-Din et al., 2017). In view of the unresponsiveness of participants to Nexvax2 900 μg in the present study, and that daily consumption of gluten is about 10 to 14 g in Europe and the United States (Hoppe et al., 2017, Kasarda, 2013), the optimized dose regimens determined in the present study would be attractive to further assess in clinical studies. Collectively, these results support the safety and tolerability of up-dosing and have allowed higher maintenance doses of Nexvax2 to be tested in efficacy trials.
Funding Source
ImmusanT, Inc. funded the study and was involved in the study design, data collection, data analysis, data interpretation, and the writing of this report.
Conflicts of Interests
JMA is a consultant to Abbvie, Abbott, Bayer, Celgene, Ferring, Janssen, MSD, Nestle, Hospira, Pfizer, Takeda, and Shire. RPA is a co-inventor of patents, owned or licensed, by ImmusanT, Inc. RPA has received royalties from patents licensed to ImmusanT, Inc. covering the composition of Nexvax2 and utilization of gluten-derived T cell epitopes for use in therapeutics. RPA has additional patents covering the uses of epitopes in diagnostics, food tests, and non-toxic cereals, all of which are owned or licensed by ImmusanT, Inc. KEG, JLD, LJW, AT, MPC, and RPA are employees of ImmusanT, Inc. The other authors declared no conflicts of interest.
Author Contributions
RPA and the medical writer, TES, wrote the first draft of the manuscript. AJMD, HCE, JMA, and TK, KEG, JLD, and AT collected data. MPC, AT, LJW, and RPA planned and managed the study. JAM was responsible for the statistical analysis. Data integration and analysis was performed by RPA and JAM. All authors reviewed and approved the manuscript, tables, and figures. The authors made the decision to submit the manuscript for publication and vouch for the accuracy of the data and analyses and for the fidelity of this report to the trial protocol. RPA had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
ImmusanT, Inc.
Acknowledgements
The authors thank Theresa E Singleton, PhD of Singleton Science, LLC (Beverly, Massachusetts, USA) for providing medical writing support, which was funded by ImmusanT Inc. (Cambridge, Massachusetts, USA) in accordance with Good Publication Practice (GPP3) guidelines.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.11.018.
Appendix A. Supplementary data
Supplementary material
References
- Alhadj A.M., Liu Y.F., Arif S., Tatovic D., Shariff H., Gibson V.B., Yusuf N., Baptista R., Eichmann M., Petrov N., Heck S., Yang J.H.M., Tree T.I.M., Pujol-Autonell I., Yeo L., Baumard L.R., Stenson R., Howell A., Clark A., Boult Z., Powrie J., Adams L., Wong F.S., Luzio S., Dunseath G., Green K., O'Keefe A., Bayly G., Thorogood N., Andrews R., Leech N., Joseph F., Nair S., Seal S., Cheung H., Beam C., Hills R., Peakman M., Dayan C.M. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaf7779. (pii, eaaf7779) [DOI] [PubMed] [Google Scholar]
- Anderson R.P., Jabri B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr. Opin. Immunol. 2013;25:410–417. doi: 10.1016/j.coi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.J., Daveson J., Marjason J.K., Ffrench R.A., Smith D., Sullivan M., Tye-Din J.A., Anderson R.P. A phase I study to determine safety, tolerability and bioactivity of Nexvax2 in HLA DQ2 + volunteers with celiac disease following a long-term, strict gluten-free diet. Gastroenterology. 2011;140:S437–8. [Google Scholar]
- Burks A.W., Calderon M.A., Casale T., Cox L., Demoly P., Jutel M., Nelson H., Akdis C.A. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J. Allergy Clin. Immunol. 2013;131 doi: 10.1016/j.jaci.2013.01.049. (1288-96.e3) [DOI] [PubMed] [Google Scholar]
- Burton B.R., Britton G.J., Fang H., Verhagen J., Smithers B., Sabatos-Peyton C.A., Carney L.J., Gough J., Strobel S., Wraith D.C. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat. Commun. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L., Ferran C., Legendre C., Thouard I., Merite S., Reuter A., Gevaert Y., Kreis H., Franchimont P., Bach J.F. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation. 1990;49:697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Goel G., Mayassi T., Qiao S.-W., Ciszewski C., King T., Daveson A.J., Andrews J.M., Krishnarajah J., Krause R., Brown G.J., Fogel R., Barish C.F., Epstein R., Kinney T., Miner P.B., Tye-Din J.A., Girardin A., Goldstein K., Dzuris J.L., Williams L.J., Xavier R., Sollid L.M., Jabri B., Anderson R.P. A single intradermal (ID) injection of Nexvax2, a peptide composition with dominant epitopes for gluten-reactive CD4 + T cells, activates T cells and tiggers acute gastrointestinal symptoms in HLA-DQ2.5 + people with celiac disease (CeD) Gastroenterology. 2016;150:S304. [Google Scholar]
- Goel G., King T., Daveson A.J., Andrews J.M., Krishnarajah J., Krause R., Brown G.J.E., Fogel R., Barish C.F., Epstein R., Kinney T.P., Miner P.B., Jr., Tye-Din J.A., Girardin A., Taavela J., Popp A., Sidney J., Mäki M., Goldstein K.E., Griffin P.H., Wang S., Dzuris J.L., Williams L.J., Sette A., Xavier R.J., Sollid L.M., Jabri B., Anderson R.P. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol. Hepatol. 2017;2:479–493. doi: 10.1016/S2468-1253(17)30110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselden B.M., Kay A.B., Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J. Exp. Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe C., Gobel R., Kristensen M., Lind M.V., Matthiessen J., Christensen T., Trolle E., Fagt S., Madsen M.L., Husby S. Intake and sources of gluten in 20- to 75-year-old Danish adults: a national dietary survey. Eur. J. Nutr. 2017;56:107–117. doi: 10.1007/s00394-015-1062-3. [DOI] [PubMed] [Google Scholar]
- Karell K., Louka A.S., Moodie S.J., Ascher H., Clot F., Greco L., Ciclitira P.J., Sollid L.M., Partanen J., European Genetics Cluster on Celiac Disease HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum. Immunol. 2003;64:469–477. doi: 10.1016/s0198-8859(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Kasarda D.D. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J. Agric. Food Chem. 2013;61:1155–1159. doi: 10.1021/jf305122s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Patel K.G., Orosz E., Kothari N., Demyen M.F., Pyrsopoulos N., Ahlawat S.K. Time trends in the prevalence of celiac disease and gluten-free diet in the US population: results from the National Health and Nutrition Examination Surveys 2009-2014. JAMA Intern. Med. 2016;176:1716–1717. doi: 10.1001/jamainternmed.2016.5254. [DOI] [PubMed] [Google Scholar]
- Kurada S., Yadav A., Leffler D.A. Current and novel therapeutic strategies in celiac disease. Expert. Rev. Clin. Pharmacol. 2016;9:1211–1223. doi: 10.1080/17512433.2016.1200463. [DOI] [PubMed] [Google Scholar]
- Larche M., Wraith D.C. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- Leffler D.A., Dennis M., Edwards George J.B., Jamma S., Magge S., Cook E.F., Schuppan D., Kelly C.P. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009;7:530–536. doi: 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J.F., Bai J.C., Biagi F., Card T.R., Ciacci C., Ciclitira P.J., Green P.H., Hadjivassiliou M., Holdoway A., van Heel D.A., Kaukinen K., Leffler D.A., Leonard J.N., Lundin K.E., McGough N., Davidson M., Murray J.A., Swift G.L., Walker M.M., Zingone F., Sanders D.S., BSG Coeliac Disease Guidelines Development Group, British Society of Gastroenterology Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–1228. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson R.C., Konkel J.E., Prendergast C.T., Thomson J.P., Ottaviano R., Leech M.D., Kay O., Zandee S.E., Sweenie C.H., Wraith D.C., Meehan R.R., Drake A.J., Anderton S.M. Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4(+) T cells tolerized by peptide immunotherapy. elife. 2014;29:3. doi: 10.7554/eLife.03416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar P.B. Immunological tolerance. Science. 1961;133:303–306. doi: 10.1126/science.133.3449.303. [DOI] [PubMed] [Google Scholar]
- Milewski M., Manser K., Nissley B.P., Mitra A. Analysis of the absorption kinetics of macromolecules following intradermal and subcutaneous administration. Eur. J. Pharm. Biopharm. 2015;89:134–144. doi: 10.1016/j.ejpb.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Murray J.A., Moore S.B., Van Dyke C.T., Lahr B.D., Dierkhising R.A., Zinsmeister A.R., Melton L.J., 3rd, Kroning C.M., El-Yousseff M., Czaja A.J. HLA DQ gene dosage and risk and severity of celiac disease. Clin. Gastroenterol. Hepatol. 2007;5(12):1406. doi: 10.1016/j.cgh.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape K.A., Merica R., Mondino A., Khoruts A., Jenkins M.K. Direct evidence that functionally impaired CD4 + T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- Ráki M., Fallang L.E., Brottveit M., Bergseng E., Quarsten H., Lundin K.E., Sollid L.M. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2831–2836. doi: 10.1073/pnas.0608610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatos-Peyton C.A., Verhagen J., Wraith D.C. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr. Opin. Immunol. 2010;22:609–615. doi: 10.1016/j.coi.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See J.A., Kaukinen K., Makharia G.K., Gibson P.R., Murray J.A. Practical insights into gluten-free diets. Nat. Rev. Gastroenterol. Hepatol. 2015;12:580–591. doi: 10.1038/nrgastro.2015.156. [DOI] [PubMed] [Google Scholar]
- Sollid L.M., Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat. Rev. Immunol. 2013;13:294–302. doi: 10.1038/nri3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter H.B., Rigden R., Martin K.F., Scolding N.J., Wraith D.C. Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS. Neurol. Neuroimmunol. Neuroinflammation. 2015;2 doi: 10.1212/NXI.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedlund J., Sjodin I., Dotevall G. GSRS – a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- Tye-Din J.A., Stewart J.A., Dromey J.A., Beissbarth T., van Heel D.A., Tatham A., Henderson K., Mannering S.I., Gianfrani C., Jewell D.P., Hill A.V., McCluskey J., Rossjohn J., Anderson R.P. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001012. (41ra51) [DOI] [PubMed] [Google Scholar]
- Tye-Din J.A., Dzuris J.L., Russell A.K., Wang S., Goldstein K.E., Williams L.J., Anderson R.P. Gluten ingestion and intradermal injection of peptides that activate gluten-specific CD4 + T cells elicit a cytokine signature dominated by interleukin-2 in celiac disease. United European Gastroenterol. J. 2017;5:A26–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material